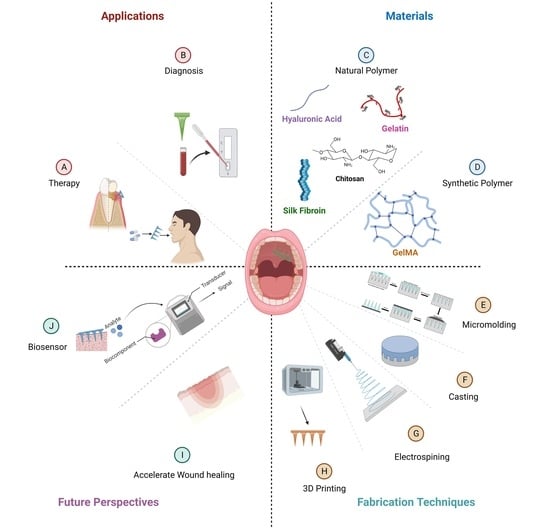
Hydrogel-Forming Microneedles with Applications in Oral Diseases Management
Abstract
:1. Introduction
2. Materials Used for Hydrogel-Forming Microneedles: Fabrication and Characteristics
2.1. Natural Polymer Fabricated Hydrogel-Forming Microneedles
2.1.1. Carbohydrate-Based Microneedles
Chitosan or Chitosan Derivatives
Hyaluronic Acid
Sodium Alginate
Pullulan
Cellulose
Combined Carbohydrate-Based Microneedles
2.1.2. Protein-Based Microneedles
Water-Soluble Silk Fibroin
Gelatin
2.1.3. Mixed Carbohydrate-Protein Microneedles
2.2. Synthetic Polymer Fabricated Hydrogel-Forming Microneedles
2.2.1. Gelatin Methacryloyl
2.2.2. Methacrylate-Based Hyaluronic Acid
2.2.3. Polyvinyl Alcohol
2.3. Combined Natural and Synthetic Polymer-Fabricated Hydrogel-Forming Microneedles
3. Fabrication of Hydrogel-Forming Microneedles
3.1. Micro-Molding Method
3.2. Casting
3.3. Electrospinning
3.4. Three-Dimensional-Printed Hydrogel-Filled Microneedle Array
4. Application of Hydrogel-Forming Microneedles in the Diagnosis and Treatment of Diseases
4.1. Diagnosis
4.1.1. Interstitial Fluid Extraction by Hydrogel-Forming Microneedles
4.1.2. In Vivo Interstitial Fluid Real-Time Analysis
4.1.3. Epstein-Barr Virus Detection
4.2. Diseases Management
4.2.1. Cancer
4.2.2. Diabetes
4.2.3. Rheumatoid Arthritis
4.2.4. Psoriasis
4.2.5. Eye Infection
4.2.6. Microencapsulation Cell Delivery
5. Applications of Hydrogel-Forming Microneedles in Dental Therapy
5.1. Collecting Oral Fluid for Diagnosis
5.2. Early Detection of Oral Cancer
5.3. Oral Cancer Treatment
5.4. Oral Vaccination
5.5. Local Anesthesia
5.6. Caries Prevention
5.7. Oral Ulcer Management
5.8. Periodontal Treatment
6. Advantages of Hydrogel-Forming Microneedles in Dentistry
7. Limitations of Hydrogel-Forming Microneedles in Dentistry
8. Future Perspectives
8.1. Hydrogel-Forming Microneedle Biosensing
8.2. Accelerate Dentoalveolar/Periodontal Wound Healing
8.3. Anti-Bacterial Effects
8.4. Future Research to Evaluate the Efficacy and Safety of HFMs in Dental Applications
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Donnelly, R.F.; Raj Singh, T.R.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010, 17, 187–207. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Liu, X.; Fu, Y.; Song, Y. Recent advances of microneedles for biomedical applications: Drug delivery and beyond. Acta Pharm. Sin. B 2019, 9, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Park, J.H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, K.; Vora, L.K.; Domínguez-Robles, J.; Naser, Y.A.; Li, M.; Larrañeta, E.; Donnelly, R.F. Hydrogel-forming microneedles for rapid and efficient skin deposition of controlled release tip-implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112226. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Morrow, D.I.; McCrudden, M.T.; Alkilani, A.Z.; Vicente-Pérez, E.M.; O’Mahony, C.; González-Vázquez, P.; McCarron, P.A.; Woolfson, A.D. Hydrogel-forming and dissolving microneedles for enhanced delivery of photosensitizers and precursors. Photochem. Photobiol. 2014, 90, 641–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damiri, F.; Kommineni, N.; Ebhodaghe, S.O.; Bulusu, R.; Jyothi, V.G.S.S.; Sayed, A.A.; Awaji, A.A.; Germoush, M.O.; Al-malky, H.S.; Nasrullah, M.Z.; et al. Microneedle-based natural polysaccharide for drug delivery systems (DDS): Progress and challenges. Pharmaceuticals 2022, 15, 190. [Google Scholar] [CrossRef]
- Turner, J.G.; White, L.R.; Estrela, P.; Leese, H.S. Hydrogel-forming microneedles: Current advancements and future trends. Macromol. Biosci. 2021, 21, e2000307. [Google Scholar] [CrossRef]
- Nele, V.; Wojciechowski, J.P.; Armstrong, J.P.; Stevens, M.M. Tailoring gelation mechanisms for advanced hydrogel applications. Adv. Funct. Mater. 2020, 30, 2002759. [Google Scholar] [CrossRef]
- Elisseeff, J. Structure starts to gel. Nat. Mate. 2008, 7, 271–273. [Google Scholar] [CrossRef]
- Jamaledin, R.; Makvandi, P.; Yiu, C.K.Y.; Agarwal, T.; Vecchione, R.; Sun, W.; Maiti, T.K.; Tay, F.R.; Netti, P.A. Engineered microneedle patches for controlled release of active compounds: Recent advances in release rrofile tuning. Adv. Ther. 2020, 3, 2000171. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; McCrudden, M.T.; Donnelly, R.F. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caffarel-Salvador, E.; Brady, A.J.; Eltayib, E.; Meng, T.; Alonso-Vicente, A.; Gonzalez-Vazquez, P.; Torrisi, B.M.; Vicente-Perez, E.M.; Mooney, K.; Jones, D.S.; et al. Hydrogel-forming microneedle arrays allow detection of drugs and glucose in vivo: Potential for use in diagnosis and therapeutic drug monitoring. PLoS ONE 2015, 10, e0145644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, E.Y.; Lee, J.; Kim, B.J.; Joo, K.I.; Kim, K.H.; Lim, G.; Cha, H.J. Bio-inspired swellable hydrogel-forming double-layered adhesive microneedle protein patch for regenerative internal/external surgical closure. Biomaterials 2019, 222, 119439. [Google Scholar] [CrossRef] [PubMed]
