Erosion–Corrosion Behavior of Friction Stud Welded Joints of X65 Pipelines in Simulated Seawater under Different Flow Rates
Abstract
:1. Introduction
2. Experimental
2.1. Material and Sample Preparation
2.2. Electrochemical Measurements
2.3. Surface Characteristics
3. Result and Discussion
3.1. Electrochemical Characterization
3.1.1. Analysis of EIS
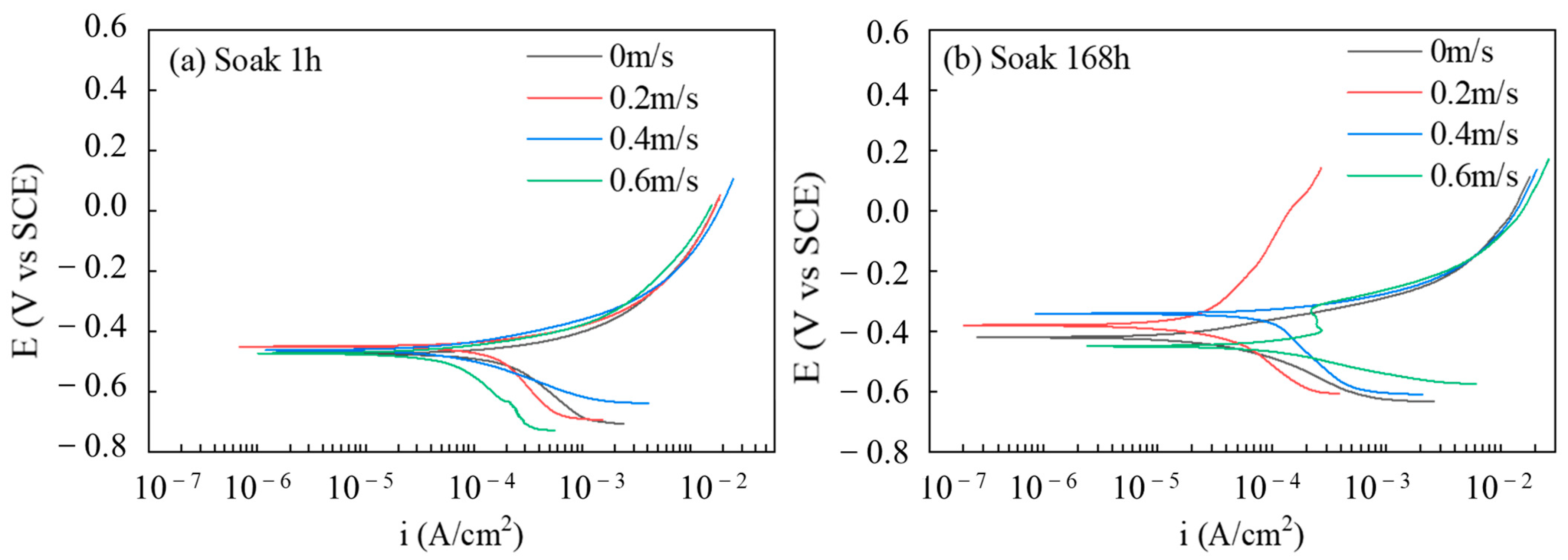
3.1.2. Analysis of PDP
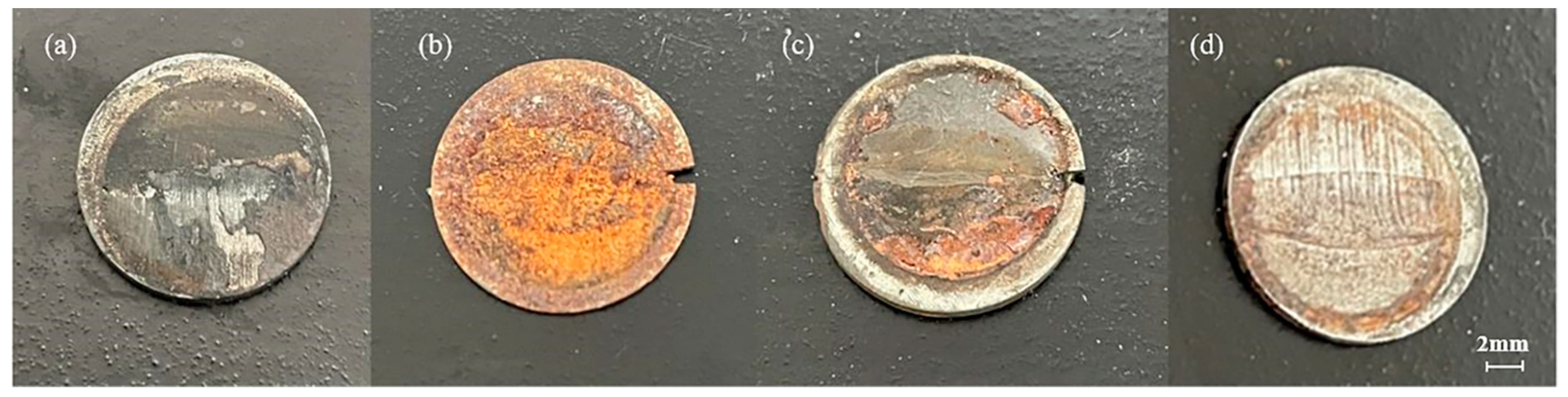
