Macroporous Mannitol Granules Produced by Spray Drying and Sacrificial Templating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Aqueous Suspension of PS Beads
2.3. Spray Drying of Mannitol/PS Granules
2.4. Etching of the PS Beads to Form Porous Mannitol Granules
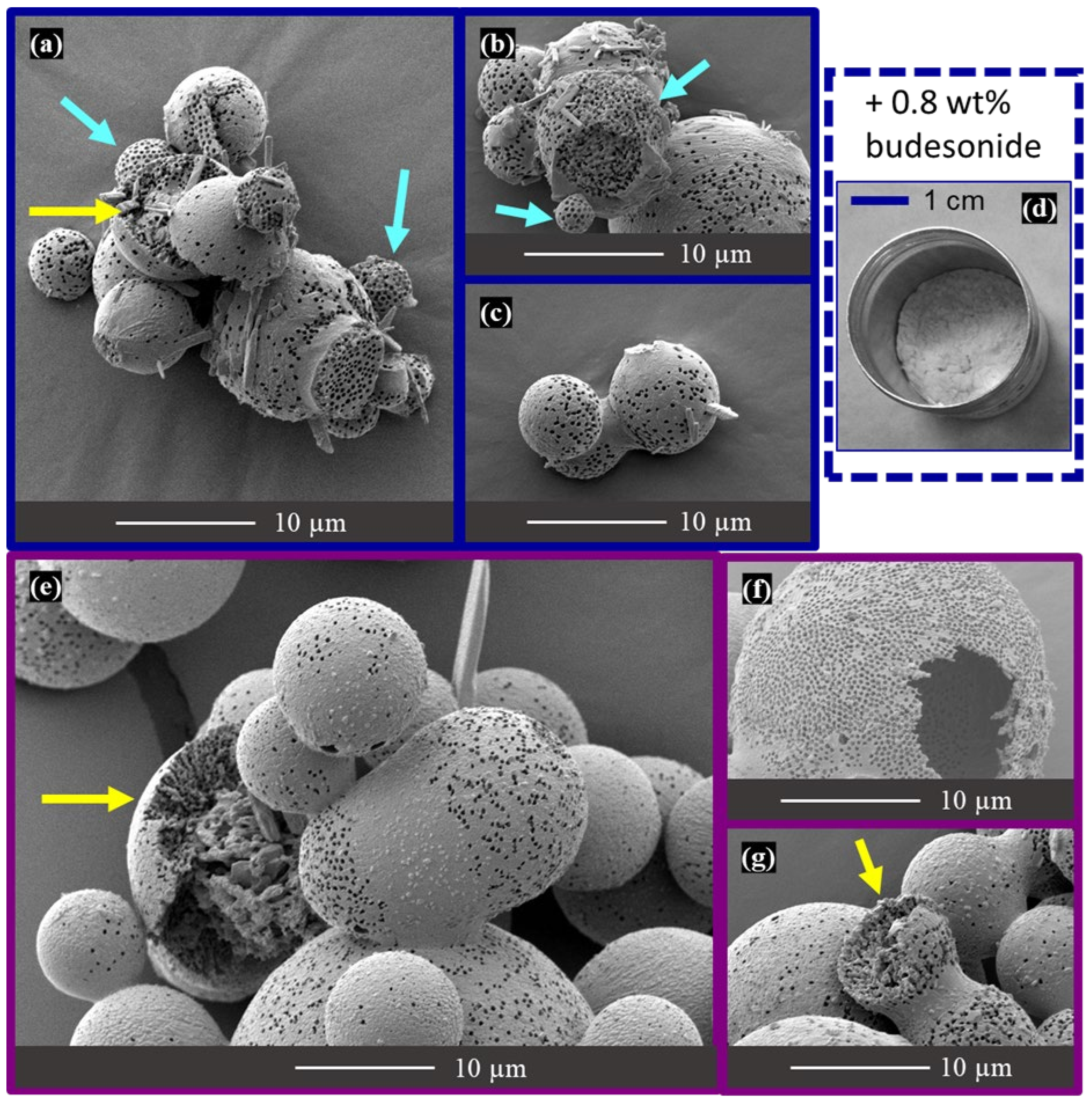
2.5. Mannitol-Budesonide Formulations
2.6. Characterization Techniques
3. Results and Discussion
3.1. Preparation of the Aqueous Suspension of PS Beads
3.2. Preparation of Porous Mannitol Granules by Spray Drying and PS Etching
3.3. Influence of the Mannitol/PS Ratio on the Etching Efficiency and on the Mannitol Polymorphism
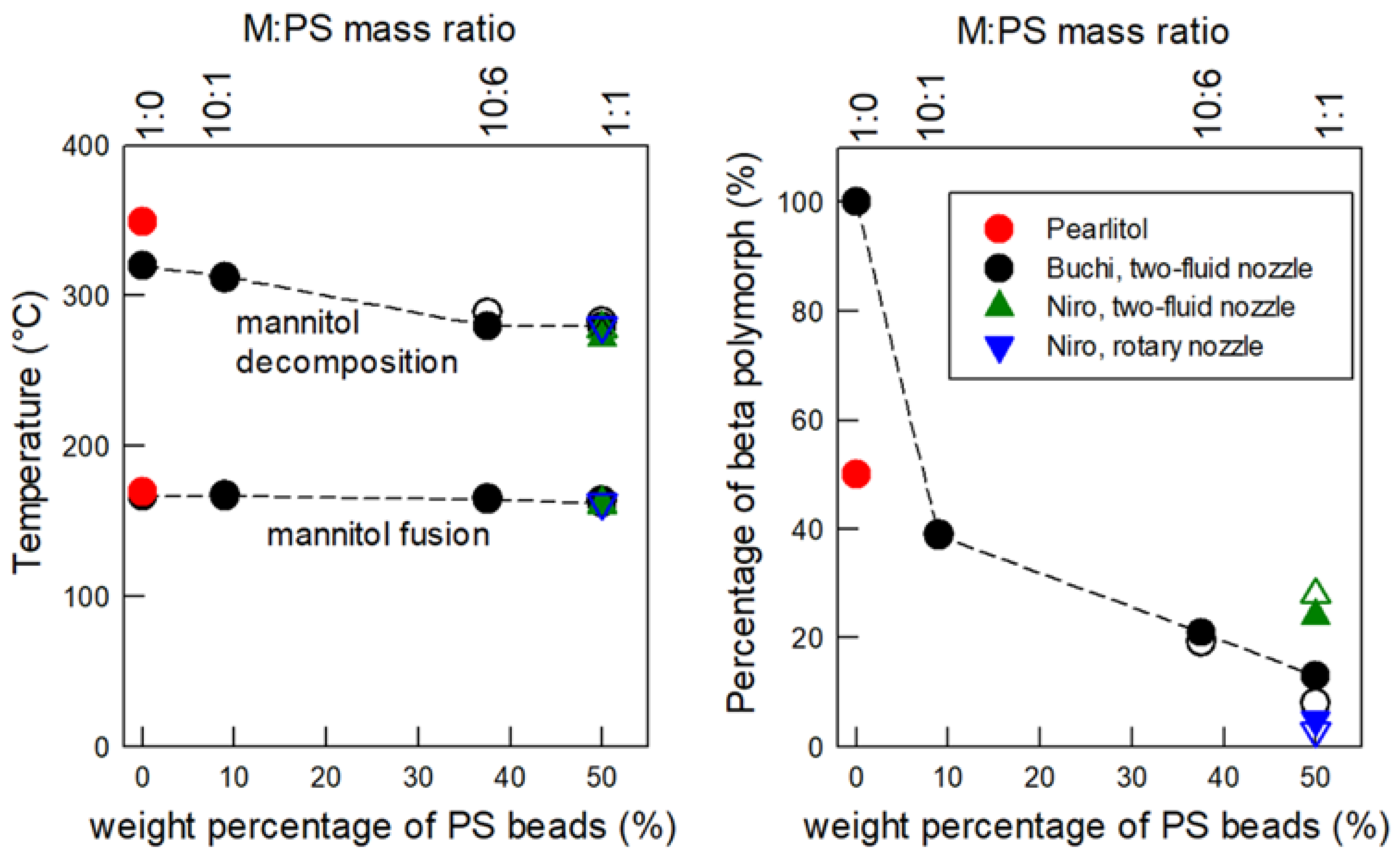
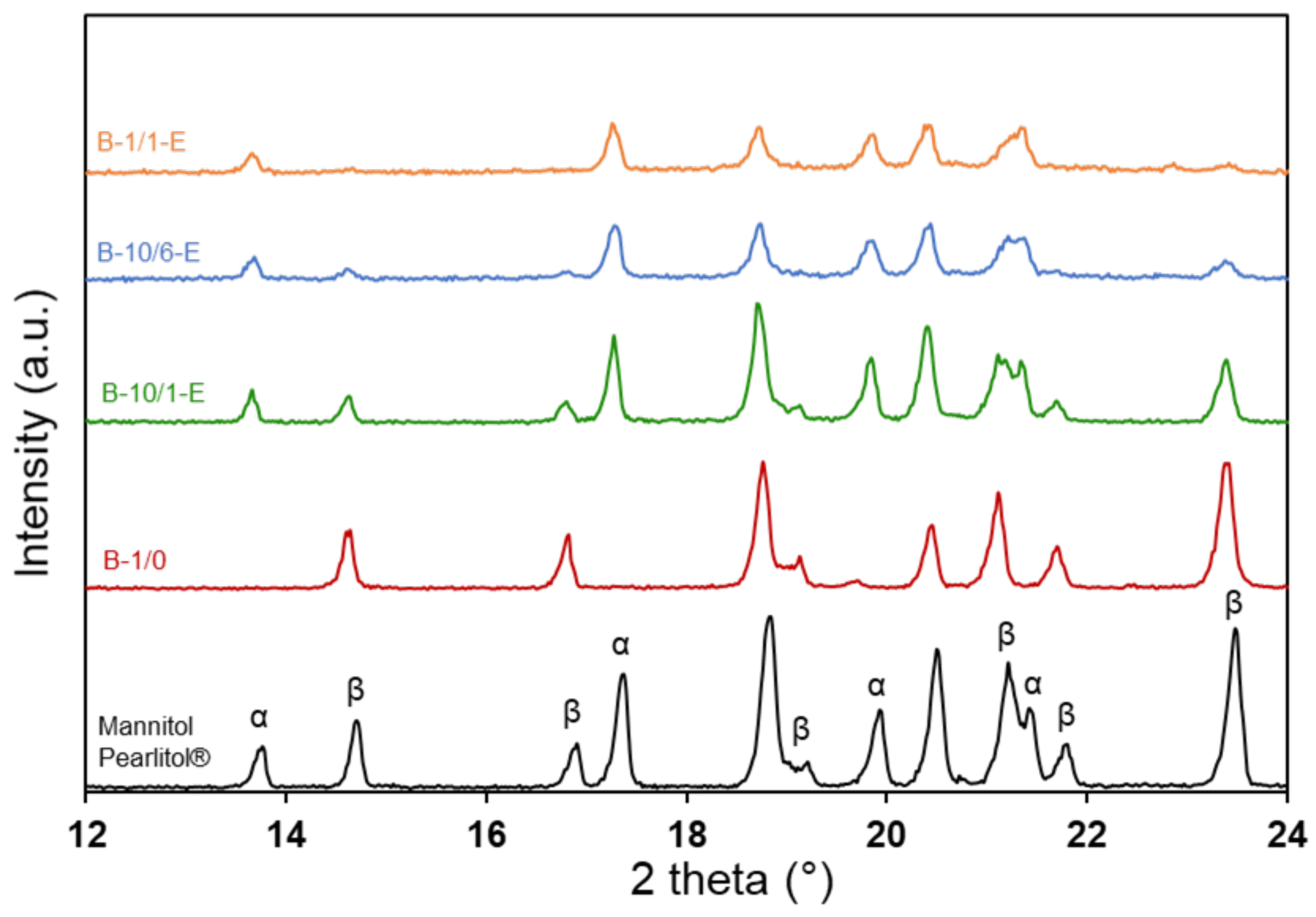
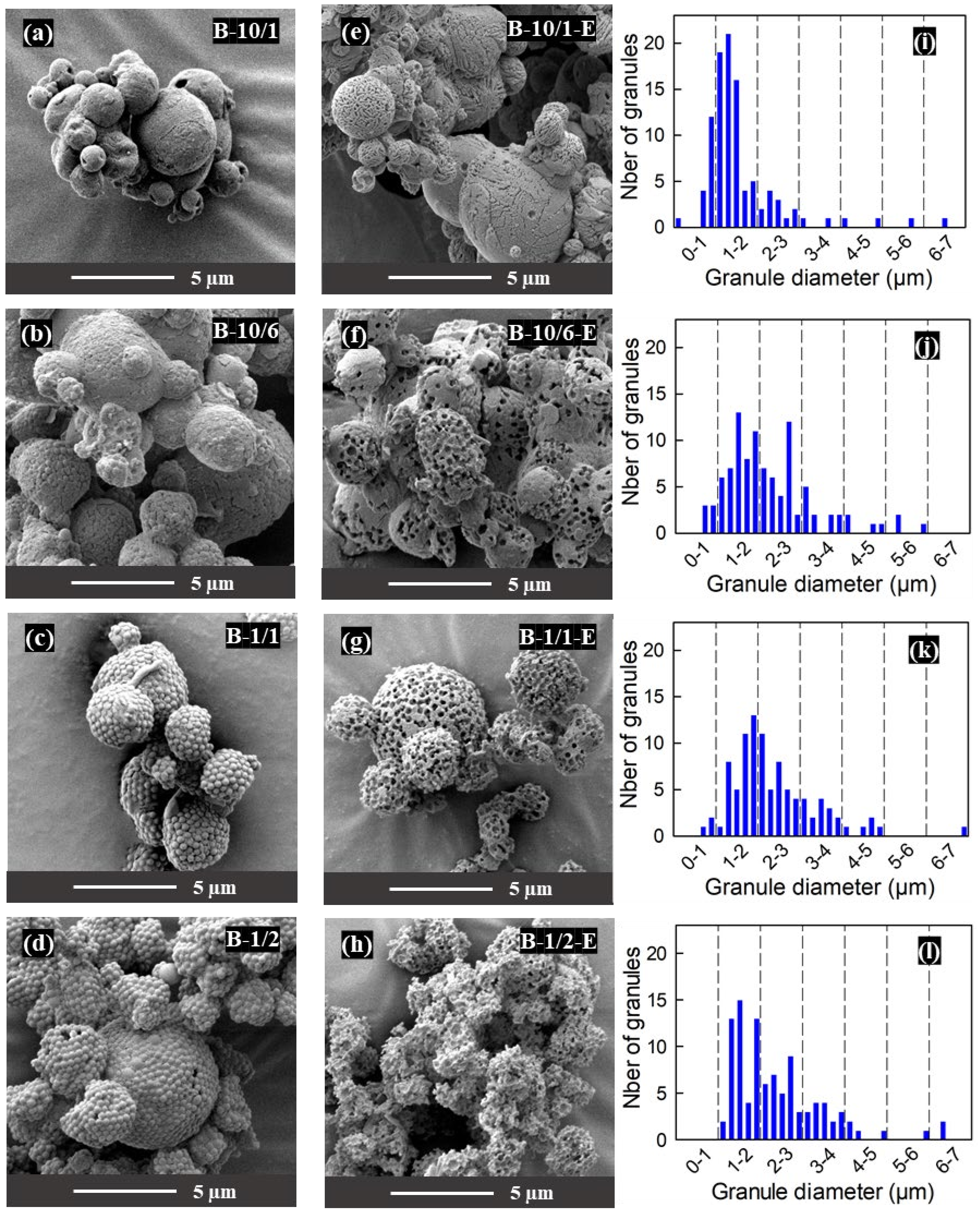
3.4. Influence of the Mannitol/PS Ratio on the Granule Microstructure
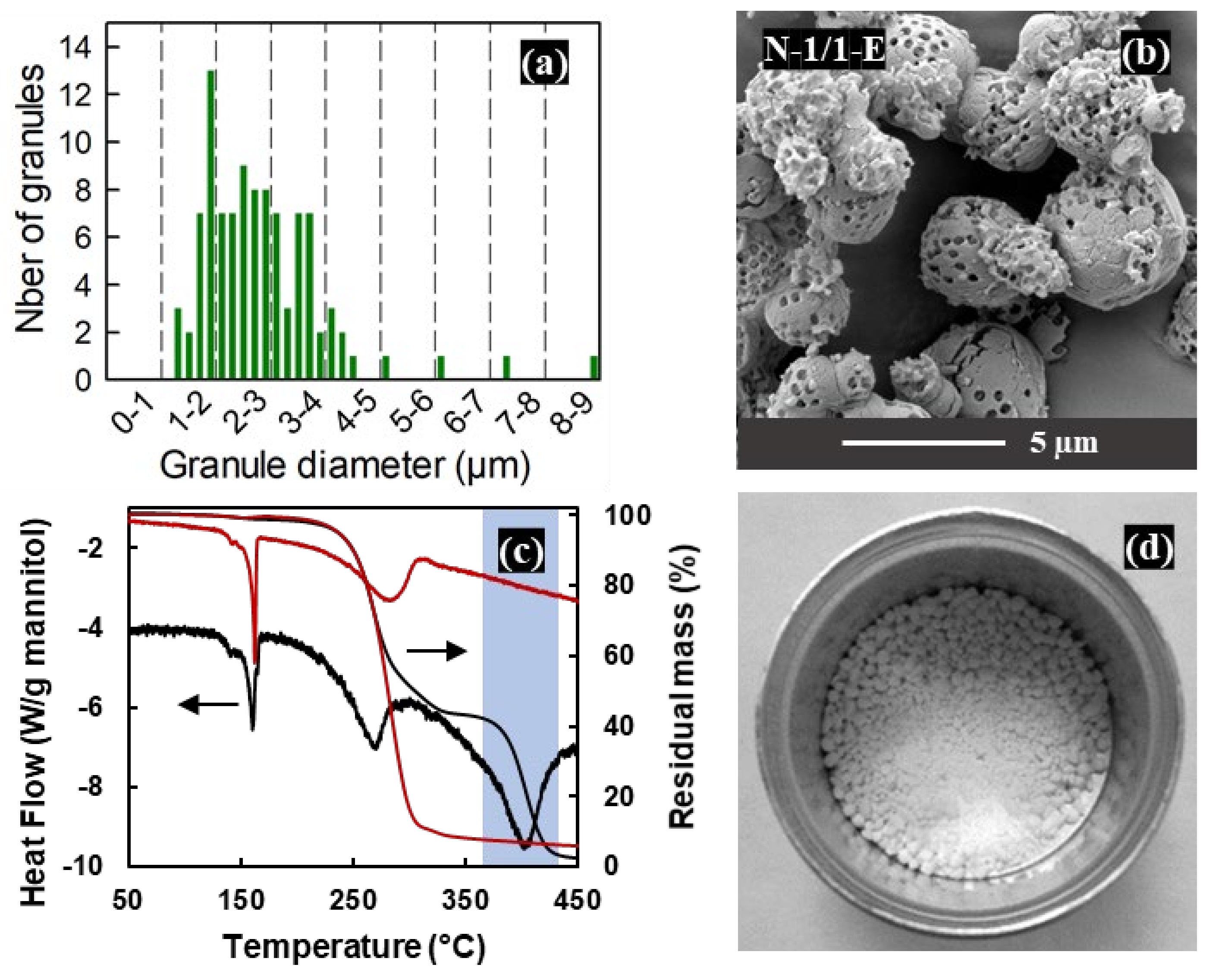
3.5. Upscaled Production for the 1:1 Mannitol/PS Ratio
3.6. Larger Porous Granules Prepared with Rotary Nozzle Atomisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, M.; Shen, L.; Lin, X.; Hong, Y.; Feng, Y. Design and Pharmaceutical Applications of Porous Particles. RSC Adv. 2017, 7, 39490–39501. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, G.; Pathak, K. Porous Carriers for Controlled/Modulated Drug Delivery. Indian J. Pharm. Sci. 2009, 71, 599–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thananukul, K.; Kaewsaneha, C.; Opaprakasit, P.; Lebaz, N.; Errachid, A.; Elaissari, A. Smart Gating Porous Particles as New Carriers for Drug Delivery. Adv. Drug Deliv. Rev. 2021, 174, 425–446. [Google Scholar] [CrossRef] [PubMed]
