Reaction Behavior and Formation Mechanism of ZrB2 and ZrC from the Ni-Zr-B4C System during Self-Propagating High-Temperature Synthesis
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Reaction Behavior of the Ni-Zr-B4C System
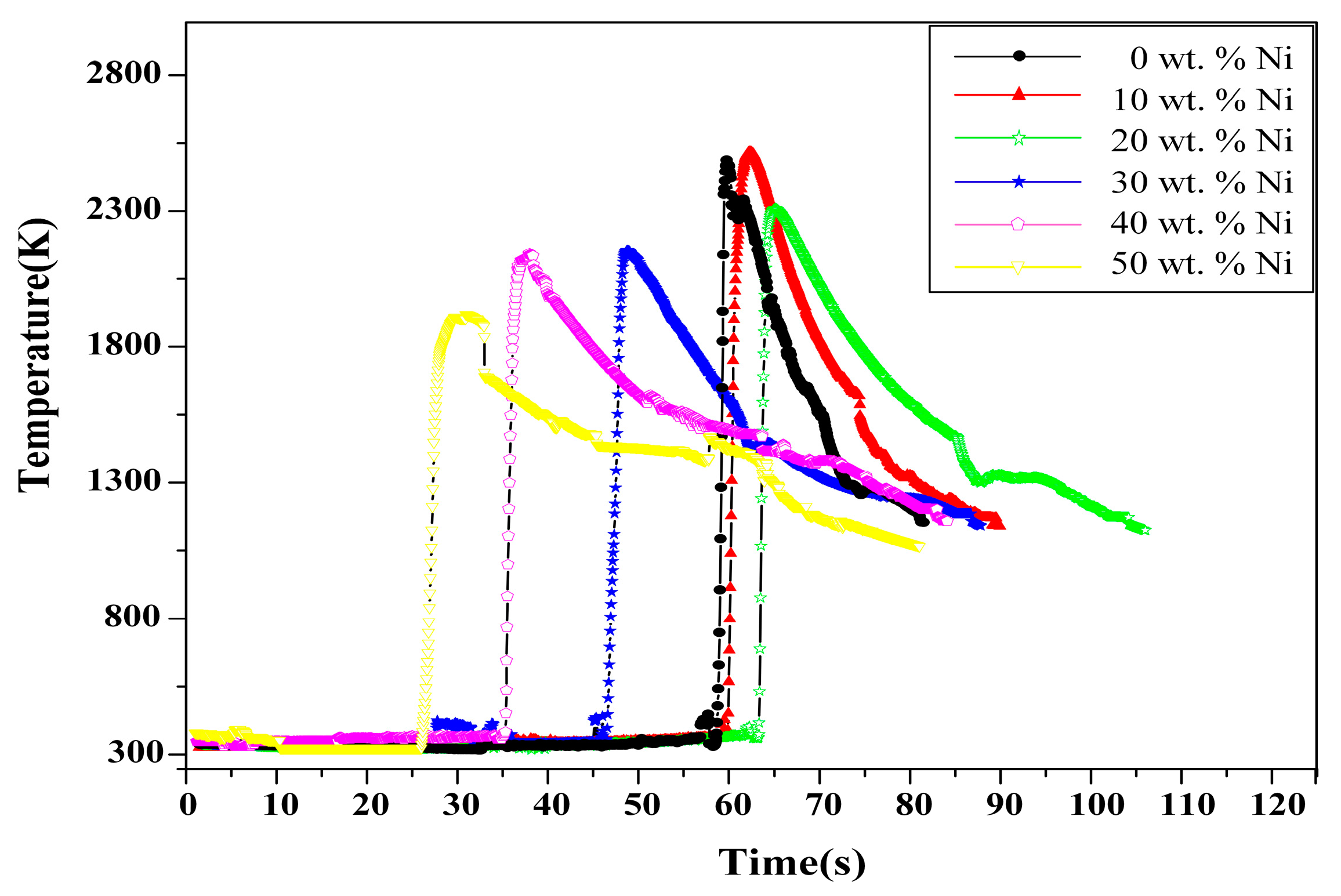
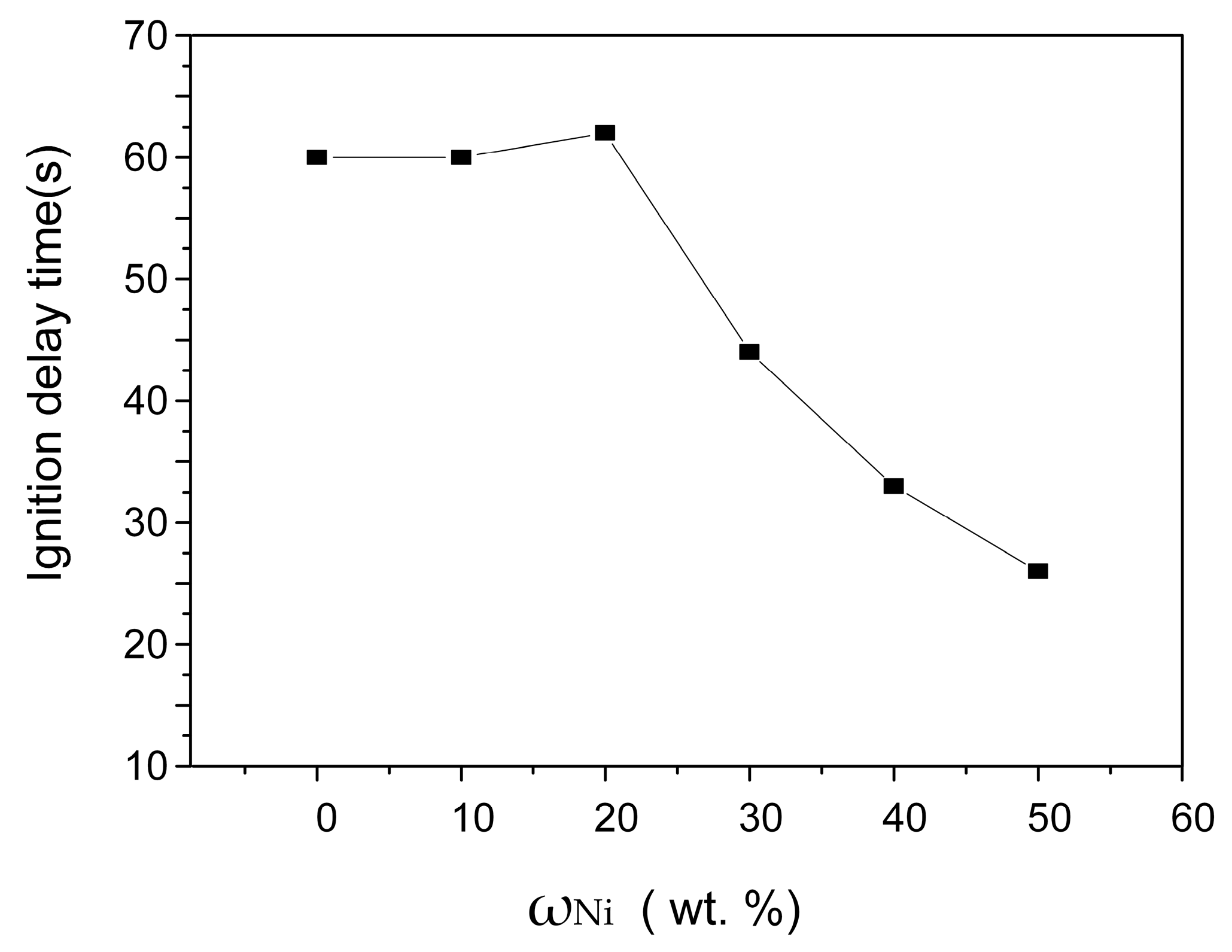
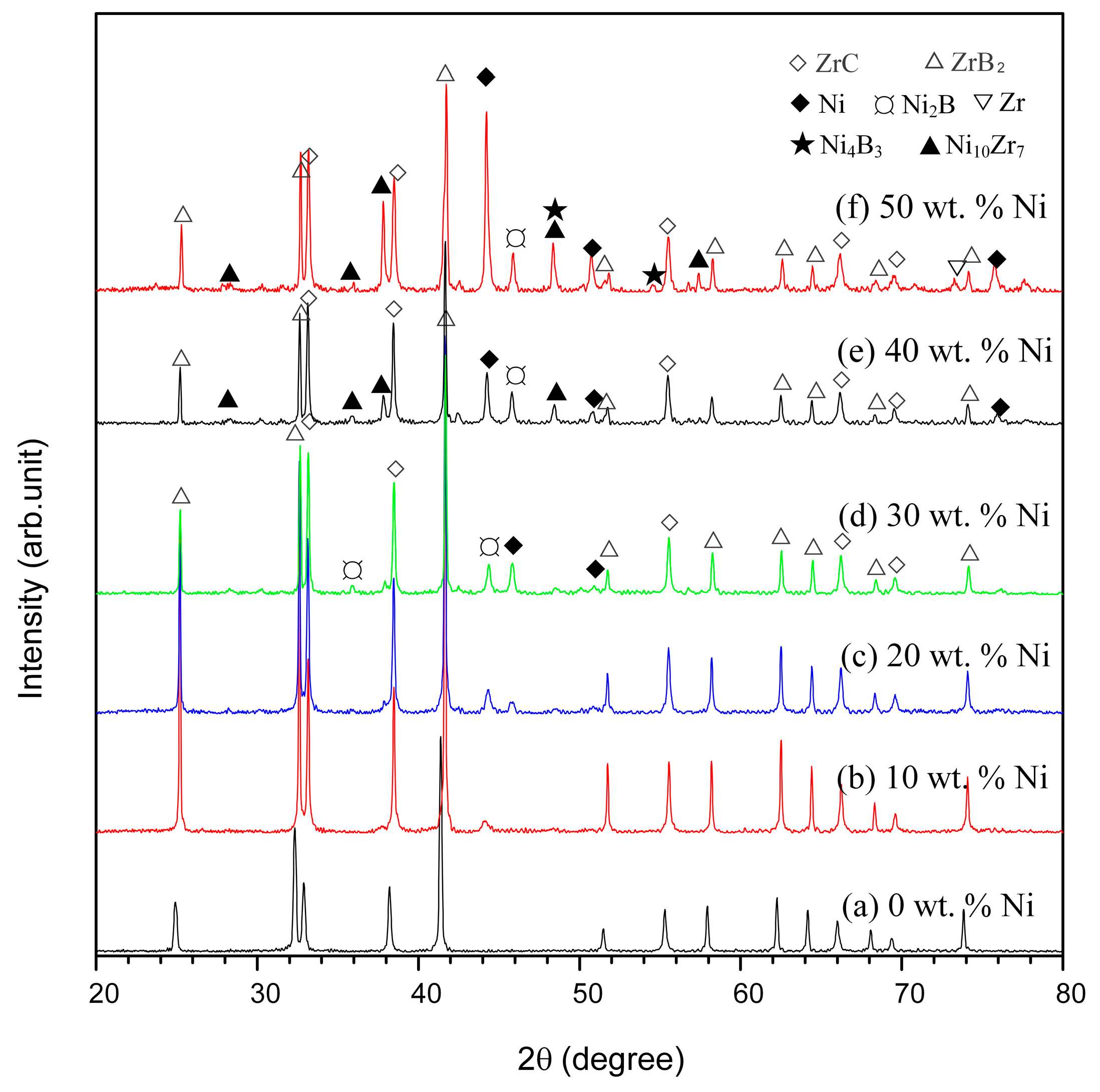
3.1.1. Effect of Ni Content on the SHS Reaction
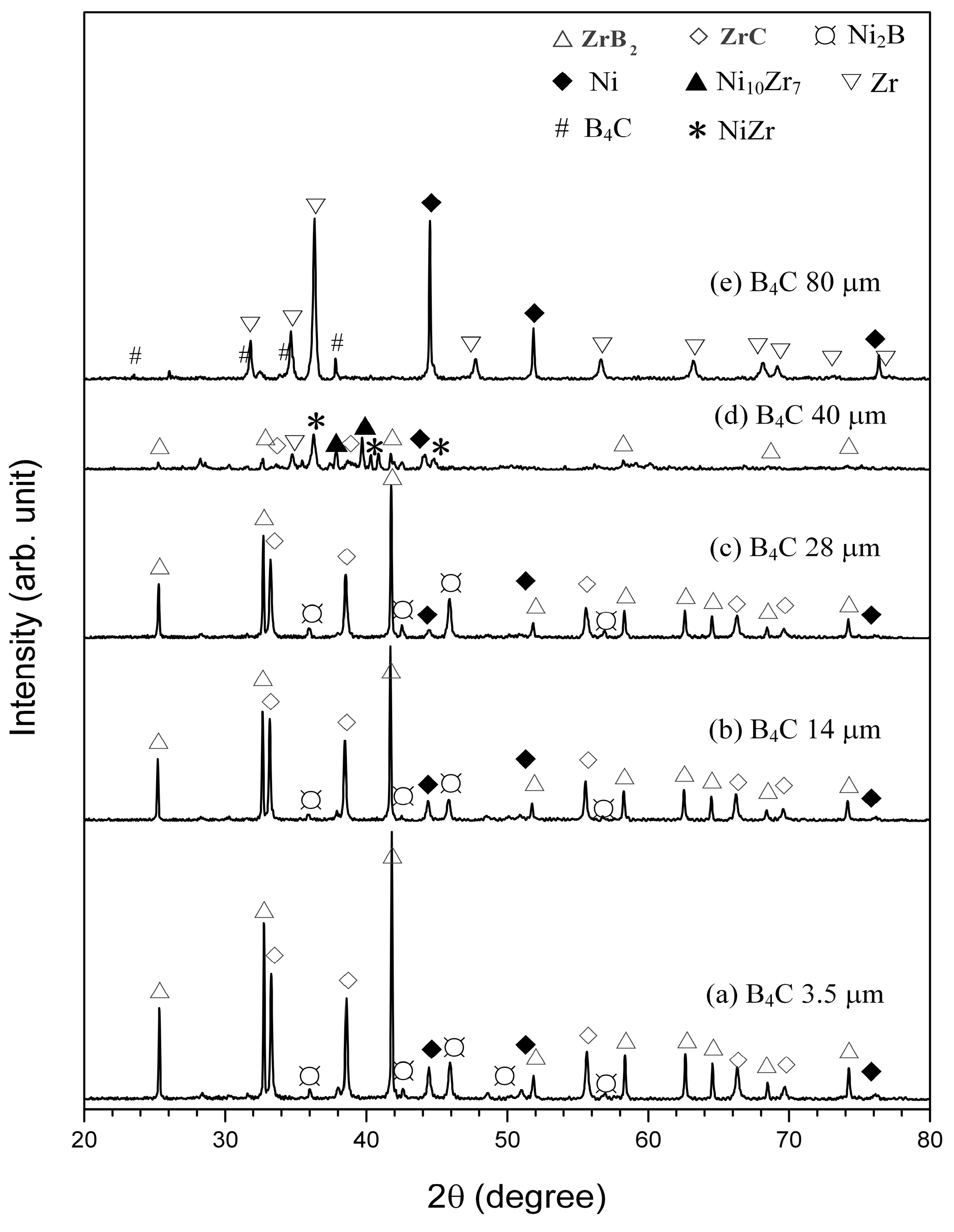
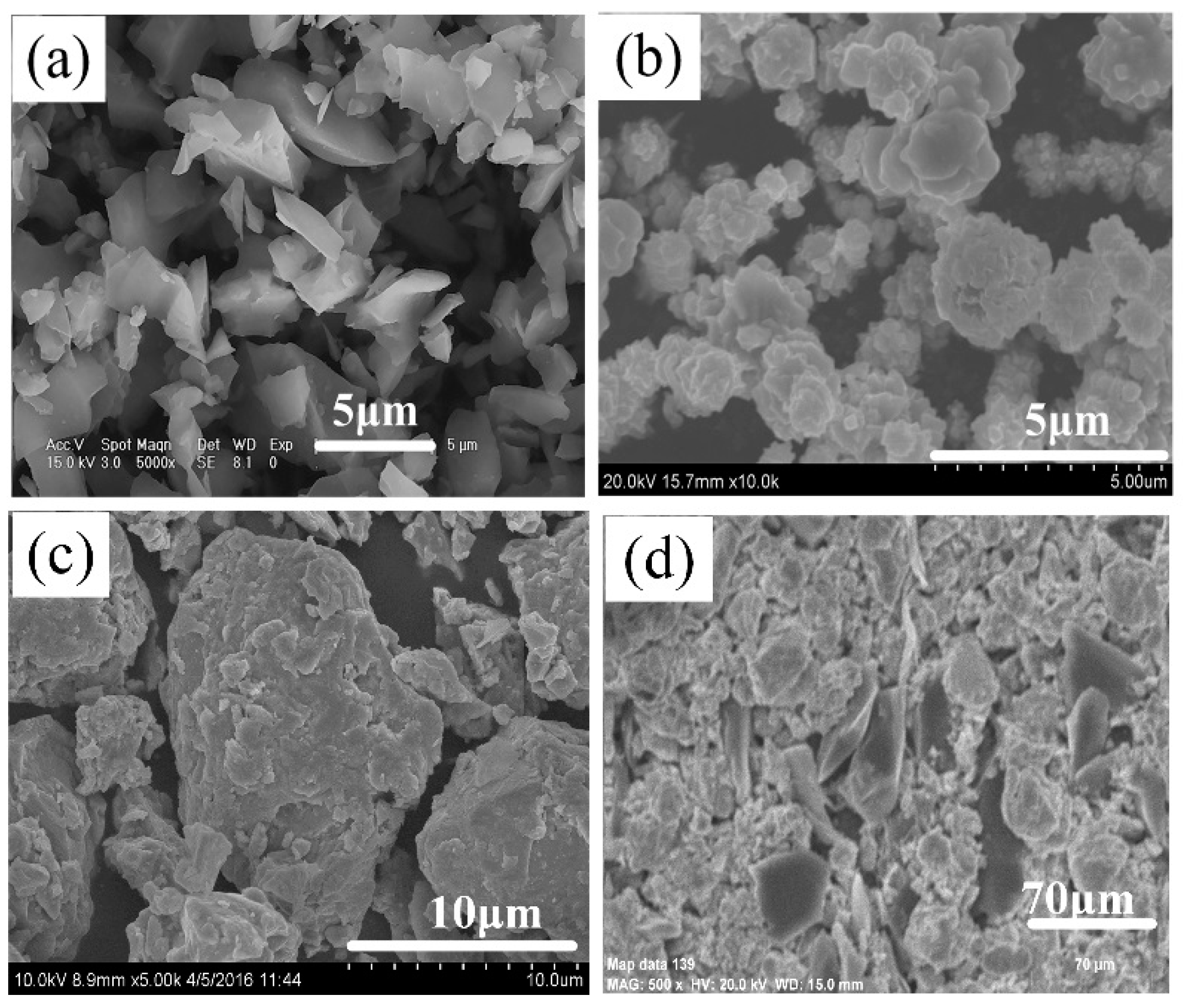
3.1.2. Effect of B4C Particle Sizes on the SHS Reaction
3.2. Formation Mechanism of ZrB2 and ZrC during the SHS Process
3.2.1. DSC Analysis
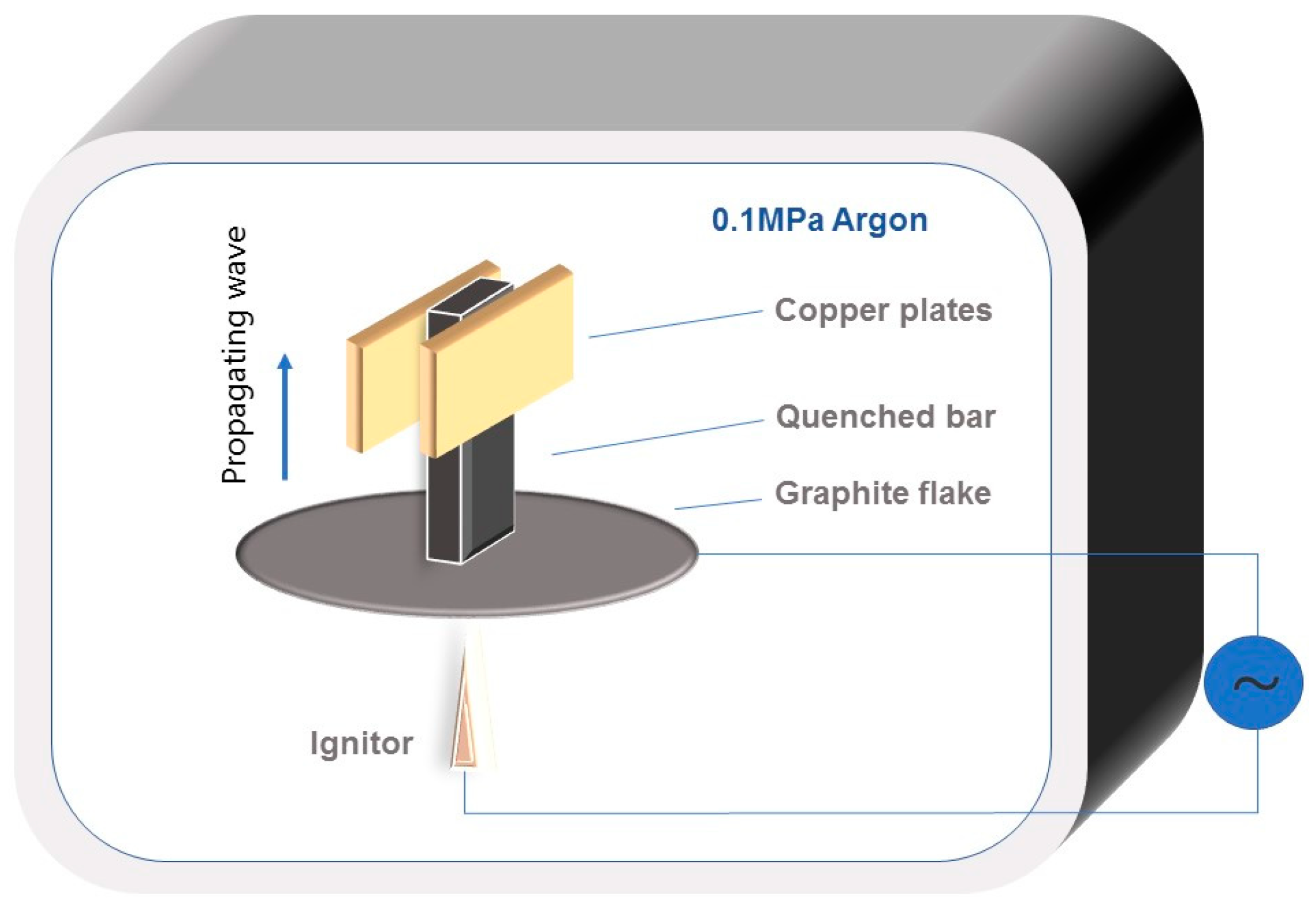
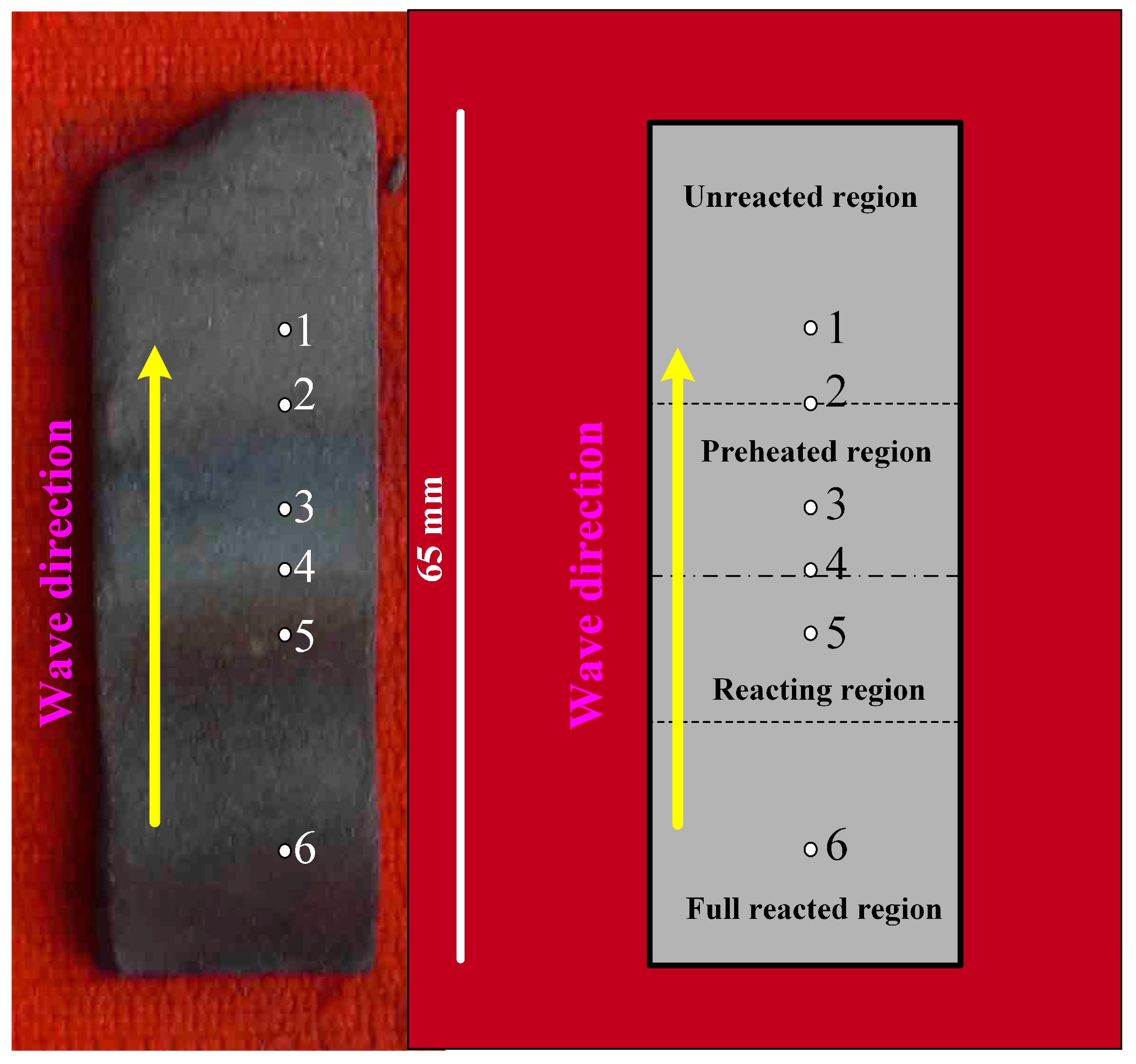
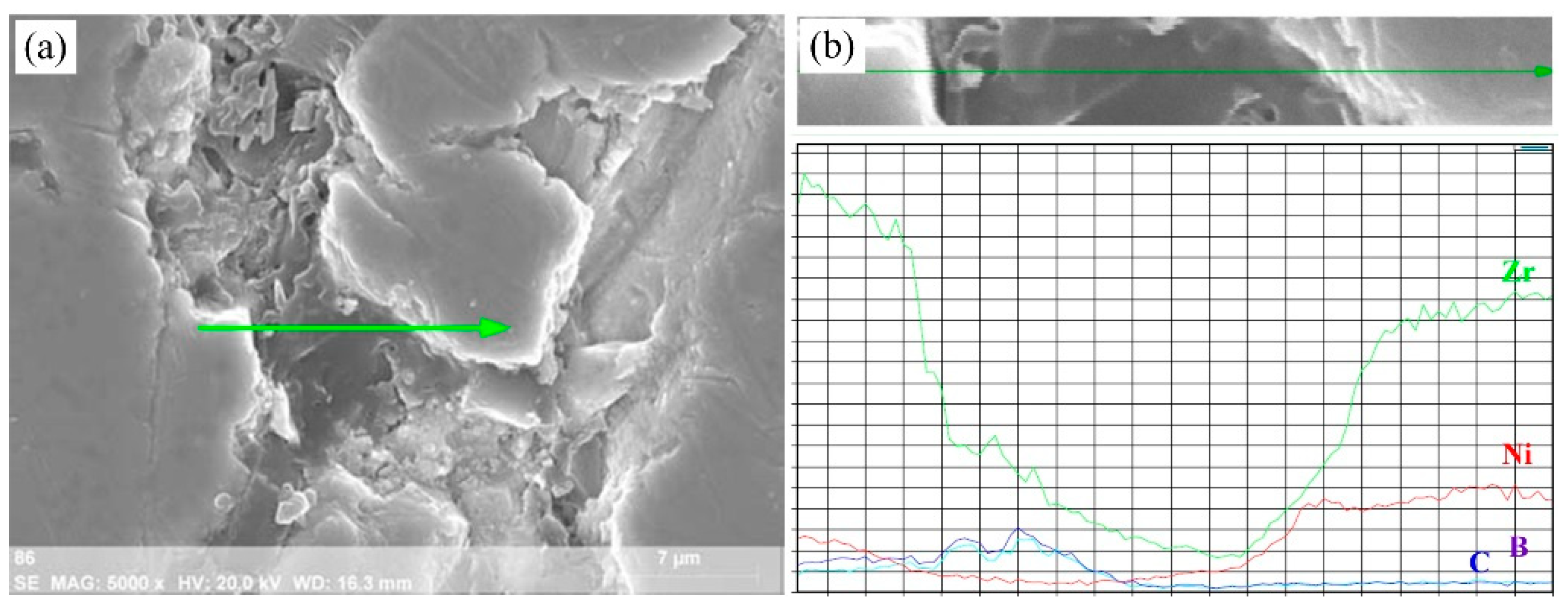