3.2. Corrosion Morphology Analysis
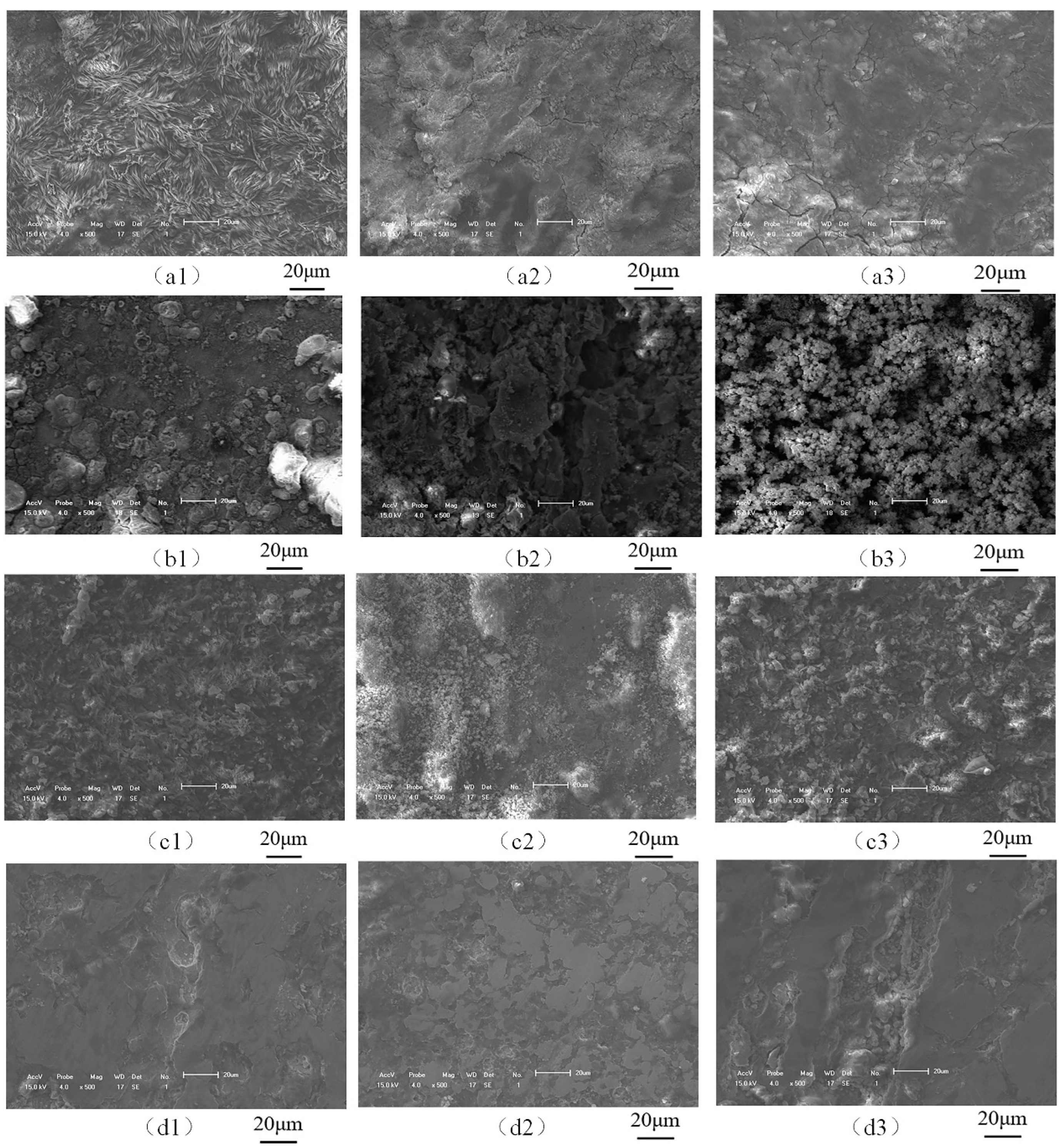
3.3. Corrosion Product Analysis
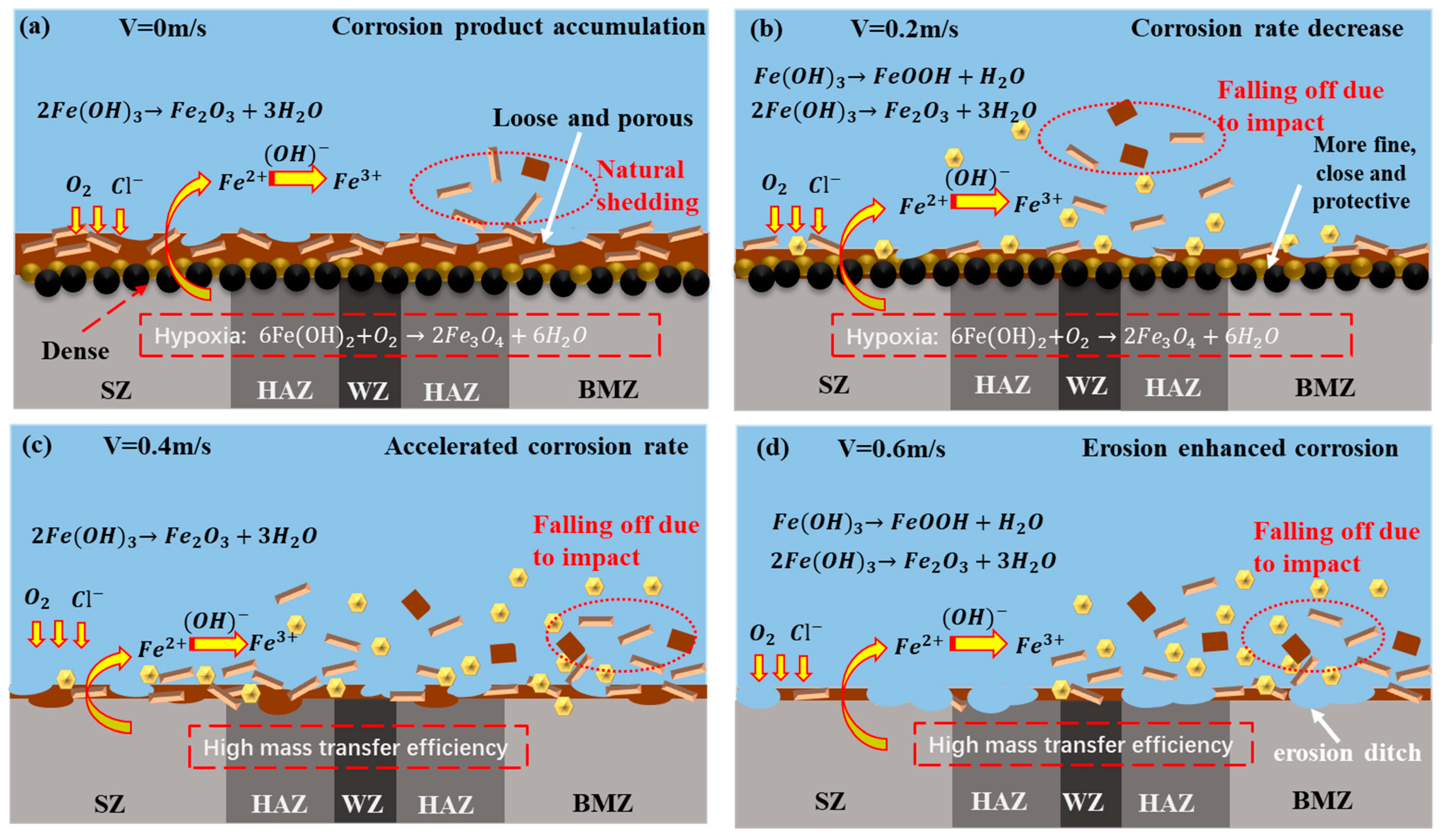
3.4. Erosion–Corrosion Mechanism
4. Conclusions
- This article conducts a thorough study of the erosion–corrosion behavior of X65 friction stir welded joints under different flow rates in simulated seawater through electrochemical experiments, surface analysis, and corrosion product detection.
- The results of the electrochemical experiments show that the corrosion product film of the friction stud welded joints in simulated seawater has a certain protective effect on the substrate under static corrosion conditions. Under the coupling effect of corrosion erosion, the corrosion rate of the specimen decreases first and then increases with the increase in flow rate. After 168 h, the self-corrosion current of the specimen at a flow rate of 0.6 m/s is about 2.73 times that of the static corrosion.