- Saffari, M.; Ebrahimi, A.; Langrish, T. A Novel Formulation for Solubility and Content Uniformity Enhancement of Poorly Water-Soluble Drugs Using Highly-Porous Mannitol. Eur. J. Pharm. Sci. 2016, 83, 52–61. [Google Scholar] [CrossRef]
- Duddu, S.P.; Sisk, S.A.; Walter, Y.H.; Tarara, T.E.; Trimble, K.R.; Clark, A.R.; Eldon, M.A.; Elton, R.C.; Pickford, M.; Hirst, P.H.; et al. Improved Lung Delivery from a Passive Dry Powder Inhaler Using an Engineered PulmoSphere? Powder. Pharm. Res. 2002, 19, 689–695. [Google Scholar] [CrossRef]
- Geller, D.E.; Weers, J.; Heuerding, S. Development of an Inhaled Dry-Powder Formulation of Tobramycin Using PulmosphereTM Technology. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 175–182. [Google Scholar] [CrossRef]
- Dellamary, L.A.; Tarara, T.E.; Smith, D.J.; Woelk, C.H.; Adractas, A.; Costello, M.L.; Gill, H.; Weers, J.G. Hollow Porous Particles in Metered Dose Inhalers. Pharm. Res. 2000, 17, 168–174. [Google Scholar] [CrossRef]
- Xu, S.; Shi, Y.; Wen, Z.; Liu, X.; Zhu, Y.; Liu, G.; Gao, H. Applied Catalysis B: Environmental Polystyrene Spheres-Templated Mesoporous Carbonous Frameworks Implanted with Cobalt Nanoparticles for Highly Efficient Electrochemical Nitrate Reduction to Ammonia. Appl. Catal. B Environ. 2023, 323, 122192. [Google Scholar] [CrossRef]
- Liu, X.; Verma, G.; Chen, Z.; Hu, B.; Huang, Q.; Yang, H.; Ma, S.; Wang, X.; Liu, X.; Verma, G.; et al. Metal-Organic Framework Nanocrystal-Derived Hollow Porous Materials: Synthetic Strategies and Emerging Applications Metal-Organic Framework Nanocrystal-Derived Hollow Porous Materials: Synthetic Strategies and Emerging Applications. Innovation 2022, 3, 100281. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Z.; Vargun, E.; Li, Y.; Saha, P.; Cheng, Q. Atomic Fe on Hierarchically Ordered Porous Carbon towards High- Performance Lithium-Sulfur Batteries. J. Electroanal. Chem. 2023, 928, 117046. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, M.; Huang, Y.; Ma, Y.; Yan, L.; Zhou, X.; Ma, X.; Zhao, X.; Chen, C.; Bai, J.; et al. Enhanced Ethanol Oxidation over Pd Nanoparticles Supported Porous Graphene-Doped MXene Using Polystyrene Particles as Sacrificial Templates. Rare Met. 2022, 41, 3170–3179. [Google Scholar] [CrossRef]
- Shakeel, A.; Rizwan, K.; Farooq, U.; Iqbal, S.; Ali, A. Chemosphere Advanced Polymeric/Inorganic Nanohybrids: An Integrated Platform for Gas Sensing Applications. Chemosphere 2022, 294, 133772. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Gucci, F.; Cairns, G.; Chianella, I.; Leighton, G.J.T. Hollow Silica Nano and Micro Spheres with Polystyrene Templating: A Mini-Review. Materials 2022, 15, 8578. [Google Scholar] [CrossRef] [PubMed]
- Daem, N.; Mayer, A.; Spronck, G.; Colson, P.; Loicq, J.; Henrist, C.; Cloots, R.; Maho, A.; Dewalque, J. Inverse Opal Photonic Nanostructures for Enhanced Light Harvesting in CH 3 NH 3 PbI 3 Perovskite Solar Cells. Appl. Nano Mater. 2022, 5, 13583–13593. [Google Scholar] [CrossRef]
- Lv, K.; Zhang, J.; Zhao, X.; Kong, N.; Tao, J.; Zhou, J. Understanding the Effect of Pore Size on Electrochemical Capacitive Performance of MXene Foams. Small 2022, 18, 1–11. [Google Scholar] [CrossRef]
