Biosynthesis of Copper Oxide and Silver Nanoparticles by Bacillus Spores and Evaluation of the Feasibility of Their Use in Antimicrobial Paints
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biosynthesis of AgNPs and CuONPs Using Bacillus atrophaeus Spores
2.2. Biosynthesis of AgNPs Using Commercial and Crude Dipicolinic Acid (DPA)
2.3. Characterization of Synthesized NPs
2.4. Determination of Minimum Inhibitory Concentration (MIC) of NPs
2.5. Preparation of Paint–NP Complexes
2.6. Examination of Antibacterial Effects of Prepared Paint–NP Complexes
3. Results and Discussion
3.1. Biosynthesis and Characterization of AgNPs and CuONPs
3.2. MIC Determination of NPs
3.3. Evaluation of Antibacterial Effects of Prepared Paint–NP Complexes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, R.; Hou, E.; Cheng, W.; Yan, X.; Zhang, T.; Li, S.; Yao, H.; Liu, J.; Guo, Y. Membrane-Targeting Neolignan-Antimicrobial Peptide Mimic Conjugates to Combat Methicillin-Resistant Staphylococcus aureus (MRSA) Infections. J. Med. Chem. 2022, 56, 16879–16892. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhu, J.; Qiao, R.; Zhao, N.; Zhao, M.; Kong, L. Facile Preparation and Controllable Absorption of a Composite Based on PMo12/Ag Nanoparticles: Photodegradation Activity and Mechanism. Chemistryselect 2022, 7, e202103668. [Google Scholar] [CrossRef]
- Maqbool, Q.; Czerwinska, N.; Giosue, C.; Sabbatini, S.; Ruello, M.L.; Tittarelli, F. New waste-derived TiO2 nanoparticles as a potential photocatalytic additive for lime based indoor finishings. J. Clean. Prod. 2022, 373, 133853. [Google Scholar] [CrossRef]