- Farias, C.; Lyman, R.; Hemingway, C.; Chau, H.; Mahacek, A.; Bouzos, E.; Mobed-Miremadi, M. Three-dimensional (3D) printed microneedles for microencapsulated cell extrusion. Bioengineering 2018, 5, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.C.; Erothu, H. Synthetic polymer hydrogels. In Biomedical Applications of Polymeric Materials and Composites; Francis, R., Kumar, D.S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; Chapter 6; pp. 141–162. [Google Scholar] [CrossRef]
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural polymer-based hydrogels with enhanced mechanical performances: Preparation, structure, and property. Adv. Healthc. Mater. 2019, 8, 1900670. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Morrow, D.I.; Singh, T.R.; Migalska, K.; McCarron, P.A.; O’Mahony, C.; Woolfson, A.D. Processing difficulties and instability of carbohydrate microneedle arrays. Drug Dev. Ind. Pharm. 2009, 35, 1242–1254. [Google Scholar] [CrossRef]
- Guo, R.; Chen, M.; Ding, Y.; Yang, P.; Wang, M.; Zhang, H.; He, Y.; Ma, H. Polysaccharides as potential anti-tumor biomacromolecules -A review. Front. Nutr. 2022, 9, 838179. [Google Scholar] [CrossRef]
- Bian, Y.; Zeng, H.; Tao, H.; Huang, L.; Du, Z.; Wang, J.; Ding, K. A pectin-like polysaccharide from Polygala tenuifolia inhibits pancreatic cancer cell growth in vitro and in vivo by inducing apoptosis and suppressing autophagy. Int. J. Biol. Macromol. 2020, 162, 107–115. [Google Scholar] [CrossRef]
- Zong, A.; Cao, H.; Wang, F. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, B.; Gao, Y. Controlled transdermal delivery of model drug compounds by MEMS microneedle array. Nanomedicine 2005, 1, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Creighton, R.L.; Woodrow, K.A. Microneedle-mediated vaccine delivery to the oral mucosa. Adv. Healthc. Mater. 2019, 8, e1801180. [Google Scholar] [CrossRef] [PubMed]
- Bobbala, S.; Hook, S. Is there an optimal formulation and delivery strategy for subunit vaccines? Pharm. Res. 2016, 33, 2078–2097. [Google Scholar] [CrossRef]
- Shen, Z.; Kuang, S.; Zhang, Y.; Yang, M.; Qin, W.; Shi, X.; Lin, Z. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact. Mater. 2020, 5, 1113–1126. [Google Scholar] [CrossRef]
- McCrudden, M.T.; McAlister, E.; Courtenay, A.J.; González-Vázquez, P.; Singh, T.R.; Donnelly, R.F. Microneedle applications in improving skin appearance. Exp. Dermatol. 2015, 24, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Prausnitz, M.R. Coating formulations for microneedles. Pharm. Res. 2007, 24, 1369–1380. [Google Scholar] [CrossRef]
- Liu, S.; Jin, M.-n.; Quan, Y.-s.; Kamiyama, F.; Katsumi, H.; Sakane, T.; Yamamoto, A. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J. Control. Release 2012, 161, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Yu, X.; Yi, X.; Guo, X.; Li, L.; Hao, Y.; Wang, W. Lidocaine-loaded hyaluronic acid adhesive microneedle patch for oral mucosal topical anesthesia. Pharmaceutics 2022, 14, 686. [Google Scholar] [CrossRef]
- Rubel, B.S. Impression materials: A comparative review of impression materials most commonly used in restorative dentistry. Dent. Clin. N. Am. 2007, 51, 629–642. [Google Scholar] [CrossRef]
- Demir, Y.K.; Akan, Z.; Kerimoglu, O. Characterization of polymeric microneedle arrays for transdermal drug delivery. PLoS ONE 2013, 8, e77289. [Google Scholar] [CrossRef] [Green Version]
- Rossaint, R.; Bouillon, B.; Cerny, V.; Coats, T.J.; Duranteau, J.; Fernández-Mondéjar, E.; Hunt, B.J.; Komadina, R.; Nardi, G.; Neugebauer, E.; et al. Management of bleeding following major trauma: An updated European guideline. Crit. Care 2010, 14, R52. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Gao, P.; He, F.; Zhang, C. Application of alginate-based hydrogels in hemostasis. Gels 2022, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Xu, K.; Mei, L.; Wu, C.; Liu, J.; Liu, Z.; Wan, L.; Zhong, W. Co-assembled supramolecular hydrogels of cell adhesive peptide and alginate for rapid hemostasis and efficacious wound healing. Soft Matter 2019, 15, 8603–8610. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Z.; Wei, S.; Sun, P.; Bai, H. Hydrogel-coated needles prevent puncture site bleeding in arteriovenous fistula and arteriovenous grafts in rats. Biomed. Pharmacother. 2021, 143, 112113. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, C.; Zhao, Y.; Jin, X. Preparation and characterization of nanoparticle reinforced alginate fibers with high porosity for potential wound dressing application. RSC Adv. 2017, 7, 39349–39358. [Google Scholar] [CrossRef] [Green Version]
