Natural Cellulose from Ziziphus jujuba Fibers: Extraction and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of Cellulose
2.3. Characterization Methods
2.3.1. Chemical Analysis
2.3.2. Fourier Transform Infrared (FTIR) Spectroscopy
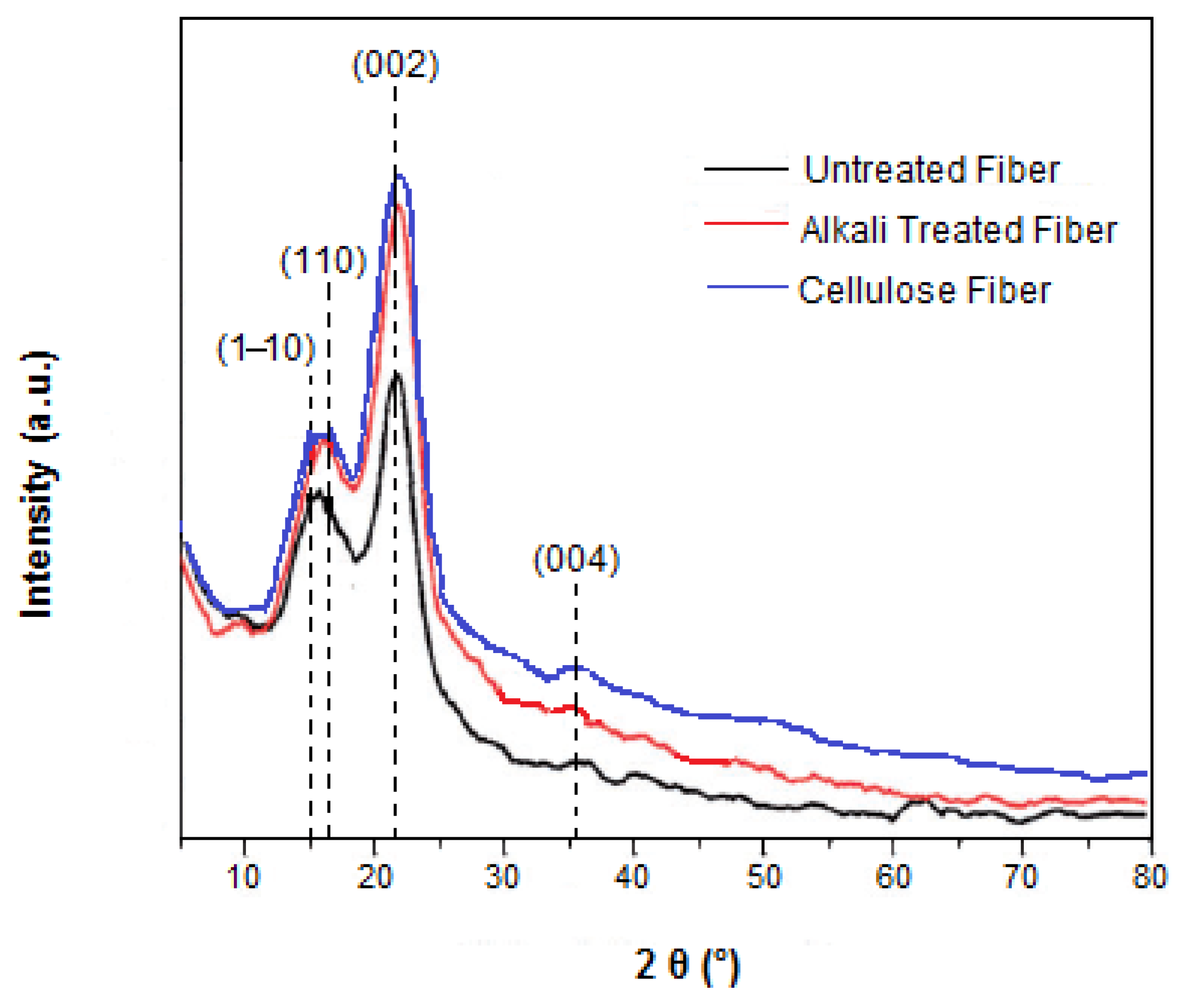
2.3.3. X-ray Diffraction Analysis (XRD)
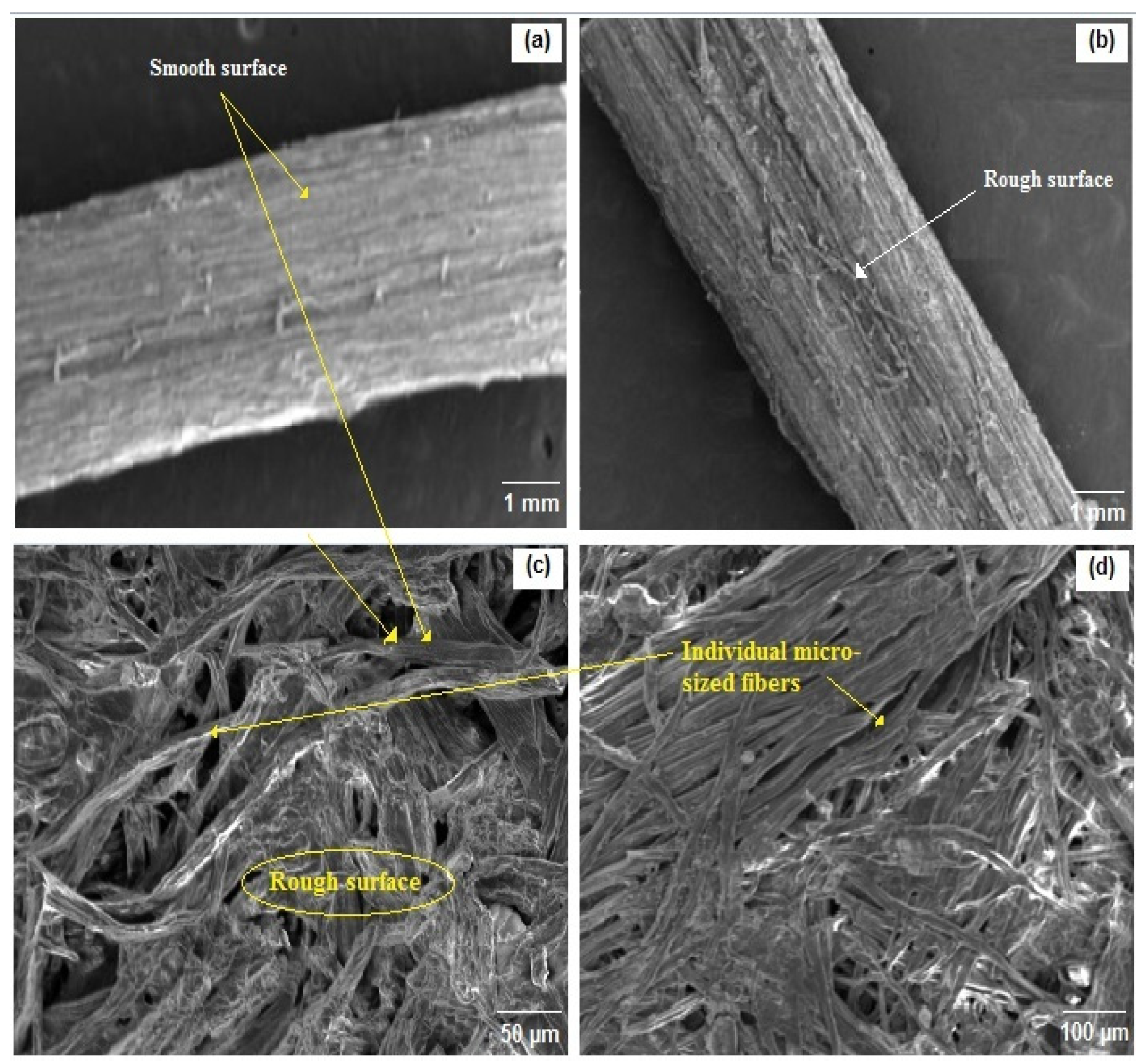
2.3.4. Scanning Electron Microscopy (SEM)
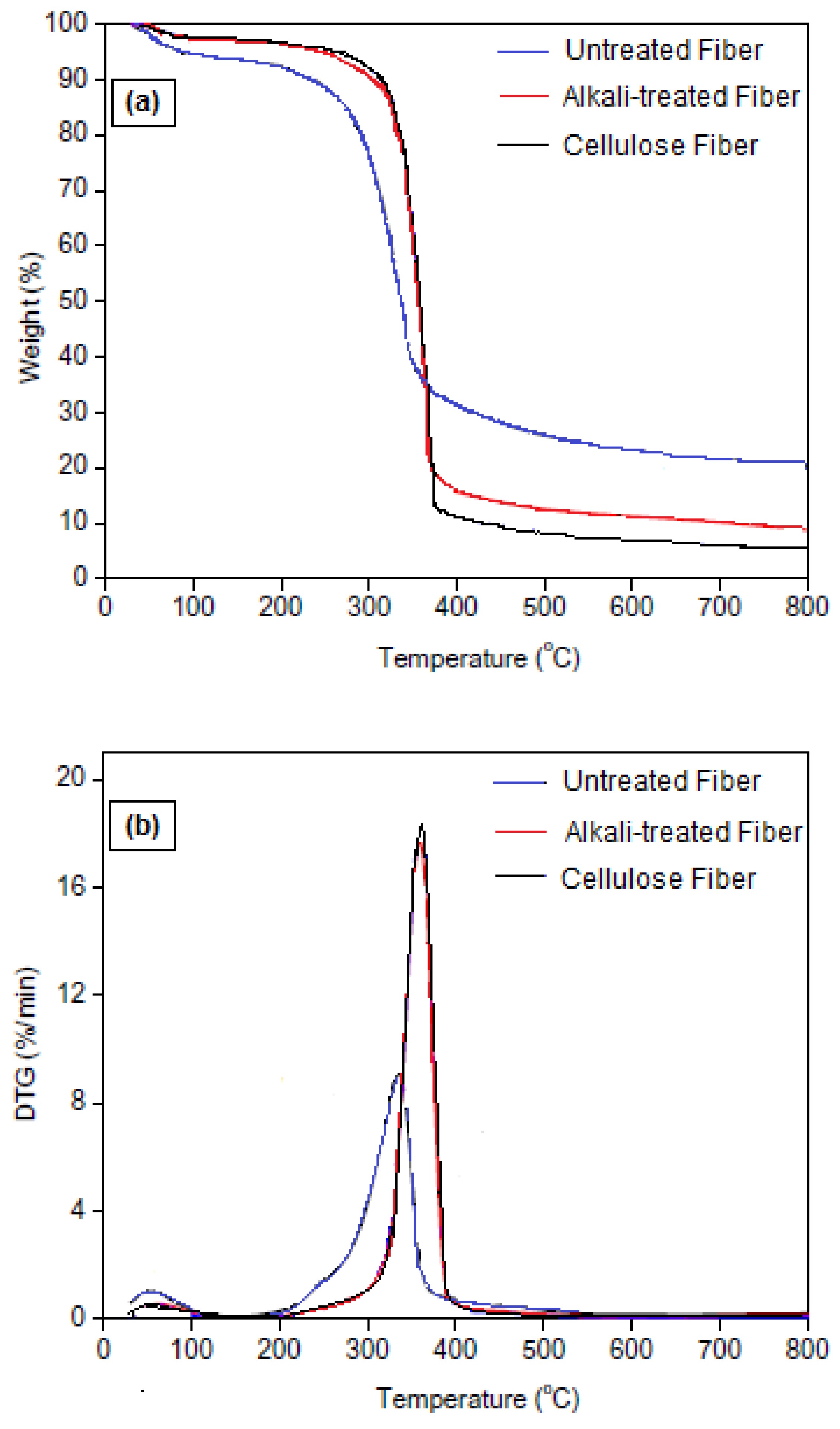
2.3.5. Thermogravimetric Analysis (TGA)
3. Results
3.1. Chemical Composition
| Fiber Name | Cellulose (wt.%) | Hemicellulose (wt.%) | Lignin (wt.%) | Reference |
|---|---|---|---|---|
| Zizyphus jujube | 43.0 | 10.2 | 5.1 | Present study |
| 5% alkali-treated Zizyphus jujube | 52.0 | 5.7 | 2.2 | |
| Leucas Aspera | 50.7 | 13.2 | 9.7 | [29] |
| Catharanthus roseus | 47.3 | 9.1 | 15.1 | [30] |
| Eleusine indica grass | 61.3 | 14.7 | 11.1 | [31] |
| Abaca | 56.0–63.0 | 15–17 | 7–10 | [26] |
| Jute | 72.0 | 13.0 | 13.0 | [27] |
| Hemp | 74.0 | 18.0 | 4.0 | [27] |
| Ramie | 68.6–72.6 | 13.1–16.7 | 0.6–0.7 | [26] |
| Saharan aloe vera | 67.4 | 8.2 | 13.7 | [32] |
| Cotton | 85–90 | 1–3 | 0.7–1.6 | [33] |
| Sisal | 78.0 | 19.0 | 8.0 | [34] |
| Bamboo | 26–43 | 30 | 21–31 | [35] |
| Shwetark | 69.6 | 0.2 | 16.8 | [36] |
| Flax | 81.0 | 14.0 | 3.0 | [27] |
| Aerial roots of banyan tree | 67.3 | 13.5 | 15.6 | [37] |
| Manau rattan (Calamus manan) | 42.0 | 20.0 | 27.0 | [3] |
| Dracaena reflexa | 70.3 | 11.0 | 11.4 | [38] |
| Ficus religiosa tree | 55.6 | 13.9 | 10.1 | [39] |
| Napier grass | 45.7 | 33.7 | 20.6 | [40] |
| Bagasse | 55.2 | 16.8 | 25.3 | [41] |
| Cabuya | 68–77 | 4–8 | 13.0 | [42] |
| Manicaria saccifera palm | 74.1 | 12.0 | 31.1 | [43] |
| Linum usitatissimum | 85.0 | 9.0 | 4.0 | [44] |
3.2. Physical Appearance
3.3. FTIR Analysis
3.4. Crystallinity Analysis
3.5. Thermal Properties
3.6. Morphological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siva, R.; Valarmathi, T.N.; Palanikumar, K.; Samrot, A.V. Study on a novel natural cellulosic fiber from Kigelia africana fruit: Characterization and analysis. Carbohydr. Polym. 2020, 244, 116494. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, M.; Palanikumar, K.; Reddy, H.K. Plant fibre based bio-composites: Sustainable and renewable green materials. Renew. Sustain. Energy Rev. 2017, 79, 558–584. [Google Scholar] [CrossRef]