- The surface layer of the corrosion product film of the friction stud welded joint under the static corrosion condition is loose and the inner layer is dense, which has a certain protective effect on the matrix. The corrosion products of the welded joints in a seawater environment are γ-FeOOH, α-FeOOH, Fe3O4, and Fe2O3 when the flow rate is less than 0.2 m/s; when the flow rate is greater than 0.4 m/s, the corrosion products affected by erosion are only γ-FeOOH and Fe2O3.
- When the flow rate is less than 0.2 m/s, the peel strength of the corrosion products is low, and the accumulation of the corrosion products leads to the formation of a dense corrosion product film in a low oxygen environment, which reduces the corrosion rate. When the flow rate is greater than 0.4 m/s, the peel strength of the corrosion product film increases with the increase in flow rate. A large number of corrosion products were stripped, and further increased the corrosion rate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jebaraj, D.J.J.; Sankaranarayanan, R. Friction stud welding—An overview. In Proceedings of the 3rd International Conference on Condensed Matter and Applied Physics (Icc-2019), Bikaner, India, 14–15 October 2019. [Google Scholar]
- Ma, G.; Gao, H.; Sun, C.; Gu, Y.; Zhao, J.; Fang, Z. Probing the Anti-Corrosion Behavior of X65 Steel Underwater FSW Joint in Simulated Seawater. Front. Mater. 2022, 8, 723986. [Google Scholar] [CrossRef]
- Jesudoss Hynes, N.R.; Nagaraj, P.; Jennifa Sujana, J.A. Investigation on Joining of Aluminum and Mild Steel by Friction Stud Welding. Mater. Manuf. Process. 2012, 27, 1409–1413. [Google Scholar] [CrossRef]
- Ma, H.; Gu, Y.; Gao, H.; Jiao, X.; Che, J.; Zeng, Q. Microstructure, Chemical Composition and Local Corrosion Behavior of a Friction Stud Welding Joint. J. Mater. Eng. Perform. 2018, 27, 666–676. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Cai, S.; Shang, X.; Peng, S.; Shu, Y.; Xiao, J.; Xie, X.; Zhang, Z.; Liu, Z.; et al. Advances in research of the mid-deep South China Sea circulation. Sci. China Earth Sci. 2019, 62, 1992–2004. [Google Scholar] [CrossRef]
