Effects of Heterogenization Treatment on the Hot-Working Temperature and Mechanical Properties of Al-Cu-Mg-Mn-(Zr) Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Melting and Casting
2.2. Heat Treatment Cycle of the As-Cast Alloys
2.3. Microstructure Characterization
2.4. Hardness and Tensile Testing
3. Results and Discussion
3.1. Scanning Electron Microscope Observation of the As-Cast and Heterogenized Alloys
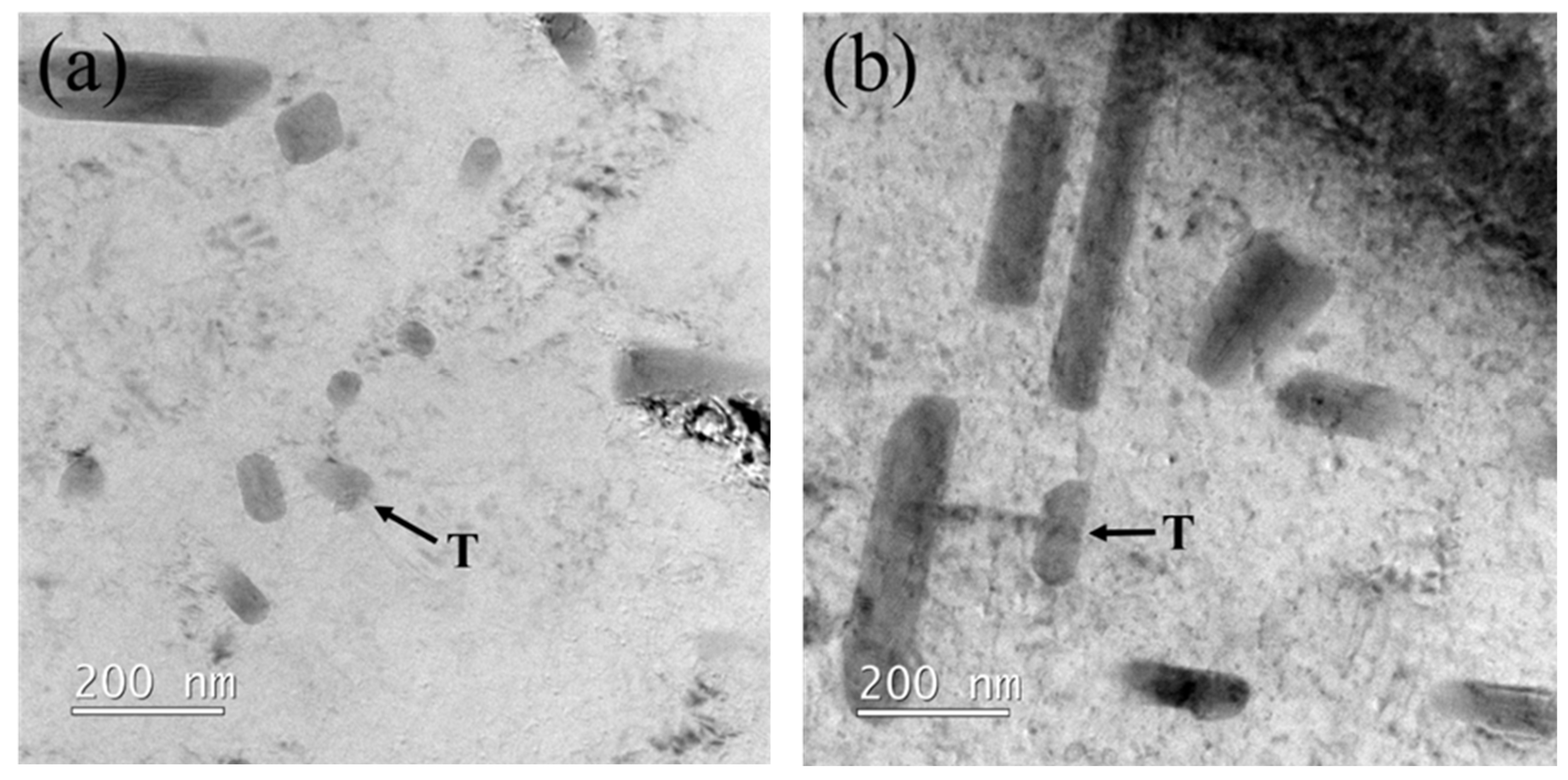
3.2. Transmission Electron Microscope Observation of Heterogenized Alloys
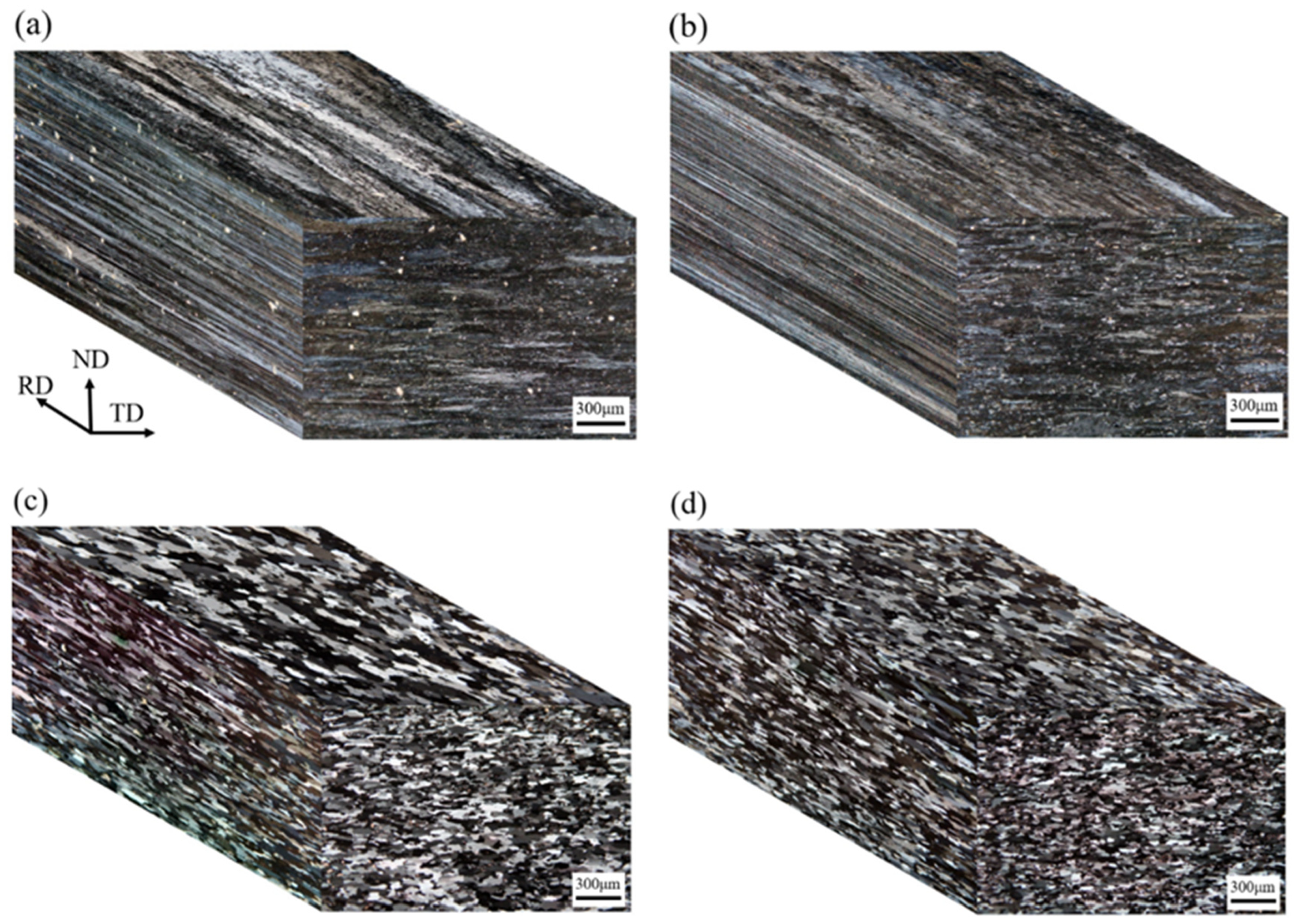
3.3. Optical Microstructure of Cold-Rolled and T4-Tempered Alloys