- Ding, L.; Han, X.; Cao, L.; Chen, Y.; Ling, Z.; Han, J.; He, S.; Jiang, S. Characterization of natural fiber from manau rattan (Calamus manan) as a potential reinforcement for polymer-based composites. J. Bioresour. Bioprod. 2022, 7, 190–200. [Google Scholar] [CrossRef]
- Satha, H.; Kouadri, I.; Benachour, D. Thermal, Structural and Morphological Studies of Cellulose and Cellulose Nanofibers Extracted from Bitter Watermelon of the Cucurbitaceae Family. J. Polym. Environ. 2020, 28, 1914–1920. [Google Scholar] [CrossRef]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Ahmed, D.; Ahmad, N.; Qama, M.T.; Alruwaili, N.K.; Bukhari, S.N.A. Extraction and characterization of microcrystalline cellulose from Lagenaria Siceraria fruit predicles. Polymers 2022, 14, 1867. [Google Scholar] [CrossRef] [PubMed]
- Khandanlou, R.; Ngoh, G.C.; Chong, W.T. Feasibility Study and Structural Analysis of Cellulose Isolated from Rice Husk: Microwave Irradiation, Optimization, and Treatment Process Scheme. BioResources 2016, 11, 5751–5766. [Google Scholar] [CrossRef] [Green Version]
- Melikoğlu, A.Y.; Bilek, S.E.; Cesur, S. Optimum alkaline treatment parameters for the extraction of cellulose and production of cellulose nanocrystals from apple pomace. Carbohydr. Polym. 2019, 215, 330–337. [Google Scholar] [CrossRef]
- Sai Prasanna, N.; Mitra, J. Isolation and characterization of cellulose nanocrystals from Cucumis sativus peels. Carbohydr. Polym. 2020, 247, 116706. [Google Scholar] [CrossRef]
- Wang, J.; Han, X.; Zhang, C.; Liu, K.; Duan, G. Source of Nanocellulose and Its Application in Nanocomposite Packaging Material: A Review. Nanomaterials 2022, 12, 3158. [Google Scholar] [CrossRef]
- Han, X.; Ding, L.; Tian, Z.; Song, Y.; Xiong, R.; Zhang, C.; Han, J.; Jiang, S. Potential new material for optical fiber: Preparation and characterization of transparent fiber based on natural cellulosic fiber and epoxy. Int. J. Biol. Macromol. 2022, 224, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Reshmy, R.; Philip, E.; Paul, S.A.; Madhavan, A.; Sindhu, R.; Binod, P.; Pandey, A.; Sirohi, R. Nanocellulose-based products for sustainable applications–recent trends and possibilities. Rev. Environ. Sci. Biotechnol. 2020, 19, 779–806. [Google Scholar] [CrossRef]
