Cetuximab-Conjugated Magnetic Poly(Lactic-co-Glycolic Acid) Nanoparticles for Dual-Targeted Delivery of Irinotecan in Glioma Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
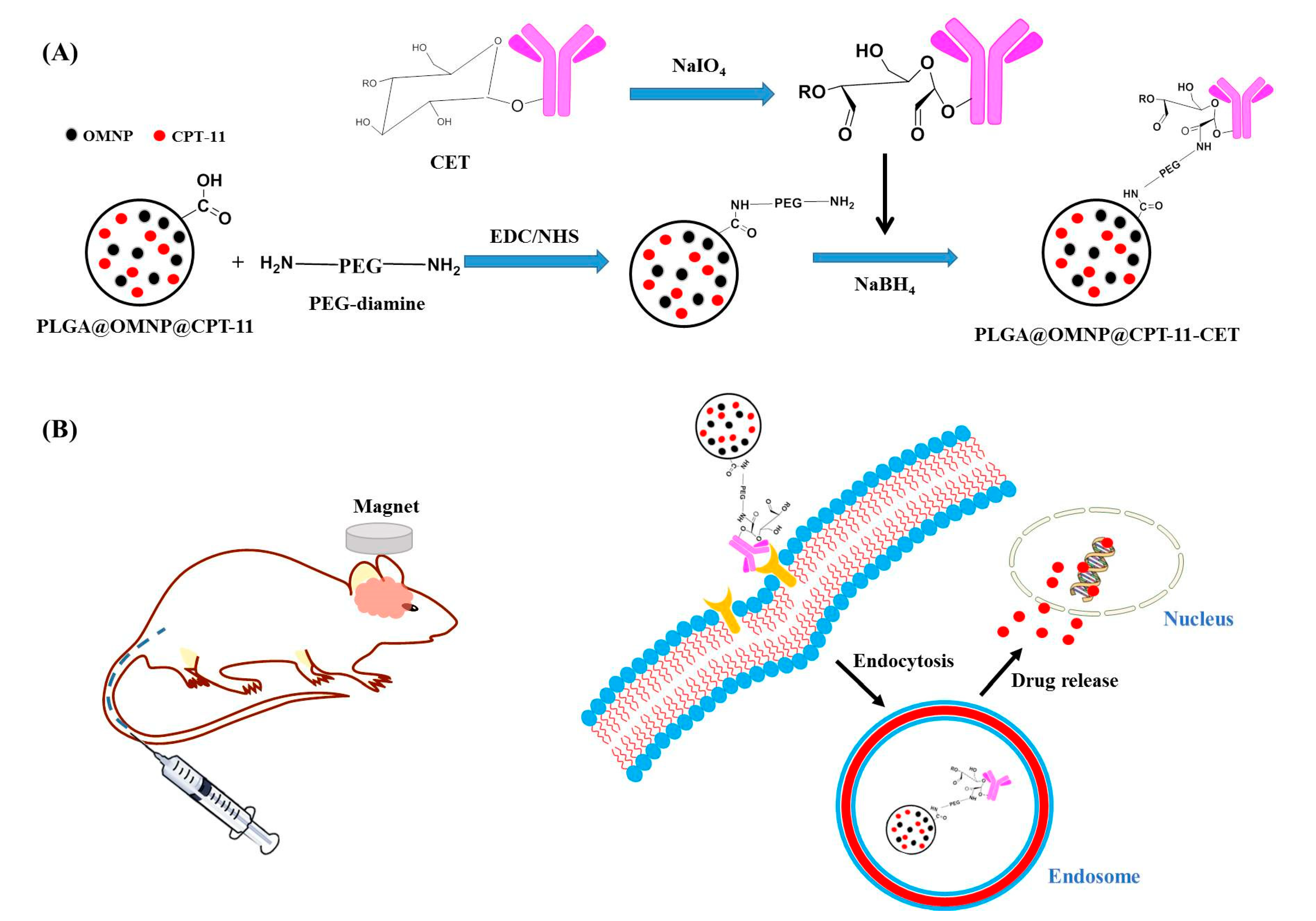
2.2. Preparation of Drug-Loaded PLGA Magnetic Nanoparticles (PLGA@OMNP@CPT-11)
2.3. Preparation of CET-Conjugated PLGA@OMNP@CPT-11 (PLGA@OMNP@CPT-11-CET)
2.4. Physicochemical Characterization
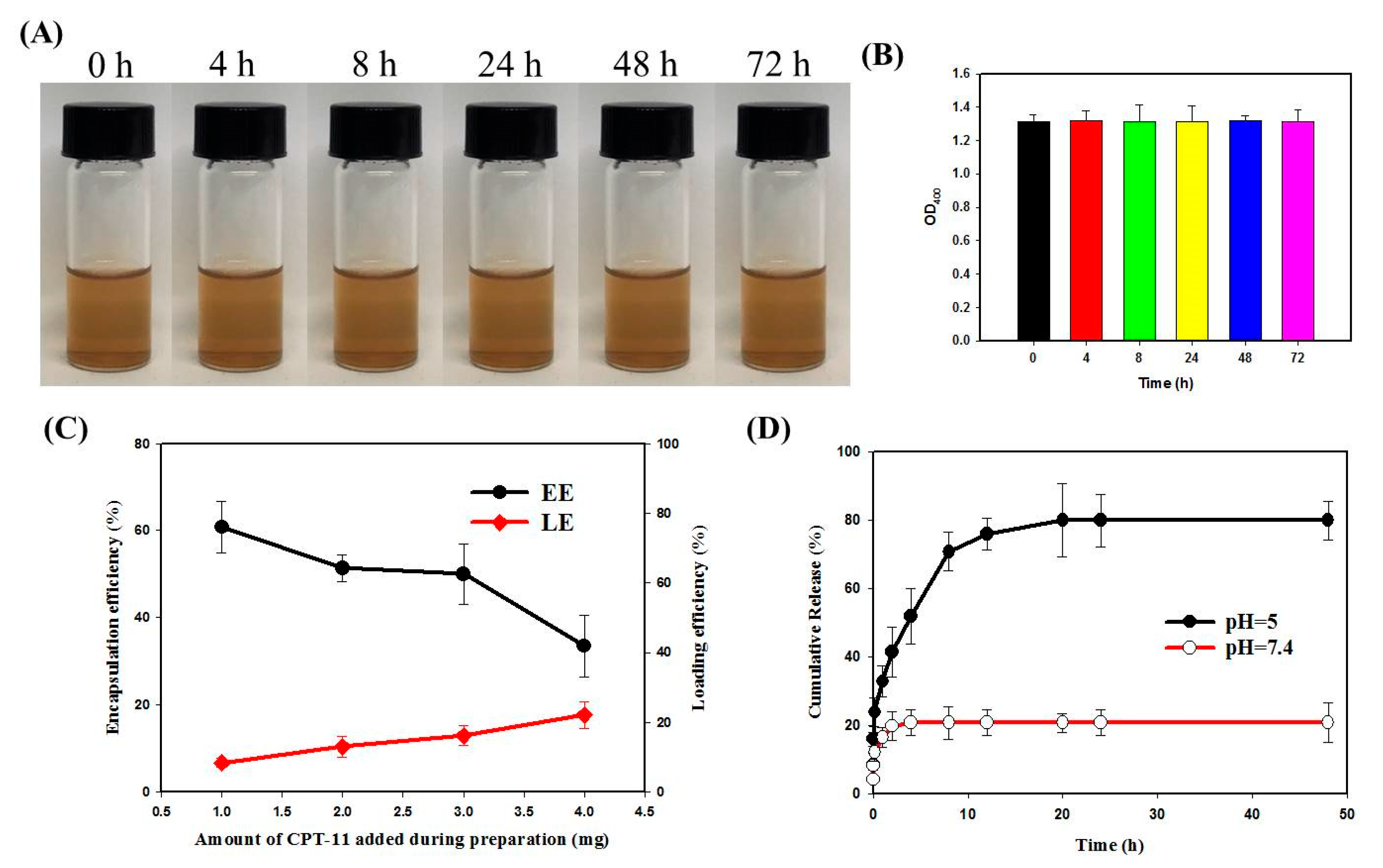
2.5. Drug Loading and Release
2.6. In Vitro Cell Culture
2.6.1. Cell Culture and Intracellular Uptake
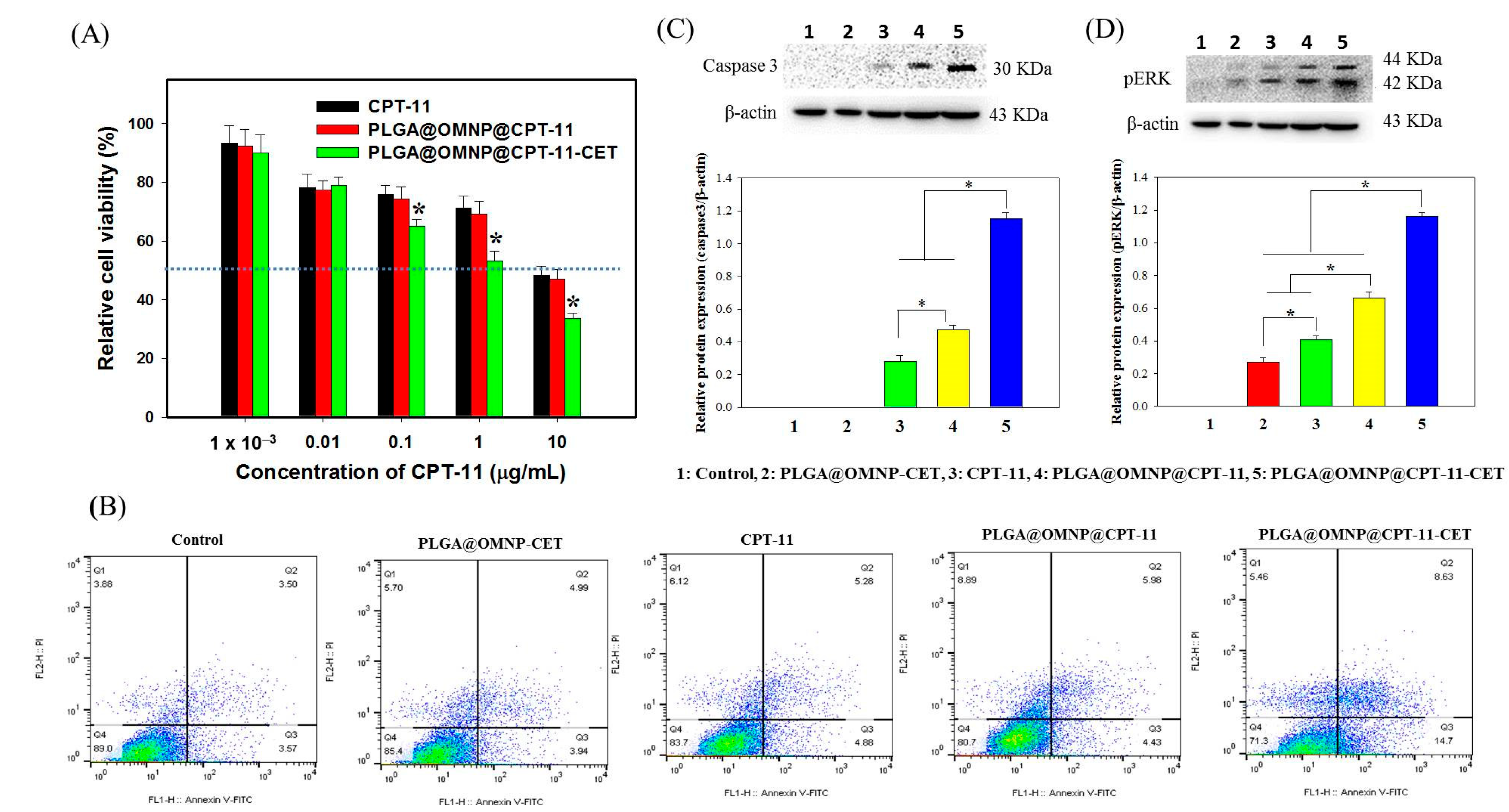
2.6.2. Cytotoxicity
2.6.3. Live/Dead Assays and Cell Apoptosis
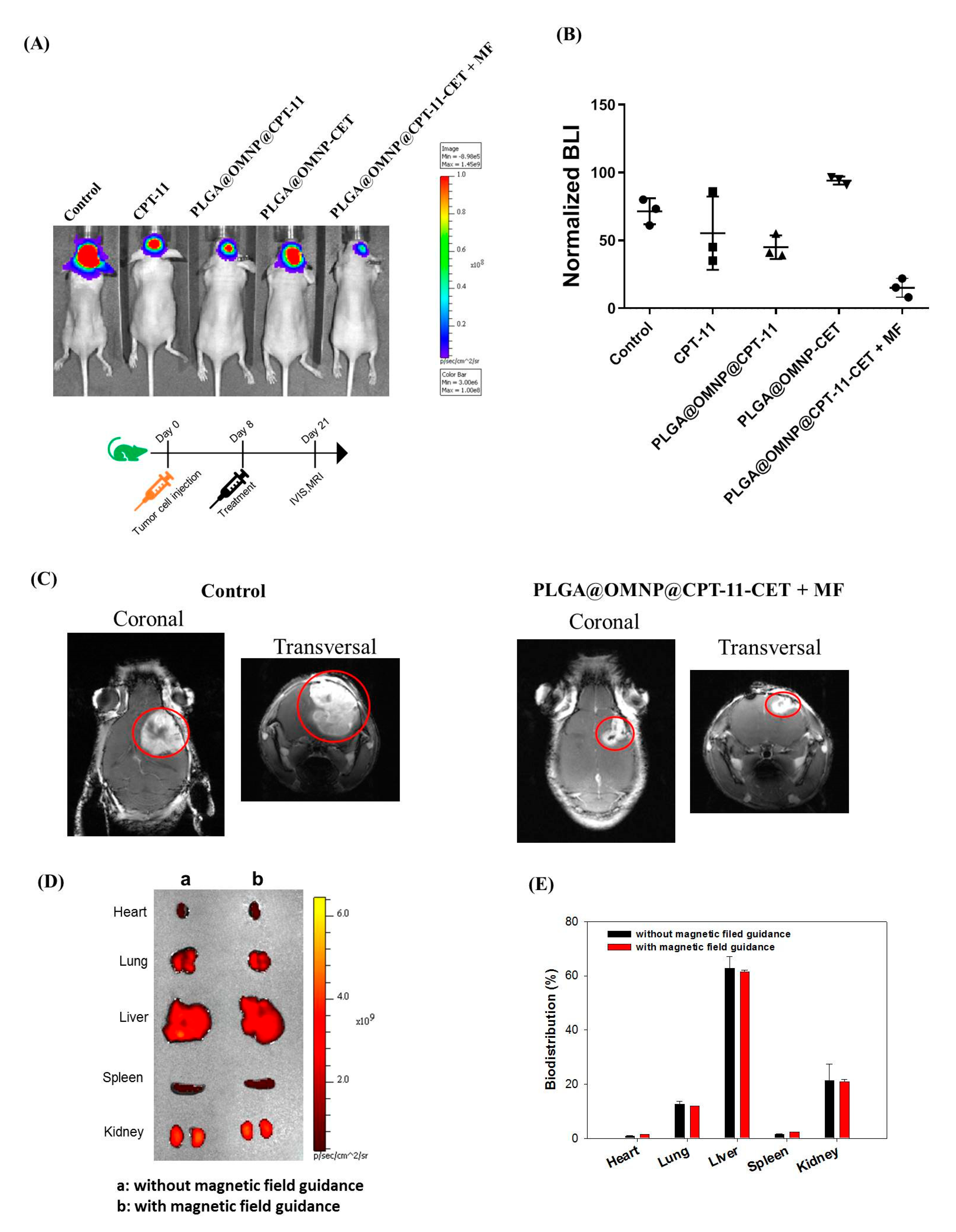
2.7. Xenograft Tumor Model in Nude Mice
2.8. Statistical Analysis
3. Results
3.1. Preparation and Characterization of Nanoparticles
3.2. In Vitro Studies
3.3. In Vivo Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koshy, M.; Villano, J.L.; Dolecek, T.A.; Howard, A.; Mahmood, U.; Chmura, S.J.; Weichselbaum, R.R.; McCarthy, B.J. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J. Neuro-Oncol. 2012, 107, 207–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, H.S.; Prados, M.D.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.K.; Paleologos, N.; Nicholas, M.K.; Jensen, R.; et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 2009, 27, 4733–4740. [Google Scholar] [CrossRef] [Green Version]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, C.-C.; Lan, Y.-H.; Lu, Y.-J.; Weng, Y.-L.; Chen, J.-P. Targeted delivery of irinotecan and SLP2 shRNA with GRP-conjugated magnetic graphene oxide for glioblastoma treatment. Biomater. Sci. 2022, 10, 3201–3222. [Google Scholar] [CrossRef] [PubMed]
