Study of Modified Magnesium Phosphate Cement for Fluoride Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
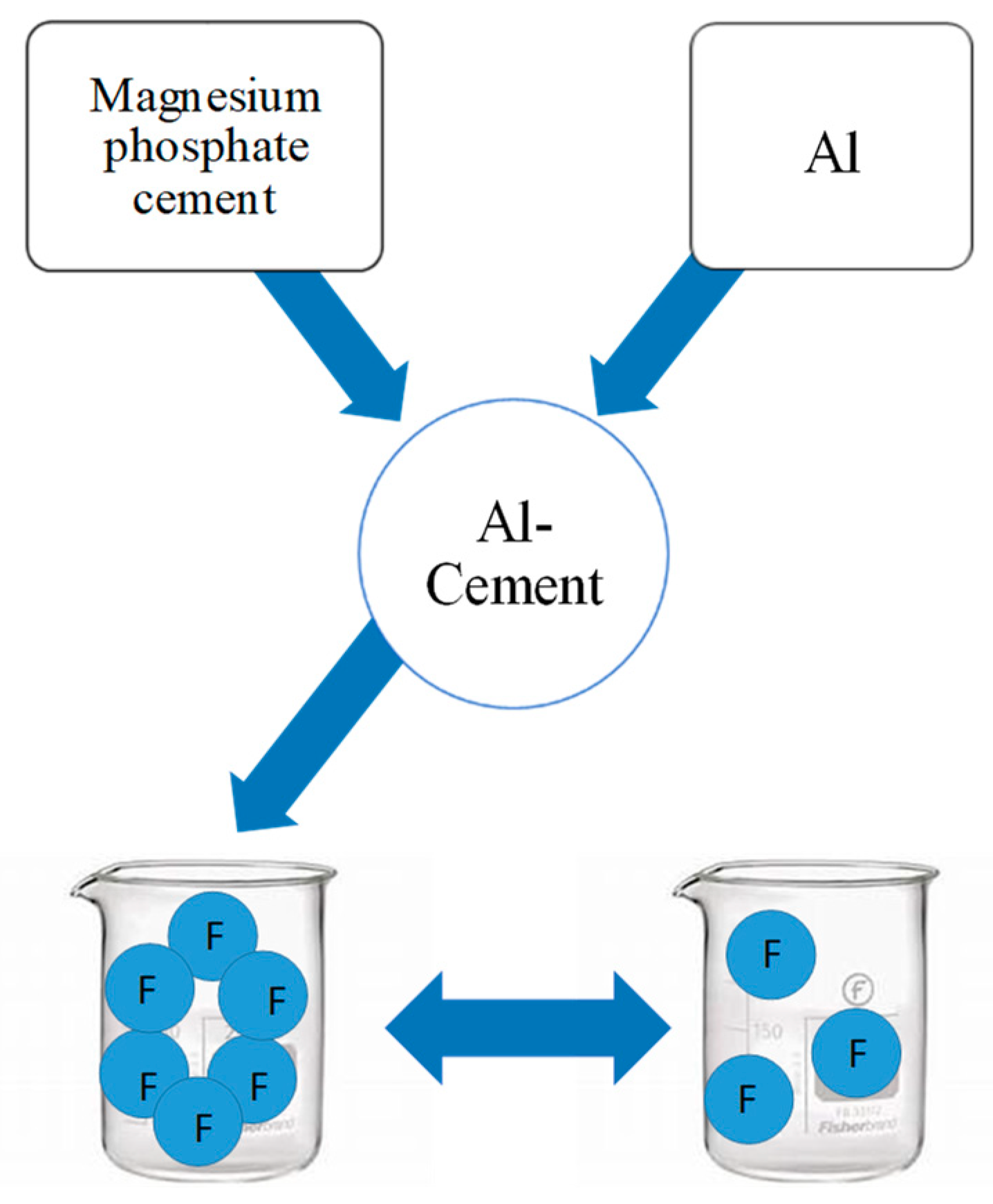
2.2. Preparation
2.3. Analysis Method
3. Results
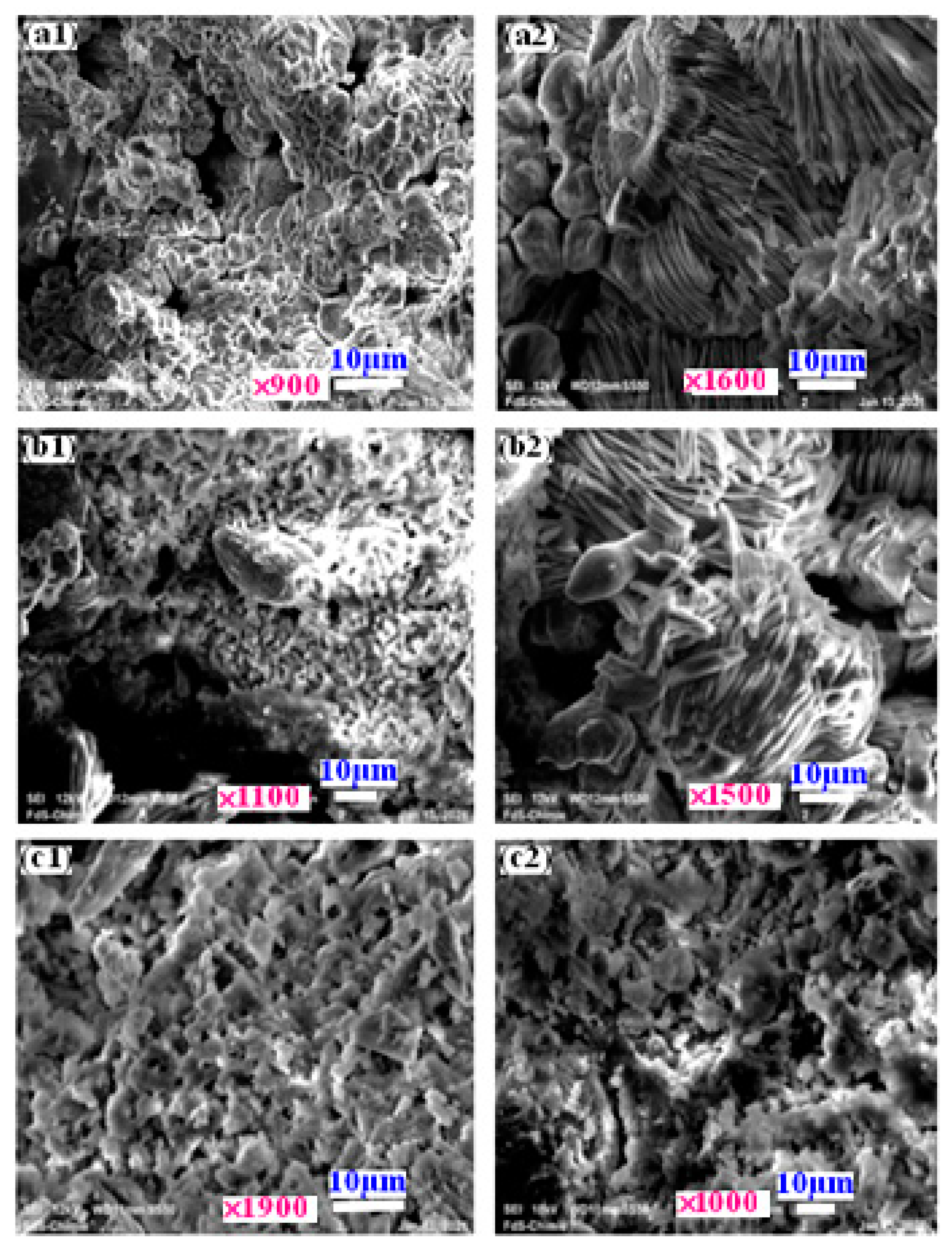
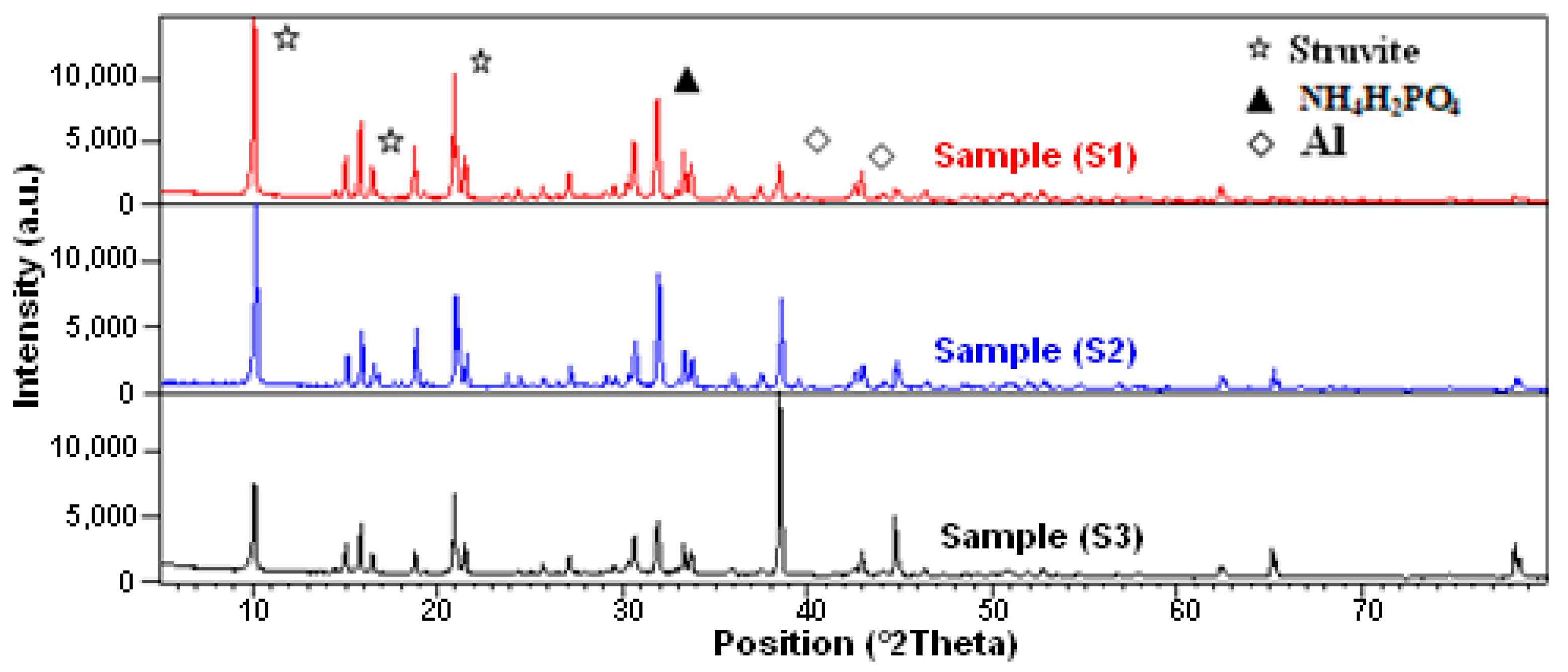