3.2.2. Combustion Wave Quenching Experiment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, Q.D.; Luo, P.; Zhang, M.X.; Song, M.S.; Li, J.G. Combustion and formation behavior of hybrid ZrB2 and ZrC particles in Al-Zr-B4C system during self-propagation high temperature synthesis. Int. J. Refract. Met. Hard Mater. 2012, 31, 89–95. [Google Scholar] [CrossRef]
- Zhang, M.X.; Huo, Y.Q.; Huang, M.; Fang, Y.H.; Zou, B.L. In situ synthesis and formation mechanism of ZrC and ZrB2 by combustion synthesis from the Co-Zr-B4C system. J. Asian Ceram. Soc. 2015, 3, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.X.; Zou, B.L.; Xu, J.Y.; Cai, X.L.; Wang, Y.; Huang, M.; Fang, Y.H.; Huo, Y.Q.; Cao, X.Q. Reaction behavior, microstructure and application in coating of in situ ZrC-ZrB2 ceramic composites powders from a Co-Zr-B4C system. Mater. Des. 2015, 81, 65–72. [Google Scholar] [CrossRef]
- Zhang, M.X.; Huo, Y.Q.; Hu, Q.D.; Zhang, P.; Zou, B.L. Reaction behavior and formation mechanism of ZrC and ZrB2 in the Cu-Zr-B4C system. Int. J. Refract. Met. Hard Mater. 2014, 43, 102–108. [Google Scholar] [CrossRef]
- Zhang, M.X.; Huo, Y.Q.; Huang, M.; Fang, Y.H.; Wang, G.P. The effect of B4C particle size on the reaction process and product in the Cu-Zr-B4C system. J. Asian Ceram. Soc. 2015, 3, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.L.; Wang, W.; Wang, R.; Zhao, C.B.; Pan, W.T.; Huang, H.J.; Du, D.F.; Wang, D.H.; Shu, D.; Dong, A.P.; et al. Formation mechanism of spherical TiC in Ni-Ti-C system during combustion synthesis. Materials 2017, 10, 1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.H.; Wang, H.Y.; Yang, Y.F.; Du, Y.L.; Jiang, Q.C. Reaction path of the synthesis of TiC-TiB2 in Cu-Ti-B4C system. Int. J. Refract. Met. Hard Mater. 2008, 26, 383–388. [Google Scholar] [CrossRef]
