Interdependence of Surface Roughness on Icephobic Performance: A Review
Abstract
:1. Introduction
2. Hydrophobicity-Induced Icephobicity
2.1. Theory of Hydrophobicity
2.2. Hydrophobicity-Induced Icephobicity
2.3. Limitations and the Role of Surface Roughness in Hydrophobicity-Induced Icephobicity
3. Role of Surface Roughness in Ice Formation
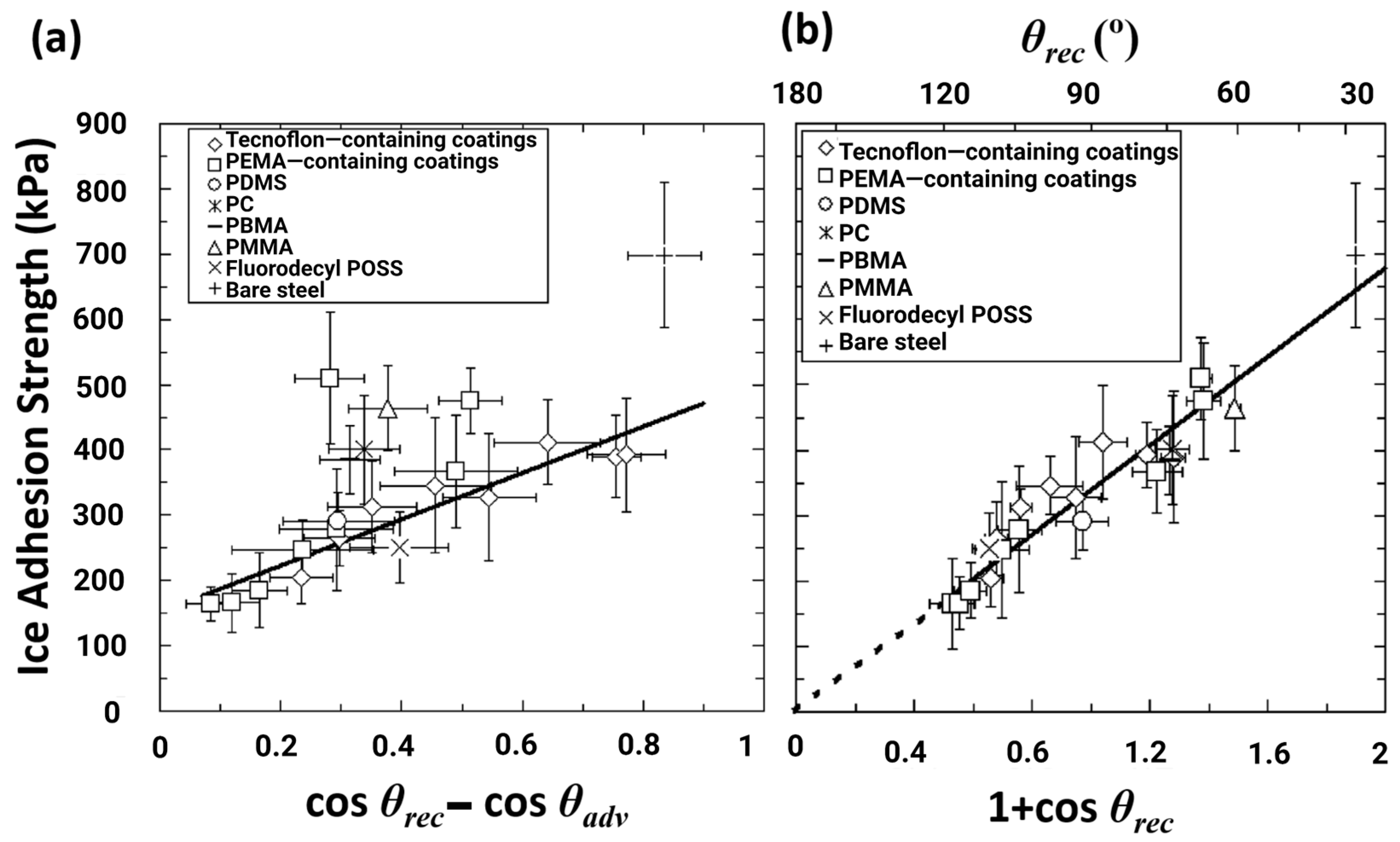
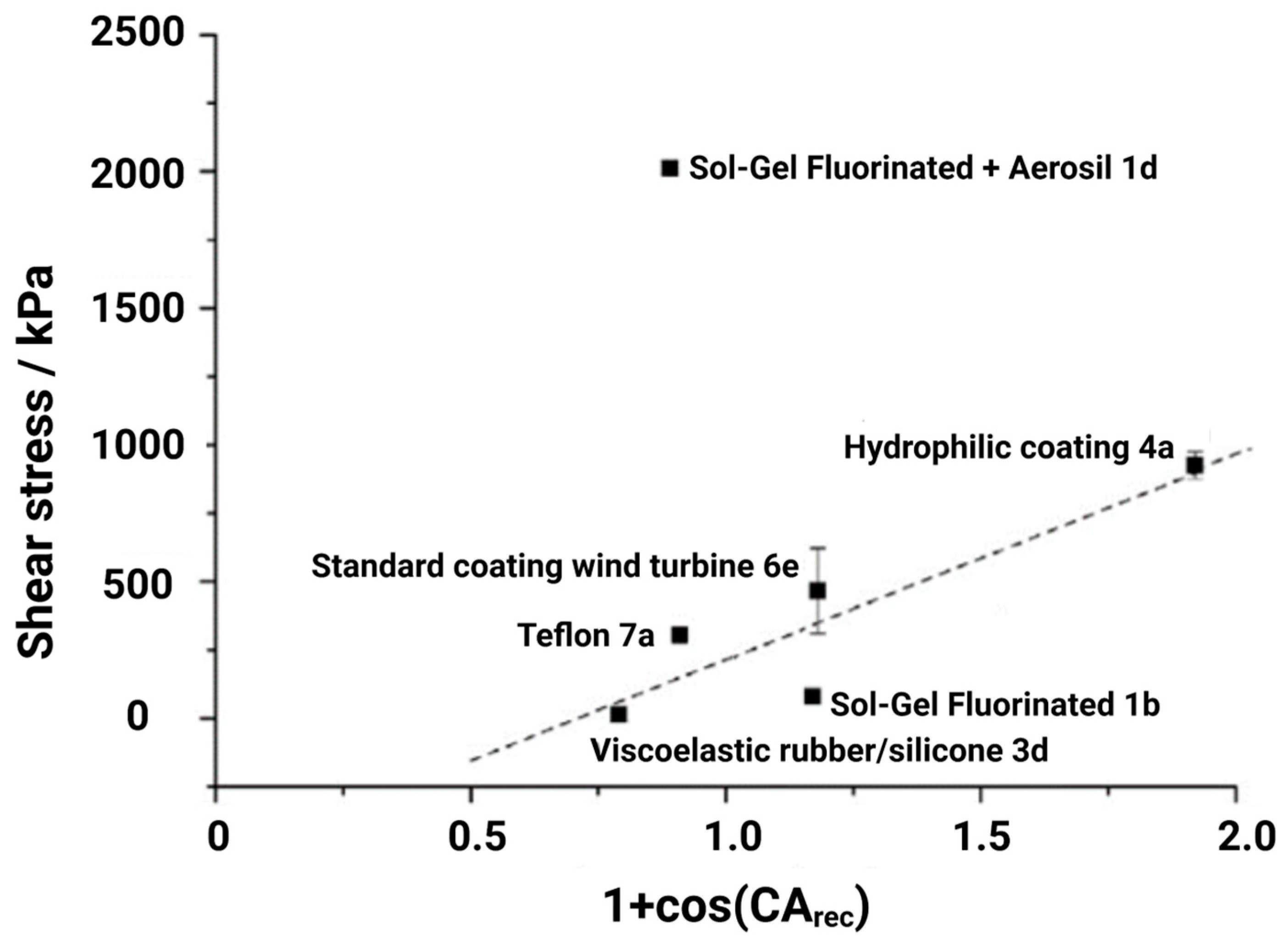
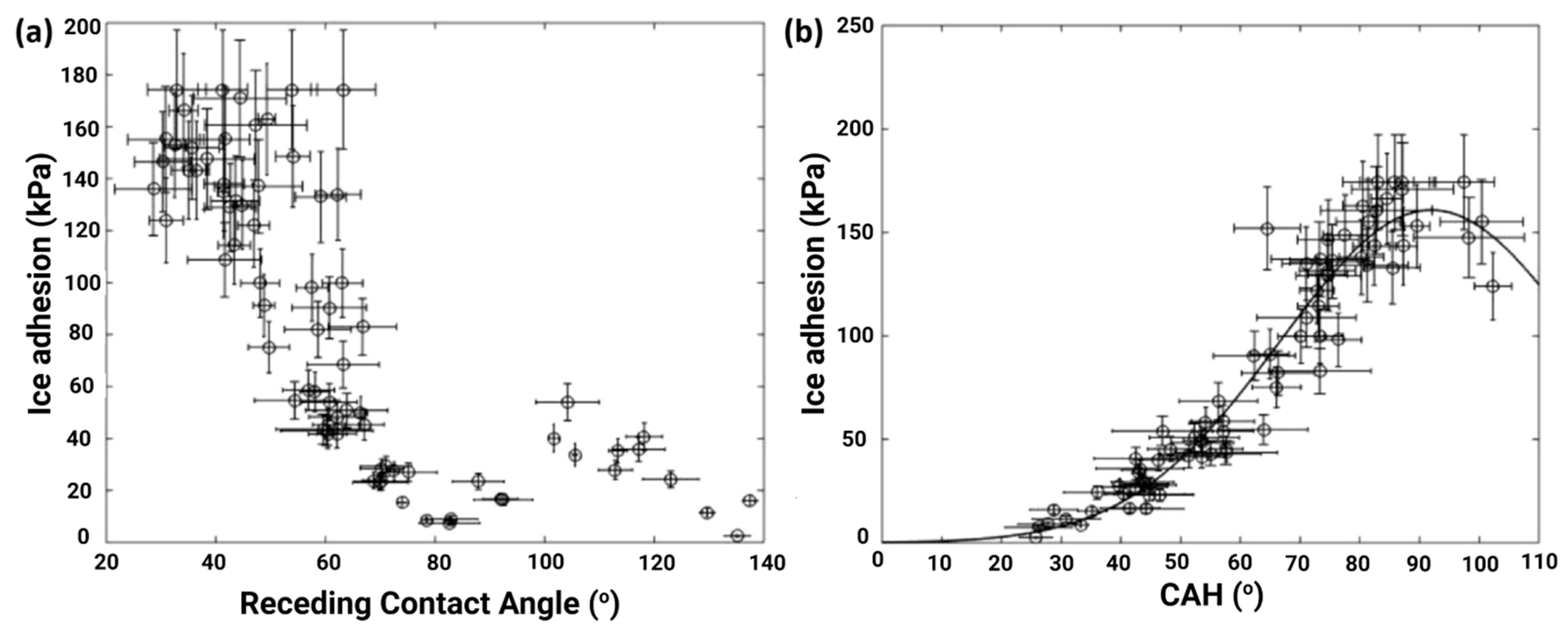
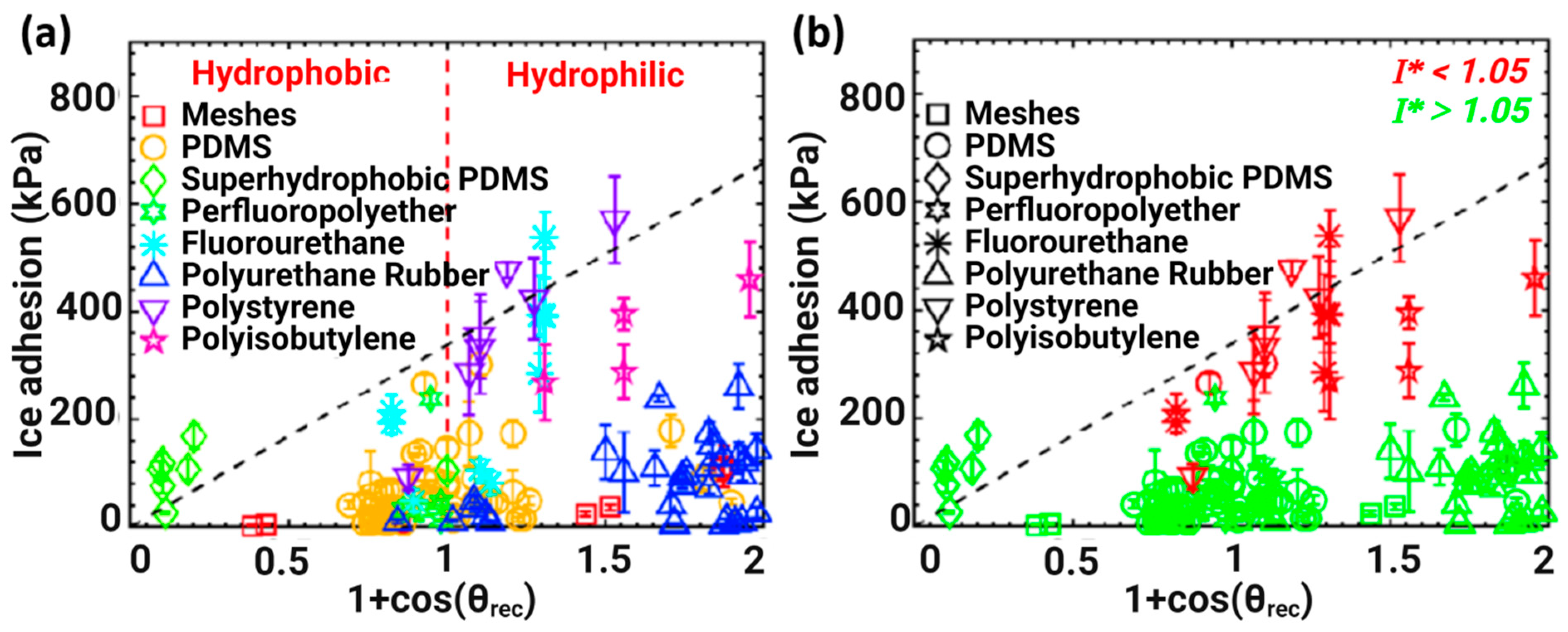
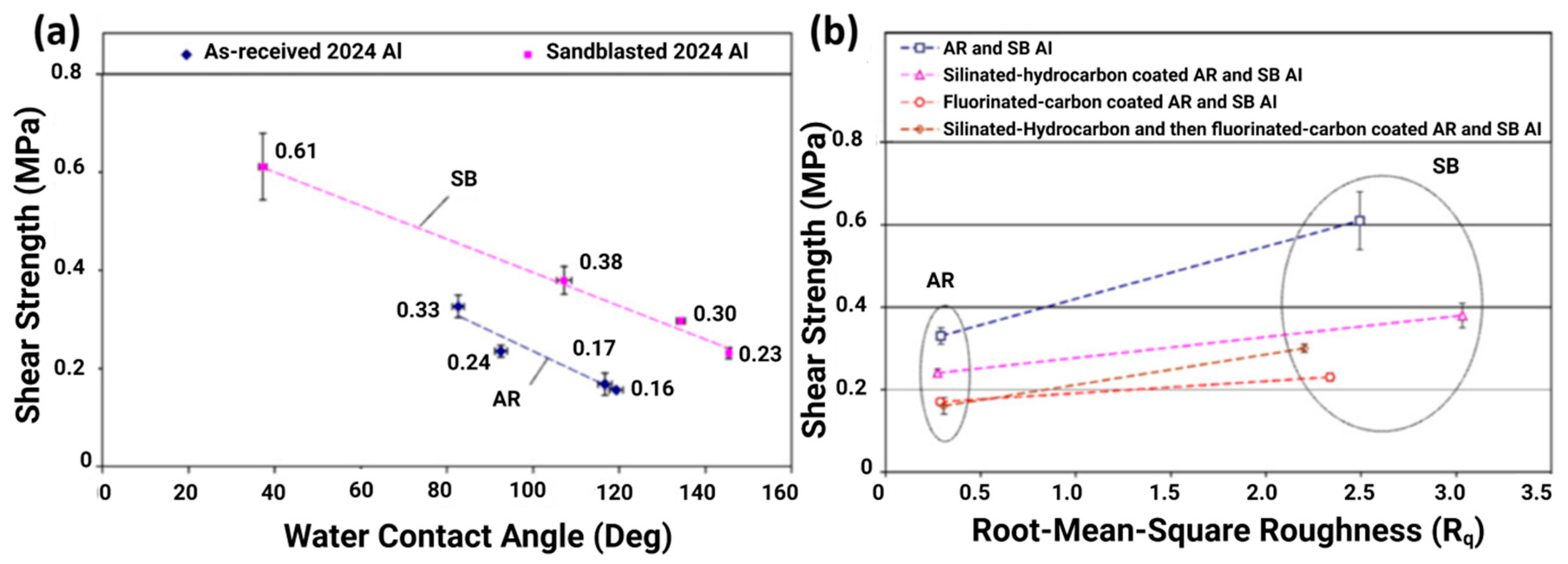
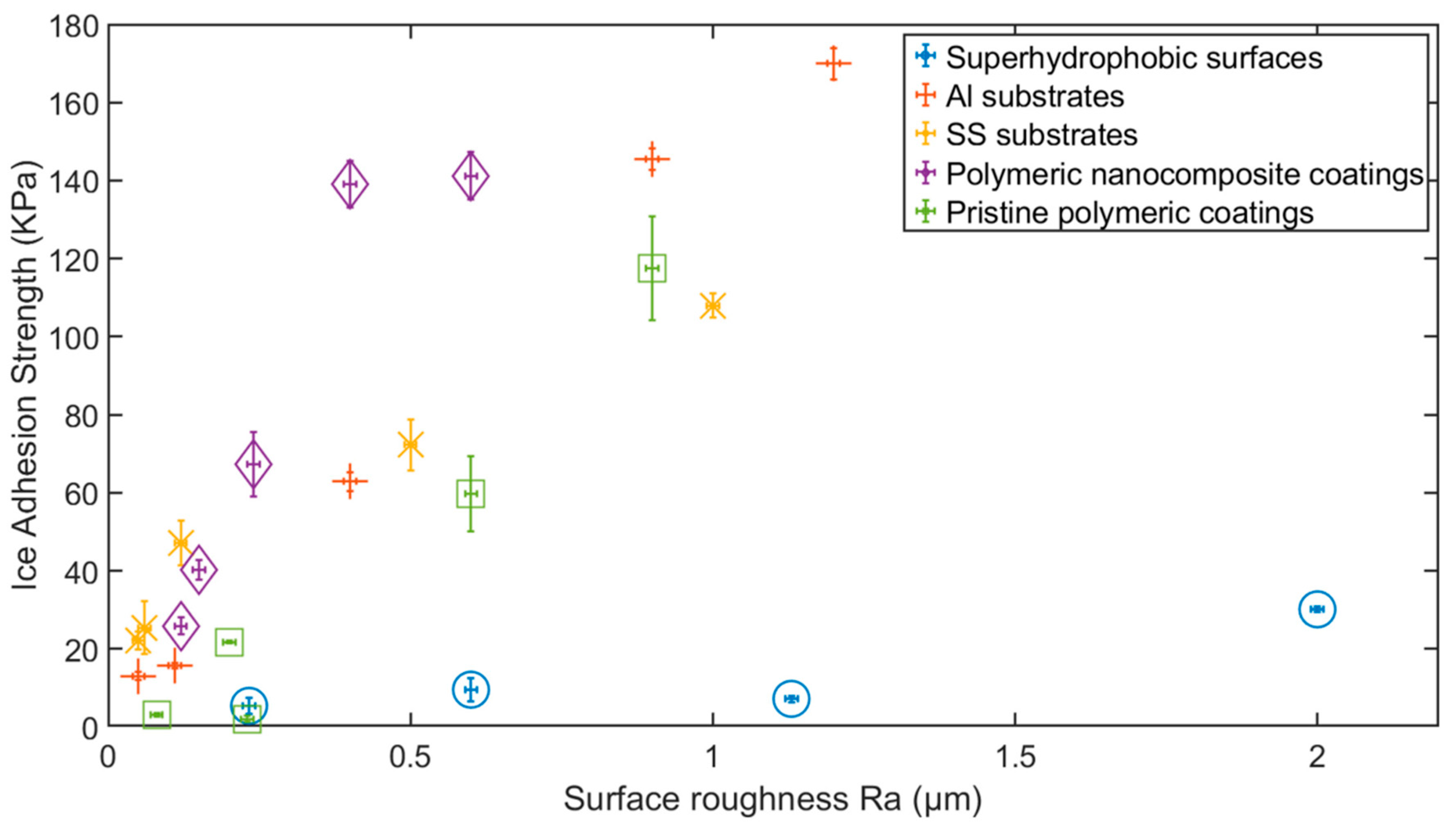
3.1. Influence of Surface Roughness on Ice Adhesion and Hydrophobicity-Induced Icephobicity
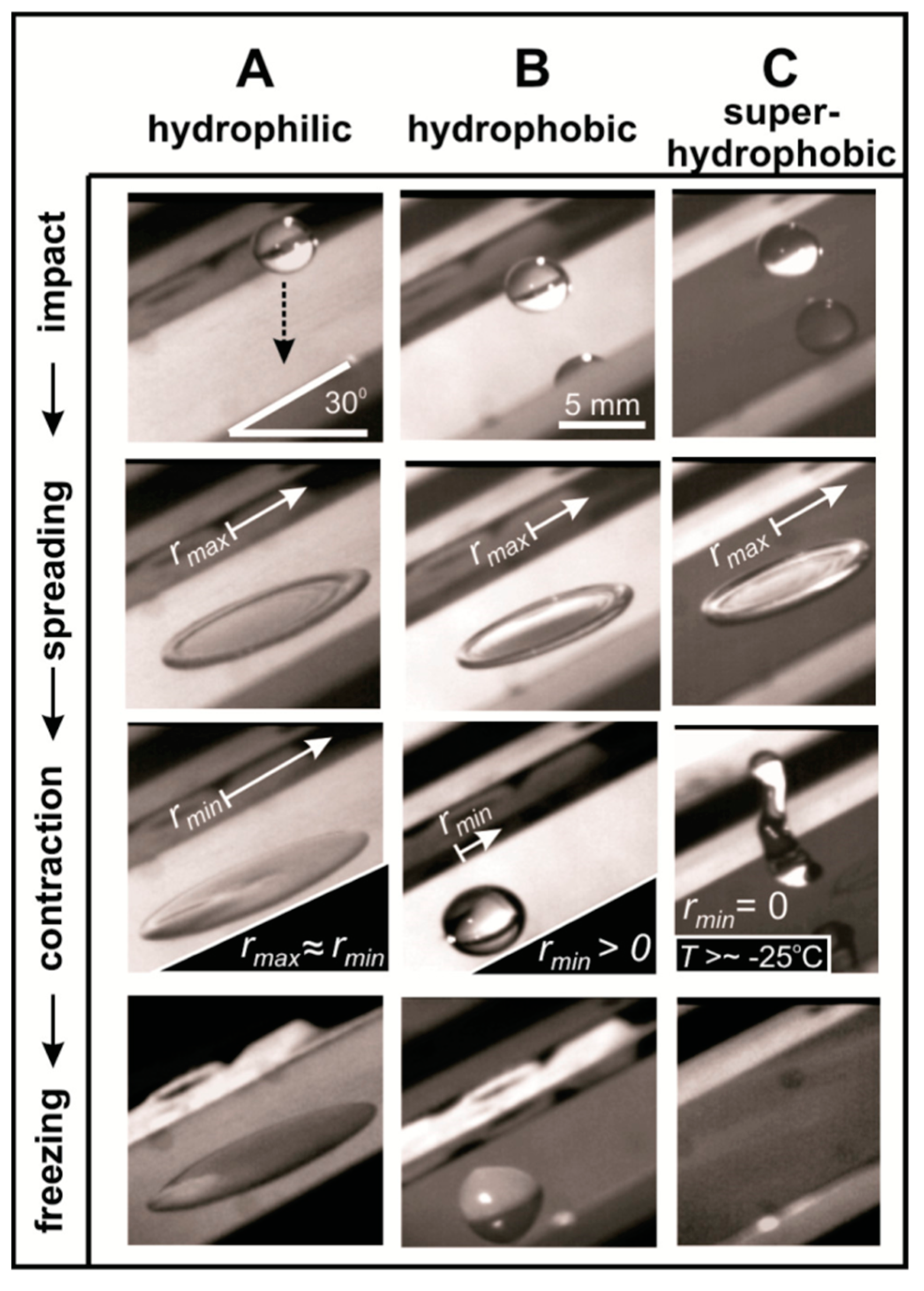
3.2. The Role of Surface Roughness on Ice Nucleation and Thermodynamics
3.3. Nanostructured Surfaces and Surface Texturing
4. Mechanism of Roughness-Dependent Ice Breakage on Elastomers and SLIPSs
4.1. Understanding Low-Ice-Adhesion Polymeric Surfaces and SLIPSs
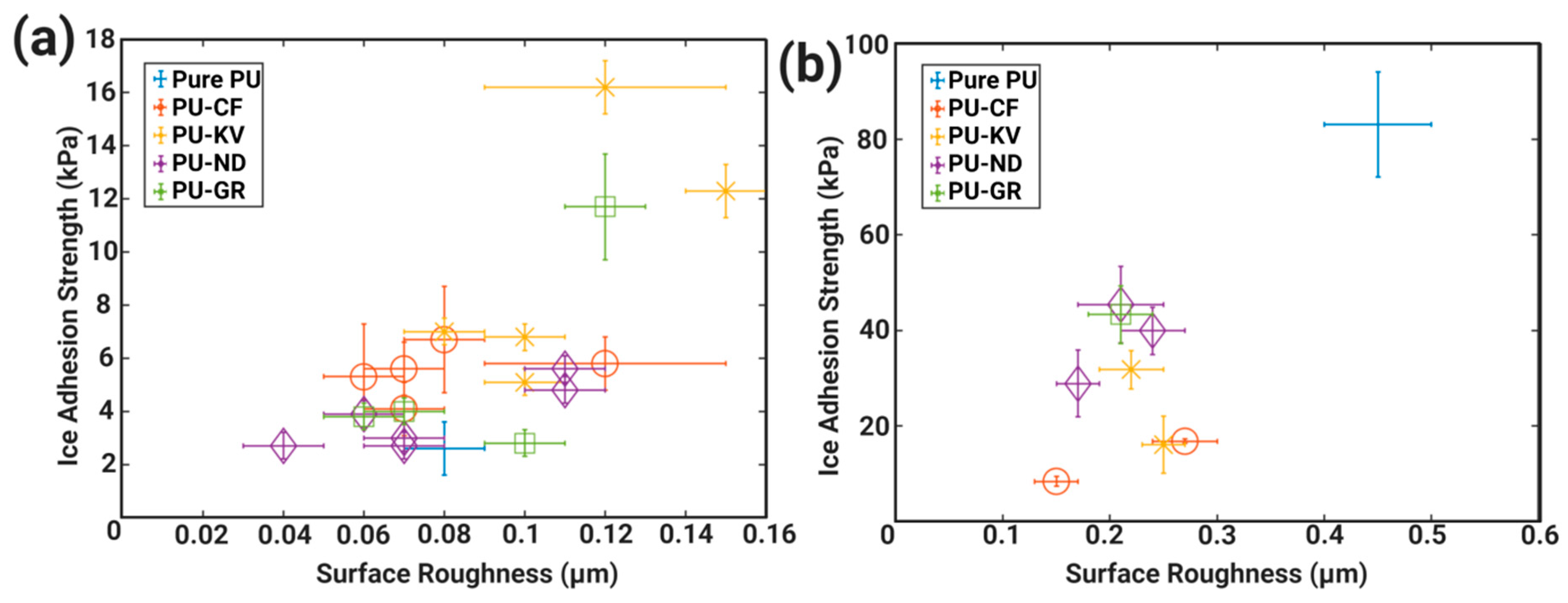
4.2. Altering Polymeric Cross-Link Densities and Elasticity
5. Sensitivity of Surface Roughness in De-Icing Evaluation
5.1. Current Situation of De-Icing Evaluation