- Adeli, M.; Samavati, V. Studies on the steady shear flow behavior and chemical properties of water-soluble polysaccharide from Ziziphus lotus fruit. Int. J. Biol. Macromol. 2015, 72, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Basyony, M.M.; Elsheikh, H.A.; Abdel Salam, H.S.; Mohamed, K.I.; Zedan, A.H. Utilization of ziziphus spina-christi leaves as a natural growth promoter in rabbit’s rations. EJRS 2017, 27, 427–446. [Google Scholar] [CrossRef]
- Hassainia, A.; Satha, H.; Boufi, S. Chitin from Agaricus bisporus: Extraction and characterization. Int. J. Biol. Macromol. 2018, 117, 1334–1342. [Google Scholar] [CrossRef]
- Hassainia, A.; Satha, H.; Boufi, S. Two Routes to Produce Chitosan from Agaricus bisporus. J. Renew. Mater. 2020, 8, 101–111. [Google Scholar] [CrossRef]
- Kouadri, I.; Satha, H. Extraction and characterization of cellulose and cellulose nanofibers from Citrullus colocynthis seeds. Ind Crops Prod. 2018, 124, 787–796. [Google Scholar] [CrossRef]
- Boureghda, Y.; Satha, H.; Bendebane, F. Chitin–Glucan Complex from Pleurotus ostreatus Mushroom: Physicochemical Characterization and Comparison of Extraction Methods. J. Waste Biomass Valorization 2021, 12, 6139–6153. [Google Scholar] [CrossRef]
- Sluiter, A. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2011. [Google Scholar]
- Segal, L.; Creely, L.; Martin, A.E.; Conrad, M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1958, 29, 786–794. [Google Scholar] [CrossRef]
- Makhlouf, A.; Layachi, A.; Kouadri, I.; Belaadi, A.; Satha, H. Structural study and thermal behavior of composites: Polyamide 66/glass fibers: The reinforcement ration effect on the kinetics of crystallization. J. Compos. Mater. 2020, 54, 1467–1481. [Google Scholar] [CrossRef]
- Maepa, C.E.; Jayaramudu, J.; Okonkwo, J.O.; Ray, S.S.; Sadiku, E.R.; Ramontja, J. Extraction and characterization of natural cellulose fibers from maize tassel. Int. J. Polym. Anal. Charact. 2015, 20, 99–109. [Google Scholar] [CrossRef]