- Ansari, S.; Diniz, I.M.; Chen, C.; Sarrion, P.; Tamayol, A.; Wu, B.M.; Moshaverinia, A. Human periodontal ligament- and gingiva-derived mesenchymal stem cells promote nerve regeneration when encapsulated in alginate/hyaluronic acid 3D scaffold. Adv. Healthc. Mater. 2017, 6, 1700670. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, A.; Chen, C.; Akiyama, K.; Xu, X.; Chee, W.W.; Schricker, S.R.; Shi, S. Encapsulated dental-derived mesenchymal stem cells in an injectable and biodegradable scaffold for applications in bone tissue engineering. J. Biomed. Mater. Res. A 2013, 101, 3285–3294. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, A.; Chen, C.; Akiyama, K.; Ansari, S.; Xu, X.; Chee, W.W.; Schricker, S.R.; Shi, S. Alginate hydrogel as a promising scaffold for dental-derived stem cells: An in vitro study. J. Mater. Sci. Mater. Med. 2012, 23, 3041–3051. [Google Scholar] [CrossRef] [PubMed]
- Darge, H.F.; Lee, C.-Y.; Lai, J.-Y.; Lin, S.-Z.; Harn, H.-J.; Chen, Y.-S.; Tsai, H.-C. Separable double-layered microneedle-based transdermal codelivery of DOX and LPS for synergistic immunochemotherapy of a subcutaneous glioma tumor. Chem. Eng. J. 2022, 433, 134062. [Google Scholar] [CrossRef]
- Tian, Y.; Lee, J.; Bhide, Y.C.; van der Maaden, K.; Huckriede, A.L.; Jiskoot, W.; Frijlink, H.W.; Hinrichs, W.L.; Bouwstra, J.A. Intradermal administration of influenza vaccine with trehalose and pullulan-based dissolving microneedle arrays. J. Pharm. Sci. 2022, 111, 1070–1080. [Google Scholar] [CrossRef]
- Fonseca, D.F.S.; Costa, P.C.; Almeida, I.F.; Dias-Pereira, P.; Correia-Sá, I.; Bastos, V.; Oliveira, H.; Duarte-Araújo, M.; Morato, M.; Vilela, C.; et al. Pullulan microneedle patches for the efficient transdermal administration of insulin envisioning diabetes treatment. Carbohydr. Polym. 2020, 241, 116314. [Google Scholar] [CrossRef]
- Vora, L.K.; Courtenay, A.J.; Tekko, I.A.; Larrañeta, E.; Donnelly, R.F. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int. J. Biol. Macromol. 2020, 146, 290–298. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Song, J.E.; Jun, S.H.; Park, S.G.; Kang, N.G. A semi-dissolving microneedle patch incorporating TEMPO-oxidized bacterial cellulose nanofibers for enhanced transdermal delivery. Polymers 2020, 12, 1873. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Park, S.C.; Lee, S.-J.; Kim, J.-C. Cellulose nanofiber-reinforced dissolving microneedles for transdermal delivery of a water-soluble compound. Cellulose 2022, 29, 9881–9897. [Google Scholar] [CrossRef]
- Wei, H.; Liu, S.; Tong, Z.; Chen, T.; Yang, M.; Guo, Y.; Sun, H.; Wu, Y.; Chu, Y.; Fan, L. Hydrogel-based microneedles of chitosan derivatives for drug delivery. React. Funct. Polym. 2022, 172, 105200. [Google Scholar] [CrossRef]
- Younas, A.; Dong, Z.; Hou, Z.; Asad, M.; Li, M.; Zhang, N. A chitosan/fucoidan nanoparticle-loaded pullulan microneedle patch for differential drug release to promote wound healing. Carbohydr. Polym. 2023, 306, 120593. [Google Scholar] [CrossRef]
- Shabnoor, N.S.; Hema Bindu, A.; Patil, A.G.; Aishwarya, S.; More, S.S.; Khan, K.; Padyana, S.; Madhavi, J.; Yadav, A.N.; Ravish, H.; et al. Peptide and protein-based hydrogels for the encapsulation of bioactive compounds and tissue engineering applications. In Protein-Based Biopolymers. From Source to Biomedical Applications; Kalia, S., Sharma, S., Eds.; Woodhead Publishing: Sawston, UK, 2023; Chapter 13; pp. 301–331. [Google Scholar] [CrossRef]
- Mukherjee, S.; Krishnan, A.; Athira, R.K.; Kasoju, N.; Sah, M.K. Silk fibroin and silk sericin in skin tissue engineering and wound healing: Retrospect and prospects. In Natural Polymers in Wound Healing and Repair; Sah, M.K., Kasoju, N., Mano, J.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Tsioris, K.; Raja, W.K.; Pritchard, E.M.; Panilaitis, B.; Kaplan, D.L.; Omenetto, F.G. Fabrication of silk microneedles for controlled-release drug delivery. Adv. Funct. Mater. 2012, 22, 330–335. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. Silk fibroin as a functional biomaterial for drug and gene delivery. Pharmaceutics 2019, 11, 494. [Google Scholar] [CrossRef] [Green Version]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Osathanon, T.; Nowwarote, N.; Pavasant, P. Basic fibroblast growth factor inhibits mineralization but induces neuronal differentiation by human dental pulp stem cells through a FGFR and PLCγ signaling pathway. J. Cell. Biochem. 2011, 112, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-W.; Zhang, Y.-F.; Sun, Z.-Y.; Song, G.-T.; Chen, Z. Dental pulp tissue engineering with bFGF-incorporated silk fibroin scaffolds. J. Biomater. Appl. 2015, 30, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.H.; Chen, M.C. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater. 2013, 9, 8952–8961. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Kim, Y.; Lee, S.H.; Yoon, Y.; Kim, W.H.; Kweon, O.K. Effect of gelatin on osteogenic cell sheet formation using canine adipose-derived mesenchymal stem cells. Cell Transplant. 2017, 26, 115–123. [Google Scholar] [CrossRef]