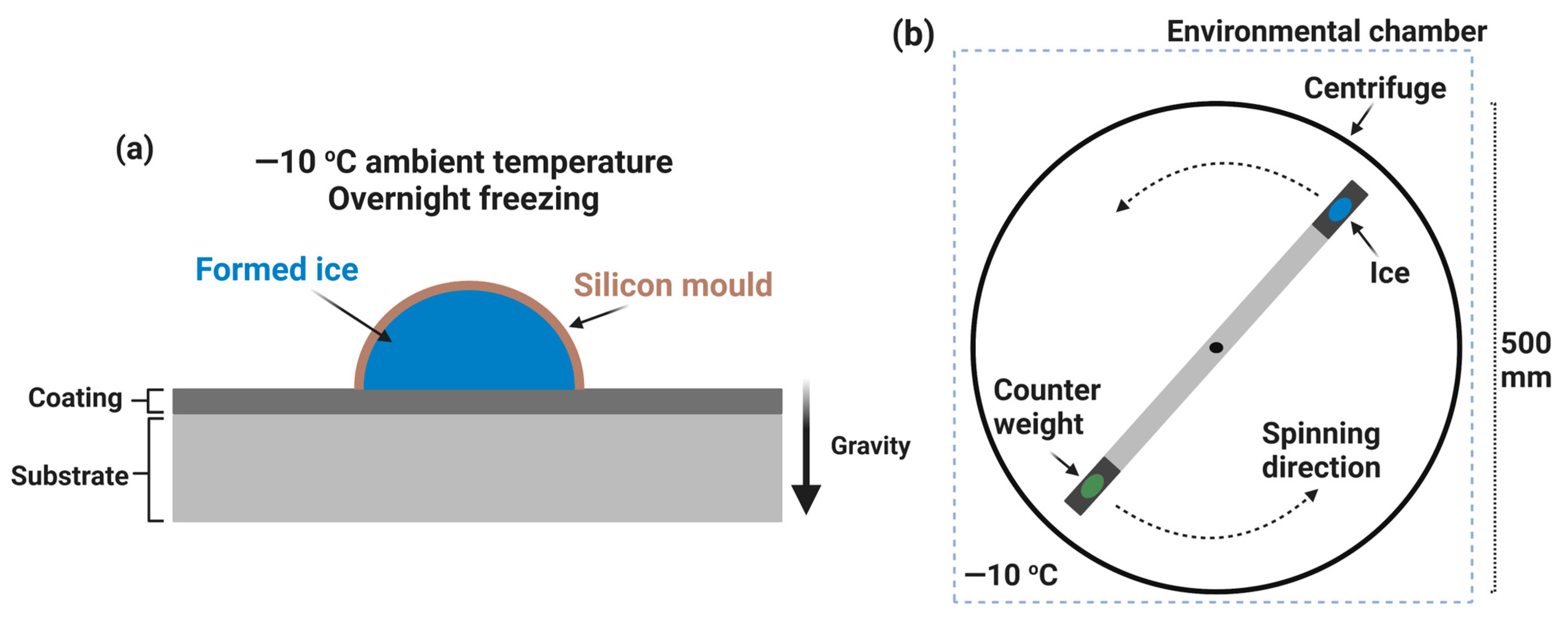
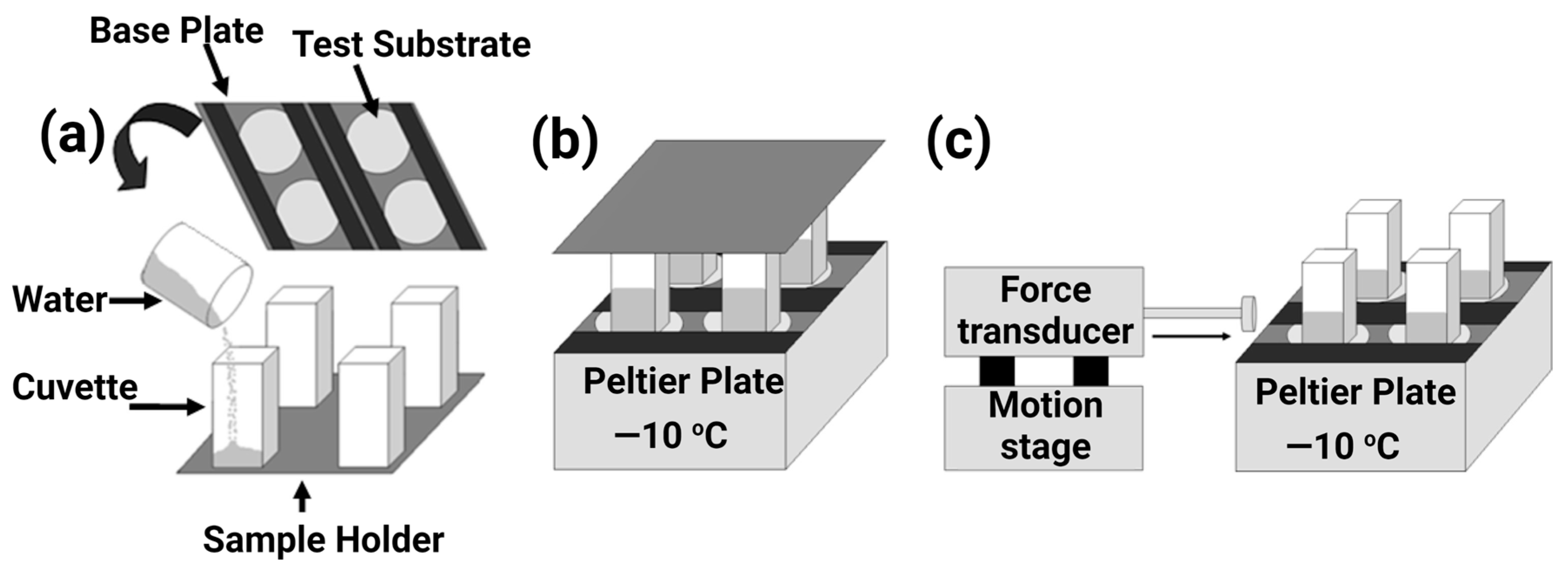
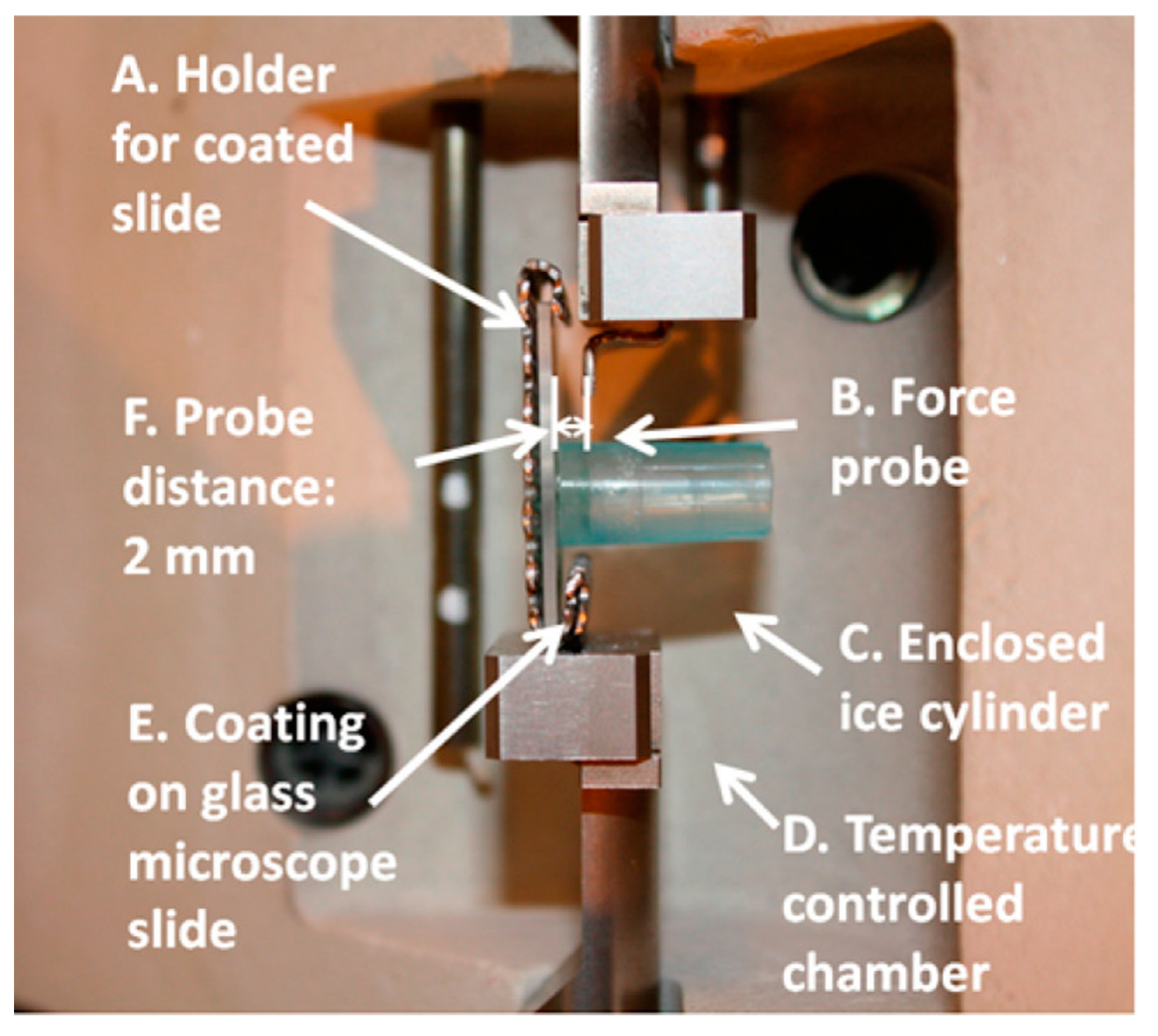
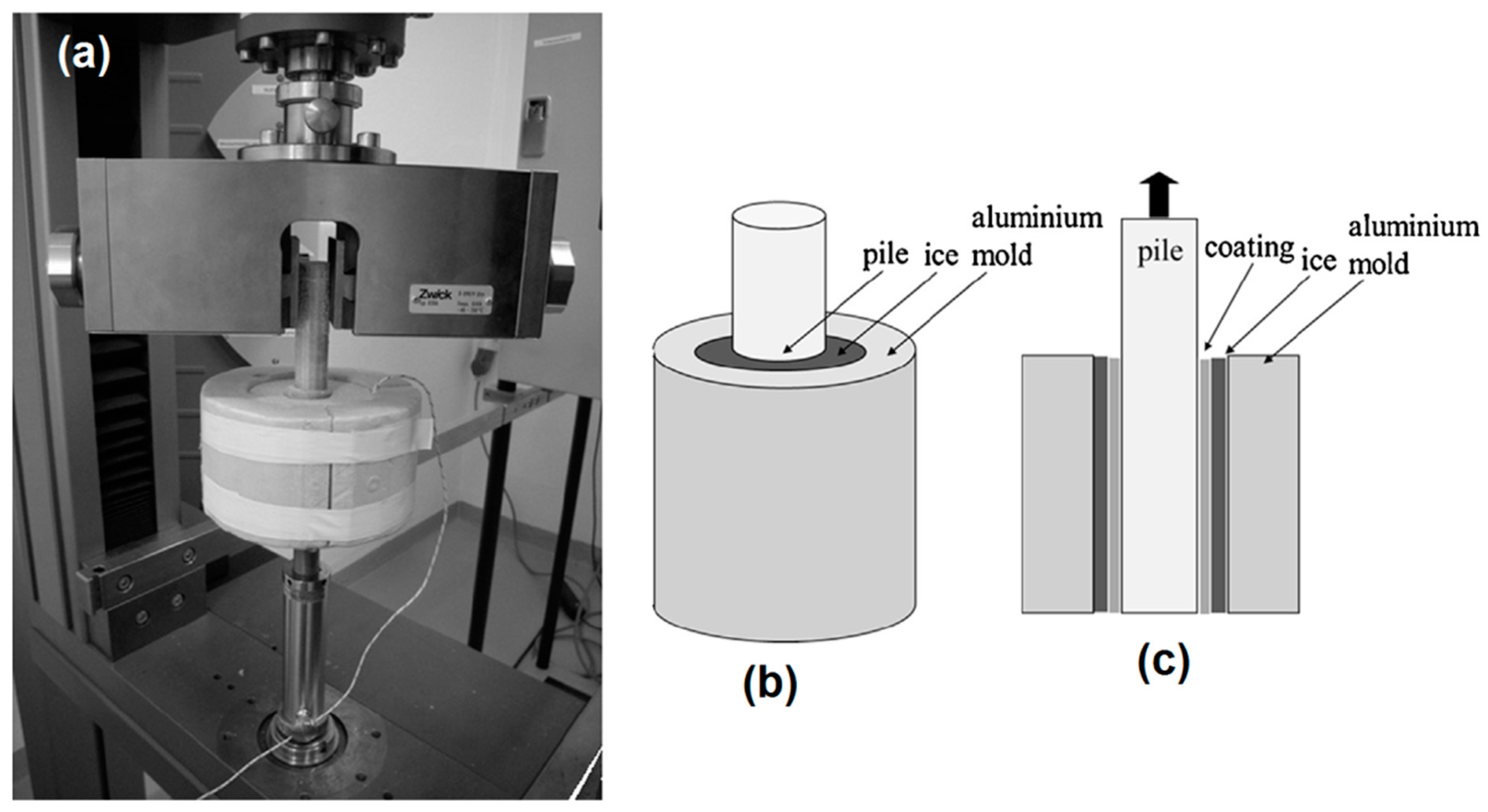
5.2. Major De-Icing Evaluation Methodologies
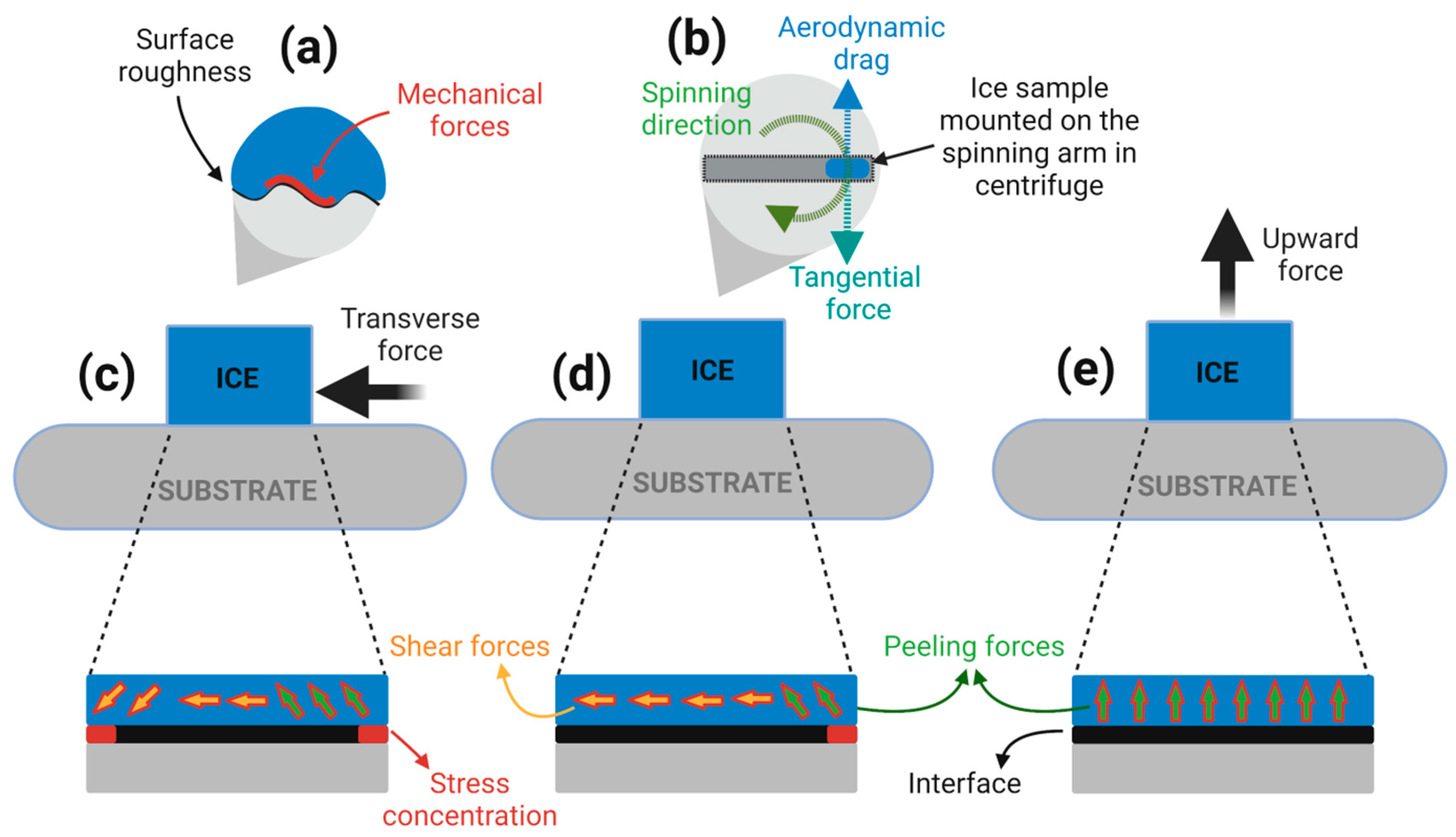
5.3. Ice/Surface Interfacial Fracture Mechanics and the Sensitivity on Surface Roughness
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhuo, Y.; Xiao, S.; Amirfazli, A.; He, J.; Zhang, Z. Polysiloxane as icephobic materials—The past, present and the future. Chem. Eng. J. 2021, 405, 127088. [Google Scholar] [CrossRef]
- Dhyani, A.; Choi, W.; Golovin, K.; Tuteja, A. Surface design strategies for mitigating ice and snow accretion. Matter 2022, 5, 1423–1454. [Google Scholar] [CrossRef]
- Bragg, M. Aircraft aerodynamic effects due to large droplet ice accretions. In Proceedings of the 34th Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 15–18 January 1996; Volume 932. [Google Scholar] [CrossRef] [Green Version]
- Mossayebi, Z.; Jafari, V.F.; Gurr, P.A.; Simons, R.; Qiao, G.G. Reduced Ice Adhesion Using Amphiphilic Poly (Ionic Liquid)-Based Surfaces. ACS Appl. Mater. Interfaces 2023, 15, 7454–7465. [Google Scholar] [CrossRef]