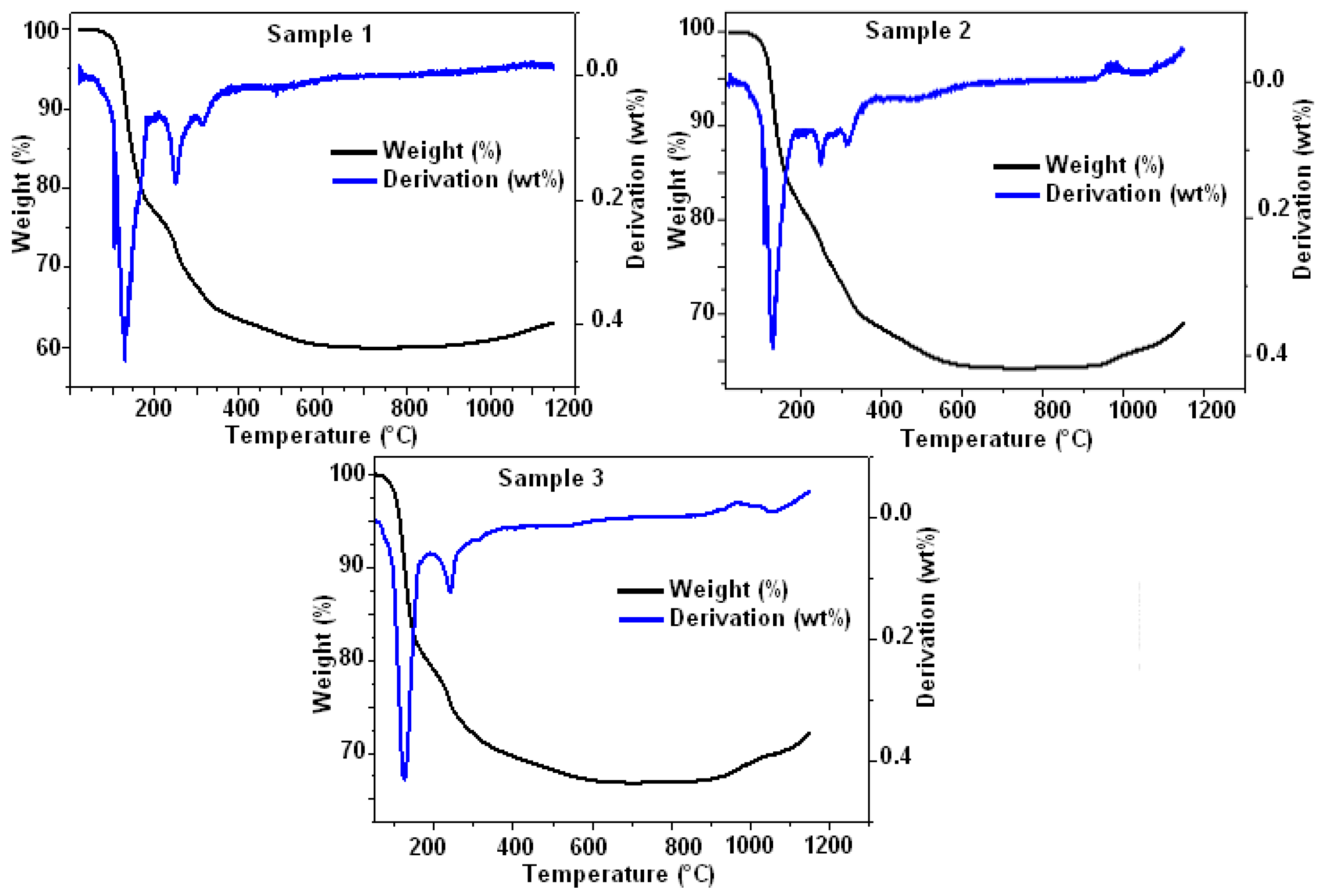
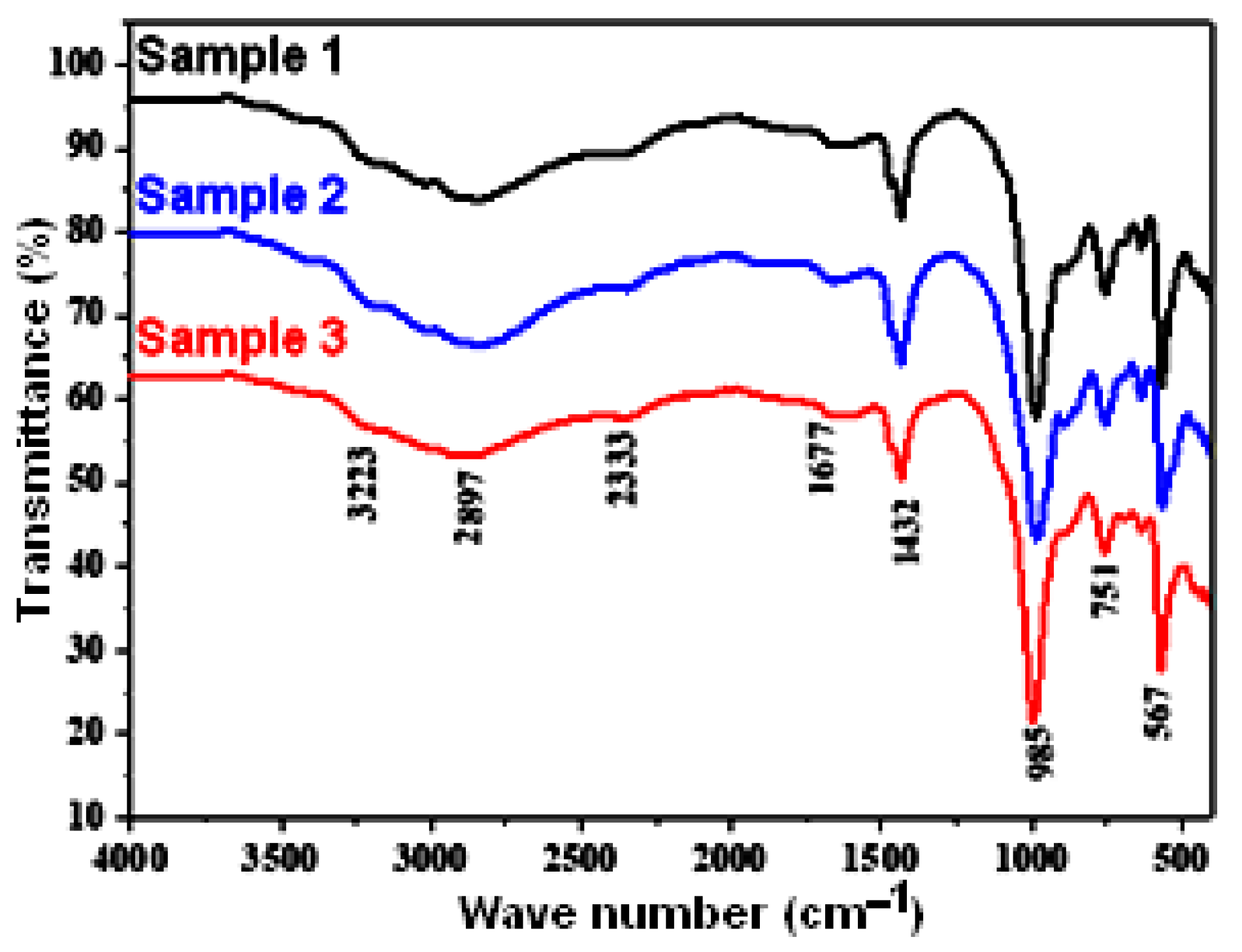
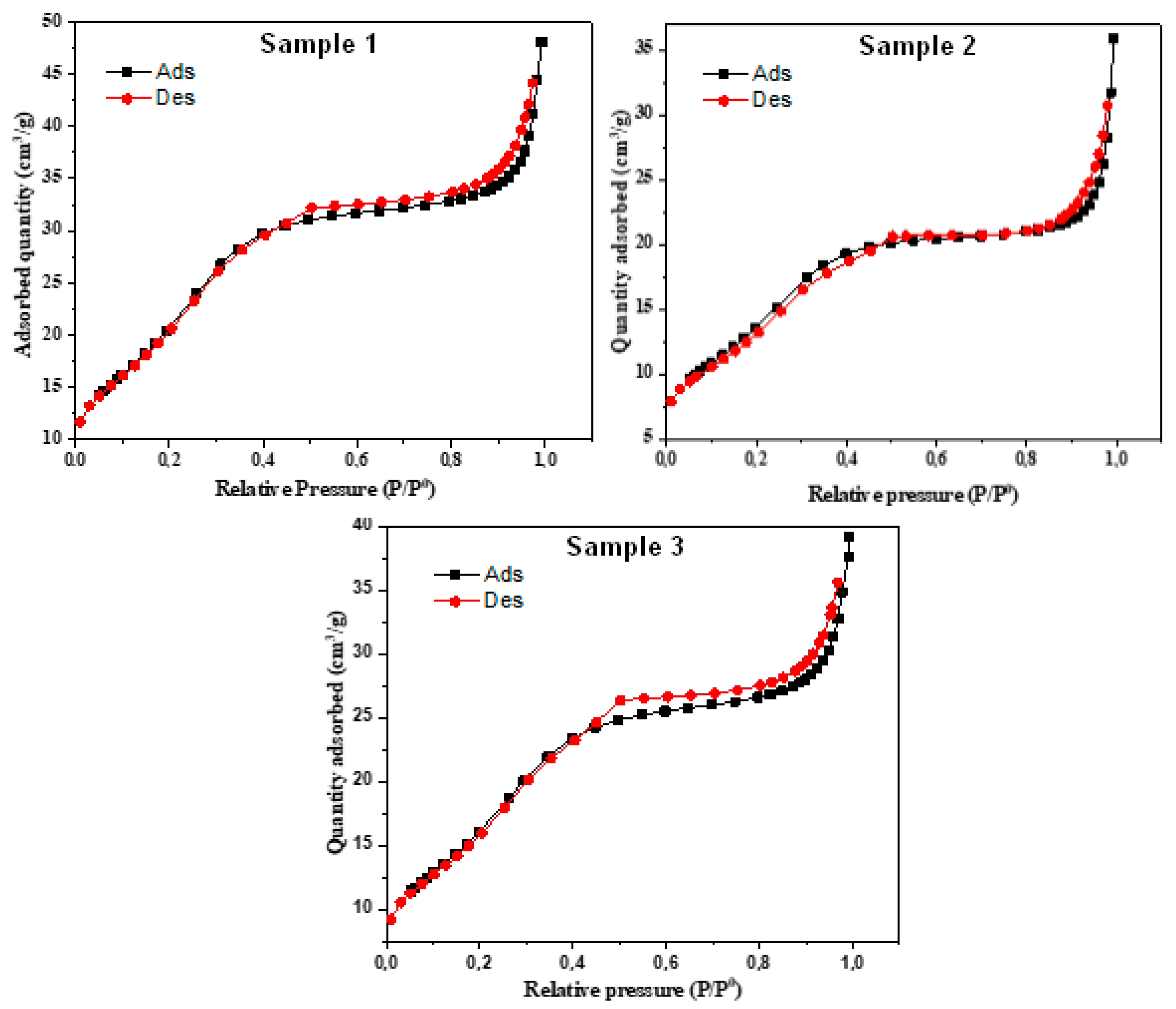
3.1. Composites Analysis
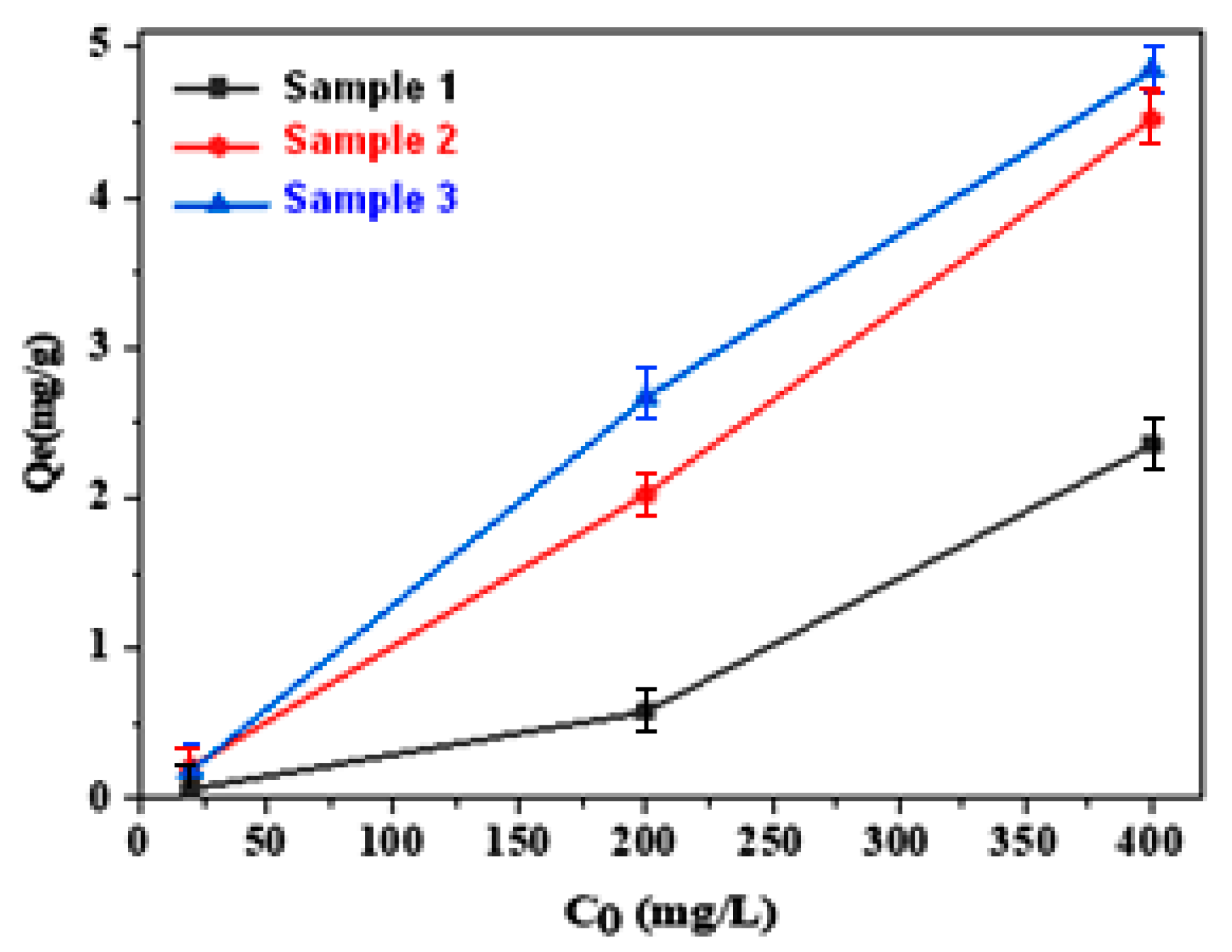
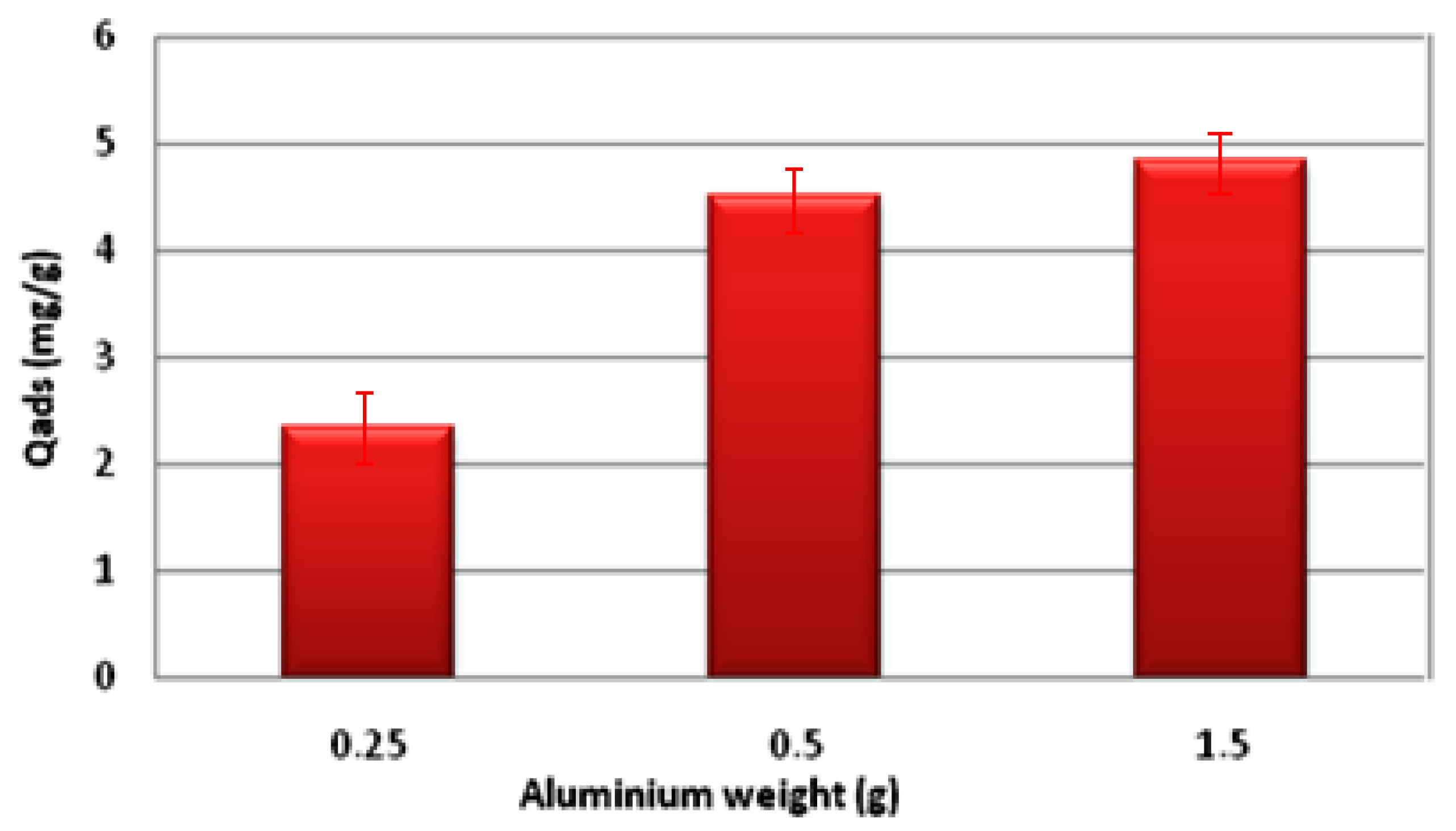
3.2. Adsorption Study
3.3. Comparison with Other Used Adsorbents
4. Conclusions
- ○
- In the current research works, new composite material using magnesium phosphate cement was used as a matrix and different amounts of aluminum introduced in order to improve the resistance of cement in water and to use them in the adsorption of fluorine. We can conclude that the incorporation of different amounts of aluminum has a positive effect on the resistance of the material, which becomes harder and more resistant dry than in water, even after days. These materials have affinities for fluorine whose adsorption capacities we tested. The optimal adsorption values found are qmax = 4.84 mg/g. Moreover, the adsorption increases with the increase in the quantity of aluminum: from 2.35 mg/g for 0.25 g of Al to 4.84 mg/g for 1.5 g of Al. Furthermore, the Langmuir model was identified as the most appropriate fit for the synthesized materials, as it displayed the highest regression coefficients (R2 values closest to 1). Likewise, the observed isotherm corresponds to a monolayer adsorption process, and the calculated value of the separation factor RL indicates that the adsorption is favorable.