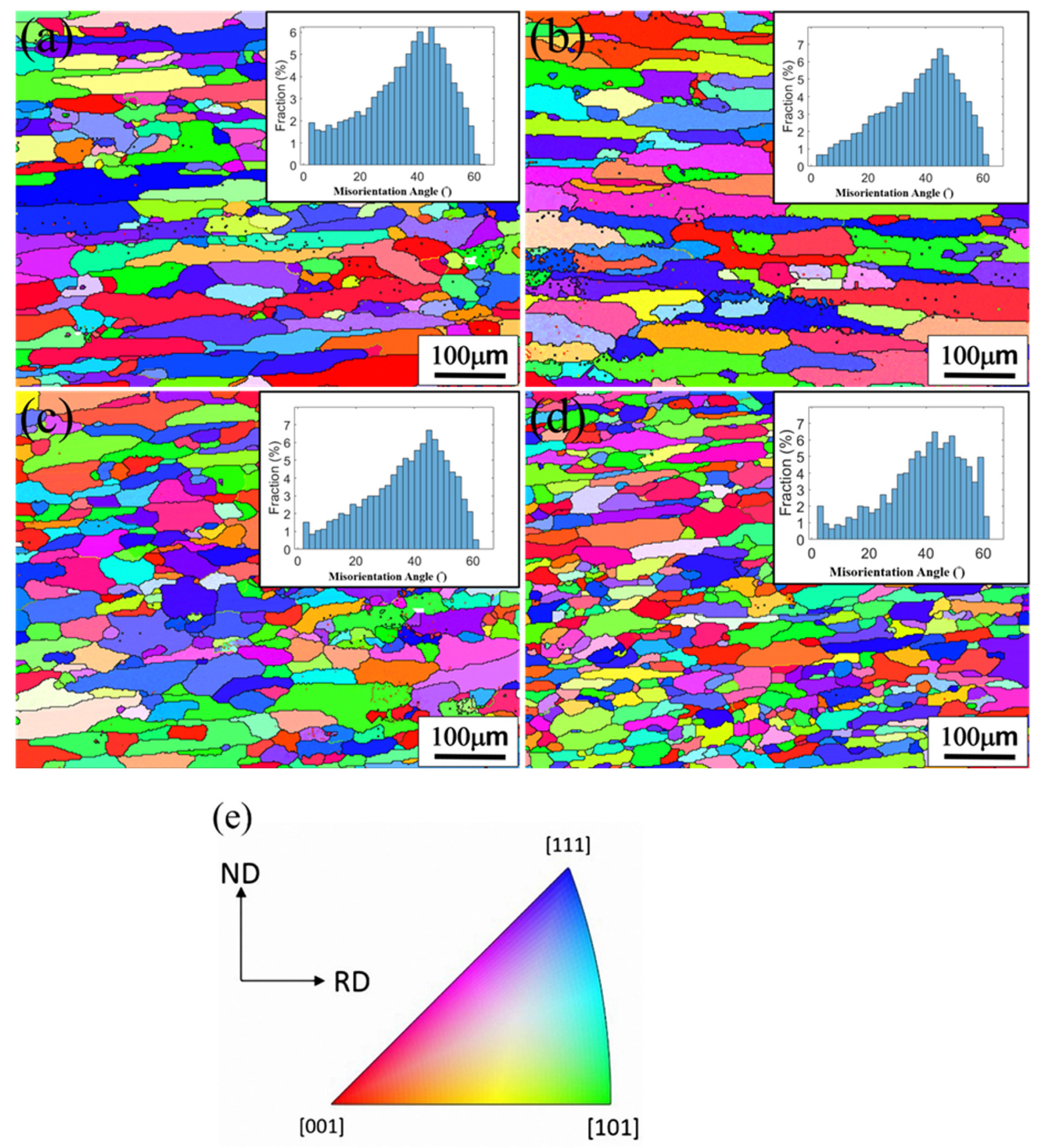
3.4. EBSD Analysis of the T4-Tempered Alloys
3.5. DSC Analysis of the As-Cast and Heterogenized Alloys
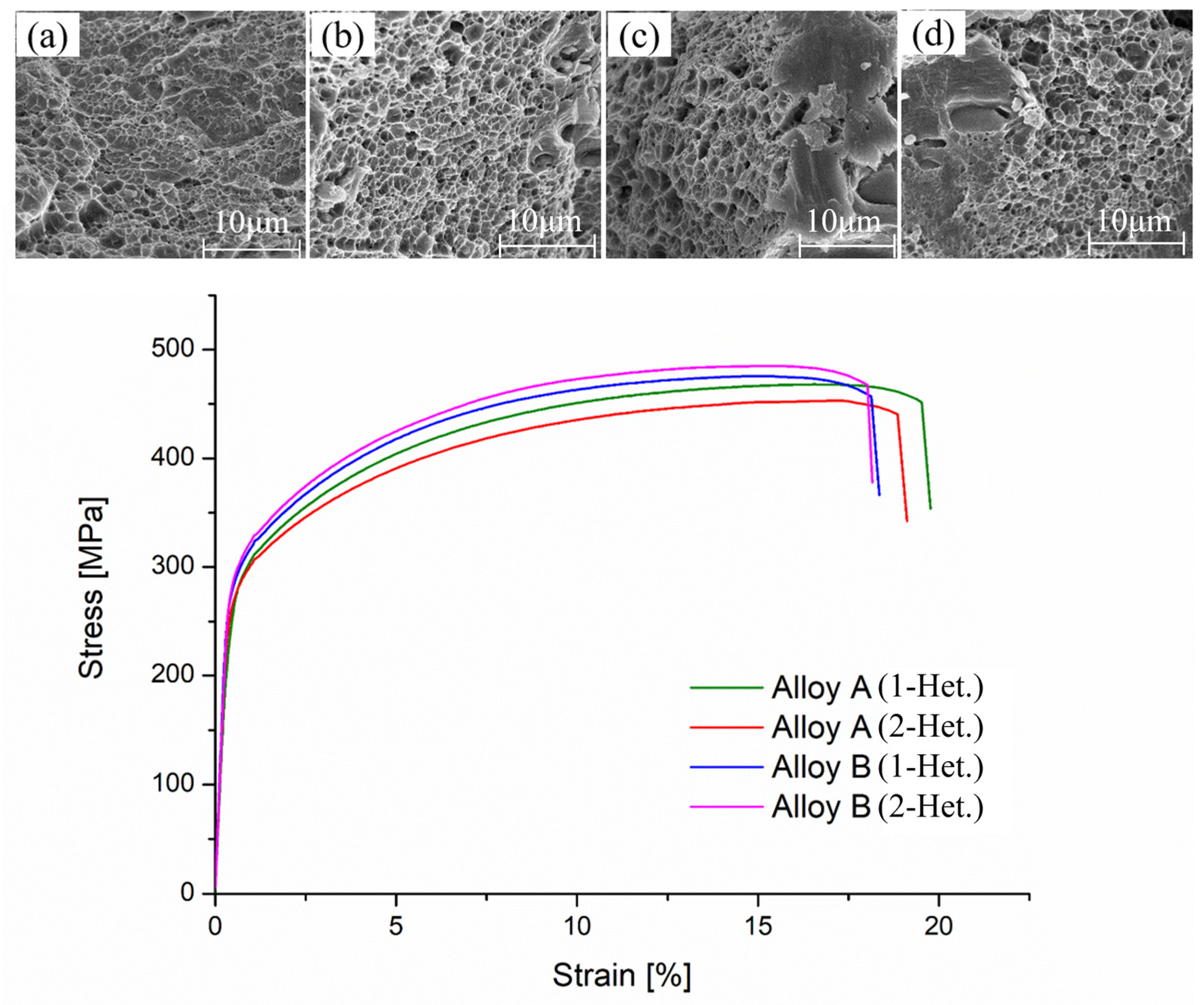
3.6. Hardness and Tensile Properties
4. Conclusions
- (1)
- Through heterogenizing heat treatment, the eutectic phases (α + θ + S) could dissolve into the α-matrix, with an elimination percentage greater than 99%. As a result, the onset melting temperature of the heterogenized alloy decreased by 17 °C compared to the as-cast alloy, thereby increasing the hot-working temperature of the Al-4.9Cu-1.2Mg-0.9Mn alloy.