- Alhajj, N.; O’Reilly, N.J.; Cathcart, H. Designing Enhanced Spray Dried Particles for Inhalation: A Review of the Impact of Excipients and Processing Parameters on Particle Properties. Powder Technol. 2021, 384, 313–331. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical Particle Engineering via Spray Drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef] [Green Version]
- Nandiyanto, A.B.D.; Okuyama, K. Progress in Developing Spray-Drying Methods for the Production of Controlled Morphology Particles: From the Nanometer to Submicrometer Size Ranges. Adv. Powder Technol. 2011, 22, 1–19. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Ogi, T.; Wang, W.N.; Gradon, L.; Okuyama, K. Template-Assisted Spray-Drying Method for the Fabrication of Porous Particles with Tunable Structures. Adv. Powder Technol. 2019, 30, 2908–2924. [Google Scholar] [CrossRef]
- Gervelas, C.; Serandour, A.L.; Geiger, S.; Grillon, G.; Fritsch, P.; Taulelle, C.; Le Gall, B.; Benech, H.; Deverre, J.R.; Fattal, E.; et al. Direct Lung Delivery of a Dry Powder Formulation of DTPA with Improved Aerosolization Properties: Effect on Lung and Systemic Decorporation of Plutonium. J. Control Release 2007, 118, 78–86. [Google Scholar] [CrossRef]
- Straub, J.A.; Chickering, D.E.; Lovely, J.C.; Zhang, H.; Shah, B.; Waud, W.R.; Bernstein, H. Intravenous Hydrophobic Drug Delivery: A Porous Particle Formulation of Paclitaxel (AI-850). Pharm. Res. 2005, 22, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Kaplin, I.Y.; Lokteva, E.S.; Golubina, E.V.; Lunin, V.V. Template Synthesis of Porous Ceria-Based Catalysts for Environmental Application. Molecules 2020, 25, 4242. [Google Scholar] [CrossRef] [PubMed]