- Hayashi, K.; Kubo, T.; Doi, K.; Tabata, Y.; Akagawa, Y. Development of new drug delivery system for implant bone augmentation using a basic fibroblast growth factor-gelatin hydrogel complex. Dent. Mater. J. 2007, 26, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Jana, B.A.; Osmani, R.A.; Jaiswal, S.; Banerjee, R.; Karri, V.V.S.R.; Wadhwani, A. Fabrication of carboxymethylcellulose-gelatin dissolving microneedle patch for pain-free, efficient, and controlled transdermal delivery of insulin. J. Pharm. Innov. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and properties of gelatin methacryloyl (GelMA) hydrogels and their recent applications in load-bearing tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef] [Green Version]
- Piao, Y.; You, H.; Xu, T.; Bei, H.-P.; Piwko, I.Z.; Kwan, Y.Y.; Zhao, X. Biomedical applications of gelatin methacryloyl hydrogels. Eng. Regen. 2021, 2, 47–56. [Google Scholar] [CrossRef]
- Luo, Z.; Sun, W.; Fang, J.; Lee, K.; Li, S.; Gu, Z.; Dokmeci, M.R.; Khademhosseini, A. Biodegradable gelatin methacryloyl microneedles for transdermal drug delivery. Adv. Healthc. Mater. 2019, 8, e1801054. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, K.; Jiang, T.; Li, S.; Chen, J.; Wu, Z.; Li, W.; Tan, R.; Wei, W.; Yang, X.; et al. GelMA/PEGDA microneedles patch loaded with HUVECs-derived exosomes and tazarotene promote diabetic wound healing. J. Nanobiotechnol. 2022, 20, 147. [Google Scholar] [CrossRef]
- Chang, W.-C.; Tai, A.-Z.; Tsai, N.-Y.; Li, Y.-C.E. An injectable hybrid gelatin methacryloyl (GelMA)/phenyl isothiocyanate-modified gelatin (gel-phe) bioadhesive for oral/dental hemostasis applications. Polymers 2021, 13, 2386. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Matyjaszewski, K.; Washburn, N.R. Influence of the degree of methacrylation on hyaluronic acid hydrogels properties. Biomaterials 2008, 29, 1739–1749. [Google Scholar] [CrossRef]
- Alaohali, A.; Salzlechner, C.; Zaugg, L.K.; Suzano, F.; Martinez, A.; Gentleman, E.; Sharpe, P.T. GSK3 inhibitor-induced dentinogenesis using a hydrogel. J. Dent. Res. 2021, 101, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, X.; Zhang, X.; Zu, Y.; Tan, Q.; Zhao, Y. Living microneedle patch with adipose-derived stem cells embedding for diabetic ulcer healing. Adv. Funct. Mater. 2023, 33, 2209986. [Google Scholar] [CrossRef]
- Liu, Y.; Vrana, N.E.; Cahill, P.A.; McGuinness, G.B. Physically crosslinked composite hydrogels of PVA with natural macromolecules: Structure, mechanical properties, and endothelial cell compatibility. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Nho, Y.C. Synthesis of PVA/PVP hydrogels having two-layer by radiation and their physical properties. Radiat. Phys. Chem. 2003, 67, 361–365. [Google Scholar] [CrossRef]
- Leachman, S.A.; Hickerson, R.P.; Schwartz, M.E.; Bullough, E.E.; Hutcherson, S.L.; Boucher, K.M.; Hansen, C.D.; Eliason, M.J.; Srivatsa, G.S.; Kornbrust, D.J. First-in-human mutation-targeted siRNA phase Ib trial of an inherited skin disorder. Mol. Ther. 2010, 18, 442–446. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, E.; Speaker, T.J.; Hickerson, R.P.; Spitler, R.; Flores, M.A.; Leake, D.; Contag, C.H.; Kaspar, R.L. Silencing of reporter gene expression in skin using siRNAs and expression of plasmid DNA delivered by a soluble protrusion array device (PAD). Mol. Ther. 2010, 18, 1667–1674. [Google Scholar] [CrossRef]
- Gonçalves da Costa Sousa, M.; Conceição de Almeida, G.; Martins Mota, D.C.; Andrade da Costa, R.; Dias, S.C.; Limberger, S.N.; Ko, F.; Lin, L.T.; Haney, E.F.; Etayash, H.; et al. Antibiofilm and immunomodulatory resorbable nanofibrous filing for dental pulp regenerative procedures. Bioact. Mater. 2022, 16, 173–186. [Google Scholar] [CrossRef]
- Sugiaman, V.K.; Jeffrey; Naliani, S.; Pranata, N.; Djuanda, R.; Saputri, R.I. Polymeric scaffolds used in dental pulp regeneration by tissue engineering approach. Polymers 2023, 15, 1082. [Google Scholar] [CrossRef]
- Pan, X.; Li, Y.; Pang, W.; Xue, Y.; Wang, Z.; Jiang, C.; Shen, C.; Liu, Q.; Liu, L. Preparation, characterisation and comparison of glabridin-loaded hydrogel-forming microneedles by chemical and physical cross-linking. Int. J. Pharm. 2022, 617, 121612. [Google Scholar] [CrossRef]
- Kolahdoozan, M.; Rahimi, T.; Taghizadeh, A.; Aghaei, H. Preparation of new hydrogels by visible light cross-linking of dextran methacrylate and poly (ethylene glycol)-maleic acid copolymer. Int. J. Biol. Macromol. 2023, 227, 1221–1233. [Google Scholar] [CrossRef]
- Dardano, P.; Caliò, A.; Di Palma, V.; Bevilacqua, M.F.; Di Matteo, A.; De Stefano, L. A Photolithographic approach to polymeric microneedles array fabrication. Materials 2015, 8, 8661–8673. [Google Scholar] [CrossRef] [Green Version]
- Lynch, S.; Liu, C.; Morgan, N.; Xiao, X.; Gomella, A.; Mazilu, D.; Bennett, E.; Assoufid, L.; De Carlo, F.; Wen, H. Fabrication of 200 nm period centimeter area hard x-ray absorption gratings by multilayer deposition. J. Micromech. Microeng. 2012, 22, 105007. [Google Scholar] [CrossRef] [Green Version]