- Zhao, G.; Du, J.; Chen, W.; Pan, M.; Chen, D. Preparation and thermostability of cellulose nanocrystals and nanofibrils from two sources of biomass: Rice straw and poplar wood. Cellulose 2019, 26, 8625–8643. [Google Scholar] [CrossRef]
- Fareez, I.M.; Ibrahim, N.A.; Yaacob, W.; Razali, N.A.M.; Jasni, A.H.; Aziz, F.A. Characteristics of cellulose extracted from Josapine pineapple leaf fibre after alkali treatment followed by extensive bleaching. Cellulose 2018, 25, 4407–4421. [Google Scholar] [CrossRef]
- Yue, Y.; Han, J.; Han, G.; Aita, G.M.; Wu, Q. Cellulose fibers isolated from energycane bagasse using alkaline and sodium chlorite treatments: Structural, chemical and thermal properties. Ind. Crops Prod. 2015, 76, 355–363. [Google Scholar] [CrossRef]
- Sarikanat, M.; Seki, Y.; Sever, K.; Durmuskahya, C. Determination of properties of Althaea officinalis L. (Marshmallow) fibers as a potential plant fiber in polymeric composite materials. Compos. B. Eng. 2014, 57, 180–186. [Google Scholar] [CrossRef]
- Senthamaraikannan, P.; Saravanakumar, S.S.; Arthanarieswaran, V.P.; Sugumaran, P. Physicochemical properties of new cellulosic fibers from bark of Acacia planifrons. Int. J. Polym. Anal. Char. 2016, 21, 207–213. [Google Scholar] [CrossRef]
- Tanpichai, S.; Witayakran, S. All-cellulose composite laminates prepared from pineapple leaf fibers treated with steam explosion and alkaline treatment. J. Reinf. Plast. Compos. 2017, 36, 1146–1155. [Google Scholar] [CrossRef]
- Vijay, R.; Manoharan, S.; Arjun, S.; Vinod, A.; Singaravelu, D.L. Characterization of silane-treated and untreated natural fibers from stem of Leucasaspera. J. Nat. Fibers 2020, 18, 1–17. [Google Scholar]
- Vinod, A.; Vijay, R.; Lenin Singaravelu, D.; Sanjay, M.R.; Siengchin, S.; Moure, M.M. Characterization of untreated and alkali treated natural fibers extracted from the stem of Catharanthusroseus. Mater. Res. Express. 2019, 6, 085406. [Google Scholar] [CrossRef]
- Khan, A.; Vijay, R.; Singaravelu, D.L.; Sanjay, M.R.; Siengchin, S.; Verpoort, F.; Alamry, K.A.; Asiri, A.M. Extraction and characterization of natural fiber from eleusine indica grass as reinforcement of sustainable fiber-reinforced polymer composites. J. Nat. Fibers 2019, 18, 1–9. [Google Scholar] [CrossRef]