- Doane, T.L.; Burda, C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem. Soc. Rev. 2012, 41, 2885–2911. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Teng, F.; Shi, C.; Chen, J.; Wu, S.; Wang, B.; Meng, X.; Essiet Imeh, A.; Li, W. Polymeric nanoparticles-Promising carriers for cancer therapy. Front. Bioeng. Biotechnol. 2022, 10, 1024143. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Grumezescu, A.M. Novel Tumor-Targeting Nanoparticles for Cancer Treatment—A Review. Int. J. Mol. Sci. 2022, 23, 5253. [Google Scholar]
- Pandey, A.; Jain, D.S. Poly Lactic-Co-Glycolic Acid (PLGA) copolymer and its pharmaceutical application. In Handbook of Polymers for Pharmaceutical Technologies: Processing and Applications; Wiley: Hoboken, NJ, USA; Scrivener Publishing: Salem, MA, USA, 2015; Volume 2, pp. 151–172. [Google Scholar]
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of hybrid PLGA nanoparticles: Future of smart drug delivery and theranostics medicine. Mater. Des. 2020, 193, 108805. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [Green Version]
- Alvi, M.; Yaqoob, A.; Rehman, K.; Shoaib, S.M.; Akash, M.S.H. PLGA-based nanoparticles for the treatment of cancer: Current strategies and perspectives. AAPS Open 2022, 8, 12. [Google Scholar] [CrossRef]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef] [PubMed]