- Eivazzadeh-Keihan, R.; Noruzi, E.B.; Mehrban, S.F.; Aliabadi, H.A.M.; Karimi, M.; Mohammadi, A.; Maleki, A.; Mahdavi, M.; Larijani, B.; Shalan, A.E. The latest advances in biomedical applications of chitosan hydrogel as a powerful natural structure with eye-catching biological properties. J. Mater. Sci. 2022, 57, 3855–3891. [Google Scholar] [CrossRef]
- Gholami, S.; Mohebi, M.-M.; Hajizadeh-Saffar, E.; Ghanian, M.-H.; Zarkesh, I.; Baharvand, H. Fabrication of microporous inorganic microneedles by centrifugal casting method for transdermal extraction and delivery. Int. J. Pharm. 2019, 558, 299–310. [Google Scholar] [CrossRef]
- Krieger, K.J.; Bertollo, N.; Dangol, M.; Sheridan, J.T.; Lowery, M.M.; O’Cearbhaill, E.D. Simple and customizable method for fabrication of high-aspect ratio microneedle molds using low-cost 3D printing. Microsyst. Nanoeng. 2019, 5, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiang, N.; Liu, Z.; Lu, M.; Yang, Y.; Liao, F.; Feng, Y.; Liu, G.; Qiu, S. Preparation and properties of polyvinylpyrrolidone/Sodium Carboxymethyl pellulose poluble microneedles. Materials 2023, 16, 3417. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, S.; Huh, I.; Kim, S.; Lahiji, S.F.; Kim, M.; Jung, H. Rapid implantation of dissolving microneedles on an electrospun pillar array. Biomaterials 2015, 64, 70–77. [Google Scholar] [CrossRef]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, B.; Venugopal, J.; Ramakrishna, S.; Lim, C. Biomimetic and bioactive nanofibrous scaffolds from electrospun composite nanofibers. Int. J. Nanomed. 2007, 2, 623–638. [Google Scholar] [CrossRef]
- Barnum, L.; Quint, J.; Derakhshandeh, H.; Samandari, M.; Aghabaglou, F.; Farzin, A.; Abbasi, L.; Bencherif, S.; Memic, A.; Mostafalu, P.; et al. 3D-printed hydrogel-filled microneedle arrays. Adv. Healthc. Mater. 2021, 10, 2001922. [Google Scholar] [CrossRef]
- Kundu, A.; Arnett, P.; Bagde, A.; Azim, N.; Kouagou, E.; Singh, M.; Rajaraman, S. DLP 3D printed “intelligent” microneedle array (iμNA) for stimuli responsive release of drugs and its in vitro and ex vivo characterization. J. Microelectromech. Syst. 2020, 29, 685–691. [Google Scholar] [CrossRef]
- Amarnani, R.; Shende, P. Microneedles in diagnostic, treatment and theranostics: An advancement in minimally-invasive delivery system. Biomed. Microdevices 2021, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liu, H.; Huang, S.; Fu, T.; Xue, W.; Guo, R. Dextran methacrylate hydrogel microneedles loaded with doxorubicin and trametinib for continuous transdermal administration of melanoma. Carbohydr. Polym. 2020, 246, 116650. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Matsumoto, H.; Moro-oka, Y.; Tanaka, M.; Miyahara, Y.; Suganami, T.; Matsumoto, A. Smart microneedle fabricated with silk fibroin combined semi-interpenetrating network hydrogel for glucose-responsive insulin delivery. ACS Biomater. Sci. Eng. 2019, 5, 5781–5789. [Google Scholar] [CrossRef]
- Cui, M.; Zheng, M.; Wiraja, C.; Chew, S.W.T.; Mishra, A.; Mayandi, V.; Lakshminarayanan, R.; Xu, C. Ocular delivery of predatory bacteria with cryomicroneedles against eye infection. Adv. Sci. 2021, 8, 2102327. [Google Scholar] [CrossRef]
- Friedel, M.; Thompson, I.A.P.; Kasting, G.; Polsky, R.; Cunningham, D.; Soh, H.T.; Heikenfeld, J. Opportunities and challenges in the diagnostic utility of dermal interstitial fluid. Nat. Biomed. Eng. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Samant, P.P.; Prausnitz, M.R. Mechanisms of sampling interstitial fluid from skin using a microneedle patch. Proc. Natl. Acad. Sci. USA 2018, 115, 4583–4588. [Google Scholar] [CrossRef] [Green Version]
- Mandal, A.; Boopathy, A.V.; Lam, L.K.W.; Moynihan, K.D.; Welch, M.E.; Bennett, N.R.; Turvey, M.E.; Thai, N.; Van, J.H.; Love, J.C.; et al. Cell and fluid sampling microneedle patches for monitoring skin-resident immunity. Sci. Transl. Med. 2018, 10, eaar2227. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, Z.; Chang, H.; Wang, L.; Chew, S.W.T.; Lio, D.C.S.; Cui, M.; Liu, L.; Tee, B.C.K.; Xu, C. Osmosis-powered hydrogel microneedles for microliters of skin interstitial fluid extraction within minutes. Adv. Healthc. Mater. 2020, 9, 1901683. [Google Scholar] [CrossRef] [PubMed]
- Al Sulaiman, D.; Chang, J.Y.H.; Bennett, N.R.; Topouzi, H.; Higgins, C.A.; Irvine, D.J.; Ladame, S. Hydrogel-coated microneedle arrays for minimally invasive sampling and sensing of specific circulating nucleic acids from skin interstitial fluid. ACS Nano 2019, 13, 9620–9628. [Google Scholar] [CrossRef] [PubMed]
- Rebrin, K.; Steil, G.M. Can interstitial glucose assessment replace blood glucose measurements? Diabetes Technol. Ther. 2000, 2, 461–472. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Niu, Y.; Li, Z.; Li, A.; Yang, H.; Xu, F.; Li, F. A hydrogel microneedle patch for point-of-care testing based on skin interstitial fluid. Adv. Healthc. Mater. 2020, 9, 1901201. [Google Scholar] [CrossRef]
