Preparation of HfCxN1−x Nanoparticles Derived from a Multifunction Precursor with Hf-O and Hf-N Bonds
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Precursors
2.3. Characterization Methods
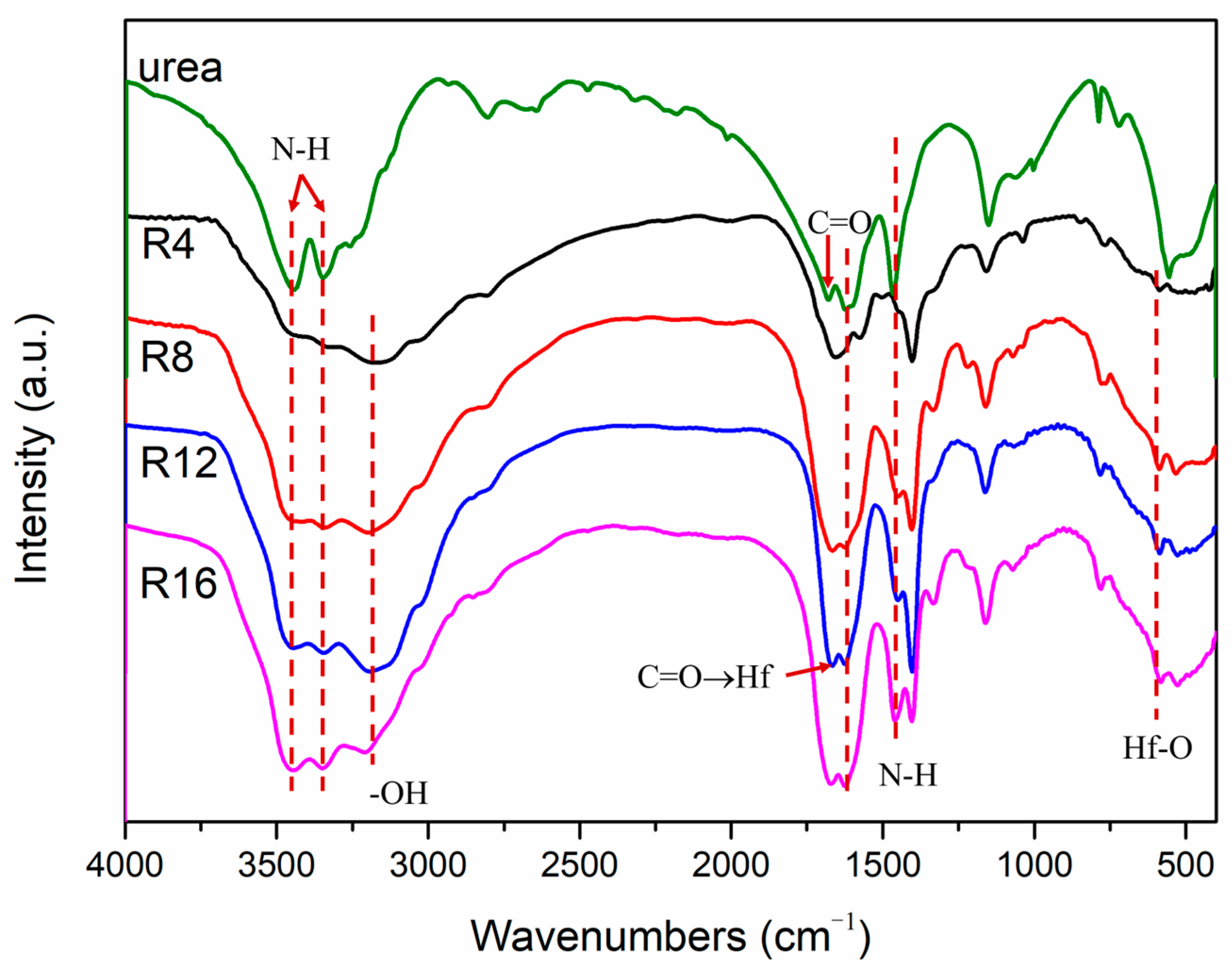
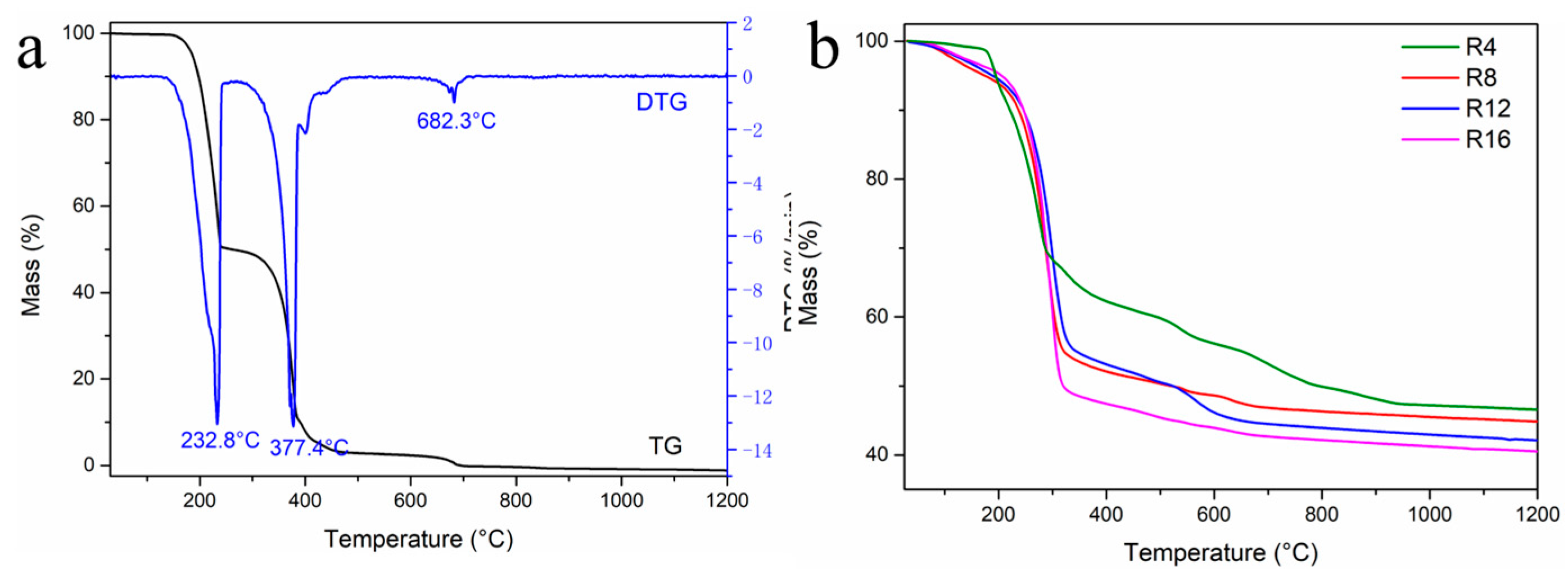
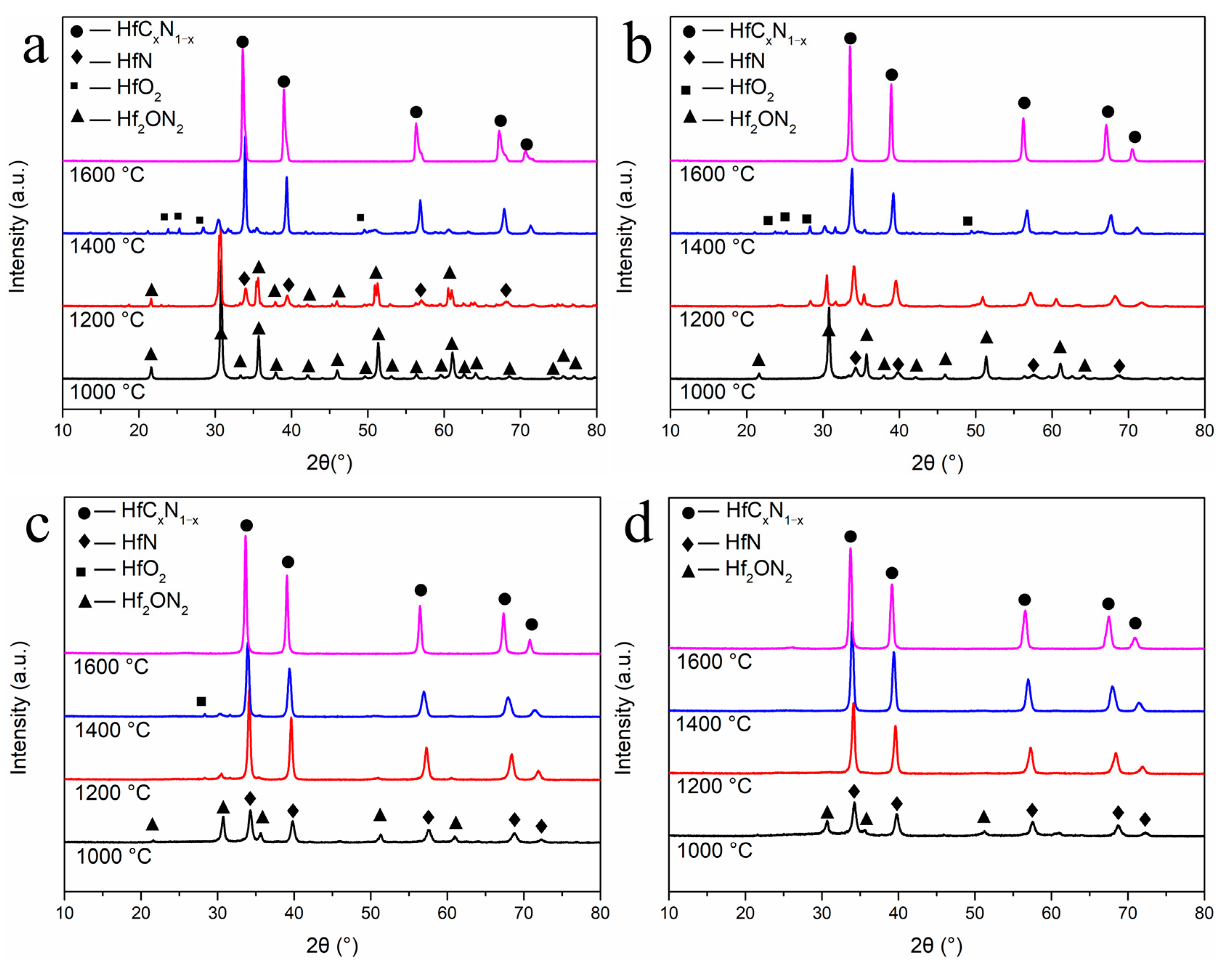
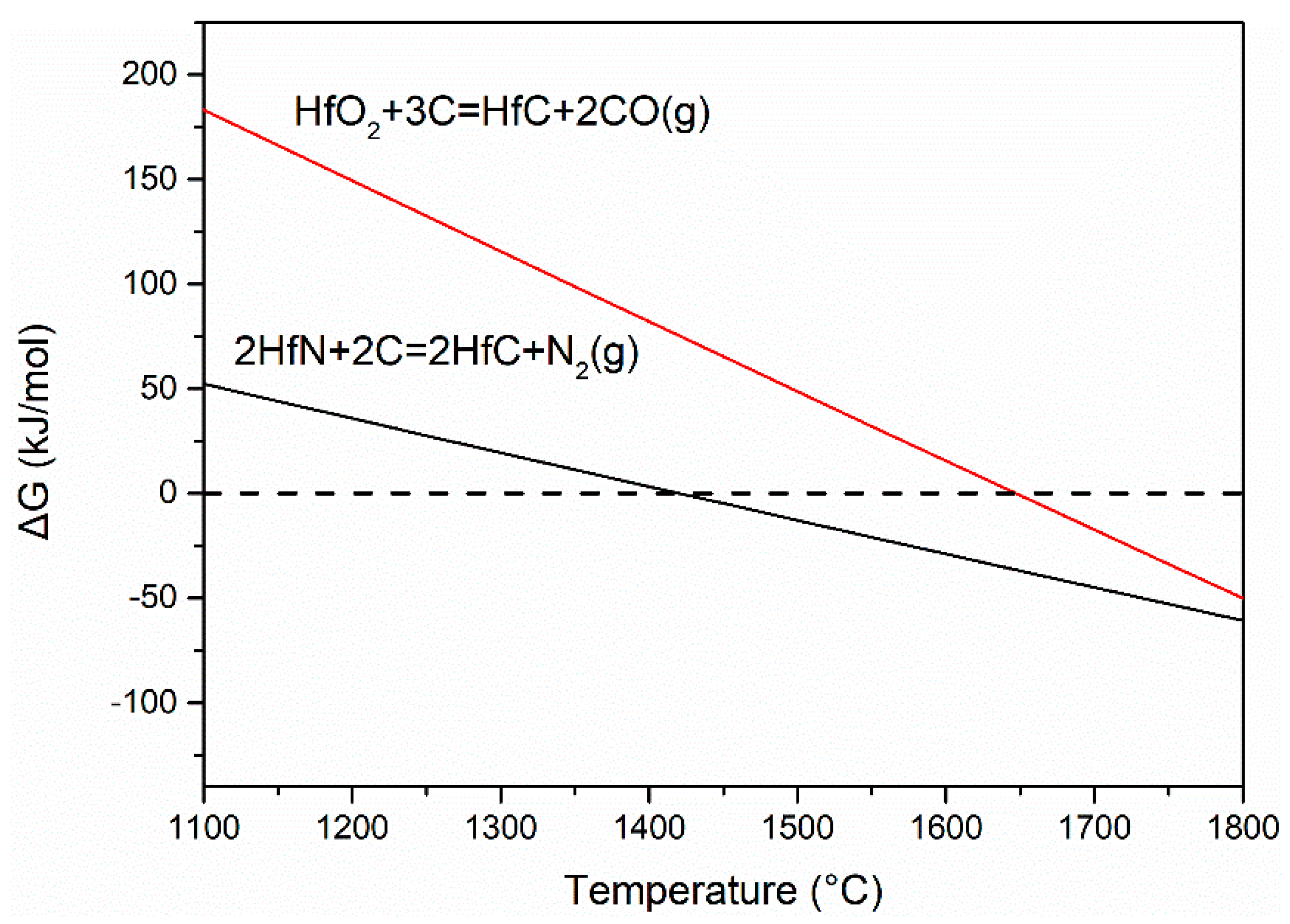
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Golla, B.R.; Mukhopadhyay, A.; Basu, B.; Thimmappa, S.K. Review on ultra-high temperature boride ceramics. Prog. Mater. Sci. 2020, 111, 100651. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G.E. Ultra-high temperature ceramics: Materials for extreme environments. Scr. Mater. 2017, 129, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Sacks, M.D.; Wang, C.-A.; Yang, Z.; Jain, A. Carbothermal reduction synthesis of nanocrystalline zirconium carbide and hafnium carbide powders using solution-derived precursors. J. Mater. Sci. 2004, 39, 6057–6066. [Google Scholar] [CrossRef]