- Chatterjee, R.; Bararnia, H.; Anand, S. A Family of Frost-Resistant and Icephobic Coatings. Adv. Mater. 2022, 34, 2109930. [Google Scholar] [CrossRef]
- Shi, K.; Duan, X. A review of ice protection techniques for structures in the Arctic and offshore harsh environments. J. Offshore Mech. Arct. Eng. 2021, 143, 064502. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.S.; Cho, H.J.; Oh, S.; Shim, J.; Nam, Y.; Sohn, S.H.; Jung, Y.C.; Han, S.C.; Lee, S.Y. Evaluation of icephobic and dissipation performance on coating materials for preventing overhead transmission line from ice/snow damages. Polymer 2020, 44, 342–348. [Google Scholar]
- Gao, L.; Liu, Y.; Ma, L.; Hu, H. A hybrid strategy combining minimized leading-edge electric-heating and superhydro-/ice-phobic surface coating for wind turbine icing mitigation. Renew. Energy 2019, 140, 943–956. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Z.; Gao, L.; Liu, Y.; Hu, H. An exploratory study on using Slippery-Liquid-Infused-Porous-Surface (SLIPS) for wind turbine icing mitigation. Renew. Energy 2020, 162, 2344–2360. [Google Scholar] [CrossRef]
- Fillion, R.; Riahi, A.; Edrisy, A. A review of icing prevention in photovoltaic devices by surface engineering. Renew. Sustain. Energy Rev. 2014, 32, 797–809. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, G.; Li, C.; Wu, J. Functional microdroplet self-dislodging icephobic surfaces: A review from mechanism to synergic morphology. Appl. Therm. Eng. 2022, 215, 118928. [Google Scholar] [CrossRef]
- Huang, X.; Tepylo, N.; Pommier-Budinger, V.; Budinger, M.; Bonaccurso, E.; Villedieu, P.; Bennani, L. A survey of icephobic coatings and their potential use in a hybrid coating/active ice protection system for aerospace applications. Prog. Aerosp. Sci. 2019, 105, 74–97. [Google Scholar] [CrossRef] [Green Version]
- Cober, S.G.; Isaac, G.A.; Strapp, J.W. Characterizations of aircraft icing environments that include supercooled large drops. J. Appl. Meteorol. 2001, 40, 1984–2002. [Google Scholar] [CrossRef]
- Kharina, A.; Rutherford, D. Fuel Efficiency Trends for New Commercial Jet Aircraft: 1960 to 2014; International Council on Clean Transportation: Washington DC, USA, 2015. [Google Scholar]
- Wood, L. Global Aircraft De-Icing Market (2022 to 2027)—Industry Trends, Share, Size, Growth, Opportunity and Forecasts. 2022. Available online: ResearchAndMarkets.com (accessed on 24 February 2023).