- Maqbool, Q.; Yigit, N.; Stöger-Pollach, M.; Ruello, M.L.; Tittarelli, F.; Rupprechter, G. Operando monitoring of a room temperature nanocomposite methanol sensor. Catal. Sci. Technol. 2022, 13, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Miao, X.; Ali, J.; Lyu, T.; Pan, G. Quantification of Oxygen Nanobubbles in Particulate Matters and Potential Applications in Remediation of Anaerobic Environment. ACS Omega 2018, 3, 10624–10630. [Google Scholar] [CrossRef]
- Mittal, A.K.; Bhaumik, J.; Kumar, S.; Banerjee, U.C. Biosynthesis of silver nanoparticles: Elucidation of prospective mechanism and therapeutic potential. J. Colloid Interface Sci. 2014, 415, 39–47. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef] [Green Version]
- Selvarajan, E.; Mohanasrinivasan, V. Biosynthesis and characterization of ZnO nanoparticles using Lactobacillus plantarum VITES07. Mater. Lett. 2013, 112, 180–182. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Roopan, S.M.; Kirthi, A.V.; Venkatesan, J.; Kim, S.-K.; Iyappan, M.; Siva, C. Biological approach to synthesize TiO2 nanoparticles using Aeromonas hydrophila and its antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 107, 82–89. [Google Scholar] [CrossRef]
- Sundaram, P.A.; Augustine, R.; Kannan, M. Extracellular biosynthesis of iron oxide nanoparticles by Bacillus subtilis strains isolated from rhizosphere soil. Biotechnol. Bioprocess Eng. 2012, 17, 835–840. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, S.; Zhang, Y.; He, S.; Tian, Y. Biosynthesis, characterization and antimicrobial activity of silver nanoparticles by a halotolerant Bacillus endophyticus SCU-L. Prep. Biochem. Biotechnol. 2018, 48, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, B.; Li, T.; Dai, S.; Weng, Y.; Lu, J.; Xu, X.; Jin, Y.; Pang, R.; Hua, Y. Biosynthesis of Au, Ag and Au–Ag bimetallic nanoparticles using protein extracts of Deinococcus radiodurans and evaluation of their cytotoxicity. Int. J. Nanomed. 2018, 13, 1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyene, H.D.; Werkneh, A.A.; Bezabh, H.K.; Ambaye, T.G. Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain. Mater. Technol. 2017, 13, 18–23. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Waris, A.; Din, M.; Ali, A.; Ali, M.; Afridi, S.; Baset, A.; Khan, A.U. A comprehensive review of green synthesis of copper oxide nanoparticles and their diverse biomedical applications. Inorg. Chem. Commun. 2021, 123, 108369. [Google Scholar] [CrossRef]
- West, G.H.; Lippy, B.E.; Cooper, M.R.; Marsick, D.; Burrelli, L.G.; Griffin, K.N.; Segrave, A.M. Toward responsible development and effective risk management of nano-enabled products in the U.S. construction industry. J. Nanopart. Res. 2016, 18, 49. [Google Scholar] [CrossRef]
- Hosseini-Abari, A.; Emtiazi, G.; Lee, S.-H.; Kim, B.-G.; Kim, J.-H. Biosynthesis of Silver Nanoparticles by Bacillus stratosphericus Spores and the Role of Dipicolinic Acid in This Process. Appl. Biochem. Biotechnol. 2014, 174, 270–282. [Google Scholar] [CrossRef]