- Gohl, D.; Ruess, H.; Pander, M.; Zeradjanin, A.R.; Mayrhofer, K.J.J.; Schneider, J.M.; Erbe, A.; Ledendecker, M. Transition Metal—Carbon Bond Enthalpies as Descriptor for the Electrochemical Stability of Transition Metal Carbides in Electrocatalytic Applications. J. Electrochem. Soc. 2020, 167, 021501. [Google Scholar] [CrossRef]
- Defilippi, C.; Shinde, D.V.; Dang, Z.; Manna, L.; Hardacre, C.; Greer, A.J.; D’Agostino, C.; Giordano, C. HfN Nanoparticles: An Unexplored Catalyst for the Electrocatalytic Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2019, 58, 15464–15470. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, J.; Adimi, S.; Shen, H.; Thomas, T.; Ma, R.; Attfield, J.P.; Yang, M. Zirconium nitride catalysts surpass platinum for oxygen reduction. Nat. Mater. 2020, 19, 282–286. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, B.; Chen, H.; Xiang, H.; Dai, F.-Z.; Wu, S.; Xu, W. Electromagnetic wave absorbing properties of TMCs (TM=Ti, Zr, Hf, Nb and Ta) and high entropy (Ti0.2Zr0.2Hf0.2Nb0.2Ta0.2)C. J. Mater. Sci. Technol. 2021, 74, 105–118. [Google Scholar] [CrossRef]