- BusinessTraveller. Snow disruption to cost BA £50 m; BusinessTraveller: London, UK, 2011. [Google Scholar]
- Department for Business, Energy & Industrial Strategy. British Energy Security Strategy; Department for Business, Energy & Industrial Strategy: London, UK, 2022.
- Shinkafi, A.; Lawson, C. Enhanced method of conceptual sizing of aircraft electro-thermal de-icing system. Int. J. Aerosp. Mech. Eng. 2014, 8, 1073–1080. [Google Scholar]
- Liu, M.; Wang, S.; Jiang, L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Wu, X.; Yang, F.; Lu, G.; Zhao, X.; Chen, Z.; Qian, S. A breathable and environmentally friendly superhydrophobic coating for anti-condensation applications. Chem. Eng. J. 2021, 412, 128725. [Google Scholar] [CrossRef]
- Wu, X.; Silberschmidt, V.V.; Hu, Z.-T.; Chen, Z. When superhydrophobic coatings are icephobic: Role of surface topology. Surf. Coat. Technol. 2019, 358, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, J.; Dodiuk, H.; Kenig, S.; Ratto, J.A.; Barry, C.; Mead, J. The reduction in ice adhesion using controlled topography superhydrophobic coatings. J. Coat. Technol. Res. 2023, 20, 469–483. [Google Scholar] [CrossRef]
- Yeong, Y.H.; Milionis, A.; Loth, E.; Sokhey, J. Self-lubricating icephobic elastomer coating (SLIC) for ultralow ice adhesion with enhanced durability. Cold Reg. Sci. Technol. 2018, 148, 29–37. [Google Scholar] [CrossRef]
- Irajizad, P.; Al-Bayati, A.; Eslami, B.; Shafquat, T.; Nazari, M.; Jafari, P.; Kashyap, V.; Masoudi, A.; Araya, D.; Ghasemi, H. Stress-localized durable icephobic surfaces. Mater. Horiz. 2019, 6, 758–766. [Google Scholar] [CrossRef]
- Idriss, H.; Guselnikova, O.; Postnikov, P.; Kolská, Z.; Haušild, P.; Lyutakov, O.; Švorčík, V. Polymer icephobic surface by graphite coating and chemical grafting with diazonium salts. Surf. Interfaces 2021, 25, 101226. [Google Scholar] [CrossRef]
- Elzaabalawy, A.; Meguid, S.A. Development of novel icephobic surfaces using siloxane-modified epoxy nanocomposites. Chem. Eng. J. 2022, 433, 133637. [Google Scholar] [CrossRef]
- Wang, P.; Li, Z.; Xie, Q.; Duan, W.; Zhang, X.; Han, H. A passive anti-icing strategy based on a superhydrophobic mesh with extremely low ice adhesion strength. J. Bionic Eng. 2021, 18, 55–64. [Google Scholar] [CrossRef]
- Shen, Y.; Xie, X.; Xie, Y.; Tao, J.; Jiang, J.; Chen, H.; Lu, Y.; Xu, Y. Statistically understanding the roles of nanostructure features in interfacial ice nucleation for enhancing icing delay performance. Phys. Chem. Chem. Phys. 2019, 21, 19785–19794. [Google Scholar] [CrossRef]
- Golovin, K.; Kobaku, S.P.; Lee, D.H.; DiLoreto, E.T.; Mabry, J.M.; Tuteja, A. Designing durable icephobic surfaces. Sci. Adv. 2016, 2, e1501496. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Qi, H.; Fan, D.; Yang, J.; Zhang, L. Beetle and mussel-inspired chimeric protein for fabricating anti-icing coating. Colloids Surf. B Biointerfaces 2022, 210, 112252. [Google Scholar] [CrossRef]
- Gao, H.; Jian, Y.; Yan, Y. The effects of bio-inspired micro/nano scale structures on anti-icing properties. Soft Matter 2021, 17, 447–466. [Google Scholar] [CrossRef]
- Ozbay, S.; Erbil, H.Y. Ice accretion by spraying supercooled droplets is not dependent on wettability and surface free energy of substrates. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 210–218. [Google Scholar] [CrossRef]
- Jeon, J.; Jang, H.; Chang, J.; Lee, K.-S.; Kim, D.R. Fabrication of micro-patterned aluminum surfaces for low ice adhesion strength. Appl. Surf. Sci. 2018, 440, 643–650. [Google Scholar] [CrossRef]
- Jung, S.; Dorrestijn, M.; Raps, D.; Das, A.; Megaridis, C.M.; Poulikakos, D. Are superhydrophobic surfaces best for icephobicity? Langmuir 2011, 27, 3059–3066. [Google Scholar] [CrossRef]
- Eberle, P.; Tiwari, M.K.; Maitra, T.; Poulikakos, D. Rational nanostructuring of surfaces for extraordinary icephobicity. Nanoscale 2014, 6, 4874–4881. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.-Y. Control of ice nucleation: Freezing and antifreeze strategies. Chem. Soc. Rev. 2018, 47, 7116–7139. [Google Scholar] [CrossRef]
- Dong, J.; Yao, Z.; Yang, T.; Jiang, L.; Shen, C. Control of superhydrophilic and superhydrophobic graphene interface. Sci. Rep. 2013, 3, 1733. [Google Scholar] [CrossRef] [Green Version]
- Meuler, A.J.; McKinley, G.H.; Cohen, R.E. Exploiting topographical texture to impart icephobicity. ACS Nano 2010, 4, 7048–7052. [Google Scholar] [CrossRef] [Green Version]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic surfaces: Insights from theory and experiment. J. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef] [Green Version]
- Semprebon, C.; McHale, G.; Kusumaatmaja, H. Apparent contact angle and contact angle hysteresis on liquid infused surfaces. Soft Matter 2017, 13, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irajizad, P.; Nazifi, S.; Ghasemi, H. Icephobic surfaces: Definition and figures of merit. Adv. Colloid Interface Sci. 2019, 269, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, J.; Ling, S.; Wheatley, R.; Hou, X. Microporous metallic scaffolds supported liquid infused icephobic construction. J. Colloid Interface Sci. 2023, 634, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Karmouch, R.; Ross, G.G. Experimental study on the evolution of contact angles with temperature near the freezing point. J. Phys. Chem. C 2010, 114, 4063–4066. [Google Scholar] [CrossRef]
- Sojoudi, H.; Wang, M.; Boscher, N.; McKinley, G.; Gleason, K. Durable and scalable icephobic surfaces: Similarities and distinctions from superhydrophobic surfaces. Soft Matter 2016, 12, 1938–1963. [Google Scholar] [CrossRef] [Green Version]
- Sojoudi, H.; Arabnejad, H.; Raiyan, A.; Shirazi, S.A.; McKinley, G.H.; Gleason, K.K. Scalable and durable polymeric icephobic and hydrate-phobic coatings. Soft Matter 2018, 14, 3443–3454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, D.; Clanet, C.; Quéré, D. Surface phenomena: Contact time of a bouncing drop. Nature 2002, 417, 811. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.K.; Bico, J.; Teo, K.B.; Chhowalla, M.; Amaratunga, G.A.; Milne, W.I.; McKinley, G.H.; Gleason, K.K. Superhydrophobic carbon nanotube forests. Nano Lett. 2003, 3, 1701–1705. [Google Scholar] [CrossRef] [Green Version]
- Kulinich, S.; Farzaneh, M. How wetting hysteresis influences ice adhesion strength on superhydrophobic surfaces. Langmuir 2009, 25, 8854–8856. [Google Scholar] [CrossRef] [PubMed]
- Dotan, A.; Dodiuk, H.; Laforte, C.; Kenig, S. The relationship between water wetting and ice adhesion. J. Adhes. Sci. Technol. 2009, 23, 1907–1915. [Google Scholar] [CrossRef]
- Petrenko, V.; Peng, S. Reduction of ice adhesion to metal by using self-assembling monolayers (SAMs). Can. J. Phys. 2003, 81, 387–393. [Google Scholar] [CrossRef]
- Meuler, A.J.; Smith, J.D.; Varanasi, K.K.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Relationships between water wettability and ice adhesion. ACS Appl. Mater. Interfaces 2010, 2, 3100–3110. [Google Scholar] [CrossRef]
- Gao, L.; McCarthy, T.J. Wetting 101°. Langmuir 2009, 25, 14105–14115. [Google Scholar] [CrossRef]
- Furmidge, C. Studies at phase interfaces. I. The sliding of liquid drops on solid surfaces and a theory for spray retention. J. Colloid Sci. 1962, 17, 309–324. [Google Scholar] [CrossRef]
- Young, T., III. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- Dupre, A. Chapter IX, Actions moleculaires (suite). In Theorie Mecanique de la Chaleur; Gauthier-Villars: Paris, France, 1869. [Google Scholar]
- Shen, Y.; Wu, X.; Tao, J.; Zhu, C.; Lai, Y.; Chen, Z. Icephobic materials: Fundamentals, performance evaluation, and applications. Prog. Mater. Sci. 2019, 103, 509–557. [Google Scholar] [CrossRef]
- Gao, L.; McCarthy, T.J. Teflon is hydrophilic. Comments on definitions of hydrophobic, shear versus tensile hydrophobicity, and wettability characterization. Langmuir 2008, 24, 9183–9188. [Google Scholar] [CrossRef] [PubMed]
- Mittal, K.L. Adhesion Measurement of Thim Films, Thick Films and Bulk Coatings; American Society for Testing and Materials (ASTM): Philadelphia, PA, USA, 1978; pp. 7–8. [Google Scholar]
- Cassie, A.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Della Volpe, C.; Maniglio, D.; Siboni, S.; Morra, M. An experimental procedure to obtain the equilibrium contact angle from the Wilhelmy method. Oil Gas Sci. Technol. 2001, 56, 9–22. [Google Scholar] [CrossRef]
- Marmur, A. Soft contact: Measurement and interpretation of contact angles. Soft Matter 2006, 2, 12–17. [Google Scholar] [CrossRef]
- Kulinich, S.; Farzaneh, M. Ice adhesion on super-hydrophobic surfaces. Appl. Surf. Sci. 2009, 255, 8153–8157. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing superoleophobic surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef] [Green Version]
- Bharathidasan, T.; Kumar, S.V.; Bobji, M.; Chakradhar, R.; Basu, B.J. Effect of wettability and surface roughness on ice-adhesion strength of hydrophilic, hydrophobic and superhydrophobic surfaces. Appl. Surf. Sci. 2014, 314, 241–250. [Google Scholar] [CrossRef]