- Mosafer, J.; Abnous, K.; Tafaghodi, M.; Jafarzadeh, H.; Ramezani, M. Preparation and characterization of uniform-sized PLGA nanospheres encapsulated with oleic acid-coated magnetic-Fe3O4 nanoparticles for simultaneous diagnostic and therapeutic applications. Colloids Surf. A Physicochem. Eng. Asp. 2017, 514, 146–154. [Google Scholar] [CrossRef]
- Polyak, B.; Friedman, G. Magnetic targeting for site-specific drug delivery: Applications and clinical potential. Expert Opin. Drug Deliv. 2009, 6, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.A.; Mozhdehi, D.; Dzuricky, M.J.; Isaacs, F.J.; Brustad, E.M.; Chilkoti, A. Active Targeting of Cancer Cells by Nanobody Decorated Polypeptide Micelle with Bio-orthogonally Conjugated Drug. Nano Lett. 2019, 19, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, Y.; Li, Z.; Li, L.; Saint-Cricq, P.; Li, C.; Lin, J.; Wang, C.; Su, Z.; Zink, J.I. Tailored Synthesis of Octopus-type Janus Nanoparticles for Synergistic Actively-Targeted and Chemo-Photothermal Therapy. Angew. Chem. Int. Ed. 2016, 55, 2118–2121. [Google Scholar] [CrossRef] [PubMed]
- Caraway, C.A.; Gaitsch, H.; Wicks, E.E.; Kalluri, A.; Kunadi, N.; Tyler, B.M. Polymeric Nanoparticles in Brain Cancer Therapy: A Review of Current Approaches. Polymers 2022, 14, 2963. [Google Scholar] [CrossRef]
- Ahamed, J.; Jaswanth Gowda, B.H.; Almalki, W.H.; Gupta, N.; Sahebkar, A.; Kesharwani, P. Recent advances in nanoparticle-based approaches for the treatment of brain tumors: Opportunities and challenges. Eur. Polym. J. 2023, 193, 112111. [Google Scholar] [CrossRef]