- Zheng, Q. Effect of Rotating Speed and Hydrostatic Pressure on Erosion-Corrosion Behavior of X65 Pipeline Steel. Int. J. Electrochem. Sci. 2017, 12, 2593–2605. [Google Scholar] [CrossRef]
- Sliem, M.H.; Fayyad, E.M.; Abdullah, A.M.; Younan, N.A.; Al-Qahtani, N.; Nabhan, F.F.; Ramesh, A.; Laycock, N.; Ryan, M.P.; Maqbool, M.; et al. Monitoring of under deposit corrosion for the oil and gas industry: A review. J. Pet. Sci. Eng. 2021, 204, 108752. [Google Scholar] [CrossRef]
- Burson-Thomas, C.B.; Wood, R.J.K. Developments in Erosion–Corrosion Over the Past 10 Years. J. Bio- Tribo-Corros. 2017, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Aminul Islam, M.; Farhat, Z.N.; Ahmed, E.M.; Alfantazi, A.M. Erosion enhanced corrosion and corrosion enhanced erosion of API X-70 pipeline steel. Wear 2013, 302, 1592–1601. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, J.; Gu, Y.; Xiong, D.; Zeng, Q.; Tian, B. Experimental and numerical investigation into the corrosion performance of X100 pipeline steel under a different flow rate in CO2-saturated produced water. J. Solid State Electrochem. 2020, 25, 993–1006. [Google Scholar] [CrossRef]
- Yi, J.Z.; Hu, H.X.; Wang, Z.B.; Zheng, Y.G. On the critical flow velocity for erosion-corrosion in local eroded regions under liquid-solid jet impingement. Wear 2019, 422–423, 94–99. [Google Scholar] [CrossRef]
- He, S.; Zhou, X.; Zheng, Z.; Zheng, Y. Theoretical model and its verification for the effect of sand concentration on the critical flow velocity for erosion–corrosion of 304 stainless steel in 3.5 wt.% NaCl solution. Tribol.-Mater. Surf. Interfaces 2017, 11, 168–177. [Google Scholar] [CrossRef]
- Wang, Z.B.; Zheng, Y.G. Critical flow velocity phenomenon in erosion-corrosion of pipelines: Determination methods, mechanisms and applications. J. Pipeline Sci. Eng. 2021, 1, 63–73. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Q.; Song, S. Corrosion behavior of ZHMn55-3-1 copper alloy in stagnant and flowing seawater with entrained sediment. Anti-Corros. Methods Mater. 2014, 61, 96–103. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, L.; Xu, C.; Wang, X.; Tan, M.Y.; Huang, Y. Electrochemical characteristics of the dynamic progression of erosion-corrosion under different flow conditions and their effects on corrosion rate calculation. J. Solid State Electrochem. 