- Shchukin, D.G.; Caruso, R.A. Template Synthesis and Photocatalytic Properties of Porous Metal Oxide Spheres Formed by Nanoparticle Infiltration. Chem. Mater. 2004, 16, 2287–2292. [Google Scholar] [CrossRef]
- Zhang, H.; Hardy, G.C.; Khimyak, Y.Z.; Rosseinsky, M.J.; Cooper, A.I. Synthesis of Hierarchically Porous Silica and Metal Oxide Beads Using Emulsion-Templated Polymer Scaffolds. Chem. Mater. 2004, 16, 4245–4256. [Google Scholar] [CrossRef]
- Gradoń, L.; Janeczko, S.; Abdullah, M.; Iskandar, F.; Okuyama, K. Self-Organization Kinetics of Mesoporous Nanostructured Particles. AIChE J. 2004, 50, 2583–2593. [Google Scholar] [CrossRef]
- Iskandar, F.; Mikrajuddin; Okuyama, K. In Situ Production of Spherical Silica Particles Containing Self-Organized Mesopores. Nano Lett. 2001, 1, 231–234. [Google Scholar] [CrossRef]
- Balgis, R.; Ogi, T.; Arif, A.F.; Anilkumar, G.M. Morphology Control of Hierarchical Porous Carbon Particles from Phenolic Resin and Polystyrene Latex Template via Aerosol Process. Carbon N. Y. 2014, 84, 281–289. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Hagura, N.; Iskandar, F.; Okuyama, K. Design of a Highly Ordered and Uniform Porous Structure with Multisized Pores in Film and Particle Forms Using a Template-Driven Self-Assembly Technique. Acta Mater. 2010, 58, 282–289. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Okuyama, K. Influences of Size and Amount of Colloidal Template and Droplet Diameter on the Formation of Porous-Structured Hyaluronic Acid Particles. Indones. J. Sci. Technol. 2017, 2, 152–165. [Google Scholar] [CrossRef] [Green Version]