- Habban Akhter, M.; Sateesh Madhav, N.; Ahmad, J. Epidermal growth factor receptor based active targeting: A paradigm shift towards advance tumor therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1188–1198. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.-J.; Lan, Y.-H.; Chuang, C.-C.; Lu, W.-T.; Chan, L.-Y.; Hsu, P.-W.; Chen, J.-P. Injectable Thermo-Sensitive Chitosan Hydrogel Containing CPT-11-Loaded EGFR-Targeted Graphene Oxide and SLP2 shRNA for Localized Drug/Gene Delivery in Glioblastoma Therapy. Int. J. Mol. Sci. 2020, 21, 7111. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Chuang, E.-Y.; Cheng, Y.-H.; Anilkumar, T.S.; Chen, H.-A.; Chen, J.-P. Thermosensitive magnetic liposomes for alternating magnetic field-inducible drug delivery in dual targeted brain tumor chemotherapy. Chem. Eng. J. 2019, 373, 720–733. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, M.; Zeng, F.; Jin, H.; Xu, Q.; Huang, Y. Dual-Targeting Magnetic PLGA Nanoparticles for Codelivery of Paclitaxel and Curcumin for Brain Tumor Therapy. ACS Appl. Mater. Interfaces 2016, 8, 32159–32169. [Google Scholar] [CrossRef]
- Chen, H.-A.; Lu, Y.-J.; Dash, B.S.; Chao, Y.-K.; Chen, J.-P. Hyaluronic Acid-Modified Cisplatin-Encapsulated Poly(Lactic-co-Glycolic Acid) Magnetic Nanoparticles for Dual-Targeted NIR-Responsive Chemo-Photothermal Combination Cancer Therapy. Pharmaceutics 2023, 15, 290. [Google Scholar] [CrossRef] [PubMed]
- Jose, G.; Lu, Y.J.; Hung, J.T.; Yu, A.L.; Chen, J.P. Co-Delivery of CPT-11 and Panobinostat with Anti-GD2 Antibody Conjugated Immunoliposomes for Targeted Combination Chemotherapy. Cancers 2020, 12, 3211. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.H.; Chou, M.Y.; Chu, I.M. Cetuximab-conjugated iron oxide nanoparticles for cancer imaging and therapy. Int. J. Nanomed. 2015, 10, 3663–3685. [Google Scholar] [CrossRef] [Green Version]