- (2)
- Compared with one-stage heterogenization, two-stage heterogenization produces finer and denser Al3Zr dispersoids. The average size of Al3Zr decreased from 25 ± 8 nm to 15 ± 5 nm, effectively inhibiting grain growth during T4 tempering and resulting in improved mechanical properties.
- (3)
- The Al-4.9Cu-1.2Mg-0.9Mn alloy, with a minor Zr addition and treated by two-stage heterogenization, exhibited the best hot workability and the highest mechanical properties. The melting temperature increased to 522.8 °C. Additionally, the hardness, yield strength, and ultimate tensile strength of the T4-aged alloy subjected to two-stage heterogenization increased to 77.5 ± 0.7 HRB, 295 ± 2.0 MPa, and 490 ± 3.0 MPa, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Warner, T. Recently-developed aluminum solutions for aerospace applications. Mater. Sci. 2006, 519, 1271–1278. [Google Scholar]
- Saha, P.K. Aluminum Extrusion Technology; ASM International: Materials Park, OH, USA, 2000. [Google Scholar]
- Wang, J.; Xiao, X. Thermodynamic and Kinetic Calculation of High Strength Aluminum-Lithium Alloy. Crystals 2022, 12, 472. [Google Scholar] [CrossRef]
- Wang, L.; Strangwood, M.; Balinta, D.; Lin, J.; Dean, T.A. Formability and failure mechanisms of AA2024 under hot forming conditions. Mater. Sci. Eng. A 2011, 197, 2648–2656. [Google Scholar] [CrossRef]
- Xu, X.F.; Zhao, Y.G. Rapid grain refinement of AA2024 alloy through recrystallization induced by electro-pulsing. Mater. Sci. Eng. A 2014, 612, 223–226. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Wang, L.; Qiao, J. The Effect of Extrusion and Heat Treatment on the Microstructure and Tensile Properties of 2024 Aluminum Alloy. Materials 2022, 15, 7566. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, D.; Bronicki, M.; Woznicki, A. High-temperature homogenization of Al-Cu-Mg alloys for extrusion in T5 temper. Arch. Metall. Mater. 2010, 55, 500–513. [Google Scholar]
- Zlaticanin, B.; Duric, S.; Jordovic, B.; Radonjic, B. Effect of magnesium content on microstructure and properties of Al-Cu-Mg alloys. Mater. Sci. Technol. 2004, 20, 138–140. [Google Scholar] [CrossRef]
- Pan, T.A.; Tzeng, Y.C.; Bor, H.Y.; Liu, K.H.; Lee, S.L. Effects of the coherency of Al3Zr on the microstructures and quench sensitivity of Al-Zn-Mg-Cu alloys. Mater. Today Commun. 2021, 28, 102611. [Google Scholar] [CrossRef]
- Tseng, C.J.; Lee, S.L. Effects of manganese on microstructure and mechanical properties of A206 alloys containing iron. J. Mater. Res. 2002, 17, 2243–2250. [Google Scholar] [CrossRef]
- Chen, Z.W.; Chen, P.; Lee, S.H. Effect of Ce addition on microstructure of Al20Cu2Mn3 twin phase in an Al-Cu-Mn casting alloy. Mater. Sci. Eng. A. 2012, 532, 606–609. [Google Scholar] [CrossRef]
- Tsivoulas, D.; Prangnell, P.B. Effects of Combined Zr and Mn Additions on dispersoid formation and recrystallization behavior of AA2198 Sheet. Adv. Mat. Res. 2010, 89, 568–573. [Google Scholar]
- Yu, T.F.; Qian, B.Y.; Wu, R.Z.; Sun, J.F.; Zhao, H. Microstructure and Mechanical Properties of the As-Cast Al-Li-Cu-Mg-Zr Alloy with High Li Content and Different Cu/Mg Ratios. Adv. Eng. Mater. 2020, 22, 1901570. [Google Scholar] [CrossRef]