- Iskandar, F.; Nandiyanto, A.B.D.; Widiyastuti, W.; Young, L.S.; Okuyama, K.; Gradon, L. Production of Morphology-Controllable Porous Hyaluronic Acid Particles Using a Spray-Drying Method. Acta Biomater. 2009, 5, 1027–1034. [Google Scholar] [CrossRef]
- Rahimpour, Y.; Kouhsoltani, M.; Hamishehkar, H. Alternative Carriers in Dry Powder Inhaler Formulations. Drug Discov. Today 2014, 19, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.G.; Schaldach, G.; Littringer, E.M.; Mescher, A.; Griesser, U.J.; Braun, D.E.; Walzel, P.E.; Urbanetz, N.A. The Impact of Spray Drying Outlet Temperature on the Particle Morphology of Mannitol. Powder Technol. 2011, 213, 27–35. [Google Scholar] [CrossRef]
- De Boeck, K.; Haarman, E.; Hull, J.; Lands, L.C.; Moeller, A.; Munck, A.; Riethmüller, J. Inhaled Dry Powder Mannitol in Children with Cystic Fi Brosis: A Randomised Ef Fi Cacy and Safety Trial. J. Cyst. Fibros. 2017, 16, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Mönckedieck, M.; Kamplade, J.; Fakner, P.; Urbanetz, N.A.; Walzel, P.; Steckel, H.; Scherließ, R. Dry Powder Inhaler Performance of Spray Dried Mannitol with Tailored Surface Morphologies as Carrier and Salbutamol Sulphate. Int. J. Pharm. 2017, 524, 351–363. [Google Scholar] [CrossRef]
- Littringer, E.M.; Mescher, A.; Schroettner, H.; Achelis, L.; Walzel, P.; Urbanetz, N.A. Spray Dried Mannitol Carrier Particles with Tailored Surface Properties—The Influence of Carrier Surface Roughness and Shape. Eur. J. Pharm. Biopharm. 