- Susoff, M.; Siegmann, K.; Pfaffenroth, C.; Hirayama, M. Evaluation of icephobic coatings—Screening of different coatings and influence of roughness. Appl. Surf. Sci. 2013, 282, 870–879. [Google Scholar] [CrossRef] [Green Version]
- Janjua, Z.A.; Turnbull, B.; Choy, K.-L.; Pandis, C.; Liu, J.; Hou, X.; Choi, K.-S. Performance and durability tests of smart icephobic coatings to reduce ice adhesion. Appl. Surf. Sci. 2017, 407, 555–564. [Google Scholar] [CrossRef]
- Kim, P.; Wong, T.-S.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-infused nanostructured surfaces with extreme anti-ice and anti-frost performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Y.; Liu, S.; Zhou, F. Material Strategies for Ice Accretion Prevention and Easy Removal. ACS Mater. Lett. 2022, 4, 246–262. [Google Scholar] [CrossRef]
- Cui, W.; Jiang, Y.; Mielonen, K.; Pakkanen, T.A. The verification of icephobic performance on biomimetic superhydrophobic surfaces and the effect of wettability and surface energy. Appl. Surf. Sci. 2019, 466, 503–514. [Google Scholar] [CrossRef]
- Cao, L.; Jones, A.K.; Sikka, V.K.; Wu, J.; Gao, D. Anti-icing superhydrophobic coatings. Langmuir 2009, 25, 12444–12448. [Google Scholar] [CrossRef]
- Mishchenko, L.; Hatton, B.; Bahadur, V.; Taylor, J.A.; Krupenkin, T.; Aizenberg, J. Design of ice-free nanostructured surfaces based on repulsion of impacting water droplets. ACS Nano 2010, 4, 7699–7707. [Google Scholar] [CrossRef] [PubMed]
- Tourkine, P.; Le Merrer, M.; Quéré, D. Delayed freezing on water repellent materials. Langmuir 2009, 25, 7214–7216. [Google Scholar] [CrossRef]
- Kirillova, A.; Ionov, L.; Roisman, I.V.; Synytska, A. Hybrid Hairy Janus Particles for Anti-Icing and De-Icing Surfaces: Synergism of Properties and Effects. Chem. Mater 2016, 28, 6995–7005. [Google Scholar] [CrossRef]
- Ozbay, S.; Yuceel, C.; Erbil, H.Y. Improved icephobic properties on surfaces with a hydrophilic lubricating liquid. ACS Appl. Mater. Interfaces 2015, 7, 22067–22077. [Google Scholar] [CrossRef]
- Varanasi, K.K.; Deng, T.; Smith, J.D.; Hsu, M.; Bhate, N. Frost formation and ice adhesion on superhydrophobic surfaces. Appl. Phys. Lett. 2010, 97, 234102. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Z. A comparison between superhydrophobic surfaces (SHS) and slippery liquid-infused porous surfaces (SLIPS) in application. Nanoscale 2020, 12, 22398–22424. [Google Scholar] [CrossRef] [PubMed]
- Nosonovsky, M.; Hejazi, V. Why superhydrophobic surfaces are not always icephobic. ACS Nano 2012, 6, 8488–8491. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, K.K.; Hsu, M.; Bhate, N.; Yang, W.; Deng, T. Spatial control in the heterogeneous nucleation of water. Appl. Phys. Lett. 2009, 95, 094101. [Google Scholar] [CrossRef]
- Memon, H.; Liu, J.; Weston, N.; Wang, J.; De Focatiis, D.S.A.; Choi, K.-S.; Hou, X. In-situ icing and water condensation study on different topographical surfaces. Cold Reg. Sci. Technol. 2019, 165, 102814. [Google Scholar] [CrossRef]
- Memon, H.; Liu, J.; De Focatiis, D.S.A.; Choi, K.-s.; Hou, X. Intrinsic dependence of ice adhesion strength on surface roughness. Surf. Coat. Technol. 2020, 385, 125382. [Google Scholar] [CrossRef]
- Sosso, G.C.; Chen, J.; Cox, S.J.; Fitzner, M.; Pedevilla, P.; Zen, A.; Michaelides, A. Crystal Nucleation in Liquids: Open Questions and Future Challenges in Molecular Dynamics Simulations. Chem. Rev. 2016, 116, 7078–7116. [Google Scholar] [CrossRef] [Green Version]
- Vogiatzis, G.G.; Theodorou, D.N. Multiscale Molecular Simulations of Polymer-Matrix Nanocomposites. Arch. Comput. Methods Eng. 2018, 25, 591–645. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Xu, Y.; Ma, J.; He, J.; Ye, H.; Song, J.; Chen, Y.; Xu, L. Efficient, Robust, and Flame-Retardant Electrothermal Coatings Based on a Polyhedral Oligomeric Silsesquioxane-Functionalized Graphene/Multiwalled Carbon Nanotube Hybrid with a Dually Cross-Linking Structure. ACS Appl. Mater. Interfaces 2023, 15, 4430–4440. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, J.; Wu, J.; Wen, K.; Wang, S.; Han, Y.; Tian, H.; Xu, P.; Chen, X.; Shao, J. Graphene-assisted wetting transition on grooved surfaces: A molecular dynamics study. Comput. Mater. Sci. 2022, 209, 111415. [Google Scholar] [CrossRef]
- Rozmanov, D.; Kusalik, P.G. Anisotropy in the crystal growth of hexagonal ice, Ih. J. Chem. Phys. 2012, 137, 4748377. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, K. Quasi-Liquid Layer on Ice and Its Effect on the Confined Freezing of Porous Materials. Crystals 2019, 9, 250. [Google Scholar] [CrossRef] [Green Version]
- Nada, H. Analysis of Ice Crystal Growth Shape under High Pressure Using Molecular Dynamics Simulation. Cryst. Growth Des. 2011, 11, 3130–3136. [Google Scholar] [CrossRef]
- Du, Z.; de Leeuw, N.H. Molecular dynamics simulations of hydration, dissolution and nucleation processes at the α-quartz (0001) surface in liquid water. Dalton Trans. 2006, 22, 2623–2634. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Z.; Fan, Q.; Lv, J.; Wang, J. Anti-Ice Coating Inspired by Ice Skating. Small 2014, 10, 4693–4699. [Google Scholar] [CrossRef]
- Hancer, M.; Arkaz, H. A facile fabrication of superhydrophobic nanocomposite coating with contact angles approaching the theoretical limit. Appl. Surf. Sci. 2015, 354, 342–346. [Google Scholar] [CrossRef]
- Hao, P.; Lv, C.; Zhang, X. Freezing of sessile water droplets on surfaces with various roughness and wettability. Appl. Phys. Lett. 2014, 104, 161609. [Google Scholar] [CrossRef]
- Lyu, J.; Wu, B.; Wu, N.; Peng, C.; Yang, J.; Meng, Y.; Xing, S. Green preparation of transparent superhydrophobic coatings with persistent dynamic impact resistance for outdoor applications. Chem. Eng. J. 2021, 404, 126456. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Wang, R.; Long, F.; Zhao, P.; Liu, L. A mosquito-eye-like superhydrophobic coating with super robustness against abrasion. Mater. Des. 2021, 203, 109552. [Google Scholar] [CrossRef]
- Schutzius, T.M.; Jung, S.; Maitra, T.; Eberle, P.; Antonini, C.; Stamatopoulos, C.; Poulikakos, D. Physics of icing and rational design of surfaces with extraordinary icephobicity. Langmuir 2014, 31, 4807–4821. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Li, H.; Wang, J.; Song, Y. Superhydrophobic surface at low surface temperature. Appl. Phys. Lett. 2011, 98, 093118. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Z.; Jiang, G.; Luo, X.; Chen, C.; Hu, X.; Zhang, H.; Zhong, M. Spontaneous dewetting transitions of droplets during icing & melting cycle. Nat. Commun. 2022, 13, 378. [Google Scholar] [CrossRef]
- Kreder, M.J.; Alvarenga, J.; Kim, P.; Aizenberg, J. Design of anti-icing surfaces: Smooth, textured or slippery? Nat. Rev. Mater. 2016, 1, 15003. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, A.; Yamada, M.; Li, R.; Shang, W.; Otta, S.; Zhong, S.; Ge, L.; Dhinojwala, A.; Conway, K.R.; Bahadur, V. Dynamics of ice nucleation on water repellent surfaces. Langmuir 2012, 28, 3180–3186. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, V.; Mishchenko, L.; Hatton, B.; Taylor, J.A.; Aizenberg, J.; Krupenkin, T. Predictive model for ice formation on superhydrophobic surfaces. Langmuir 2011, 27, 14143–14150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heydari, G.; Thormann, E.; Järn, M.; Tyrode, E.; Claesson, P.M. Hydrophobic surfaces: Topography effects on wetting by supercooled water and freezing delay. J. Phys. Chem. C 2013, 117, 21752–21762. [Google Scholar] [CrossRef]
- Fletcher, N. Size effect in heterogeneous nucleation. J. Chem. Phys. 1958, 29, 572–576. [Google Scholar] [CrossRef]
- He, Z.; Zhuo, Y.; He, J.; Zhang, Z. Design and preparation of sandwich-like polydimethylsiloxane (PDMS) sponges with super-low ice adhesion. Soft Matter 2018, 14, 4846–4851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ye, F.; Tao, H.; Lin, J. Effects of frost formation on the ice adhesion of micro-nano structure metal surface by femtosecond laser. J. Colloid Interface Sci. 2021, 603, 233–242. [Google Scholar] [CrossRef]
- Campbell, J.M.; Meldrum, F.C.; Christenson, H.K. Is Ice Nucleation from Supercooled Water Insensitive to Surface Roughness? J. Phys. Chem. C 2015, 119, 1164–1169. [Google Scholar] [CrossRef] [Green Version]
- Zeng, D.; Li, Y.; Liu, H.; Yang, Y.; Peng, L.; Zhu, C.; Zhao, N. Superhydrophobic coating induced anti-icing and deicing characteristics of an airfoil. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130824. [Google Scholar] [CrossRef]
- Hou, W.; Shen, Y.; Tao, J.; Xu, Y.; Jiang, J.; Chen, H.; Jia, Z. Anti-icing performance of the superhydrophobic surface with micro-cubic array structures fabricated by plasma etching. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124180. [Google Scholar] [CrossRef]