- Tsivoulas, D.; Prangnell, P.B. The effect of Mn and Zr dispersoid-forming additions on recrystallization resistance in Al-Cu-Li AA2198 sheet. Acta Mater. 2014, 77, 1–16. [Google Scholar] [CrossRef]
- Ye, J.; Pan, Q.L.; Liu, B.; Hu, Q.; Qu, L.F.; Wang, W.Y.; Wang, X.D. Effects of co-addition of minor Sc and Zr on aging precipitates and mechanical properties of Al-Zn-Mg-Cu alloys. J. Mater. Res. Technol. 2023, 22, 2944–2954. [Google Scholar] [CrossRef]
- Beigi Kheradmand, A.; Mirdamadi, S.; Lalegani, Z.; Hamawandi, B. Effect of Thermomechanical Treatment of Al-Zn-Mg-Cu with Minor Amount of Sc and Zr on the Mechanical Properties. Materials 2022, 15, 589. [Google Scholar] [CrossRef]
- Starink, M.J.; Gao, N.; Kamp, N. Relations between microstructure, precipitation, age-formability and damage tolerance of Al-Cu-Mg-Li (Mn, Zr, Sc) alloys for age forming. Mater. Sci. Eng. A 2006, 418, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Humphreys, F.J.; Hatherly, M. Recrystallization and Related Annealing Phenomena-Recrystallization of Two Phase Alloys; Elsevier Ltd.: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Wu, L.M.; Wang, W.H.; Hsu, Y.F.; Trong, S. Effects of homogenization treatment on recrystallization behavior and dispersoid distribution in an Al-Zn-Mg-Sc-Zr alloy. J. Alloys Compd. 2008, 456, 163–169. [Google Scholar] [CrossRef]
- Robson, J.D. A new model for prediction of dispersoid precipitation in aluminum alloys containing zirconium and scandium. Acta Mater. 2004, 52, 1409–1421. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Precipitation evolution in Al-Zr and Al-Zr-Ti alloys during isothermal aging at 375–425 °C. Acta Mater. 2008, 56, 114–127. [Google Scholar] [CrossRef]
- Davis, J.R. ASM Specialty Handbook: Aluminum and Aluminum Alloys; ASM International: Materials Park, OH, USA, 1993. [Google Scholar]
- ASTM E1558-09; Standard Guide for Electrolytic Polishing of Metallographic Specimens. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM E8; Standard Test Methods for Tension Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2020.
- Effenberg, G.; Ilyenko, S. Ternary Alloy Systems: Phase Diagrams, Crystallographic and Thermodynamic Data; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Nowotnik, G.M.; Sieniawski, J. Analysis of intermetallic phases in 2024 aluminum alloy. Solid State Phenom. 2013, 197, 238–243. [Google Scholar] [CrossRef]
- Rinderer, B. The metallurgy of homogenization. Mater. Sci. Forum. 2011, 693, 264–275. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Zhao, G. Effects of two-step homogenization on precipitation behavior of Al3Zr dispersoids and recrystallization resistance in 7150 aluminum alloy. Mater Charact. 2015, 102, 122–130. [Google Scholar] [CrossRef]
- Jia, Z.H. Precipitation behavior of Al3Zr precipitate in Al-Cu-Zr and Al-Cu-Zr-Ti-V alloys. Trans. Nonferrous Met. Soc. China. 2012, 22, 1860–1865. [Google Scholar] [CrossRef]
- Belov, N.A.; Alabin, A.N. Effect of Zr additions and annealing temperature on electrical conductivity and hardness of hot rolled Al sheets. Trans. Nonferrous Met. Soc. China 2015, 25, 2817–2826. [Google Scholar] [CrossRef] [Green Version]
- Buranova, Y.; Kulitskiy, V.; Peterlechner, M.; Mogucheva, A.; Kaibyshev, R.; Divinski, S.V.; Wilde, G. Al3(Sc,Zr)-based precipitates in Al-Mg alloy: Effect of severe deformation. Acta Mater. 2017, 124, 210–224. [Google Scholar] [CrossRef]