- Banala, R.R.; Nagati, V.B.; Karnati, P.R. Green synthesis and characterization of Carica papaya leaf extract coated silver nanoparticles through X-ray diffraction, electron microscopy and evaluation of bactericidal properties. Saudi J. Biol. Sci. 2015, 22, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.-A.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In Vitro Antimicrobial Activity of Green Synthesized Silver Nanoparticles Against Selected Gram-negative Foodborne Pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Venkataa, A.L.K.; Anthony, S.P.; Muthuraman, M.S. Synthesis of Solanum nigrum mediated copper oxide nanoparticles and their photocatalytic dye degradation studies. Mater. Res. Express 2019, 6, 125402. [Google Scholar] [CrossRef]
- Kokila, T.; Ramesh, P.; Geetha, D. Biosynthesis of AgNPs using Carica Papaya peel extract and evaluation of its antioxidant and antimicrobial activities. Ecotoxicol. Environ. Saf. 2016, 134, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; He, D.; Qian, Y.; Guan, B.; Gao, S.; Cui, Y.; Yokoyama, K.; Wang, L. Fungus-Mediated Green Synthesis of Silver Nanoparticles Using Aspergillus terreus. Int. J. Mol. Sci. 2011, 13, 466–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karunakaran, G.; Jagathambal, M.; Gusev, A.; Torres, J.A.L.; Kolesnikov, E.; Kuznetsov, D. Rapid Biosynthesis of AgNPs Using Soil Bacterium Azotobacter vinelandii With Promising Antioxidant and Antibacterial Activities for Biomedical Applications. JOM 2017, 69, 1206–1212. [Google Scholar] [CrossRef]