2012, 82, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.D.; Price, R. The Influence of Fine Excipient Particles on the Performance of Carrier-Based Dry Powder Inhalation Formulations. Pharm. Res. 2006, 23, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.Y.T.; Qiu, Y.; Lo, F.F.K.; Lin, H.H.S.; Chan, H.K.; Kwok, P.C.L.; Lam, J.K.W. Inhaled Powder Formulation of Naked SiRNA Using Spray Drying Technology with L-Leucine as Dispersion Enhancer. Int. J. Pharm. 2017, 530, 40–52. [Google Scholar] [CrossRef]
- Arzi, R.S.; Sosnik, A. Electrohydrodynamic Atomization and Spray-Drying for the Production of Pure Drug Nanocrystals and Co-Crystals ☆. Adv. Drug Deliv. Rev. 2018, 131, 79–100. [Google Scholar] [CrossRef]
- Walsh, D.; Serrano, D.R.; Marie, A.; Norris, B.A. Production of Cocrystals in an Excipient Matrix by Spray Drying. Int. J. Pharm. 2018, 536, 467–477. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Rowe, R.C., Sheskey, P.J., Quinn, M.E., Eds.; The Pharmaceutical Press: London, UK; The American Pharmacists Association: Washington, DC, USA, 2009. [Google Scholar]
- Grodowska, K.; Parczewski, A. Organic Solvents in the Pharmaceutical Industry. Acta Pol. Pharm. Drug Res. 2010, 67, 3–12. [Google Scholar]
- Dimian, A.C.; Bildea, C.S.; Kiss, A.A. Chemical Product Design; Elsevier Science: Amsterdam, The Netherlands, 2014; Volume 35, ISBN 9780444627001. [Google Scholar]
- Food and Drug Administration, Center for Drug Evaluation and Research. Q3C—Tables and List Guidance for Industry Q3C—Tables and List Guidance for Industry. Available online: https://www.fda.gov/media/71737/download (accessed on 15 December 2022).
- Food and Drug Administration, Center for Drug Evaluation and Research. Appendix 6. Toxicological Data For Class 3 Solvents. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q3c-appendix-6 (accessed on 15 December 2022).