- Balaji, A.N.; Nagarajan, K.J. Characterization of alkali treated and untreated new cellulosic fiber from Saharan Aloe vera cactus leaves. Carbohydr. Polym. 2017, 174, 200–208. [Google Scholar]
- Reddy, K.O.; Ashok, B.; Reddy, K.R.N.; Feng, Y.E.; Zhang, J.; Rajulu, A.V. Extraction and characterization of novel lignocellulosic fibers from Thespesia lampas plant. Int. J. Polym. Anal. Char. 2014, 19, 48–61. [Google Scholar] [CrossRef]
- Suryanto, H.; Marsyahyo, E.; Irawan, Y.S.; Soenoko, R. Morphology, structure, and mechanical properties of natural cellulose fiber from mending grass (Fimbristylisglobulosa). J. Nat. Fibers 2014, 11, 333–351. [Google Scholar] [CrossRef]
- Fiore, V.; Scalici, T.; Valenza, A. Characterization of a new natural fiber from Arundodonax L. as potential reinforcement of polymer composites. Carbohydr. Polym. 2014, 106, 77–83. [Google Scholar] [CrossRef]
- Raja, K.; Prabu, B.; Ganeshan, P.; Chandra Sekar, V.S.; NagarajaGanesh, B. Characterization studies of natural cellulosic fibers extracted from shwetark stem. J. Nat. Fibers 2020, 18, 1–14. [Google Scholar] [CrossRef]
- Ganapathy, T.; Sathiskumar, R.; Senthamaraikannan, P.; Saravanakumar, S.S.; Khan, A. Characterization of raw and alkali treated new natural cellulosic fibres extracted from the aerial roots of banyan tree. Int. J. Biol. Macromol. 2019, 138, 573–581. [Google Scholar] [CrossRef]
- Manimaran, P.; Saravanan, S.P.; Sanjay, M.R.; Siengchin, S.; Jawaid, M.; Khan, A. Characterization of new cellulosic fiber: Dracaena reflexa as a reinforcement for polymer composite structures. J. Mater. Res. Technol. 2019, 8, 1952–1963. [Google Scholar] [CrossRef]
- Moshi, A.A.M.; Ravindran, D.; Bharathi, S.R.S.; Indran, S.; Saravanakumar, S.S.; Liu, Y. Characterization of a new cellulosic natural fiber extracted from the root of Ficus religiosa tree. Int. J. Biol. Macromol. 2020, 142, 212–221. [Google Scholar] [CrossRef]
- Kommula, V.P.; Reddy, K.O.; Shukla, M.; Marwala, T.; Reddy, E.V.S.; Rajulu, A.V. Extraction, modification, and characterization of natural ligno-cellulosic fiber strands from napier grass. Int. J. Polym. Anal. Char. 2016, 21, 18–28. [Google Scholar] [CrossRef]