- Zou, B.L.; Xu, J.Y.; Wang, Y.; Zhao, S.M.; Fan, X.Z.; Hui, Y.; Zhou, X.; Huang, W.Z.; Cai, X.L.; Tao, S.Y.; et al. Self-propagating high-temperature synthesis of TiC-TiB2-based Co cermets from a Co-Ti-B4C system and fabrication of coatings using the cermet powders. Chem. Eng. J. 2013, 233, 138–148. [Google Scholar] [CrossRef]
- Yang, Y.F.; Wang, H.Y.; Zhao, R.Y.; Liang, Y.H.; Jiang, Q.C. Effect of Ni content on the reaction behaviors of self-propagating high-temperature synthesis in the Ni-Ti-B4C system. Int. J. Refract. Met. Hard Mater. 2008, 26, 77–83. [Google Scholar] [CrossRef]
- Levashov, E.A.; Mukasyan, A.S.; Rogachev, A.S.; Shtansky, D.V. Self-propagating high-temperature synthesis of advanced materials and coatings. Int. Mater. Rev. 2017, 62, 203–239. [Google Scholar] [CrossRef]
- Jin, S.B.; Su, H.K.; Sha, G. Atom Probe tomography analysis of TiCx powders synthesized by SHS in Al/Fe/Cu-Ti-C systems. Materials 2019, 12, 4095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matveev, A.E.; Promakhov, V.; Nikitin, P.; Babaev, A.; Vorozhtsov, A. Effect of mechanical activation of Al-Ti-B powder mixture on phase composition and structure of Al-TiB2 composite materials obtained by self-propagating high-temperature synthesis (SHS). Materials 2022, 12, 2668. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Y.; Zou, B.L.; Zhao, S.M.; Hui, Y.; Huang, W.Z.; Zhou, X.; Wang, Y.; Cai, X.L.; Cao, X.Q. Fabrication and properties of ZrC-ZrB2/Ni cermet coatings on a magnesium alloy by atmospheric plasma spraying of SHS powders. Ceram. Int. 2014, 40, 15537–15544. [Google Scholar] [CrossRef]