- Endres, S.C.; Ciacchi, L.C.; Mädler, L. A review of contact force models between nanoparticles in agglomerates, aggregates, and films. J. Aerosol Sci. 2021, 153, 105719. [Google Scholar] [CrossRef]
- Yang, L.; Tian, J.; Meng, J.; Zhao, R.; Li, C.; Ma, J.; Jin, T. Modification and Characterization of Fe3O4 Nanoparticles for Use in Adsorption of Alkaloids. Molecules 2018, 23, 562. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Lee, C.-H.; Park, J.; Seo, S.; Lim, E.-K.; Song, Y.J.; Suh, J.-S.; Yoon, H.-G.; Huh, Y.-M.; Haam, S. Antibody conjugated magnetic PLGA nanoparticles for diagnosis and treatment of breast cancer. J. Mater. Chem. 2007, 17, 2695–2699. [Google Scholar] [CrossRef]
- Salehizadeh, H.; Hekmatian, E.; Sadeghi, M.; Kennedy, K. Synthesis and characterization of core-shell Fe3O4-gold-chitosan nanostructure. J. Nanobiotechnol. 2012, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- Marques, D.; Dos Santos, L.A.; Schopf, L.; Fraga, J. Analysis of Poly(Lactic-co-Glycolic Acid)/Poly(Isoprene) Polymeric Blend for application as biomaterial. Polímeros 2013, 23, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Peng, H.; Wen, Y.; Li, N. Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2010, 256, 3093–3097. [Google Scholar] [CrossRef]
- Pednekar, P.A.; Venkata Raman, B. The FT-IR spectrometric studies of vibrational bands of Semecarpus anacardium Linn.F. Leaf, stem powder and extracts. Asian J. Pharm. Clin. Res. 2013, 6, 159–168. [Google Scholar]