- Teleshov, V.V.; Kaputkin, E.Y.; Golovleva, A.P.; Kosmacheva, N.P. Temperature ranges of phase transformations and mechanical properties of alloys of the Al-Cu-Mg-Ag system with various Cu/Mg ratio. Met. Sci. Heat Treat. 2005, 47, 139–144. [Google Scholar] [CrossRef]
- Wang, G.J.; Xiong, B.Q.; Zhang, Y.A.; Li, P.Y. Microstructural characterization of as-cast and homogenized 2D70 aluminum alloy. Int. J. Miner. Metall. Mater. 2009, 16, 427–431. [Google Scholar] [CrossRef]
- Ivanov, R.; Deschamps, A.; Geuser, F.D. Clustering kinetics during natural ageing of Al-Cu based alloys with (Mg, Li) additions. Acta Mater. 2018, 157, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Yang, Z.; Zhang, G. Nanostructure Characteristics of Al3Sc1−xZrx Nanoparticles and Their Effects on Mechanical Property and SCC Behavior of Al-Zn-Mg Alloys. Materials 2020, 13, 1909. [Google Scholar] [CrossRef] [Green Version]
- Nikolay, A.B.; Dmitry, G.E. Multicomponent Phase Diagrams; Elsevier Science: Amsterdam, The Netherlands, 2005. [Google Scholar]





| Alloy | Cu | Mg | Mn | Zr | Fe | Si | Al |
|---|---|---|---|---|---|---|---|
| A (0Zr) | 4.91 | 1.22 | 0.92 | - | 0.09 | 0.04 | Balance |
| B (0.15Zr) | 4.88 | 1.21 | 0.91 | 0.16 | 0.09 | 0.04 | Balance |
| Notation. | RD | ND | Aspect Ratio | Recrystallization Fraction |
|---|---|---|---|---|
| Alloy A (1-Het.) | 117.8 (45.5) * | 24.9 (3.4) | 4.7 (0.6) | 90.84 (0.8) |
| Alloy A (2-Het.) | 163.8 (50.2) | 28.3 (3.5) | 5.8 (0.4) | 90.40 (1.0) |
| Alloy B (1-Het.) | 67.3 (15.8) | 23.7 (2.8) | 2.8 (1.0) | 90.15 (1.2) |
| Alloy B (2-Het.) | 57.0 (19.8) | 22.3 (3.2) | 2.5 (0.7) | 91.16 (2.0) |
| Notation | Peak I (α + θ + S) | Peak II (α + θ) | ||||
|---|---|---|---|---|---|---|
| |Qac | | |Qh| | (|Qh| − |Qac|)/ |Qac| × 100% | |Qac| | |Qh| | (|Qh| − |Qac|)/ |Qac| × 100% | |
| Alloy A | 7.9 (0.1) * | 0.05 (0.003) | –99.3% (0.2) | 8.5 (0.1) | 5.3 (0.1) | –37.6% (0.5) |
| Alloy B | 8.2 (0.1) | 0.05 (0.005) | –99.4% (0.3) | 9.6 (0.2) | 5.9 (0.1) | –38.5% (1.0) |
| Notation | Hardness (HRB) | YS (MPa) | UTS (MPa) | EL (%) |
|---|---|---|---|---|
| Alloy A (1-Het.) | 75.4 (0.4) * | 284 (2.0) | 466 (2.8) | 19.4 (0.5) |
| Alloy A (2-Het.) | 73.7 (0.4) | 280 (3.5) | 460 (2.2) | 19.5 (0.4) |
| Alloy B (1-Het.) | 76.5 (0.4) | 286 (4.6) | 477 (5.5) | 18.8 (0.4) |
| Alloy B (2-Het.) | 77.5 (0.7) | 295 (2.0) | 490 (3.0) | 18.0 (0.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, M.-C.; Hsu, Y.-D.; Chen, M.-C.; Yang, W.-C.; Lee, S.-L. Effects of Heterogenization Treatment on the Hot-Working Temperature and Mechanical Properties of Al-Cu-Mg-Mn-(Zr) Alloys. Materials 2023, 16, 4256. https://doi.org/10.3390/ma16124256
Wen M-C, Hsu Y-D, Chen M-C, Yang W-C, Lee S-L. Effects of Heterogenization Treatment on the Hot-Working Temperature and Mechanical Properties of Al-Cu-Mg-Mn-(Zr) Alloys. Materials. 2023; 16(12):4256. https://doi.org/10.3390/ma16124256
Chicago/Turabian StyleWen, Ming-Che, Yuan-Da Hsu, Mien-Chung Chen, Wen-Chen Yang, and Sheng-Long Lee. 2023. "Effects of Heterogenization Treatment on the Hot-Working Temperature and Mechanical Properties of Al-Cu-Mg-Mn-(Zr) Alloys" Materials 16, no. 12: 4256. https://doi.org/10.3390/ma16124256