- Jemal, K.; Sandeep, B.V.; Pola, S. Synthesis, characterization, and evaluation of the antibacterial activity of Allophylus serratus leaf and leaf derived callus extracts mediated silver nanoparticles. J. Nanomater. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Dehaj, M.S.; Mohiabadi, M.Z. Experimental study of water-based CuO nanofluid flow in heat pipe solar collector. J. Therm. Anal. Calorim. 2019, 137, 2061–2072. [Google Scholar] [CrossRef]
- Karthik, L.; Kumar, G.; Kirthi, A.V.; Rahuman, A.A.; Rao, K.V.B. Streptomyces sp. LK3 mediated synthesis of silver nanoparticles and its biomedical application. Bioprocess Biosyst. Eng. 2014, 37, 261–267. [Google Scholar] [CrossRef]
- Govarthanan, M.; Selvankumar, T.; Manoharan, K.; Rathika, R.; Shanthi, K.; Lee, K.J.; Cho, M.; Kamala-Kannan, S.; Oh, B.T. Biosynthesis and characterization of silver nanoparticles using panchakavya, an Indian traditional farming formulating agent. Int. J. Nanomed. 2014, 9, 1593–1600. [Google Scholar] [CrossRef] [Green Version]
- Lanje, A.S.; Sharma, S.J.; Pode, R.B.; Ningthoujam, R.S. Synthesis and optical characterization of copper oxide nanoparticles. Adv. Appl. Sci. Res. 2010, 1, 36–40. [Google Scholar]
- Erjaee, H.; Rajaian, H.; Nazifi, S. Synthesis and characterization of novel silver nanoparticles using Chamaemelum nobile extract for antibacterial application. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 25004. [Google Scholar] [CrossRef]