- ○
- As far as our study is concerned, the obtained experimental results revealed that, for the different materials used for adsorption, Al cement displays great promise as an adsorbent, particularly due to its exceptional capacity to effectively remove fluoride from drinking water. In addition, it proves to be an interesting and worthwhile compound that deserves further as well as deeper investigation owing to its valuable characteristics and wide range of applications. From this perspective, this synthesis may be regarded as significant in terms of laying the ground for future researchers to extend this study further and explore fully and deeply the additional features of this compound.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Togarepi, E. Deflouridation of water using physico-chemically treated sand as a low-cost adsorbent: An equilibrium study. Afr. J. Environ. Sci. Technol. 2012, 6, 176–181. [Google Scholar] [CrossRef]
- Mehta, D.; Mazumdar, S.; Singh, S.K. Magnetic adsorbents for the treatment of water/wastewater-A review. J. Water Process Eng. 2015, 7, 244–265. [Google Scholar] [CrossRef]
- Nyangi, M.J.; Chebude, Y.; Kilulya, K.F.; Salim, C.J. Comparative Study on Adsorption Isotherm and Kinetics of Defluoridation Using Aluminum and Iron Electrodes in Electrocoagulation. Chem. Afr. 2021, 4, 391–398. [Google Scholar] [CrossRef]
- Gharsallah, S.; Alsawi, A.; Hammami, B.; Khitouni, M.; Charnay, C.; Chemingui, M. Synthesis and Characterization of New Composite Materials Based on Magnesium Phosphate Cement for Fluoride Retention. Materials 2023, 16, 718. [Google Scholar] [CrossRef]
- Shivaprasad, P.; Singh, P.; Saharan, K.; George, S. Synthesis of nano alumina for defluoridation of drinking water. Nano-Struct. Nano-Objects 2018, 13, 109–120. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Y.; Hong, X.; Huang, C.; Huang, C.P. The role of fluoroaluminate complexes on the adsorption of fluoride onto hydrous alumina in aqueous solutions. J. Colloid Interface Sci. 2020, 561, 275–286. [Google Scholar] [CrossRef]
- Mondal, P.; Purkait, M.K. Preparation and characterization of novel green synthesized iron aluminum nanocomposite and studying its efficiency in fluoride removal. Chemosphere 2019, 235, 391402. [Google Scholar] [CrossRef]
- Murambasvina, G.; Mahamadi, C. Effective fluoride adsorption using water hyacinth beads doped with hydrous oxides of aluminium and iron. Groundw. Sustain. Dev. 2019, 10, 100302. [Google Scholar] [CrossRef]
- He, J.; Yang, Y.; Wu, Z.; Xie, C.; Zhang, K.; Kong, L.; Liu, J. Review of fluoride removal from water environment by adsorption. J. Environ. Chem. Eng. 2020, 8, 104516. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, Z.; Feng, C.; Sugiura, N.; Li, M.; Chen, R. Fluoride removal from water by granular ceramic adsorption. J. Colloid Interface Sci. 2010, 348, 579–584. [Google Scholar] [CrossRef]
- Borgohain, X.; Boruah, A.; Sarma, G.K.; Rashid, M.H. Rapid and extremely high adsorption performance of porous MgO nanostructures for fluoride removal from water. J. Mol. Liq. 2020, 305, 112799. [Google Scholar] [CrossRef]
- Chau, C.K.; Qiao, F.; Li, Z. Microstructure of magnesium potassium phosphate cement. Constr. Build. Mater. 2011, 25, 2911–2917. [Google Scholar] [CrossRef]
- Kononenko, O.A.; Milyutin, V.V.; Makarenkov, V.I.; Kozlitin, E.A. Immobilization of NPP evaporator bottom high salt-bearing liquid radioactive waste into struvite-based phosphate matrices. J. Hazard. Mater. 2021, 416, 125902. [Google Scholar] [CrossRef]
- Tansel, B.; Lunn, G.; Monje, O. Struvite formation and decomposition characteristics for ammonia and phosphorus recovery: A review of magnesium-ammonia-phosphate interactions. Chemosphere 2018, 194, 504–514. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, X.; Ma, R.; Sun, S.; Fang, L.; Lin, J.; Luo, J. Solid waste-based magnesium phosphate cements: Preparation, performance and solidification/stabilization mechanism. Constr. Build. Mater. 2021, 297, 123761. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, T.; Jiang, R.; Ohtake, H. Phosphate enhance recovery from wastewater by mechanism analysis and optimization of struvite settleability in fluidized bed reactor. Sci. Rep. 2016, 6, 32215. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Shi, T.; Li, J. Experimental study on mechanical properties and fracture toughness of magnesium phosphate cement. Constr. Build. Mater. 2015, 96, 346–352. [Google Scholar] [CrossRef]
- Soudee, E. Liants Phosphomagnésiens: Mécanisme de Prise et Durabilité. Ph.D. Thesis, INSA Lyon, Villeurbanne, France, 1999; p. 257. [Google Scholar]
- Ding, Z.; Dong, B.; Xing, F.; Han, N.; Li, Z. Cementing mechanism of potassium phosphate based magnesium phosphate cement. Ceram. Int. 2012, 38, 6281–6288. [Google Scholar] [CrossRef]
- Le Rouzic, M. Conditions de formation de la k-struvite dans les ciments phosphomagnésiens. In Proceedings of the 31ème rencontres Universitaire de l’AUGC, Cachan, France, 29–31 May 2013; HAL Open Science: Lyon, France, 2013. Available online: https://hal.science/hal-00903603 (accessed on 11 August 2023).