- Wen, Q.; Feng, Y.; Yu, Z.; Peng, D.-L.; Nicoloso, N.; Ionescu, E.; Riedel, R. Microwave Absorption of SiC/HfCxN1−x/C Ceramic Nanocomposites with HfCxN1−x-Carbon Core-Shell Particles. J. Am. Ceram. Soc. 2016, 99, 2655–2663. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Z.; Meng, X.; Wang, R. MXene-engineered lithium–sulfur batteries. J. Mater. Chem. A 2019, 7, 22730–22743. [Google Scholar] [CrossRef]
- Yu, Y.D.; Zhou, J.; Sun, Z.M. Novel 2D Transition-Metal Carbides: Ultrahigh Performance Electrocatalysts for Overall Water Splitting and Oxygen Reduction. Adv. Funct. Mater. 2020, 30, 2000570. [Google Scholar] [CrossRef]
- Liang, H.; Fang, L.; Guan, S.; Peng, F.; Zhang, Z.; Chen, H.; Zhang, W.; Lu, C. Insights into the Bond Behavior and Mechanical Properties of Hafnium Carbide under High Pressure and High Temperature. Inorg. Chem. 2021, 60, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Silvestroni, L.; Bellosi, A.; Melandri, C.; Sciti, D.; Liu, J.; Zhang, G. Microstructure and properties of HfC and TaC-based ceramics obtained by ultrafine powder. J. Eur. Ceram. Soc. 2011, 31, 619–627. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Zhang, J.; Fu, Y.; Tian, S. Effects of HfC nanowire amount on the microstructure and ablation resistance of CVD-HfC coating. Ceram. Int. 2018, 44, 11340–11349. [Google Scholar] [CrossRef]
- Baklanova, N.; Zima, T.; Boronin, A.; Kosheev, S.; Titov, A.; Isaeva, N.; Graschenkov, D.; Solntsev, S. Protective ceramic multilayer coatings for carbon fibers. Surf. Coat. Technol. 2006, 201, 2313–2319. [Google Scholar] [CrossRef]
- Tong, M.; Fu, Q.; Zhou, L.; Feng, T.; Li, H.; Li, T.; Li, K. Ablation behavior of a novel HfC-SiC gradient coating fabricated by a facile one-step chemical vapor co-deposition. J. Eur. Ceram. Soc. 2018, 38, 4346–4355. [Google Scholar] [CrossRef]
- Li, F.; Huang, X.; Liu, J.-X.; Zhang, G.-J. Sol-gel derived porous ultra-high temperature ceramics. J. Adv. Ceram. 2020, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Wang, J.; Wang, X.; Wang, H. Preparation and high-temperature performance of HfC-based nanocomposites derived from precursor with Hf-(O,N) bonds. Ceram. Int. 2017, 43, 7159–7165. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, X.; Wang, J.; Wang, H. Synthesis of a novel single-source precursor for HfC ceramics and its feasibility for the preparation of Hf-based ceramic fibres. Ceram. Int. 2018, 44, 7305–7309. [Google Scholar] [CrossRef]
- Inzenhofer, K.; Schmalz, T.; Wrackmeyer, B.; Motz, G. The preparation of HfC/C ceramics via molecular design. Dalton Trans. 2011, 40, 4741–4745. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, F.; Han, W.; Zhao, T. Synthesis and pyrolysis of non-oxide precursors for ZrC/SiC and HfC/SiC composite ceramics. Ceram. Int. 2020, 46, 22102–22107. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Yin, X.; Han, L.; Fu, Q.; Li, H.; Riedel, R. Two birds with one stone: Simultaneous fabrication of HfC nanowires and CNTs through efficient utilization of polymer-derived ceramics. J. Mater. Sci. Technol. 2022, 129, 163–172. [Google Scholar] [CrossRef]
- Zeng, G.; Xu, P.; Zeng, C.; Fang, C.; Wang, Y.; Yang, X.; Zhang, M.; Su, Z.; Huang, Q. Synthesis and pyrolysis of single-source precursor for HfCxN1−x-SiC ceramic with different SiC contents. Ceram. Int. 2022, 48, 22967–22974. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Wang, H. Synthesis and properties of a precursor derived nano Zr(C, N)-carbon composite coating on SiC fibers. Mater. Des. 2016, 94, 214–220. [Google Scholar] [CrossRef]
- Yuan, F.-Y.; Deng, N.; Shih, C.-C.; Tseng, Y.-T.; Chang, T.-C.; Chang, K.-C.; Wang, M.-H.; Chen, W.-C.; Zheng, H.-X.; Wu, H.; et al. Conduction Mechanism and Improved Endurance in HfO2-Based RRAM with Nitridation Treatment. Nanoscale Res. Lett. 2017, 12, 574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, Y.-H.; Chiu, H.-T.; Kuo, T.-F.; Chi, C.-C.; Chuang, S.-H. Intriguing conducting properties of HfOxNy thin films prepared from the Hf[N(C2H5)2]4. Appl. Phys. Lett. 2006, 89, 252901. [Google Scholar] [CrossRef] [Green Version]
- Yate, L.; Coy, L.E.; Aperador, W. Robust tribo-mechanical and hot corrosion resistance of ultra-refractory Ta-Hf-C ternary alloy films. Sci. Rep. 2017, 7, 3080. [Google Scholar] [CrossRef] [Green Version]