- Gouyau, J.; Duval, R.E.; Boudier, A.; Lamouroux, E. Investigation of Nanoparticle Metallic Core Antibacterial Activity: Gold and Silver Nanoparticles against Escherichia coli and Staphylococcus aureus. Int. J. Mol. Sci. 2021, 22, 1905. [Google Scholar] [CrossRef] [PubMed]
- John, M.S.; Nagoth, J.A.; Zannotti, M.; Giovannetti, R.; Mancini, A.; Ramasamy, K.P.; Miceli, C.; Pucciarelli, S. Biogenic Synthesis of Copper Nanoparticles Using Bacterial Strains Isolated from an Antarctic Consortium Associated to a Psychrophilic Marine Ciliate: Characterization and Potential Application as Antimicrobial Agents. Mar. Drugs 2021, 19, 263. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [Green Version]
- Meghana, S.; Kabra, P.; Chakraborty, S.; Padmavathy, N. Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv. 2015, 5, 12293–12299. [Google Scholar] [CrossRef]
- Chen, M.C.; Koh, P.W.; Ponnusamy, V.K.; Lee, S.L. Titanium dioxide and other nanomaterials based antimicrobial additives in functional paints and coatings: Review. Prog. Org. Coatings 2022, 163, 106660. [Google Scholar] [CrossRef]
- Solano, R.; Patiño-Ruiz, D.; Herrera, A. Preparation of modified paints with nano-structured additives and its potential applications. Nanomater. Nanotechnol. 2020, 10, 1847980420909188. [Google Scholar] [CrossRef]
- Hajipour, P.; Eslami, A.; Bahrami, A.; Hosseini-Abari, A.; Saber, F.Y.; Mohammadi, R.; Mehr, M.Y. Surface modification of TiO2 nanoparticles with CuO for visible-light antibacterial applications and photocatalytic degradation of antibiotics. Ceram. Int. 2021, 47, 33875–33885. [Google Scholar] [CrossRef]