- Palacios Hinestroza, H.; Hernandez Diaz, J.A.; Esquivel Alfaro, M.; Toriz, G.; Rojas, O.J.; Sulbaran-Rangel, B.C. Isolation and Characterization of Nanofibrillar Cellulose from Agave tequilana Weber Bagasse. Adv. Mater. Sci. Eng. 2019, 2019, 1342547. [Google Scholar] [CrossRef] [Green Version]
- Kozłowski, R.; Władyka-Przybylak, M. Flammability and fire resistance of composites reinforced by natural fibers. Polym. Adv. Technol. 2008, 19, 446–453. [Google Scholar] [CrossRef]
- Porras, A.; Maranon, A.; Ashcroft, I.A. Characterization of a novel natural cellulose fabric from Manicariasaccifera palm as possible reinforcement of composite materials. Compos. B. Eng. 2015, 74, 66–73. [Google Scholar] [CrossRef]
- Jothibasu, S.; Mohanamurugan, S.; Vinod, A. Influence of chemical treatments on the mechanical characteristics of areca sheath flax fibres based epoxy composites. J. Chem. 2018, 11, 1255–1262. [Google Scholar]
- Kouadri, I.; Layachi, A.; Makhlouf, A.; Satha, H. Optimization of extraction process and characterization of water-soluble polysaccharide (Galactomannan) from Algerian biomass; Citrullus colocynthis seeds. Int. J. Polym. Anal. Charact. 2018, 23, 362–375. [Google Scholar] [CrossRef]
- Khenblouche, A.; Bechki, D.; Gouamid, M.; Charradi, K.; Segni, L.; Hadjadj, M.; Boughali, S. Extraction and characterization of cellulose microfibers from Retamaraetam stems. Polímeros. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Amior, A.; Kouadri, I.; Makhlouf, A.; Satha, H. Extraction process of Galactomannan from Algerian biomass; optimization and physic-chemical analysis. In Proceedings of the Fifth International Conference on Biobased Materials and Composites: ICBMC, Monastir, Tunisia, 17–20 March 2019. [Google Scholar]
- Mohamed, N.A.N.; Jai, J. Response surface methodology for optimization of cellulose extraction from banana stem using NaOH-EDTA for pulp and paper making. Heliyon 2022, 8, e09114. [Google Scholar] [CrossRef]
- Tanpichai, S.; Mekcham, S.; Kongwittaya, C.; Kiwijaroun, W.; Thongdonsun, K.; Thongdeelerd, C.; Boonmahitthisud, A. Extraction of Nanofibrillated Cellulose from Water Hyacinth Using a High Speed Homogenizer. J. Nat. Fibers 2021, 19, 5676–5696. [Google Scholar] [CrossRef]