2020, 24, 2511–2524. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, L.; Zhou, Q.; Wang, X.; Huang, Y. Understanding the influences of pre-corrosion on the erosion-corrosion performance of pipeline steel. Wear 2020, 442–443, 203151. [Google Scholar] [CrossRef]
- Parancheerivilakkathil, M.S.; Parapurath, S.; Ainane, S.; Yap, Y.F.; Rostron, P. Flow Velocity and Sand Loading Effect on Erosion–Corrosion during Liquid-Solid Impingement on Mild Steel. Appl. Sci. 2022, 12, 2530. [Google Scholar] [CrossRef]
- Hu, X.; Neville, A. CO2 erosion–corrosion of pipeline steel (API X65) in oil and gas conditions—A systematic approach. Wear 2009, 267, 2027–2032. [Google Scholar] [CrossRef]
- Elemuren, R.; Evitts, R.; Oguocha, I.; Kennell, G.; Gerspacher, R.; Odeshi, A. Slurry erosion-corrosion of 90° AISI 1018 steel elbow in saturated potash brine containing abrasive silica particles. Wear 2018, 410–411, 149–155. [Google Scholar] [CrossRef]
- Santos Martinez, J.D.; Ladino, D.H.; Calderón Hernández, J.W.; Falleiros, N.A.; de Melo, H.G. Corrosion characterization of new low manganese microalloyed X65 steels in sour and NON-SOUR synthetic seawater. J. Mater. Res. Technol. 2022, 18, 3198–3214. [Google Scholar] [CrossRef]
- Rajahram, S.S.; Harvey, T.J.; Wood, R.J.K. Electrochemical investigation of erosion–corrosion using a slurry pot erosion tester. Tribol. Int. 2011, 44, 232–240. [Google Scholar] [CrossRef]
- Rajahram, S.S.; Harvey, T.J.; Wood, R.J.K. Erosion–corrosion resistance of engineering materials in various test conditions. Wear 2009, 267, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, N.R.; Kumar, V.S.S. Experimental erosion-corrosion analysis of friction stir welding of AA 5083 and AA 6061 for sub-sea applications. Appl. Ocean. Res. 2020, 98, 102121. [Google Scholar] [CrossRef]
- Zheng, Z.B.; Zheng, Y.G. Erosion-enhanced corrosion of stainless steel and carbon steel measured electrochemically under liquid and slurry impingement. Corros. Sci. 2016, 102, 259–268. [Google Scholar] [CrossRef]