- Lu, J.; Fan, X.; Hu, J.; Li, J.; Rong, J.; Wang, W.; Chen, Y.; Liu, W.; Chen, J.; Chen, Y. Construction and function of robust and moist bilayer chitosan-based hydrogel wound dressing. Mater. Des. 2023, 226, 111604. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Ding, M.; Fan, X.; Hu, J.; Chen, Y.; Li, J.; Li, Z.; Liu, W. A 4arm-PEG macromolecule crosslinked chitosan hydrogels as antibacterial wound dressing. Carbohydr. Polym. 2022, 277, 118871. [Google Scholar] [CrossRef]
- Li, Y.; Xia, X.; Hou, W.; Lv, H.; Liu, J.; Li, X. How Effective are Metal Nanotherapeutic Platforms Against Bacterial Infections? A Comprehensive Review of Literature. Int. J. Nanomed. 2023, 18, 1109–1128. [Google Scholar] [CrossRef] [PubMed]
- Vaja (Dumitru), F.; Comanescu, C.; Oprea, O.; Ficai, D.; Guran, C. Effects of ZnO nanoparticles on the wet scrub resistance and photocatalytic properties of acrylic coatings. Rev. Chim. 2012, 63, 722–726. [Google Scholar] [CrossRef]
- Ficai, D.; Oprea, O.; Ficai, A.; Holban, A. Metal Oxide Nanoparticles: Potential Uses in Biomedical Applications. Curr. Proteom. 2014, 11, 139–149. [Google Scholar] [CrossRef]

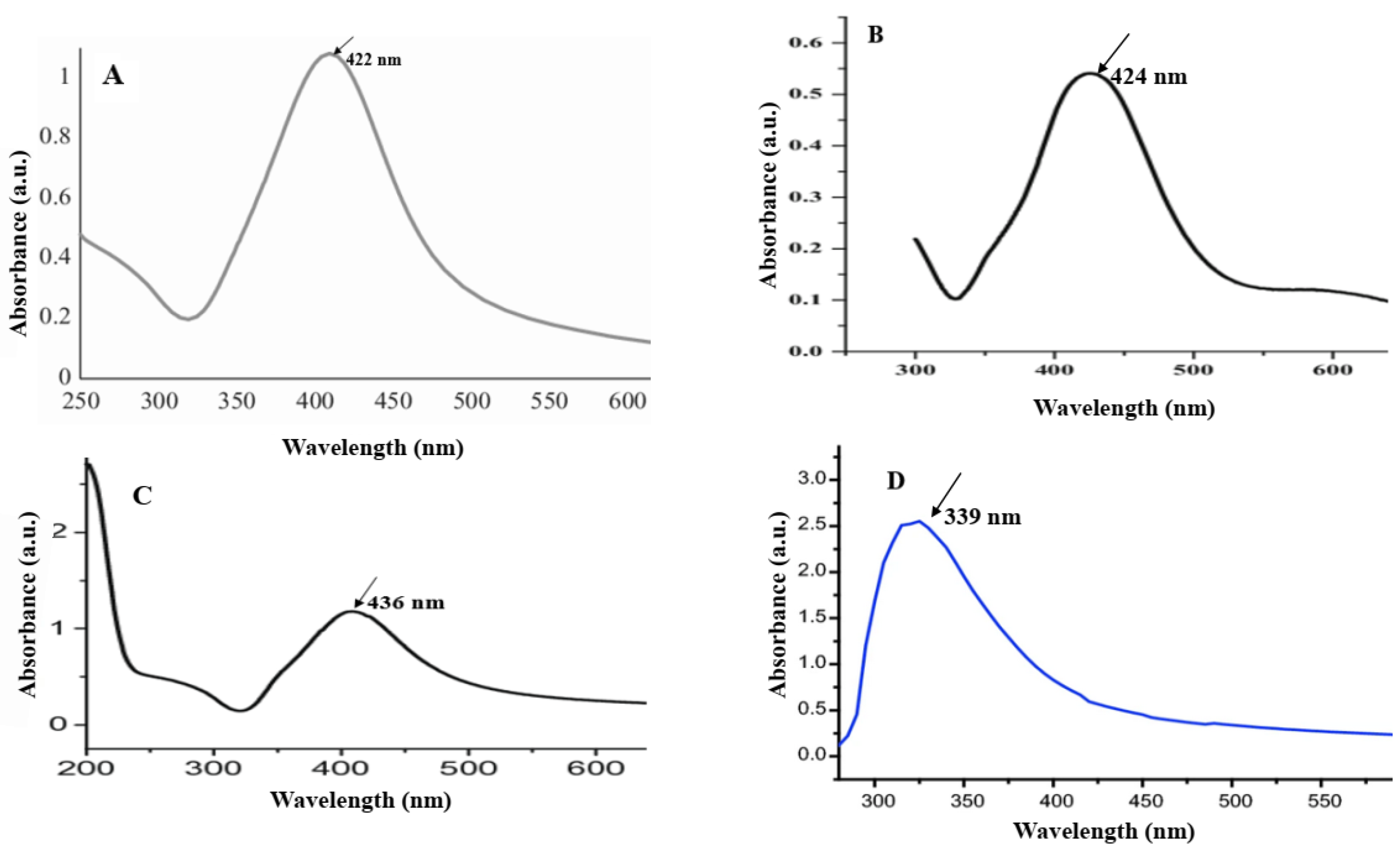
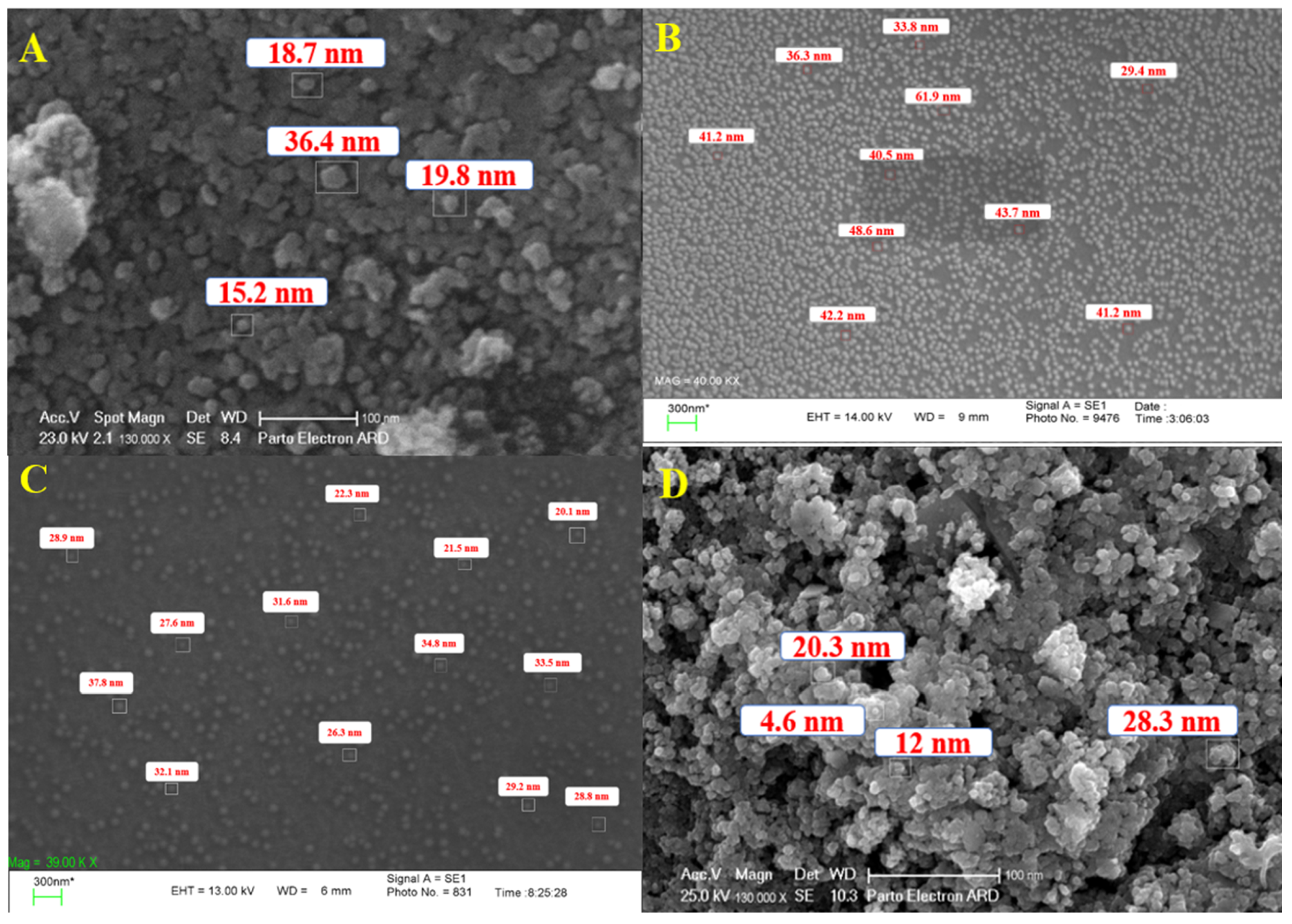

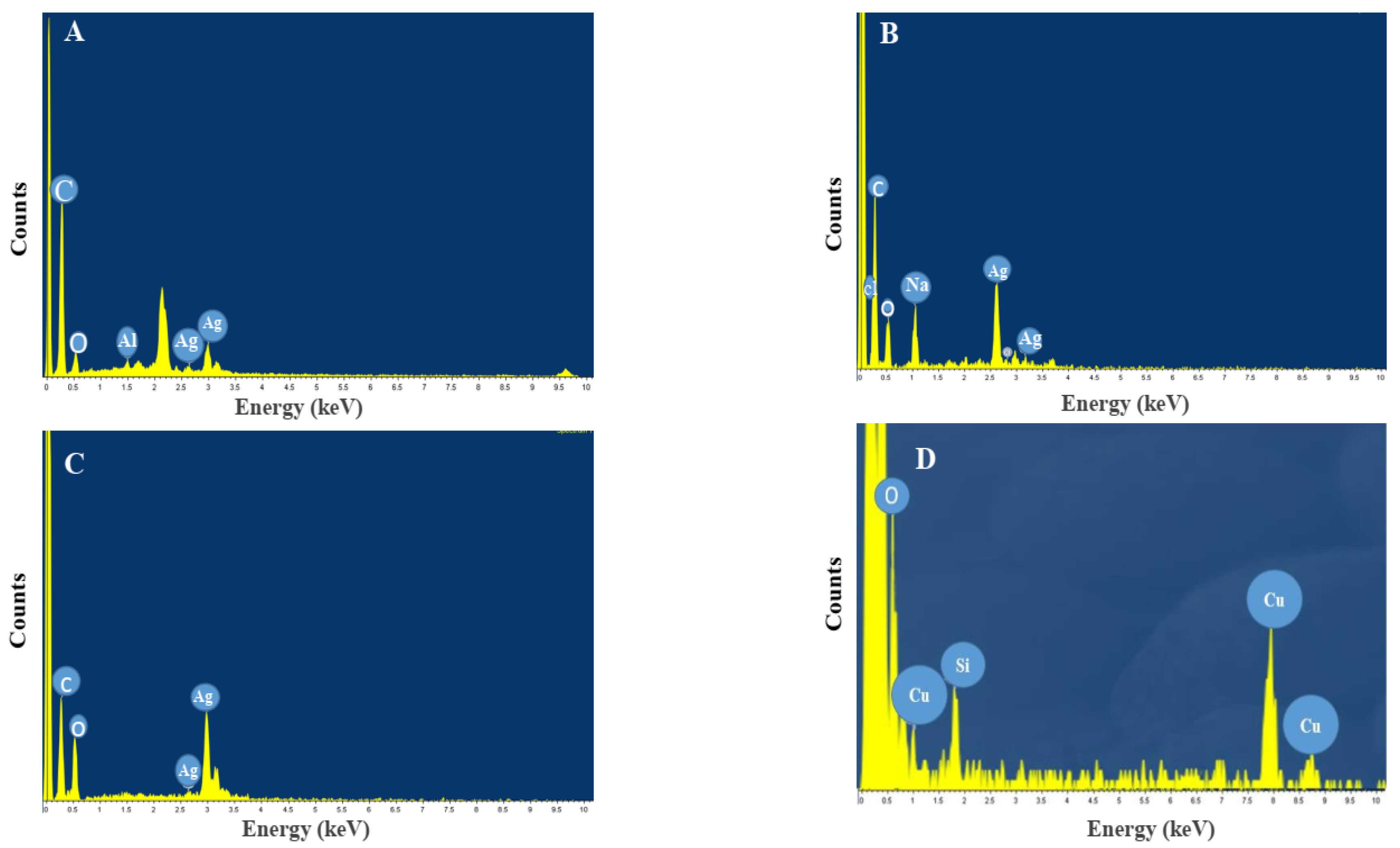
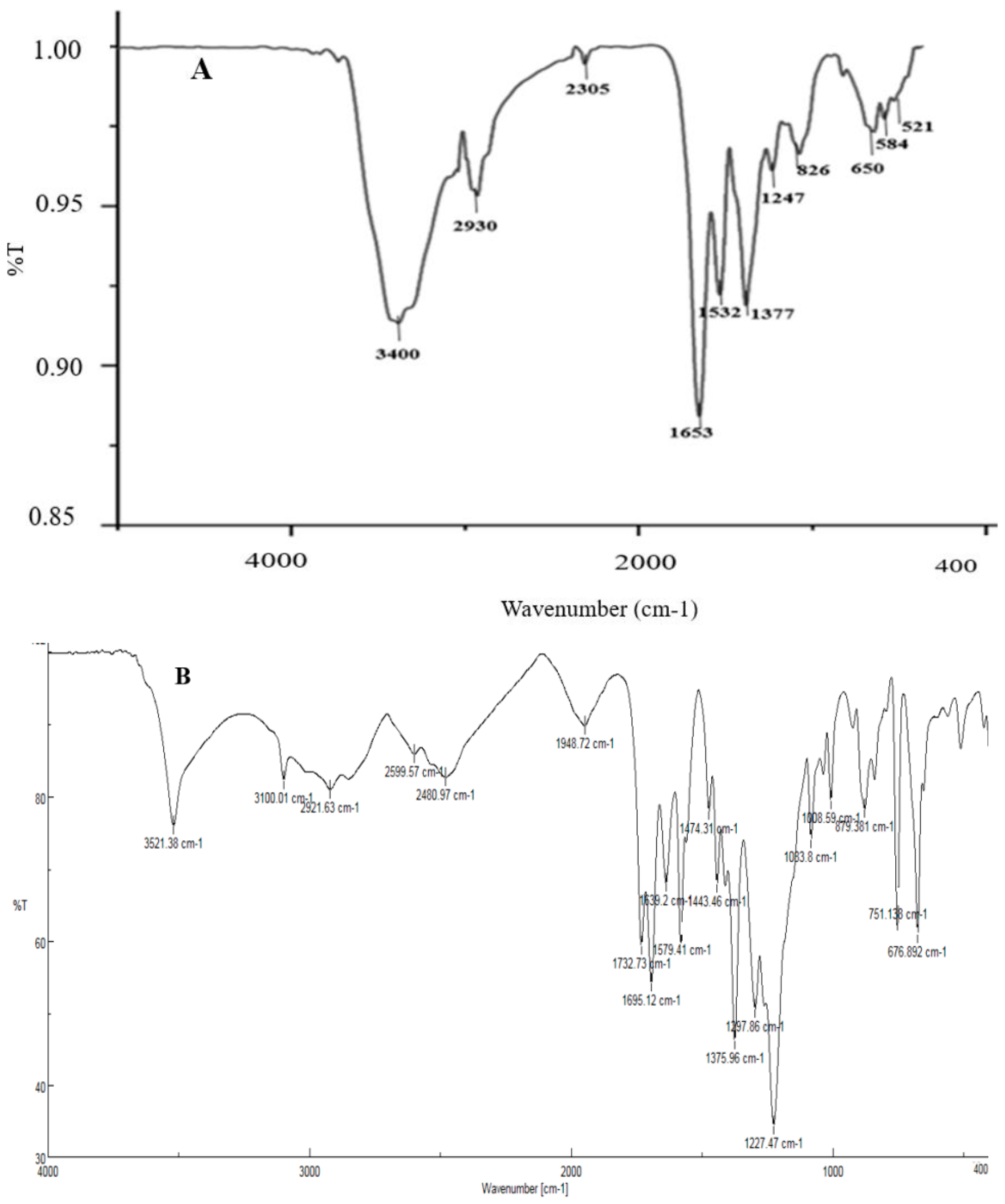
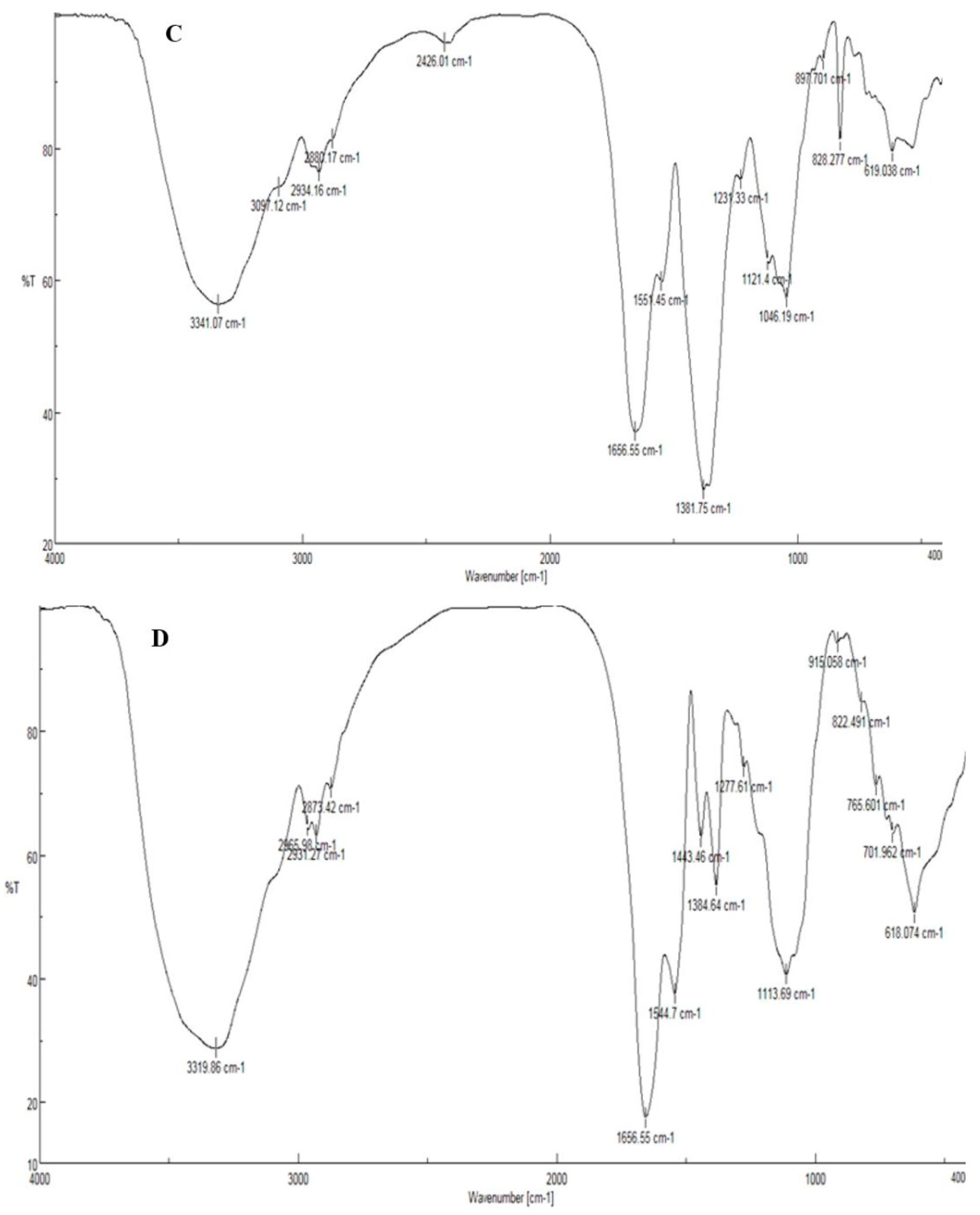
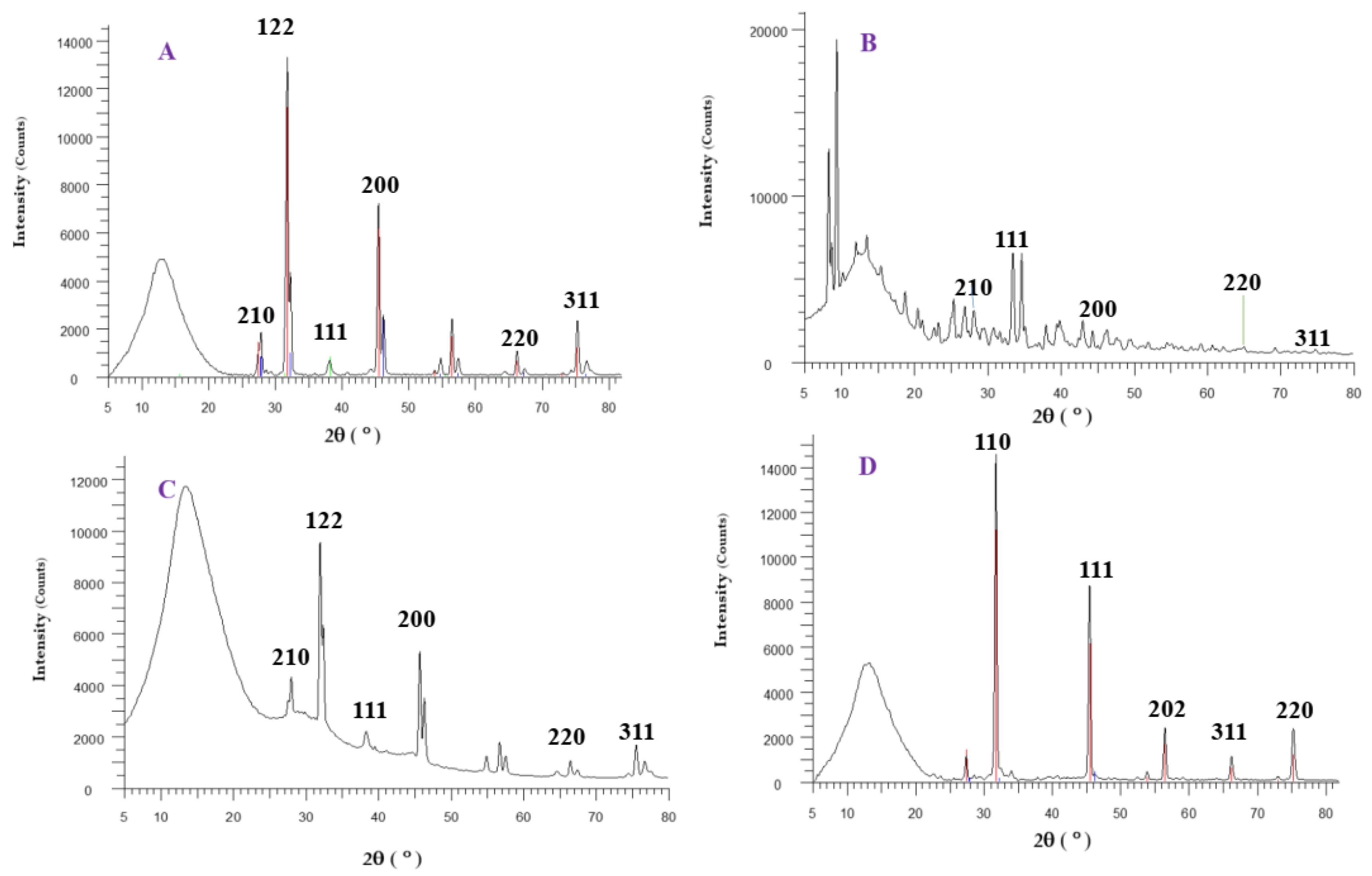
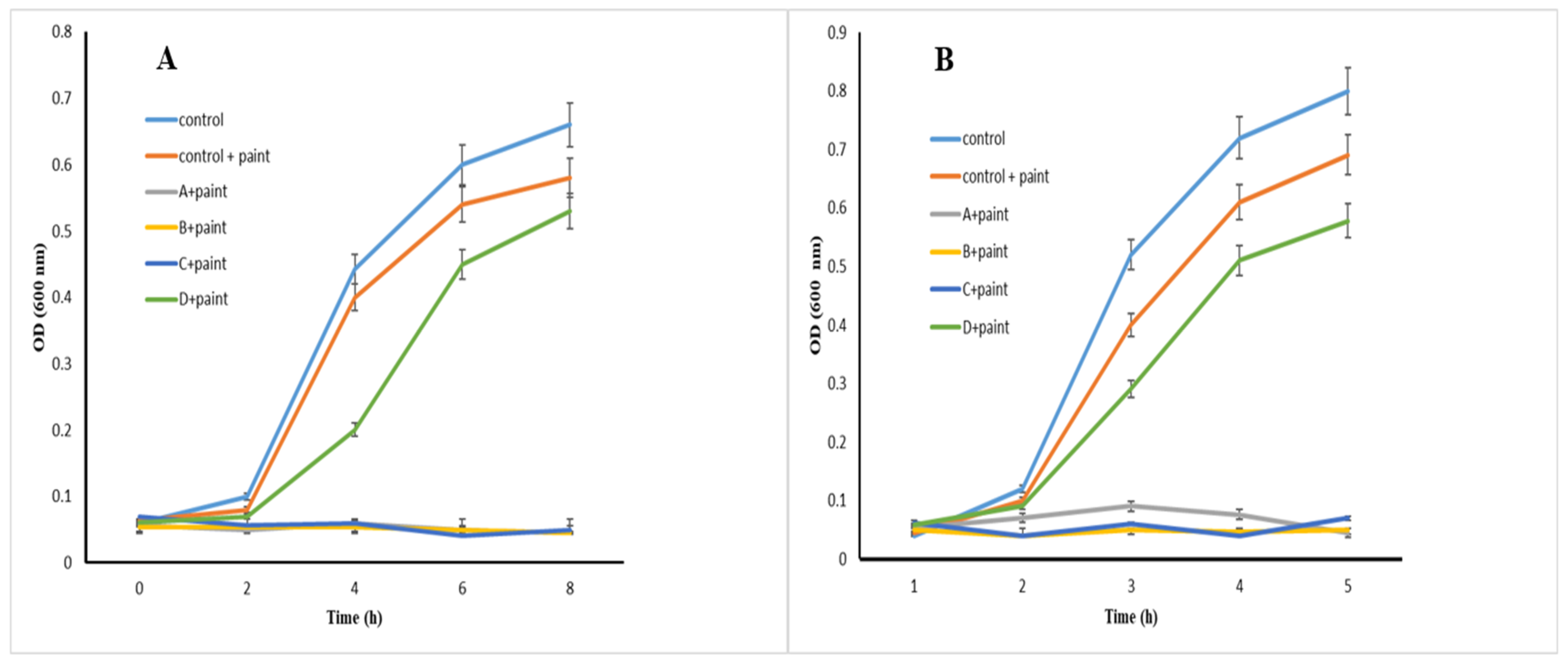
| Sample Code | Explanation |
|---|---|