- Rohadi, T.N.T.; Ridzuan, M.J.M.; Majid, M.S.A.; Khasri, A.; Sulaiman, M.H.J. Mater. Res. Technol. 2020, 9, 15057–15071. [Google Scholar]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Saravanakumar, S.S.; Kumaravel, A.; Nagarajan, T.; Moorthy, I.G. Investigation of physico-chemical properties of alkali-treated Prosopis juliflora fibers. Int. J. Polym. Anal. Charact. 2014, 19, 309–317. [Google Scholar] [CrossRef]
- Senthamaraikannan, P.; Kathiresan, M. Characterization of raw and alkali treated new natural cellulosic fiber from Coccinia grandis L. Carbohydr. Polym. 2018, 186, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Han, J.; Han, G.; Zhang, Q.; French, A.D.; Wu, Q. Characterization of cellulose I/II hybrid fibers isolated from energycane bagasse during the delignification process: Morphology, crystallinity and percentage estimation. Carbohydr. Polym. 2015, 133, 438–447. [Google Scholar] [CrossRef]
- Ciftci, D.; Flores, R.A.; Saldaña, M.D.A. Cellulose fiber isolation and characterization from sweet blue lupin hull and canola straw. J. Environ. Polym. Degrad. 2018, 26, 2773–2781. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Sudharsanaa, T.; Bharghavi, A.; Sowmeya, G.S.; Balaram, G. Insights on lignocellulosic pretreatments for biofuel production-SEM and reduction of lignin analysis. Int. J. ChemTech Res. 2014, 6, 4334–4444. [Google Scholar]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addison–Wesley: New York, NY, USA, 1978. [Google Scholar]
- NagarajaGanesh, B.; Ganeshan, P.; Ramshankar, P.; Raja, K. Assessment of natural cellulose fibers derived from Sennaauriculata for making light weight industrial biocomposites. Ind. Crops Prod. 2019, 139, 111546. [Google Scholar] [CrossRef]
- Tanpichai, S.; Witayakran, S.; Srimarut, Y.; Woraprayote, W.; Malila, Y. Porosity, density and mechanical properties of the paper of steam exploded bamboo microfibers controlled by nanofibrillated cellulose. J. Mater. Res. Technol. 2019, 8, 3612–3622. [Google Scholar] [CrossRef]