- Yu, J.; Wang, H.; Yu, Y.; Luo, Z.; Liu, W.; Wang, C. Corrosion behavior of X65 pipeline steel: Comparison of wet–Dry cycle and full immersion. Corros. Sci. 2018, 133, 276–287. [Google Scholar] [CrossRef]
- Benamor, A.; Talkhan, A.G.; Nasser, M.; Hussein, I.; Okonkwo, P.C. Effect of temperature and fluid speed on the corrosion behavior of carbon steel pipeline in Qatari oilfield produced water. J. Electroanal. Chem. 2018, 808, 218–227. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, G.; Wu, W.; Qiao, Q.; Li, Y.; Li, X. Effect of pH and chloride on the micro-mechanism of pitting corrosion for high strength pipeline steel in aerated NaCl solutions. Appl. Surf. Sci. 2015, 349, 746–756. [Google Scholar] [CrossRef]
- Sun, B.; Liao, W.; Li, Z.; Liu, Z.; Du, C. Corrosion behavior of X65 pipeline steel in coastal areas. Anti-Corros. Methods Mater. 2019, 66, 286–293. [Google Scholar] [CrossRef]
- Ukpai, J.I.; Barker, R.; Hu, X.; Neville, A. Exploring the erosive wear of X65 carbon steel by acoustic emission method. Wear 2013, 301, 370–382. [Google Scholar] [CrossRef]
- Jafery, K.M.; Embong, Z.; Othman, N.K.; Yaakob, N.; Shah, M.; Hashim, N.Z.N. Initial stage of corrosion formation for X70 pipeline external surface in acidic soil (peat) environment. Mater. Today Proc. 2022, 51, 1381–1387. [Google Scholar] [CrossRef]
- Wang, X.; Melchers, R.E. Corrosion of carbon steel in presence of mixed deposits under stagnant seawater conditions. J. Loss Prev. Process Ind. 2017, 45, 29–42. [Google Scholar] [CrossRef]
- Hou, D.; Zhang, K.; Hong, F.; Wu, S.; Wang, Z.; Li, M.; Wang, M. The corrosion deterioration of reinforced passivation Film: The impact of defects. Appl. Surf. Sci. 2022, 582, 152408. [Google Scholar] [CrossRef]
- Wang, Y.; Mu, X.; Dong, J.; Umoh, A.J.; Ke, W. Insight into atmospheric corrosion evolution of mild steel in a simulated coastal atmosphere. J. Mater. Sci. Technol. 2021, 76, 41–50. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, E.; Zhao, X.; Xue, Y.; An, Y.; Zhou, H. Effect of microstructure evolution of Ti6Al4V alloy on its cavitation erosion and corrosion resistance in artificial seawater. J. Mater. Sci. Technol. 2022, 100, 169–181. [Google Scholar] [CrossRef]