- Zhao, L.; Wen, Z.; Jiang, F.; Zheng, Z.; Lu, S. Silk/polyols/GOD microneedle based electrochemical biosensor for continuous glucose monitoring. RSC Adv. 2020, 10, 6163–6171. [Google Scholar] [CrossRef]
- Zheng, H.; Zuo, B. Functional silk fibroin hydrogels: Preparation, properties and applications. J. Mater. Chem. B 2021, 9, 1238–1258. [Google Scholar] [CrossRef]
- Shah, R.; Patel, T.; Freedman, J.E. Circulating extracellular vesicles in human disease. N. Engl. J. Med. 2018, 379, 958–966. [Google Scholar] [CrossRef]
- Yang, B.; Fang, X.; Kong, J. In situ sampling and monitoring cell-free DNA of the Epstein–Barr virus from dermal interstitial fluid using wearable microneedle patches. ACS Appl. Mater. Interfaces 2019, 11, 38448–38458. [Google Scholar] [CrossRef]
- Wang, J.; Ye, Y.; Yu, J.; Kahkoska, A.R.; Zhang, X.; Wang, C.; Sun, W.; Corder, R.D.; Chen, Z.; Khan, S.A.; et al. Core-shell microneedle gel for self-regulated insulin delivery. ACS Nano 2018, 12, 2466–2473. [Google Scholar] [CrossRef]
- Chen, C.H.; Shyu, V.B.; Chen, C.T. Dissolving microneedle patches for transdermal insulin delivery in diabetic mice: Potential for clinical applications. Materials 2018, 11, 1625. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Liu, P.; Zhu, J.; Lan, J.; Li, Y.; Zhang, L.; Zhu, J.; Tao, J. Hyaluronic acid-based dissolving microneedle patch loaded with methotrexate for improved treatment of psoriasis. ACS Appl. Mater. Interfaces 2019, 11, 43588–43598. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Lin, D.-C.; Chern, E.; Huang, Y.-Y. The use of micro-needle arrays to deliver cells for cellular therapies. Biomed. Microdevices 2020, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Alafnan, A.; Seetharam, A.A.; Hussain, T.; Gupta, M.S.; Rizvi, S.M.D.; Moin, A.; Alamri, A.; Unnisa, A.; Awadelkareem, A.M.; Elkhalifa, A.O.; et al. Development and characterization of PEGDA microneedles for localized drug delivery of gemcitabine to treat inflammatory breast cancer. Materials 2022, 15, 7693. [Google Scholar] [CrossRef]
- Gangrade, A.; Mandal, B.B. Drug delivery of anticancer drugs from injectable 3D porous silk scaffold for prevention of gastric cancer growth and recurrence. ACS Biomater. Sci. Eng. 2020, 6, 6195–6206. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Zak, J.K. Biomaterial-based regional chemotherapy: Local anticancer drug delivery to enhance chemotherapy and minimize its side-effects. Mater. Sci. Eng. C 2016, 62, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, N.; Lapointe, R.; Lerouge, S. Biomaterials for enhanced immunotherapy. APL Bioeng. 2022, 6, 041502. [Google Scholar] [CrossRef]
- Kim, J.; Choi, Y.; Kim, D.-H.; Yoon, H.Y.; Kim, K. Injectable Hydrogel-based combination cancer immunotherapy for overcoming localized therapeutic efficacy. Pharmaceutics 2022, 14, 1908. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; Wang, J.; Lanier, O.L.; Wechsler, M.E.; Peppas, N.A.; Gu, Z. Macroencapsulation devices for cell therapy. Engineering 2022, 13, 53–70. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, W.; Li, C.; Zhang, J.; Qin, L.; Lai, Y. Recent advances of microneedles and their application in disease treatment. Int. J. Mol. Sci. 2022, 23, 2401. [Google Scholar] [CrossRef]
- Cao, J.; Su, J.; An, M.; Yang, Y.; Zhang, Y.; Zuo, J.; Zhang, N.; Zhao, Y. Novel DEK-targeting aptamer delivered by a hydrogel microneedle attenuates collagen-induced arthritis. Mol. Pharma. 2021, 18, 305–316. [Google Scholar] [CrossRef]
- Espinoza, L.; Zakraoui, L.; Espinoza, C.; Gutierrez, F.; Jara, L.; Silveira, L.; Cuellar, M.; Martinez-Osuna, P. Psoriatic arthritis: Clinical response and side effects to methotrexate therapy. J. Rheumatol. 1992, 19, 872–877. [Google Scholar]
- Bi, D.; Qu, F.; Xiao, W.; Wu, J.; Liu, P.; Du, H.; Xie, Y.; Liu, H.; Zhang, L.; Tao, J. Reactive oxygen species-responsive gel-based microneedle patches for prolonged and intelligent psoriasis management. ACS Nano 2023, 17, 4346–4357. [Google Scholar] [CrossRef]
- Bachy, V.; Hervouet, C.; Becker, P.D.; Chorro, L.; Carlin, L.M.; Herath, S.; Papagatsias, T.; Barbaroux, J.-B.; Oh, S.-J.; Benlahrech, A. Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays. Proc. Natl. Acad. Sci. USA 2013, 110, 3041–3046. [Google Scholar] [CrossRef] [PubMed]
- Gualeni, B.; Coulman, S.; Shah, D.; Eng, P.; Ashraf, H.; Vescovo, P.; Blayney, G.; Piveteau, L.D.; Guy, O.; Birchall, J. Minimally invasive and targeted therapeutic cell delivery to the skin using microneedle devices. Br. J. Dermatol. 2018, 178, 731–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enggi, C.K.; Sulistiawati, S.; Stephanie, S.; Tangdilintin, F.; Achmad, A.A.; Putri, R.A.; Burhanuddin, H.; Arjuna, A.; Manggau, M.A.; Permana, A.D. Development of probiotic loaded multilayer microcapsules incorporated into dissolving microneedles for potential improvement treatment of vulvovaginal candidiasis: A proof of concept study. J. Colloid Interface Sci. 2023, 648, 203–219. [Google Scholar] [CrossRef]