- Dash, B.S.; Lu, Y.-J.; Chen, H.-A.; Chuang, C.-C.; Chen, J.-P. Magnetic and GRPR-targeted reduced graphene oxide/doxorubicin nanocomposite for dual-targeted chemo-photothermal cancer therapy. Mater. Sci. Eng. C 2021, 128, 112311. [Google Scholar] [CrossRef]
- Pirooznia, N.; Hasannia, S.; Lotfi, A.S.; Ghanei, M. Encapsulation of Alpha-1 antitrypsin in PLGA nanoparticles: In Vitro characterization as an effective aerosol formulation in pulmonary diseases. J. Nanobiotechnol. 2012, 10, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, S.; Shi, X.; Tian, Y.; Gao, F. pH-Responsive Polymer Nanomaterials for Tumor Therapy. Front. Oncol. 2022, 12, 855019. [Google Scholar] [CrossRef] [PubMed]
- Zolnik, B.S.; Burgess, D.J. Effect of acidic pH on PLGA microsphere degradation and release. J. Control. Release 2007, 122, 338–344. [Google Scholar] [CrossRef]
- Okeley, N.M.; Toki, B.E.; Zhang, X.; Jeffrey, S.C.; Burke, P.J.; Alley, S.C.; Senter, P.D. Metabolic Engineering of Monoclonal Antibody Carbohydrates for Antibody–Drug Conjugation. Bioconjug. Chem. 2013, 24, 1650–1655. [Google Scholar] [CrossRef]
- Wong, D.J.L.; Robert, L.; Atefi, M.S.; Lassen, A.; Avarappatt, G.; Cerniglia, M.; Avramis, E.; Tsoi, J.; Foulad, D.; Graeber, T.G.; et al. Antitumor activity of the ERK inhibitor SCH722984 against BRAF mutant, NRAS mutant and wild-type melanoma. Mol. Cancer 2014, 13, 194. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.-B.; Zhao, W.; Renchi, G.; Gong, Y.; Shi, Y. Customizing delivery nano-vehicles for precise brain tumor therapy. J. Nanobiotechnol. 2023, 21, 32. [Google Scholar] [CrossRef]