- Hartwig, A.M.C. Ethyl Acetate Ethyl Acetate. MAK Collect. Occup. Heal. Saf. 2019, 4, 2027–2044. [Google Scholar] [CrossRef]
- Yohanala, P.T.F.; Mulya Dewa, R.; Quarta, K.; Widiyastuti, W.; Winardi, S. Preparation of Polystyrene Spheres Using Surfactant-Free Emulsion Polymerization. Mod. Appl. Sci. 2015, 9, 121. [Google Scholar] [CrossRef]
- Cai, Z.; Teng, J.; Yan, Q.; Zhao, X.S. Solvent Effect on the Self-Assembly of Colloidal Microspheres via a Horizontal Deposition Method. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 402, 37–44. [Google Scholar] [CrossRef]
- Cheary, B.Y.R.W.; Coelho, A. A Fundamental Parameters Approach to X-Ray Line-Profile Fitting. J. Appl. Crystallogr. 1992, 25, 109–121. [Google Scholar] [CrossRef]
- Fang, J.; Xuan, Y.; Li, Q. Preparation of Polystyrene Spheres in Different Particle Sizes and Assembly of the PS Colloidal Crystals. Sci. China Technol. Sci. 2010, 53, 3088–3093. [Google Scholar] [CrossRef]
- Handscomb, C.S.; Kraft, M.; Bayly, A.E. A New Model for the Drying of Droplets Containing Suspended Solids after Shell Formation. Chem. Eng. Sci. 2009, 64, 228–246. [Google Scholar] [CrossRef]
- Zafiryadis, F.L. Numerical Modeling of Droplet Trajectories in Pilot Plant Spray Dryer Fitted with Rotary Atomizer. Masters’ Thesis, Technical University of Denmark (Department of Mechanical Engineering), Lyngby, Denmark, 2019. Available online: http://s3-eu-west-1.amazonaws.com/foreninglet-wordpress-offload-s3/wp-content/uploads/sites/53/2018/01/07112723/FrederikZafiryadis_Spray_Dryer.pdf (accessed on 5 October 2022).




| Label | M/PS Ratio (w/w) | Spray Dryer | (Mannitol) (g/L) | (PS Beads) (g/L) | Expected PS Content in Spray-Dried Granules | TGA-Measured PS Content in Spray-Dried Granules | Tmelting for Mannitol |
|---|---|---|---|---|---|---|---|
| B-1/0 | 1/0 | Büchi, Two-fluid nozzle | 36 | 0 | 0 wt% | Not applicable | 165 |
| B-10/1 | 10/1 | Büchi, Two-fluid nozzle | 33.6 | 3.36 | 9 wt% | 7.9 wt% | 165 |
| B-10/6 | 10/6 | Büchi, Two-fluid nozzle | 5.60 | 3.36 | 37.5 wt% | 34.9 wt% | 162 |
| B-1/1 | 1/1 | Büchi, Two-fluid nozzle | 3.36 | 3.36 | 50 wt% | 48.7 wt% | 160 |
| B-1/2 | 1/2 | Büchi, Two-fluid nozzle | 1.18 | 3.36 | 66.7 wt% | - | - |
| N-1/1-TF | 1/1 | Niro, Two-fluid nozzle | 20.18 | 20.18 | 50 wt% | 43.0 wt% | 161 |
| N-1/1-R | 1/1 | Niro, Rotary nozzle | 20.18 | 20.18 | 50 wt% | - | 162 |
| Spray-Drying Parameters | Büchi (Lab-Scale) | Niro (Pilot-Scale) | |
|---|---|---|---|
| Two-Fluid Nozzle | Two-Fluid Nozzle | Rotary Nozzle | |
| Inlet temperature (°C) | 120 | 120 | 120 |
| Outlet temperature (°C) | 43 | 72 | 75 |
| Atomization gas flow rate (m3/h) | 0.6 | 6.7 | 6.7 |
| Drying gas flow rate (m3/h) | 29.4 | 67 | 67 |
| Liquid flow rate (mL/min) | 5 | 25 | 25 |
| Injected volume (mL) | 25 | 250 | 250 |
| Typical experimental yield | 25% | 50% | 20% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentin, M.; Coibion, D.; Vertruyen, B.; Malherbe, C.; Cloots, R.; Boschini, F. Macroporous Mannitol Granules Produced by Spray Drying and Sacrificial Templating. Materials 2023, 16, 25. https://doi.org/10.3390/ma16010025
Valentin M, Coibion D, Vertruyen B, Malherbe C, Cloots R, Boschini F. Macroporous Mannitol Granules Produced by Spray Drying and Sacrificial Templating. Materials. 2023; 16(1):25. https://doi.org/10.3390/ma16010025
Chicago/Turabian StyleValentin, Morgane, Damien Coibion, Bénédicte Vertruyen, Cédric Malherbe, Rudi Cloots, and Frédéric Boschini. 2023. "Macroporous Mannitol Granules Produced by Spray Drying and Sacrificial Templating" Materials 16, no. 1: 25. https://doi.org/10.3390/ma16010025