- Jin, L.J.; Söder, P.O.; Leung, W.K.; Corbet, E.F.; Samaranayake, L.P.; Söder, B.; Davies, W.I. Granulocyte elastase activity and PGE2 levels in gingival crevicular fluid in relation to the presence of subgingival periodontopathogens in subjects with untreated adult periodontitis. J. Clin. Periodontol. 1999, 26, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Loe, H. Absence and presence of fluid from normal and inflamed gingivae. Periodontics 1965, 3, 171–177. [Google Scholar]
- Liang, K.-H.; Lin, Y.-Y.; Chiang, S.-H.; Tsai, E.-T.; Lo, W.-L.; Wang, C.-L.; Wang, T.-Y.; Sun, Y.-C.; Kao, S.-Y.; Wu, C.-H.; et al. Recent progress of biomarkers in oral cancers. J. Chin. Med. Assoc. 2021, 84, 987–992. [Google Scholar] [CrossRef]
- Ma, Y.; Boese, S.E.; Luo, Z.; Nitin, N.; Gill, H.S. Drug coated microneedles for minimally-invasive treatment of oral carcinomas: Development and in vitro evaluation. Biomed. Microdevices 2015, 17, 44. [Google Scholar] [CrossRef]
- Matta, A.; Janardhanam, L.S.L.; Venuganti, V.V.K. Dissolvable layered microneedle patch containing 5-fluorouracil for localized treatment of oral carcinoma. J. Chem. Sci. 2023, 135, 23. [Google Scholar] [CrossRef]
- Manimaran, R.; Patel, K.D.; Lobo, V.M.; Kumbhar, S.S.; Venuganti, V.V.K. Buccal mucosal application of dissolvable microneedle patch containing photosensitizer provides effective localized delivery and phototherapy against oral carcinoma. Int. J. Pharm. 2023, 640, 122991. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.K.; Chowdhury, M.Y.E.; Tao, W.; Gill, H.S. Mucosal vaccine delivery: Current state and a pediatric perspective. J. Control Release 2016, 240, 394–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macpherson, A.J.; Geuking, M.B.; McCoy, K.D. Immunoglobulin A: A bridge between innate and adaptive immunity. Curr. Opin. Gastroenterol. 2011, 27, 529–533. [Google Scholar] [CrossRef]
- Squier, C.A. The permeability of oral mucosa. Crit. Rev. Oral Biol. Med. 1991, 2, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tao, W.; Krebs, S.J.; Sutton, W.F.; Haigwood, N.L.; Gill, H.S. Vaccine delivery to the oral cavity using coated microneedles induces systemic and mucosal immunity. Pharm. Res. 2014, 31, 2393–2403. [Google Scholar] [CrossRef] [Green Version]
- Zhen, Y.; Wang, N.; Gao, Z.; Ma, X.; Wei, B.; Deng, Y.; Wang, T. Multifunctional liposomes constituting microneedles induced robust systemic and mucosal immunoresponses against the loaded antigens via oral mucosal vaccination. Vaccine 2015, 33, 4330–4340. [Google Scholar] [CrossRef]
- Armfield, J.M. The extent and nature of dental fear and phobia in Australia. Aust. Dent. J. 2010, 55, 368–377. [Google Scholar] [CrossRef]
- Daly, S.; Claydon, N.C.A.; Newcombe, R.G.; Seong, J.; Addy, M.; West, N.X. Randomised controlled trial of a microneedle patch with a topical anaesthetic for relieving the pain of dental injections. J. Dent. 2021, 107, 103617. [Google Scholar] [CrossRef]
- Seeni, R.Z.; Zheng, M.; Lio, D.C.S.; Wiraja, C.; Mohd Yusoff, M.F.B.; Koh, W.T.Y.; Liu, Y.; Goh, B.T.; Xu, C. Targeted delivery of anesthetic agents to bone tissues using conductive microneedles enhanced iontophoresis for painless dental anesthesia. Adv. Funct. Mater. 2021, 31, 2105686. [Google Scholar] [CrossRef]
- Loesche, W.J. Microbiology of dental decay and periodontal disease. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Chapter 99. Available online: https://www.ncbi.nlm.nih.gov/books/NBK8259/ (accessed on 3 April 2023).
- Fiyaz, M.; Ramesh, A.; Ramalingam, K.; Thomas, B.; Shetty, S.; Prakash, P. Association of salivary calcium, phosphate, pH and flow rate on oral health: A study on 90 subjects. J. Indian Soc. Periodontol. 2013, 17, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Altankhishig, B.; Okuyama, K.; Yamamoto, H.; Naito, K.; Hayashi, M.; Sano, H.; Sidhu, S.K.; Saito, T. Inhibition of demineralization of dentin by fluoride-containing hydrogel desensitizers: An in vitro study. J. Funct. Biomater. 2022, 13, 246. [Google Scholar] [CrossRef] [PubMed]
- Buzalaf, M.A.; Hannas, A.R.; Kato, M.T. Saliva and dental erosion. J. Appl. Oral Sci. 2012, 20, 493–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nizami, M.Z.I.; Xu, V.W.; Yin, I.X.; Yu, O.Y.; Chu, C.H. Metal and metal oxide nanoparticles in caries prevention: A review. Nanomaterials 2021, 11, 3446. [Google Scholar] [CrossRef]
- Yee, R.; Holmgren, C.; Mulder, J.; Lama, D.; Walker, D.; van Palenstein Helderman, W. Efficacy of silver diamine fluoride for arresting caries treatment. J. Dent. Res. 2009, 88, 644–647. [Google Scholar] [CrossRef]
- Goodson, J.M. Dental applications. In Medical Applications of Controlled Release; CRC Press: Boca Raton, FL, USA, 2019; Chapter 6; pp. 115–138. Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9780429276620-6/dental-applications-max-goodson?context=ubx&refId=9fcd4731-f1d9-42ad-99c2-cfce7132f576 (accessed on 3 April 2023).