- Wen, Q.; Yu, Z.; Xu, Y.; Lu, Y.; Fasel, C.; Morita, K.; Guillon, O.; Buntkowsky, G.; Ionescu, E.; Riedel, R. SiC/HfyTa1−yCxN1−x/C ceramic nanocomposites with HfyTa1−yCxN1−x-carbon core–shell nanostructure and the influence of the carbon-shell thickness on electrical properties. J. Mater. Chem. C 2018, 6, 855–864. [Google Scholar] [CrossRef]



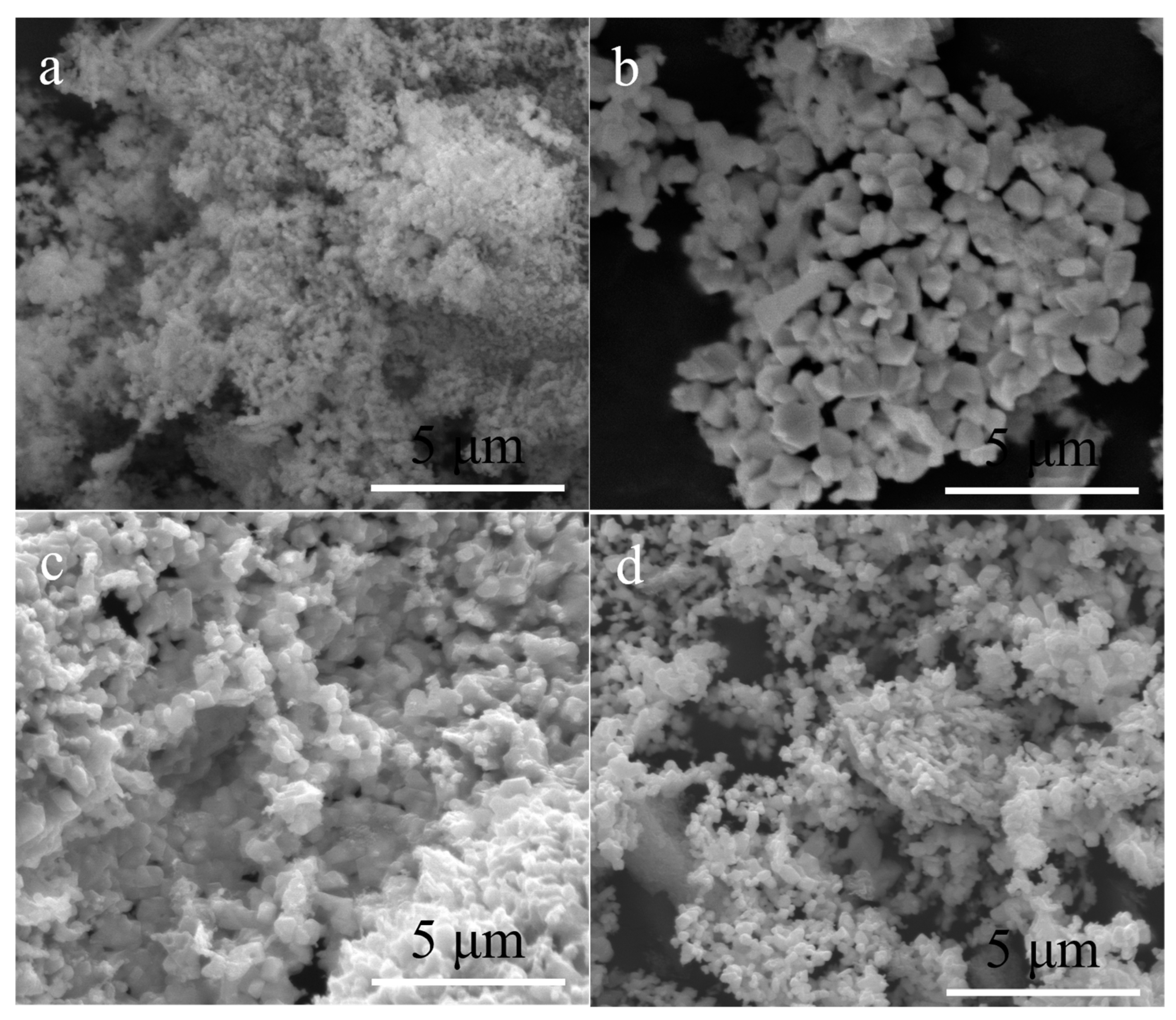
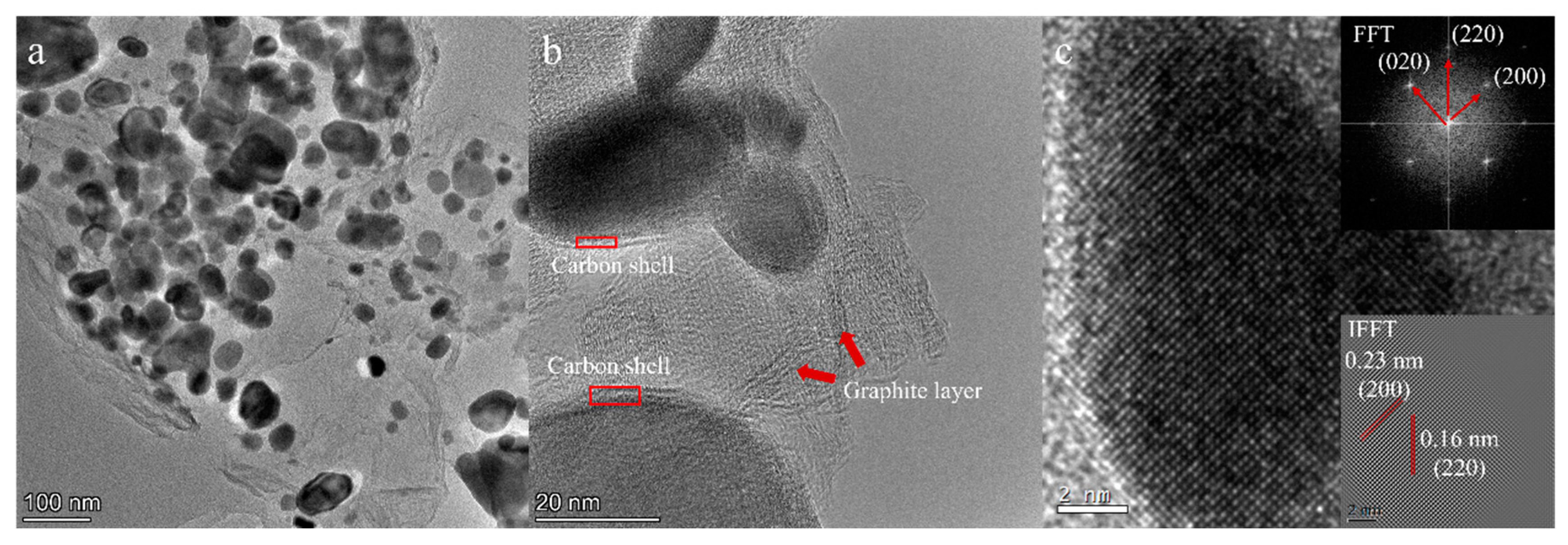
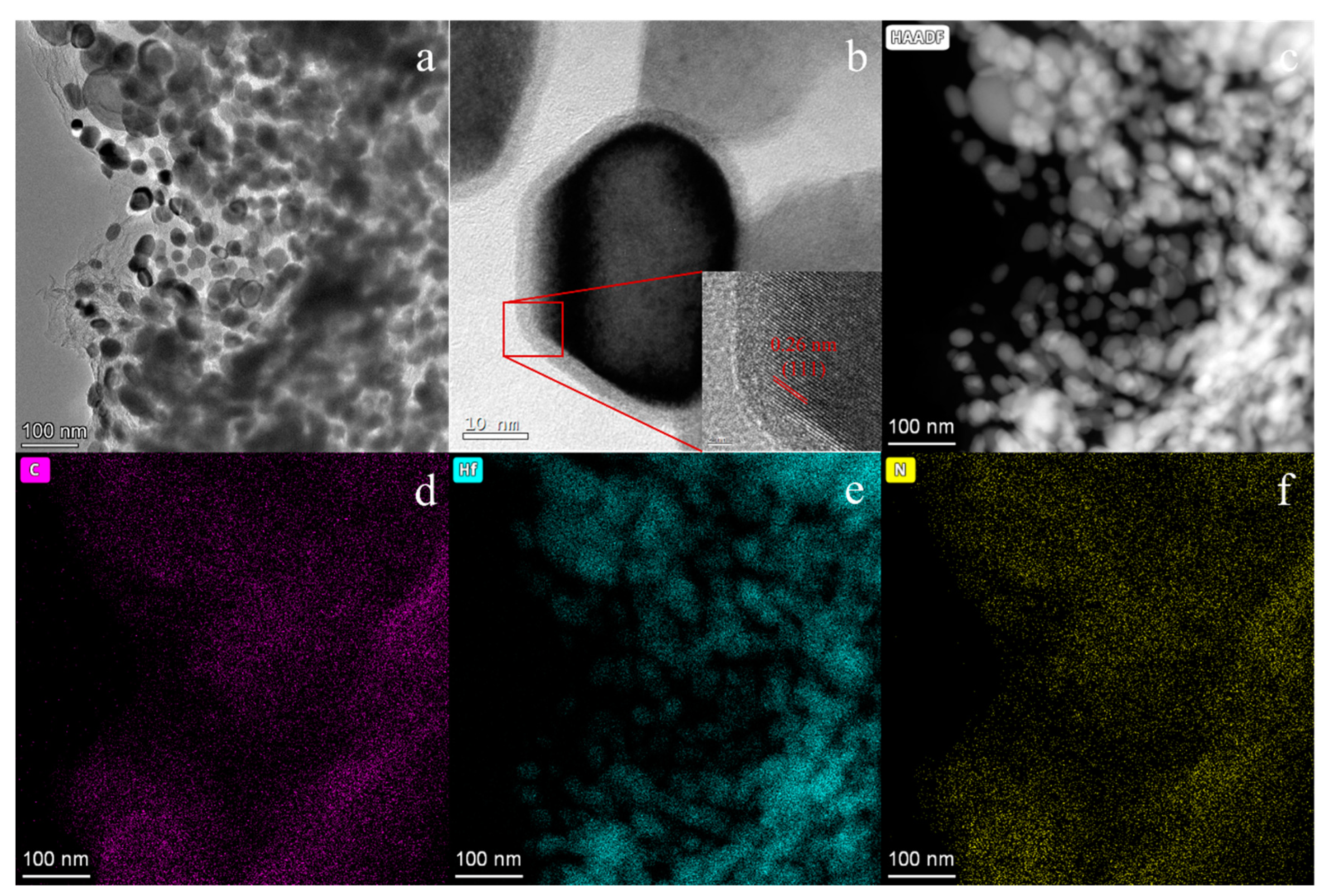
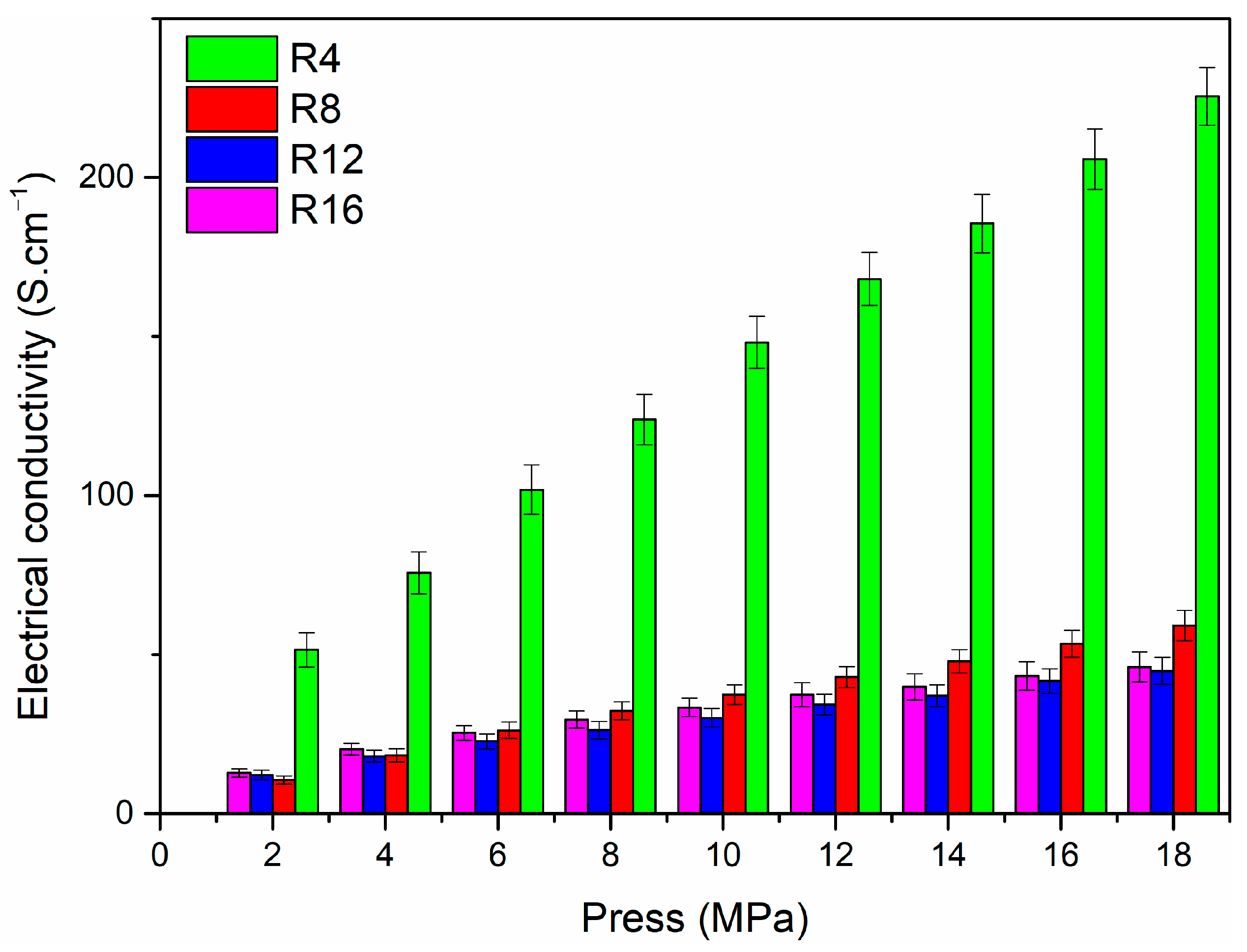
| Precursor | Urea (mol) | HfCl4 (mol) | Methanol (mL) |
|---|---|---|---|
| R4 | 0.04 | 0.01 | 20 |
| R8 | 0.08 | 0.01 | 20 |
| R12 | 0.12 | 0.01 | 20 |
| R16 | 0.16 | 0.01 | 20 |
| Sample | Lattice Parameters | HfCxN1−x | Estimated Grain Size | Elemental Analysis (wt%) | |
|---|---|---|---|---|---|
| C | N | ||||
| R4-1400 | 0.45894 | HfC0.57N0.43 | 58.7 nm | n.d. | n.d. |
| R4-1600 | 0.46264 | HfC0.90N0.10 | 25.6 nm | 5.48 | 2.0 |
| R8-1400 | 0.45824 | HfC0.51N0.49 | 47.7 nm | n.d. | n.d. |