- Saito, H.; Takai, K.; Yamauchi, G. Water- and ice-repellent coatings. Surf. Coat. Int. 1997, 80, 168–171. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, N.; Li, J.; Wu, H.; Izuchukwu, N.K.; Ahmed, S.; Han, E.-H.; Liu, F. Enhanced anti-icing and anticorrosion properties of nano-SiO2 composite superhydrophobic coating constructed by a large-scale micropillar array approach. Prog. Org. Coat. 2023, 175, 107324. [Google Scholar] [CrossRef]
- Attarzadeh, R.; Dolatabadi, A. Icephobic performance of superhydrophobic coatings: A numerical analysis. Int. J. Heat Mass Transf. 2019, 136, 1327–1337. [Google Scholar] [CrossRef]
- Huang, W.; Huang, J.; Guo, Z.; Liu, W. Icephobic/anti-icing properties of superhydrophobic surfaces. Adv. Colloid Interface Sci. 2022, 304, 102658. [Google Scholar] [CrossRef]
- Haque, M.R.; Das, S.R.; Betz, A.R. Experimental investigation of condensation and freezing phenomena on hydrophilic and hydrophobic graphene coating. Appl. Therm. Eng. 2019, 160, 113987. [Google Scholar] [CrossRef]
- Wei, C.; Jin, B.; Zhang, Q.; Zhan, X.; Chen, F. Anti-icing performance of super-wetting surfaces from icing-resistance to ice-phobic aspects: Robust hydrophobic or slippery surfaces. J. Alloys Compd. 2018, 765, 721–730. [Google Scholar] [CrossRef]
- Balordi, M.; Cammi, A.; Santucci de Magistris, G.; Chemelli, C. Role of micrometric roughness on anti-ice properties and durability of hierarchical super-hydrophobic aluminum surfaces. Surf. Coat. Technol. 2019, 374, 549–556. [Google Scholar] [CrossRef]
- Zou, M.; Beckford, S.; Wei, R.; Ellis, C.; Hatton, G.; Miller, M. Effects of surface roughness and energy on ice adhesion strength. Appl. Surf. Sci. 2011, 257, 3786–3792. [Google Scholar] [CrossRef]
- Farhadi, S.; Farzaneh, M.; Kulinich, S. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Laforte, C.; Laforte, J.-L. Deicing strains and stresses of iced substrates. J. Adhes. Sci. Technol. 2012, 26, 603–620. [Google Scholar] [CrossRef]
- Fortin, G.; Perron, J. Ice adhesion models to predict shear stress at shedding. J. Adhes. Sci. Technol. 2012, 26, 523–553. [Google Scholar] [CrossRef]
- Rønneberg, S.; Laforte, C.; Volat, C.; He, J.; Zhang, Z. The effect of ice type on ice adhesion. AIP Adv. 2019, 9, 055304. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, R.; Kozhukhova, M.; Sobolev, K.; Nosonovsky, M. Anti-Icing Superhydrophobic Surfaces: Controlling Entropic Molecular Interactions to Design Novel Icephobic Concrete. Entropy 2016, 18, 132. [Google Scholar] [CrossRef] [Green Version]
- Han, T.; Kim, J.H.; Kim, Y.D.; Ahn, D.J.; Lim, D.-K. Solution-Based One-Step Preparation of Three-Dimensional Self-Assembled Octadecyl Silica Nanosquare Plate and Microlamella Structures for Superhydrophobic and Icephobic Surfaces. Langmuir 2021, 37, 5886–5894. [Google Scholar] [CrossRef] [PubMed]
- Loho, T.; Leveneur, J.; Kennedy, J. Effects of surface topography and chemistry modifications of stainless steel through ion implantation on icephobicity. Procedia Manuf. 2019, 30, 231–238. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, K.; Tao, C.; Zhao, Y.; Li, X.; Zhu, K.; Yuan, X. Strategies for anti-icing: Low surface energy or liquid-infused? RSC Adv. 2016, 6, 70251–70260. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, L.; Wang, W.; Kota, A.K.; Hu, H. An experimental study on soft PDMS materials for aircraft icing mitigation. Appl. Surf. Sci. 2018, 447, 599–609. [Google Scholar] [CrossRef]
- Fu, Q.T.; Liu, E.J.; Wilson, P.; Chen, Z. Ice nucleation behaviour on sol–gel coatings with different surface energy and roughness. Phys. Chem. Chem. Phys. 2015, 17, 21492–21500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, X.; Toubin, C.; Habartova, A.; Pluharova, E.; Roeselova, M.; Pettersson, J.B. Rapid Water Transport through Organic Layers on Ice. J. Phys. Chem. A 2018, 122, 4861–4868. [Google Scholar] [CrossRef]
- Yancheshme, A.A.; Momen, G.; Aminabadi, R.J. Mechanisms of ice formation and propagation on superhydrophobic surfaces: A review. Adv. Colloid Interface Sci. 2020, 279, 102155. [Google Scholar] [CrossRef] [PubMed]
- Schwidetzky, R.; Kunert, A.T.; Bonn, M.; Pöschl, U.; Ramløv, H.; DeVries, A.L.; Fröhlich-Nowoisky, J.; Meister, K. Inhibition of bacterial ice nucleators is not an intrinsic property of antifreeze proteins. J. Phys. Chem. B 2020, 124, 4889–4895. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; Lu, W.; Xu, H.; Kim, P.; Kreder, M.J.; Alvarenga, J.; Aizenberg, J. Inhibition of ice nucleation by slippery liquid-infused porous surfaces (SLIPS). Phys. Chem. Chem. Phys. 2013, 15, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Samaha, M.A.; Gad-el-Hak, M. Polymeric slippery coatings: Nature and applications. Polymers 2014, 6, 1266–1311. [Google Scholar] [CrossRef] [Green Version]
- Menini, R.; Farzaneh, M. Advanced icephobic coatings. J. Adhes. Sci. Technol. 2011, 25, 971–992. [Google Scholar] [CrossRef]
- Poynor, A.; Hong, L.; Robinson, I.K.; Granick, S.; Zhang, Z.; Fenter, P.A. How water meets a hydrophobic surface. Phys. Rev. Lett. 2006, 97, 266101. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Uysal, A.; Stripe, B.; Ha, Y.-g.; Marks, T.J.; Karapetrova, E.A.; Dutta, P. How water meets a very hydrophobic surface. Phys. Rev. Lett. 2010, 105, 037803. [Google Scholar] [CrossRef]
- Björneholm, O.; Hansen, M.H.; Hodgson, A.; Liu, L.-M.; Limmer, D.T.; Michaelides, A.; Pedevilla, P.; Rossmeisl, J.; Shen, H.; Tocci, G. Water at interfaces. Chem. Rev. 2016, 116, 7698–7726. [Google Scholar] [CrossRef]
- Chen, D.; Gelenter, M.D.; Hong, M.; Cohen, R.E.; McKinley, G.H. Icephobic Surfaces Induced by Interfacial Nonfrozen Water. ACS Appl. Mater. Interfaces 2017, 9, 4202–4214. [Google Scholar] [CrossRef]
- Aghdam, A.S.; Cebeci, F.Ç. Tailoring the Icephobic Performance of Slippery Liquid-Infused Porous Surfaces through the LbL Method. Langmuir 2020, 36, 14145–14154. [Google Scholar] [CrossRef]
- Cui, W.; Pakkanen, T.A. Icephobic performance of one-step silicone-oil-infused slippery coatings: Effects of surface energy, oil and nanoparticle contents. J. Colloid Interface Sci. 2020, 558, 251–258. [Google Scholar] [CrossRef]
- Yuan, J.; Huang, Y.; Li, H.; Jiang, L.; Dan, Y. Preparation and anti-icing performance of cross-linked polysiloxane coatings containing silicone oil. React. Funct. Polym. 2022, 170, 105124. [Google Scholar] [CrossRef]
- Wang, C.; Fuller, T.; Zhang, W.; Wynne, K.J. Thickness dependence of ice removal stress for a polydimethylsiloxane nanocomposite: Sylgard 184. Langmuir 2014, 30, 12819–12826. [Google Scholar] [CrossRef] [PubMed]
- Memon, H.; De Focatiis, D.S.A.; Choi, K.-S.; Hou, X. Durability enhancement of low ice adhesion polymeric coatings. Prog. Org. Coat. 2021, 151, 106033. [Google Scholar] [CrossRef]
- Yang, D.; Bao, R.; Clare, A.T.; Choi, K.-S.; Hou, X. Hydrophobically/oleophilically guarded powder metallurgical structures and liquid impregnation for ice mitigation. Chem. Eng. J. 2022, 446, 137115. [Google Scholar] [CrossRef]
- Guo, L.; Tang, G.; Kumar, S. Droplet Morphology and Mobility on Lubricant-Impregnated Surfaces: A Molecular Dynamics Study. Langmuir 2019, 35, 16377–16387. [Google Scholar] [CrossRef] [PubMed]
- Newby, B.-m.Z.; Chaudhury, M.K.; Brown, H.R. Macroscopic evidence of the effect of interfacial slippage on adhesion. Science 1995, 269, 1407. [Google Scholar] [CrossRef]
- Mohseni, M.; Dijvejin, Z.A.; Golovin, K. Designing scalable elastomeric anti-fouling coatings: Shear strain dissipation via interfacial cavitation. J. Colloid Interface Sci. 2021, 589, 556–567. [Google Scholar] [CrossRef]
- Chaudhury, M.; Kim, K. Shear-induced adhesive failure of a rigid slab in contact with a thin confined film. Eur. Phys. J. E Soft Matter Biol. Phys. 2007, 23, 175–183. [Google Scholar] [CrossRef]
- Ghatak, A.; Mahadevan, L.; Chaudhury, M.K. Measuring the work of adhesion between a soft confined film and a flexible plate. Langmuir 2005, 21, 1277–1281. [Google Scholar] [CrossRef]
- Vorvolakos, K.; Chaudhury, M.K. The effects of molecular weight and temperature on the kinetic friction of silicone rubbers. Langmuir 2003, 19, 6778–6787. [Google Scholar] [CrossRef]
- Chernyak, Y.B.; Leonov, A. On the theory of the adhesive friction of elastomers. Wear 1986, 108, 105–138. [Google Scholar] [CrossRef]
- Griffith, A.A. The phenomena of rupture and flow in solids. Philos. Trans. R. Soc. Lond. Ser. A Contain. Pap. Math. Phys. Character 1921, 221, 163–198. [Google Scholar]
- Vogel, N.; Belisle, R.A.; Hatton, B.; Wong, T.-S.; Aizenberg, J. Transparency and damage tolerance of patternable omniphobic lubricated surfaces based on inverse colloidal monolayers. Nat. Commun. 2013, 4, 2176. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.-S.; Kang, S.H.; Tang, S.K.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, R.; Liu, D.; Weng, Z.; Song, G.; Li, W.; Wang, Z. Slippery liquid infused porous surfaces with anti-icing performance fabricated by direct laser interference lithography. Prog. Org. Coat. 2023, 175, 107308. [Google Scholar] [CrossRef]
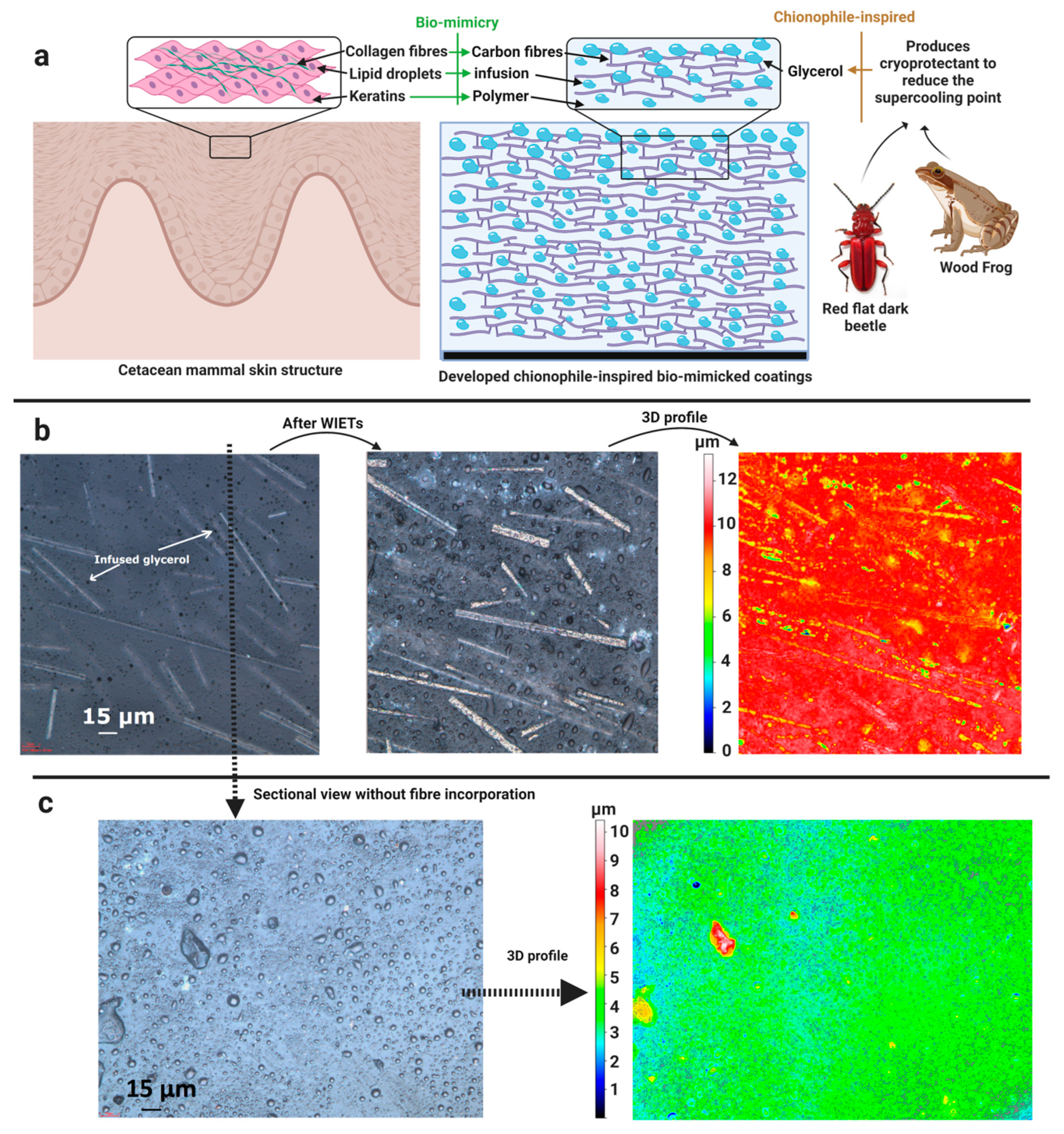