- Zhang, L.M.; Ma, A.L.; Yu, H.; Umoh, A.J.; Zheng, Y.G. Correlation of microstructure with cavitation erosion behaviour of a nickel-aluminum bronze in simulated seawater. Tribol. Int. 2019, 136, 250–258. [Google Scholar] [CrossRef]


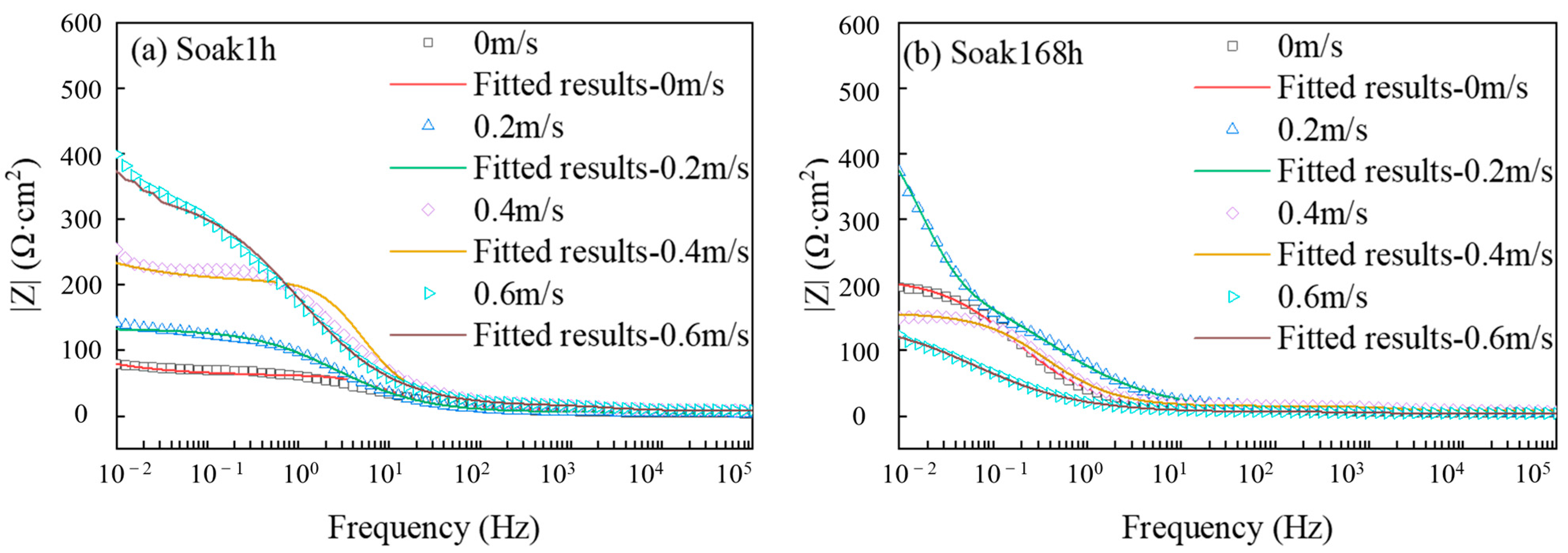
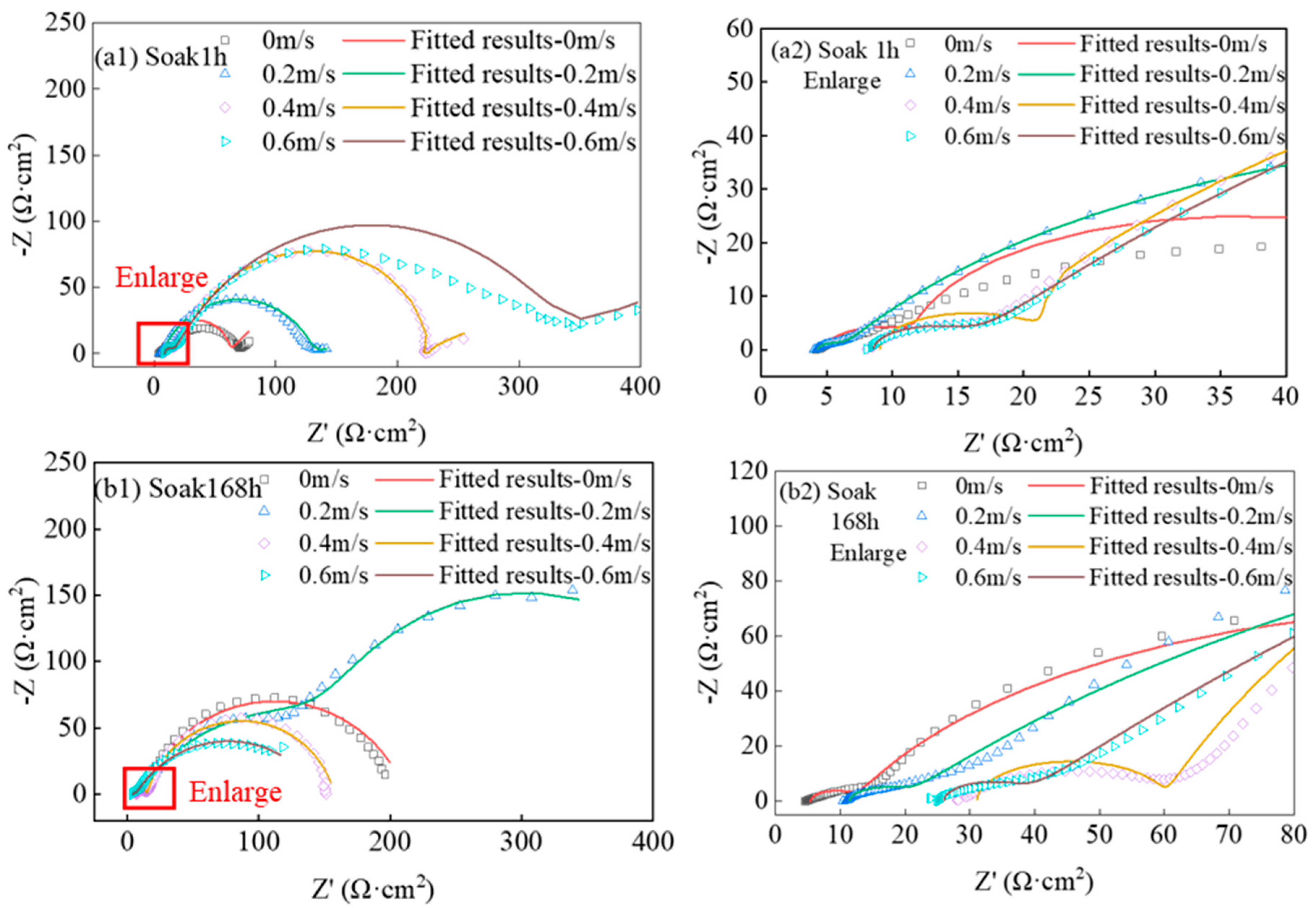
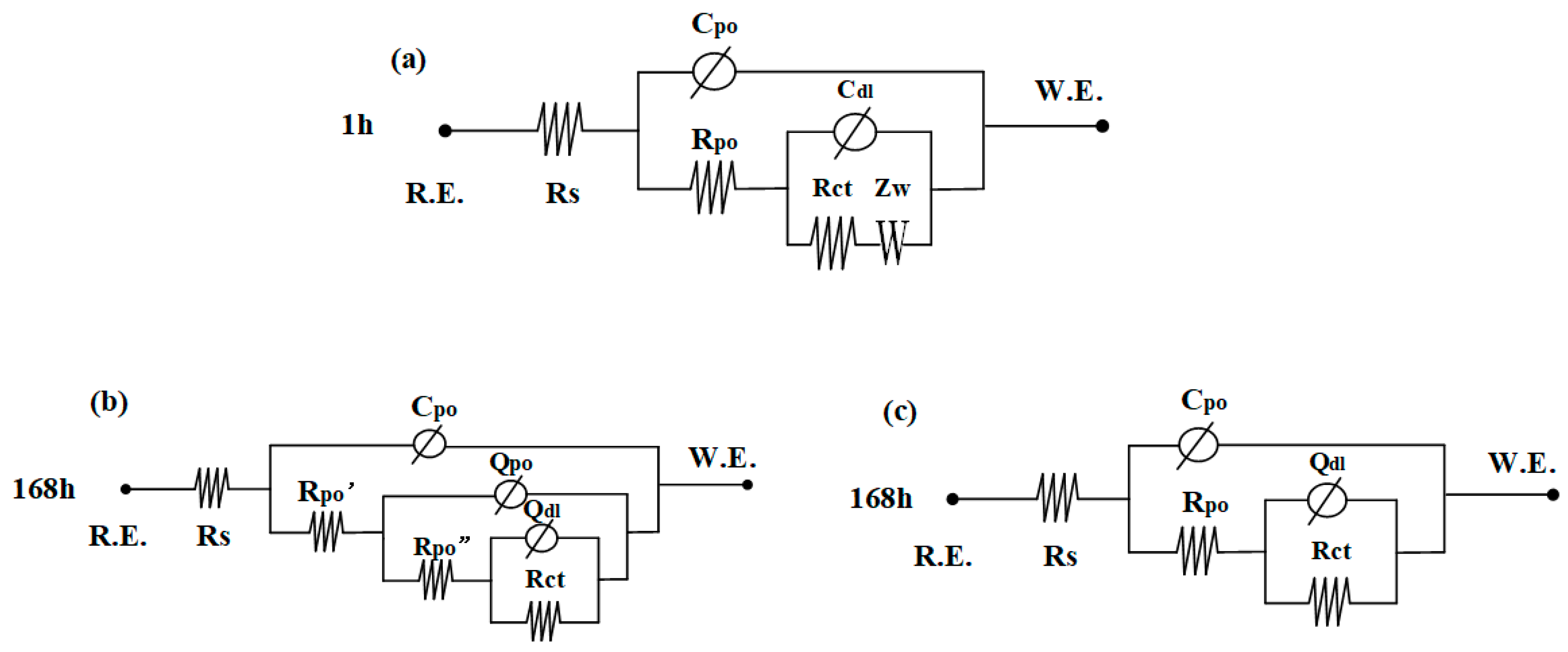



| Component | 1 h | 168 h | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 m/s | 0.2 m/s | 0.4 m/s | 0.6 m/s | 0 m/s | 0.2 m/s | 0.4 m/s | 0.6 m/s | |
| Rs (Ω·cm2) | 4.914 | 5.00 | 9.06 | 8.56 | 5.42 | 5. 62 | 7.88 | 4.33 |
| Cpo (μF/cm2) | 42.41 | 80.07 | 11.88 | 7.67 | 117.0 | 22.45 | 13.29 | 120.9 |
| Rpo (Ω·cm2) | 8.28 | 8.80 | 13.64 | 7.88 | 7.28 | - | 7.24 | 2.15 |
| Cdl (mF/cm2) | 3.87 | 4.25 | 2.30 | 1.11 | - | - | - | - |
| Rct (Ω·cm2) | 57.58 | 116.68 | 200.9 | 336.4 | 199.3 | 196.3 | 142.6 | 141.0 |
| Qdl (mF/cm2) | - | - | - | - | 6.20 | 3.23 | 4.74 | 16.27 |
| Rpo′ (Ω·cm2) | - | - | - | - | - | 4.582 | - | - |
| Qpo (mF/cm2) | - | - | - | - | - | 32.81 | - | - |
| Rpo″ (Ω·cm2) | - | - | - | - | - | 168.9 | - | - |
| Zw (Ω·cm2) | 0.170 | 0.12 | 0.10 | 0.16 | - | - | - | - |
| Parameter | 1 h | 168 h | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 m/s | 0.2 m/s | 0.4 m/s | 0.6 m/s | 0 m/s | 0.2 m/s | 0.4 m/s | 0.6 m/s | |
| Icorr (μA/cm2) | 154.24 | 120.31 | 43.87 | 35.42 | 30.81 | 20.39 | 69.16 | 84.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Feng, Y.; Gao, H.; Wang, L.; Yang, X.; Gu, Y. Erosion–Corrosion Behavior of Friction Stud Welded Joints of X65 Pipelines in Simulated Seawater under Different Flow Rates. Materials 2023, 16, 4326. https://doi.org/10.3390/ma16124326
Zhao J, Feng Y, Gao H, Wang L, Yang X, Gu Y. Erosion–Corrosion Behavior of Friction Stud Welded Joints of X65 Pipelines in Simulated Seawater under Different Flow Rates. Materials. 2023; 16(12):4326. https://doi.org/10.3390/ma16124326
Chicago/Turabian StyleZhao, Jie, Yuqi Feng, Hui Gao, Lei Wang, Xiaoyu Yang, and Yanhong Gu. 2023. "Erosion–Corrosion Behavior of Friction Stud Welded Joints of X65 Pipelines in Simulated Seawater under Different Flow Rates" Materials 16, no. 12: 4326. https://doi.org/10.3390/ma16124326