| R8-1600 | 0.46224 | HfC0.86N0.14 | 46.5 nm | 9.89 | 1.08 |
| R12-1400 | 0.45728 | HfC0.42N0.58 | 63.6 nm | n.d. | n.d. |
| R12-1600 | 0.46119 | HfC0.77N0.23 | 38.2 nm | 14.9 | 1.01 |
| R16-1200 | 0.45467 | HfC0.19N0.81 | 41.5 nm | n.d. | n.d. |
| R16-1400 | 0.45720 | HfC0.41N0.59 | 72.2 nm | n.d. | n.d. |
| R16-1600 | 0.46013 | HfC0.68N0.32 | 45.5 nm | 19.3 | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, G.; Xu, P.; Zeng, C.; Huang, Q.; Su, Z. Preparation of HfCxN1−x Nanoparticles Derived from a Multifunction Precursor with Hf-O and Hf-N Bonds. Materials 2023, 16, 4426. https://doi.org/10.3390/ma16124426
Zeng G, Xu P, Zeng C, Huang Q, Su Z. Preparation of HfCxN1−x Nanoparticles Derived from a Multifunction Precursor with Hf-O and Hf-N Bonds. Materials. 2023; 16(12):4426. https://doi.org/10.3390/ma16124426
Chicago/Turabian StyleZeng, Guang, Ping Xu, Chen Zeng, Qizhong Huang, and Zhean Su. 2023. "Preparation of HfCxN1−x Nanoparticles Derived from a Multifunction Precursor with Hf-O and Hf-N Bonds" Materials 16, no. 12: 4426. https://doi.org/10.3390/ma16124426