- Memon, H.; De Focatiis, D.S.A.; Choi, K.-S.; Hou, X. Toward Exceptional Icephobicity with Chionophile-Inspired Durable Biomimetic Coatings. ACS Appl. Polym. Mater. 2021, 3, 4184–4194. [Google Scholar] [CrossRef]
- Dou, R.; Chen, J.; Zhang, Y.; Wang, X.; Cui, D.; Song, Y.; Jiang, L.; Wang, J. Anti-icing coating with an aqueous lubricating layer. ACS Appl. Mater. Interfaces 2014, 6, 6998–7003. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Sun, F.; Pan, Q. Highly compressible and stretchable superhydrophobic coating inspired by bio-adhesion of marine mussels. J. Mater. Chem. A 2014, 2, 11365–11371. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, X.; Chen, J.; He, Z.; Liu, J.; Li, Q.; Wang, J.; Jiang, L. Organogel as durable anti-icing coatings. Sci. China Mater. 2015, 58, 559–565. [Google Scholar] [CrossRef]
- Seyfi, J.; Jafari, S.H.; Khonakdar, H.A.; Sadeghi, G.M.M.; Zohuri, G.; Hejazi, I.; Simon, F. Fabrication of robust and thermally stable superhydrophobic nanocomposite coatings based on thermoplastic polyurethane and silica nanoparticles. Appl. Surf. Sci. 2015, 347, 224–230. [Google Scholar] [CrossRef]
- Zhuo, Y.; Håkonsen, V.; He, Z.; Xiao, S.; He, J.; Zhang, Z. Enhancing the mechanical durability of icephobic surfaces by introducing autonomous self-healing function. ACS Appl. Mater. Interfaces 2018, 10, 11972–11978. [Google Scholar] [CrossRef] [PubMed]
- Valentini, L.; Bittolo Bon, S.; Hernández, M.; Lopez-Manchado, M.A.; Pugno, N.M. Nitrile butadiene rubber composites reinforced with reduced graphene oxide and carbon nanotubes show superior mechanical, electrical and icephobic properties. Compos. Sci. Technol. 2018, 166, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Liu, Y.; Hu, H. An experimental investigation of dynamic ice accretion process on a wind turbine airfoil model considering various icing conditions. Int. J. Heat Mass Transf. 2019, 133, 930–939. [Google Scholar] [CrossRef]
- Work, A.; Lian, Y. A critical review of the measurement of ice adhesion to solid substrates. Prog. Aerosp. Sci. 2018, 98, 1–26. [Google Scholar] [CrossRef]
- Rønneberg, S.; Zhuo, Y.; Laforte, C.; He, J.; Zhang, Z. Interlaboratory study of ice adhesion using different techniques. Coatings 2019, 9, 678. [Google Scholar] [CrossRef] [Green Version]
- Schulz, M.; Sinapius, M. Evaluation of Different Ice Adhesion Tests for Mechanical Deicing Systems; SAE International: Warrendale, PA, USA, 2015. [Google Scholar]
- Andrews, E.; Majid, H.; Lockington, N. Adhesion of ice to a flexible substrate. J. Mater. Sci. 1984, 19, 73–81. [Google Scholar] [CrossRef]
- Andrews, E.; Lockington, N. The cohesive and adhesive strength of ice. J. Mater. Sci. 1983, 18, 1455–1465. [Google Scholar] [CrossRef]
- Wang, F.; Ding, W.; He, J.; Zhang, Z. Phase transition enabled durable anti-icing surfaces and its DIY design. Chem. Eng. J. 2019, 360, 243–249. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Maghsoudi, K.; Jafari, R.; Momen, G. A comparative study of the icephobic and self-cleaning properties of Teflon materials having different surface morphologies. J. Mater. Process. Technol. 2020, 276, 116415. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, S.; Zhuo, Y.; Ding, W.; He, J.; Zhang, Z. Liquid layer generators for excellent icephobicity at extremely low temperatures. Mater. Horiz. 2019, 6, 2063–2072. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Xue, J.; Wang, Y.; Chen, Q.; Ding, J.; Wang, Q. Ice-phobic coatings based on silicon-oil-infused polydimethylsiloxane. ACS Appl. Mater. Interfaces 2013, 5, 4053–4062. [Google Scholar] [CrossRef]
- Work, A.H., Jr.; Gyekenyesi, A.L.; Kreeger, R.E.; Salem, J.A.; Vargas, M.M.; Drabiak, D.R. The Adhesion Strength of Impact Ice Measured Using a Modified Lap Joint Test; NASA: Washington, DC, USA, 2018.
- Soltis, J.; Palacios, J.; Eden, T.; Wolfe, D. Evaluation of ice-adhesion strength on erosion-resistant materials. AIAA J. 2015, 53, 1825–1835. [Google Scholar] [CrossRef]
- Palacios, J.; Wolfe, D.; Bailey, M.; Szefi, J. Ice testing of a centrifugally powered pneumatic deicing system for helicopter rotor blades. J. Am. Helicopter Soc. 2015, 60, 1–12. [Google Scholar] [CrossRef]
- Tetteh, E.; Loth, E. Reducing Static and Impact Ice Adhesion with a Self-Lubricating Icephobic Coating (SLIC). Coatings 2020, 10, 262. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, Y.; Xiao, S.; Håkonsen, V.; Li, T.; Wang, F.; He, J.; Zhang, Z. Ultrafast self-healing and highly transparent coating with mechanically durable icephobicity. Appl. Mater. Today 2020, 19, 100542. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Moita, A.S.; Zhang, C.; Wang, S.; Liu, Y. Fabrication of bio-inspired non-fluorinated superhydrophobic surfaces with anti-icing property and its wettability transformation analysis. Appl. Surf. Sci. 2020, 505, 144386. [Google Scholar] [CrossRef]
- Rønneberg, S.; He, J.; Zhang, Z. The need for standards in low ice adhesion surface research: A critical review. J. Adhes. Sci. Technol. 2020, 34, 319–347. [Google Scholar] [CrossRef]
- Laforte, C.; Beisswenger, A. Icephobic material centrifuge adhesion test. In Proceedings of the 11th International Workshop on Atmospheric Icing of Structures, IWAIS, Montreal, QC, Canada, 12–16 June 2005; pp. 12–16. [Google Scholar]
- Jellinek, H.; Kachi, H.; Kittaka, S.; Lee, M.; Yokota, R. Ice releasing block-copolymer coatings. Colloid Polym. Sci. 1978, 256, 544–551. [Google Scholar] [CrossRef]
- Andersson, L.-O.; Golander, C.-G.; Persson, S. Ice adhesion to rubber materials. J. Adhes. Sci. Technol. 1994, 8, 117–132. [Google Scholar] [CrossRef]
- Saito, H.; Takai, K.-I.; Yamauchi, G. A study on ice adhesiveness to water-repellent coating. J. Soc. Mater. Sci. 1997, 46, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Raraty, L.; Tabor, D. The adhesion and strength properties of ice. Proc. R. Soc. Lond. A Math. Phys. Sci. 1958, 245, 184–201. [Google Scholar]
- Bascom, W.; Cottington, R.; Singleterry, C. The Adhesion of Ice to Hydrophobic Surfaces; Naval Research Lab: Washington, DC, USA, 1966. [Google Scholar]
- Landy, M.; Freiberger, A. Studies of ice adhesion: I. Adhesion of ice to plastics. J. Colloid Interface Sci. 1967, 25, 231–244. [Google Scholar] [CrossRef]
- Murase, H.; Nanishi, K. On the relationship of thermodynamic and physical properties of polymers with ice adhesion. Ann. Glaciol. 1985, 6, 146–149. [Google Scholar] [CrossRef] [Green Version]
- Murase, H.; Nanishi, K.; Kogure, H.; Fujibayashi, T.; Tamura, K.; Haruta, N. Interactions between heterogeneous surfaces of polymers and water. J. Appl. Polym. Sci. 1994, 54, 2051–2062. [Google Scholar] [CrossRef]
- Memon, H.; Mirshahidi, K.; Zarasvand, K.A.; Golovin, K.; De Focatiis, D.S.A.; Choi, K.-S.; Hou, X. Comparative study on the influence of surface characteristics on de-icing evaluation. J. Mater. Sci. 2021, 56, 17337–17352. [Google Scholar] [CrossRef]
- Kasaai, M.R.; Farzaneh, M. A Critical Review of Evaluation Methods of Ice Adhesion Strength on the Surface of Materials. In Proceedings of the ASME 2004 23rd International Conference on Offshore Mechanics and Arctic Engineering, Vancouver, BC, Canada, 20–25 June 2004; pp. 919–926. [Google Scholar]
- Gold, L.W. Process of failure in ice. Can. Geotech. J. 1970, 7, 405–413. [Google Scholar] [CrossRef]
- Javan-Mashmool, M.; Volat, C.; Farzaneh, M. A new method for measuring ice adhesion strength at an ice–substrate interface. Hydrol. Process. 2006, 20, 645–655. [Google Scholar] [CrossRef]
- Somlo, B.; Gupta, V. A hydrophobic self-assembled monolayer with improved adhesion to aluminum for deicing application. Mech. Mater. 2001, 33, 471–480. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Stenroos, C.; Ruohomaa, R.; Bolelli, G.; Lusvarghi, L.; Vuoristo, P. Research on icing behavior and ice adhesion testing of icephobic surfaces. In Proceedings of the International Workshop on Atmospheric Icing of Structures (IWAIS), Uppsala, Sweden, 28 June–3 July 2015. [Google Scholar]
- Mirshahidi, K.; Alasvand Zarasvand, K.; Luo, W.; Golovin, K. A high throughput tensile ice adhesion measurement system. HardwareX 2020, 8, e00146. [Google Scholar] [CrossRef]
- Laroche, A.; Grasso, M.J.; Dolatabadi, A.; Bonaccurso, E. Tensile and Shear Test Methods for Quantifying the Ice Adhesion Strength to a Surface. In Ice Adhesion; Scrivener Publishing LLC: Beverly, MA, USA, 2020; pp. 237–284. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memon, H.; Wang, J.; Hou, X. Interdependence of Surface Roughness on Icephobic Performance: A Review. Materials 2023, 16, 4607. https://doi.org/10.3390/ma16134607
Memon H, Wang J, Hou X. Interdependence of Surface Roughness on Icephobic Performance: A Review. Materials. 2023; 16(13):4607. https://doi.org/10.3390/ma16134607
Chicago/Turabian StyleMemon, Halar, Jie Wang, and Xianghui Hou. 2023. "Interdependence of Surface Roughness on Icephobic Performance: A Review" Materials 16, no. 13: 4607. https://doi.org/10.3390/ma16134607