| A | AgNPs synthesized by spore |
| B | AgNPs synthesized by commercial DPA |
| C | AgNPs synthesized by crude DPA |
| D | CuONPs synthesized by spore |
| NPs | MIC (μg/mL) | |
|---|---|---|
| E. coli | S. aureus | |
| A | 125 | 62.5 |
| B | 125 | 31.2 |
| C | 62.5 | 62.5 |
| D | 200 | 180 |
| Paint | 100 mg/mL | 100 mg/mL |
| NP–Paint Complexes | Number of Colonies/mL | |
|---|---|---|
| S. aureus | E. coli | |
| Control | 3.73 × 105 | 3.11 × 105 |
| Control + paint | 2.7 × 105 | 2.13 × 105 |
| A + paint | 31.5 × 103 | 6 × 103 |
| B + paint | 21 × 103 | 5.5 × 103 |
| C + paint | 13 × 103 | No growth |
| D + paint | 69 × 103 | 15 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alali, A.; Hosseini-Abari, A.; Bahrami, A.; Yazdan Mehr, M. Biosynthesis of Copper Oxide and Silver Nanoparticles by Bacillus Spores and Evaluation of the Feasibility of Their Use in Antimicrobial Paints. Materials 2023, 16, 4670. https://doi.org/10.3390/ma16134670
Alali A, Hosseini-Abari A, Bahrami A, Yazdan Mehr M. Biosynthesis of Copper Oxide and Silver Nanoparticles by Bacillus Spores and Evaluation of the Feasibility of Their Use in Antimicrobial Paints. Materials. 2023; 16(13):4670. https://doi.org/10.3390/ma16134670
Chicago/Turabian StyleAlali, Arkan, Afrouzossadat Hosseini-Abari, Abbas Bahrami, and Maryam Yazdan Mehr. 2023. "Biosynthesis of Copper Oxide and Silver Nanoparticles by Bacillus Spores and Evaluation of the Feasibility of Their Use in Antimicrobial Paints" Materials 16, no. 13: 4670. https://doi.org/10.3390/ma16134670