- Bhowmik, A.; Chakravarti, S.; Ghosh, A.; Shaw, R.; Bhandary, S.; Bhattacharyya, S.; Sen, P.C.; Ghosh, M.K. Anti-SSTR2 peptide based targeted delivery of potent PLGA encapsulated 3,3′-diindolylmethane nanoparticles through blood brain barrier prevents glioma progression. Oncotarget 2017, 8, 65339–65358. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]




| Sample | Average Particle Size (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) |
|---|---|---|---|
| MNP | 239.6 ± 14.0 | 0.17 ± 0.04 | 16.2 ± 0.8 |
| OMNP | 221.3 ± 2.7 | 0.19 ± 0.03 | −19.8 ± 0.7 |
| PLGA@OMNP@CPT-11 | 237.2 ± 4.5 | 0.12 ± 0.02 | −20.6 ± 2.3 |
| PLGA@OMNP@CPT-11-CET | 245.2 ± 5.1 | 0.29 ± 0.02 | −13.0 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dash, B.S.; Lu, Y.-J.; Luo, S.-H.; Chen, J.-P. Cetuximab-Conjugated Magnetic Poly(Lactic-co-Glycolic Acid) Nanoparticles for Dual-Targeted Delivery of Irinotecan in Glioma Treatment. Materials 2023, 16, 5526. https://doi.org/10.3390/ma16165526
Dash BS, Lu Y-J, Luo S-H, Chen J-P. Cetuximab-Conjugated Magnetic Poly(Lactic-co-Glycolic Acid) Nanoparticles for Dual-Targeted Delivery of Irinotecan in Glioma Treatment. Materials. 2023; 16(16):5526. https://doi.org/10.3390/ma16165526
Chicago/Turabian StyleDash, Banendu Sunder, Yu-Jen Lu, Shu-Hui Luo, and Jyh-Ping Chen. 2023. "Cetuximab-Conjugated Magnetic Poly(Lactic-co-Glycolic Acid) Nanoparticles for Dual-Targeted Delivery of Irinotecan in Glioma Treatment" Materials 16, no. 16: 5526. https://doi.org/10.3390/ma16165526