- Scully, C.; Shotts, R. ABC of oral health. Mouth ulcers and other causes of orofacial soreness and pain. Br. Med. J. 2000, 321, 162–165. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, T.; Yu, X.; Yi, X.; Li, L.; Qu, X.; Zhang, Z.; Hao, Y.; Wang, W. Betamethasone-loaded dissolvable microneedle patch for oral ulcer treatment. Colloids Surf. B Biointerfaces 2023, 222, 113100. [Google Scholar] [CrossRef]
- Wang, Y.; Sheng, A.; Jiang, X.; Yang, S.; Lin, L.; Yang, M.; Zhu, F.; Hu, Y.; Li, J.; Chang, L. Multidrug dissolvable microneedle patch for the treatment of recurrent oral ulcer. Bio-Des. Manuf. 2023, 6, 255–267. [Google Scholar] [CrossRef]
- Lasserre, J.F.; Brecx, M.C.; Toma, S. Oral microbes, biofilms and their role in periodontal and peri-implant diseases. Materials 2018, 11, 1802. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Hasani-Sadrabadi, M.M.; Zarubova, J.; Dashtimighadam, E.; Haghniaz, R.; Khademhosseini, A.; Butte, M.J.; Moshaverinia, A.; Aghaloo, T.; Li, S. Immunomodulatory microneedle patch for periodontal tissue regeneration. Matter 2022, 5, 666–682. [Google Scholar] [CrossRef]
- Patil, S.B.; Inamdar, S.Z.; Reddy, K.R.; Raghu, A.V.; Akamanchi, K.G.; Inamadar, A.C.; Das, K.K.; Kulkarni, R.V. Functionally tailored electro-sensitive poly(acrylamide)-g-pectin copolymer hydrogel for transdermal drug delivery application: Synthesis, characterization, in-vitro and ex-vivo evaluation. Drug Deliv. Lett. 2020, 10, 185–196. [Google Scholar] [CrossRef]
- Liebl, H.; Kloth, L.C. Skin cell proliferation stimulated by microneedles. J. Am. Coll. Clin. Wound Spec. 2012, 4, 2–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divyashri, G.; Badhe, R.V.; Sadanandan, B.; Vijayalakshmi, V.; Kumari, M.; Ashrit, P.; Bijukumar, D.; Mathew, M.T.; Shetty, K.; Raghu, A.V. Applications of hydrogel-based delivery systems in wound care and treatment: An up-to-date review. Polym. Adv. Technol. 2022, 33, 2025–2043. [Google Scholar] [CrossRef]
- Singh, S.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Hattarki, S.; Bogar, C.; Bhat, K. Green tea catechins showed antibacterial activity on Streptococcus mutans—An in vitro study. Indian J. Dent. Res. 2021, 32, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, T.; Zhao, B.; Fan, W.; Shen, Y.; Wei, H.; Zhang, M.; Zheng, W.; Peng, J.; Wang, J.; et al. Acceleration of oral wound healing under diabetes mellitus conditions using bioadhesive hydrogel. ACS Appl. Mater. Interfaces 2023, 15, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, H.U.; Lee, Y.C.; Kim, G.H.; Park, E.C.; Han, S.H.; Lee, J.G.; Choi, S.; Heo, N.S.; Kim, D.L.; et al. Wound healing potential of antibacterial microneedles loaded with green tea extracts. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 757–762. [Google Scholar] [CrossRef]
- Batra, P.; Dawar, A.; Miglani, S. Microneedles and nanopatches-based delivery devices in dentistry. Discoveries 2020, 8, e116. [Google Scholar] [CrossRef]
- Ullah Khan, S.; Saleh, T.A.; Wahab, A.; Khan, M.H.U.; Khan, D.; Ullah Khan, W.; Rahim, A.; Kamal, S.; Ullah Khan, F.; Fahad, S. Nanosilver: New ageless and versatile biomedical therapeutic scaffold. Int. J. Nanomed. 2018, 13, 733–762. [Google Scholar] [CrossRef] [Green Version]
- González García, L.E.; MacGregor, M.N.; Visalakshan, R.M.; Ninan, N.; Cavallaro, A.A.; Trinidad, A.D.; Zhao, Y.; Hayball, A.J.D.; Vasilev, K. Self-sterilizing antibacterial silver-loaded microneedles. Chem. Commun. 2019, 55, 171–174. [Google Scholar] [CrossRef]
- Xu, J.; Danehy, R.; Cai, H.; Ao, Z.; Pu, M.; Nusawardhana, A.; Rowe-Magnus, D.; Guo, F. Microneedle patch-mediated treatment of bacterial biofilms. ACS Appl. Mater. Interfaces 2019, 11, 14640–14646. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.G.; Laabei, M.; Li, S.; Estrela, P.; Leese, H.S. Antimicrobial releasing hydrogel forming microneedles. Biomater. Adv. 2023, 151, 213467. [Google Scholar] [CrossRef] [PubMed]






| Material | Description | Applications |
|---|---|---|
| Natural Polymers | ||
| Carbohydrate-based | Polysaccharides with biocompatibility and bioactive properties |
|
| Protein-based | Gelatin and silk fibroin with biocompatibility and controllable degradation |
|
| Synthetic Polymers | ||
| Polyvinyl pyrrolidone | Synthetic polymer with good mechanical properties and biocompatibility |
|
| Poly (ethylene glycol) | Synthetic polymer with high water solubility and biocompatibility |
|
| Poly (methyl vinyl ether-co-maleic acid) | Synthetic polymer with mucoadhesive properties and biocompatibility |
|
| Poly (acrylic acid) | Synthetic polymer with pH-responsive properties and biocompatibility |
|
| Method | Description | Advantages | Disadvantages |
|---|---|---|---|
| Micro-molding method | Fabrication using a micro-molding process to create MN structures made of hydrogel materials. |
|
|
| Casting | Casting hydrogel material into MN molds |
|
|
| Electrospinning | Electrospinning hydrogel solution into MN structures |
|
|
| 3D printed hydrogel-filled microneedle array | 3D printing of hydrogel-filled MN arrays |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Bi, D.; Hu, Z.; Yang, Y.; Liu, Y.; Leung, W.K. Hydrogel-Forming Microneedles with Applications in Oral Diseases Management. Materials 2023, 16, 4805. https://doi.org/10.3390/ma16134805
Li Y, Bi D, Hu Z, Yang Y, Liu Y, Leung WK. Hydrogel-Forming Microneedles with Applications in Oral Diseases Management. Materials. 2023; 16(13):4805. https://doi.org/10.3390/ma16134805
Chicago/Turabian StyleLi, Yuqing, Duohang Bi, Zhekai Hu, Yanqi Yang, Yijing Liu, and Wai Keung Leung. 2023. "Hydrogel-Forming Microneedles with Applications in Oral Diseases Management" Materials 16, no. 13: 4805. https://doi.org/10.3390/ma16134805