- Khawas, P.; Deka, S.C. Isolation and characterization of cellulose nanofibers from culinary banana peel using high-intensity ultrasonication combined with chemical treatment. Carbohydr. Polym. 2016, 137, 608–616. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Mouhoubi, S.; Bourahli, M.E.H.; Osmani, H.; Abdeslam, S. Effect of alkali treatment on alfa fibers behavior. J. Nat. Fibers 2017, 14, 239–249. [Google Scholar] [CrossRef]
- Yan, L.; Chouw, N.; Yuan, X.J. Experimental Investigation and Analysis of Mercerized and Citric Acid Surface Treated Bamboo Fiber Reinforced Composite. Reinf. Plast. Compos. 2012, 31, 425–437. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Ouarhim, W.; Essabir, H.; Bensalah, M.; Zari, N.; Bouhfid, R. Structural laminated hybrid composites based on raffia and glass fibers: Effect of alkali treatment, mechanical and thermal properties. Compos. B 2018, 154, 128–137. [Google Scholar] [CrossRef]


| Characteristic Bands (cm−1) | Cellulose | Lignin | Hemicellulose | |
|---|---|---|---|---|
| 3424 2922 1734 1508 1429 1054 | -OH C-H C=O C=C CH2, CH3 C-O, C-O-C | ✓ ✓ | ✓ ✓ | - ✓ |
| - | ✓ | ✓ | ||
| - | ✓ | - | ||
| ✓ | ✓ | ✓ | ||
| ✓ | - | - | ||
| Samples | Crystallinity Index (%) | Crystallite Size (nm) |
|---|---|---|
| Untreated fiber | 35.70 | 16.30 |
| Alkali-treated fiber | 50.81 | 12.81 |
| Cellulose fiber | 57.51 | 10.12 |
| Samples | Tonset (°C) | Tmax (°C) | Charred Residue (%) |
|---|---|---|---|
| Untreated fiber | 175 | 335 | 19.1 |
| Alkali-treated fiber | 215 | 360 | 8.1 |
| Cellulose fiber | 220 | 362 | 5.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amior, A.; Satha, H.; Laoutid, F.; Toncheva, A.; Dubois, P. Natural Cellulose from Ziziphus jujuba Fibers: Extraction and Characterization. Materials 2023, 16, 385. https://doi.org/10.3390/ma16010385
Amior A, Satha H, Laoutid F, Toncheva A, Dubois P. Natural Cellulose from Ziziphus jujuba Fibers: Extraction and Characterization. Materials. 2023; 16(1):385. https://doi.org/10.3390/ma16010385
Chicago/Turabian StyleAmior, Aicha, Hamid Satha, Fouad Laoutid, Antoniya Toncheva, and Philippe Dubois. 2023. "Natural Cellulose from Ziziphus jujuba Fibers: Extraction and Characterization" Materials 16, no. 1: 385. https://doi.org/10.3390/ma16010385