- Xu, J.Y.; Ma, P.F.; Zou, B.L. Reaction mechanism of ZrB2-ZrC formation in Ni-Zr-B4C system analyzed by differential scanning calorimetry. Materials 2021, 14, 6467. [Google Scholar] [CrossRef]
- Moore, J.J.; Feng, H.J. Combustion synthesis of advanced materials: Part I: Reaction parameters. Prog. Mater. Sci. 1995, 39, 243–273. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substances, 3rd ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 1995. [Google Scholar]
- Zou, B.L.; Shen, P.; Jiang, Q.C. Dependence of the SHS reaction behavior and product on B4C particle size in Al-Ti-B4C and Al-TiO2-B4C systems. Mater. Res. Bull. 2009, 44, 499–504. [Google Scholar] [CrossRef]
- Matveev, A.E.; Nikitin, P.Y.; Zhukov, I.A.; Zhukov, A.S. The use of plastic waste as carbon raw materials to obtain TiC-based powders. Ceram. Int. 2021, 47, 21140–21146. [Google Scholar] [CrossRef]
- Nikitin, P.Y.; Zhukov, I.A.; Matveev, A.E.; Sokolov, S.D.; Boldin, M.S.; Vorozhtsov, A.B. AlMgB14-TiB2 composite materials obtained by self-propagating high-temperature synthesis and spark plasma sintering. Ceram. Int. 2020, 46, 22733–22737. [Google Scholar] [CrossRef]
- Choi, Y.; Rhee, S.W. Effect of aluminum addition on the combustion reaction of titanium and carbon to form TiC. J. Mater. Sci. 1993, 28, 6669–6675. [Google Scholar] [CrossRef]
- Yang, Y.F.; Wang, H.Y.; Zhao, R.Y.; Jiang, Q.C. Effect of reactant particle size on the self-propagating high-temperature synthesis reaction behaviors in the Ni-Ti-B4C system. Metall. Mater. Trans. A 2009, 40, 232–239. [Google Scholar] [CrossRef]
- Massalski, T.B.; Okamoto, H.; Subramanian, P.R. Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Metals Park, OH, USA, 1990. [Google Scholar]
- Hayes, E.T.; Roberson, A.H.; Paasche, O.G. The Zirconium-Nickel phase diagram. Trans. ASM 1953, 45, 893–900. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Ma, P.; Zou, B.; Yang, X. Reaction Behavior and Formation Mechanism of ZrB2 and ZrC from the Ni-Zr-B4C System during Self-Propagating High-Temperature Synthesis. Materials 2023, 16, 354. https://doi.org/10.3390/ma16010354
Xu J, Ma P, Zou B, Yang X. Reaction Behavior and Formation Mechanism of ZrB2 and ZrC from the Ni-Zr-B4C System during Self-Propagating High-Temperature Synthesis. Materials. 2023; 16(1):354. https://doi.org/10.3390/ma16010354
Chicago/Turabian StyleXu, Jiaying, Pengfei Ma, Binglin Zou, and Xue Yang. 2023. "Reaction Behavior and Formation Mechanism of ZrB2 and ZrC from the Ni-Zr-B4C System during Self-Propagating High-Temperature Synthesis" Materials 16, no. 1: 354. https://doi.org/10.3390/ma16010354