- Ruan, W.; Liao, J.; Li, F.; Gu, X.; Zhou, A.; Xie, Y. Effects of water purifying material waste and red mud on performance of magnesium phosphate cement. Constr. Build. Mater. 2012, 303, 124563. [Google Scholar] [CrossRef]
- Le Rouzic, M.; Chaussadent, T.; Stefan, L.; Saillio, M. On the influence of Mg/P ratio on the properties and durability of magnesium potassium phosphate cement pastes. Cem. Concr. Res. 2017, 96, 2741. [Google Scholar] [CrossRef]
- Souza, T.; Luz, A.; Pagliosa, P.; Pandolfelli, V.C. Mineralizing alumina–magnesia cement-bonded castables containing magnesium borates. Ceram. Int. 2015, 41, 11143–11152. [Google Scholar] [CrossRef]
- Sotiriadis, K.; Mácová, P.; Mazur, A.; Tolstoy, M.; Viani, A. A solid state NMR and in-situ infrared spectroscopy study on the setting reaction of magnesium sodiumphosphatecement. J. Non-Cryst. Solids 2018, 498, 4959. [Google Scholar] [CrossRef]
- Spirovski, F.; Kuzmanovski, I.; Lutz, H.D.; Engelen, B. Infrared and Raman spectra of magnesium ammonium phosphate hexahydrate (struvite) and its isomorphous analogues. I. Spectra of protiated and partially deuterated magnesium potassium phosphate hexahydrate. J. Mol. Struct. 2004, 689, 1–10. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Chaudhry, S.A.; Khan, T.A.; Ali, I. Adsorptive removal of Pb(II) and Zn(II) from water onto manganese oxide-coated sand: Isotherm, thermodynamic and kinetic studies. Egypt. J. Basic Appl. Sci. 2016, 3, 287–300. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, Z.; Feng, C.; Zhu, D.; Yang, Y.; Sugiura, N. Preparation and characterization of porous granular ceramic containing dispersed aluminum and iron oxides as adsorbents for fluoride removal from aqueous solution. J. Hazard. Mater. 2011, 186, 863–868. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Chaudhary, M.; Jain, N.; Maiti, A. A comparative adsorption kinetic modeling of fluoride adsorption by nanoparticles and its polymeric nanocomposite. J. Environ. Chem. Eng. 2011, 9, 105595. [Google Scholar] [CrossRef]
- Ouakouak, A. Elimination du Cuivre, des Nitrates et des Phosphates des Eaux par Adsorption sur Différents Matériaux. Ph.D. Thesis, Université Mohamed Khider, Biskra, Algérie, 2017. [Google Scholar]
- Fan, X.; Parker, D.; Smith, M.D. Adsorption kinetics of fluoride on low-cost materials. Water Res. 2003, 37, 4929–4937. [Google Scholar] [CrossRef]
| Composite | MgO (g) | NH4H2PO4 (g) | Borax (g) | Al (g) | H2O (g) |
|---|---|---|---|---|---|
| S1 | 1.0790 | 2.8800 | 0.3310 | 0.2560 | 2 |
| S2 | 1.0075 | 2.8250 | 0.3230 | 0.5620 | 2 |
| S3 | 1.0680 | 2.8640 | 0.3115 | 1.5320 | 2 |
| Composite | BET Surface (m²/g) | Pore Volume (cm³/g) | Pore Size (Å) |
|---|---|---|---|
| S1 | 83.52 | 0.074 | 35.58 |
| S2 | 52.27 | 0.055 | 41.24 |
| S3 | 64.03 | 0.060 | 37.79 |
| Model | Sample 1 | Sample 2 | Sample 3 |
|---|---|---|---|
| Langmuir | b = 0.205 L/g q0 = 4.87 mg/g Rl = 0.806 Kap = −0.012 R2 = 0.999 | b = 0.023 L/g q0 = 43.47 mg/g Rl = 0.993 Kap = −0.000310 R2 = 0.999 | b = −0.059 L/g q0 = −16.94 mg/g Rl = 0.988 Kap = −0.0006 R2 = 0.997 |
| Freundlich | 1/n = 0.907 Kf = 0.007 R2 = 0.999 | 1/n = 1.022 Kf = 0.011 R2 = 0.997 | 1/n = 1.082 Kf = 0.0084 R2 = 0.962 |
| Temkin | A = 0.052 B = 0.492 R2 = 0.894 | A = 0.048 B = 1.304 R2 = 0.782 | A = 0.172 B = 0.782 R2 = 0.675 |
| Dubinin-Radushkevich | qd = 0.951 B=119.4 R2 = 0.829 | qd = 1.799 B= 103.7 R2 = 0.796 | qd = 1.799 B= 126.3 R2 = 0.792 |
| Adsorbent | Removal Capacity (mg/g) | Reference |
|---|---|---|
| Activated quartz | 1.16 | Fan et al. (2003) [31] |
| Alumina cement | 1.61 | Sana et al. (2023) [4] |
| Zeolite cement | 1.76 | Sana et al. (2023) [4] |
| Porous granular ceramic | 1.79 | Nan Chen et al.(2010) [27] |
| H2O2 cement | 2.21 | Sana et al. (2023) [4] |
| Composite S1 | 2.35 | Present study |
| Composite S2 | 4.51 | Present study |
| Composite S3 | 4.84 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharsallah, S.; Mallah, A.; Alsawi, A.; Hammami, B.; Khitouni, M.; Charnay, C.; Chemingui, M. Study of Modified Magnesium Phosphate Cement for Fluoride Removal. Materials 2023, 16, 5749. https://doi.org/10.3390/ma16175749
Gharsallah S, Mallah A, Alsawi A, Hammami B, Khitouni M, Charnay C, Chemingui M. Study of Modified Magnesium Phosphate Cement for Fluoride Removal. Materials. 2023; 16(17):5749. https://doi.org/10.3390/ma16175749
Chicago/Turabian StyleGharsallah, Sana, Abdulrahman Mallah, Abdulrahman Alsawi, Bechir Hammami, Mohamed Khitouni, Clarence Charnay, and Mahmoud Chemingui. 2023. "Study of Modified Magnesium Phosphate Cement for Fluoride Removal" Materials 16, no. 17: 5749. https://doi.org/10.3390/ma16175749






