Preferential Enrichment of Enantiomer from Amino Acid Schiff Bases by Coordination Interaction and Crystallization
Abstract
:1. Introduction
2. Materials and Methods
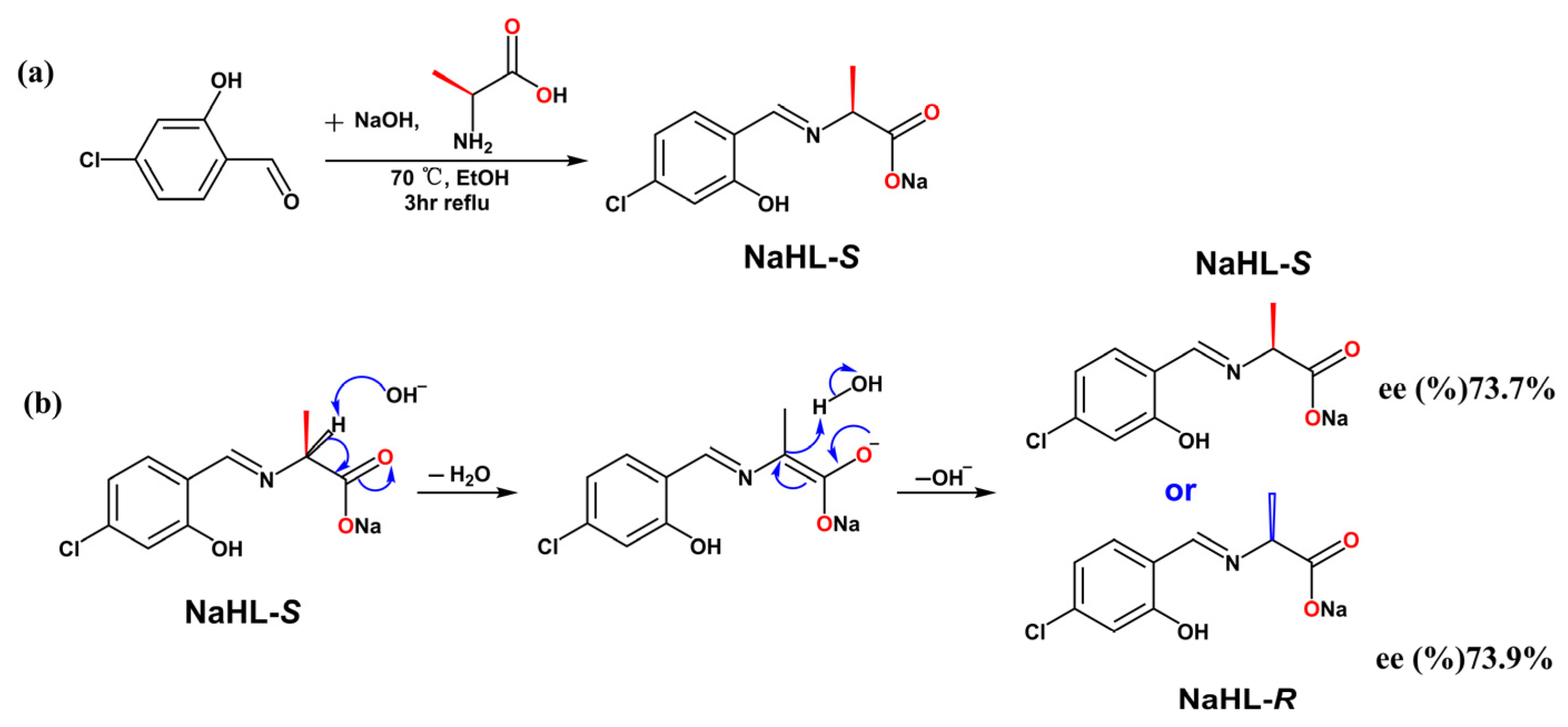
2.1. Synthesis of Ligand
2.2. Synthesis of Complexes
2.2.1. Synthesis of [Cu(L-S) (H2O)]n (1-S)
2.2.2. Synthesis of [Cu(L-R) (H2O)]n (1-R)
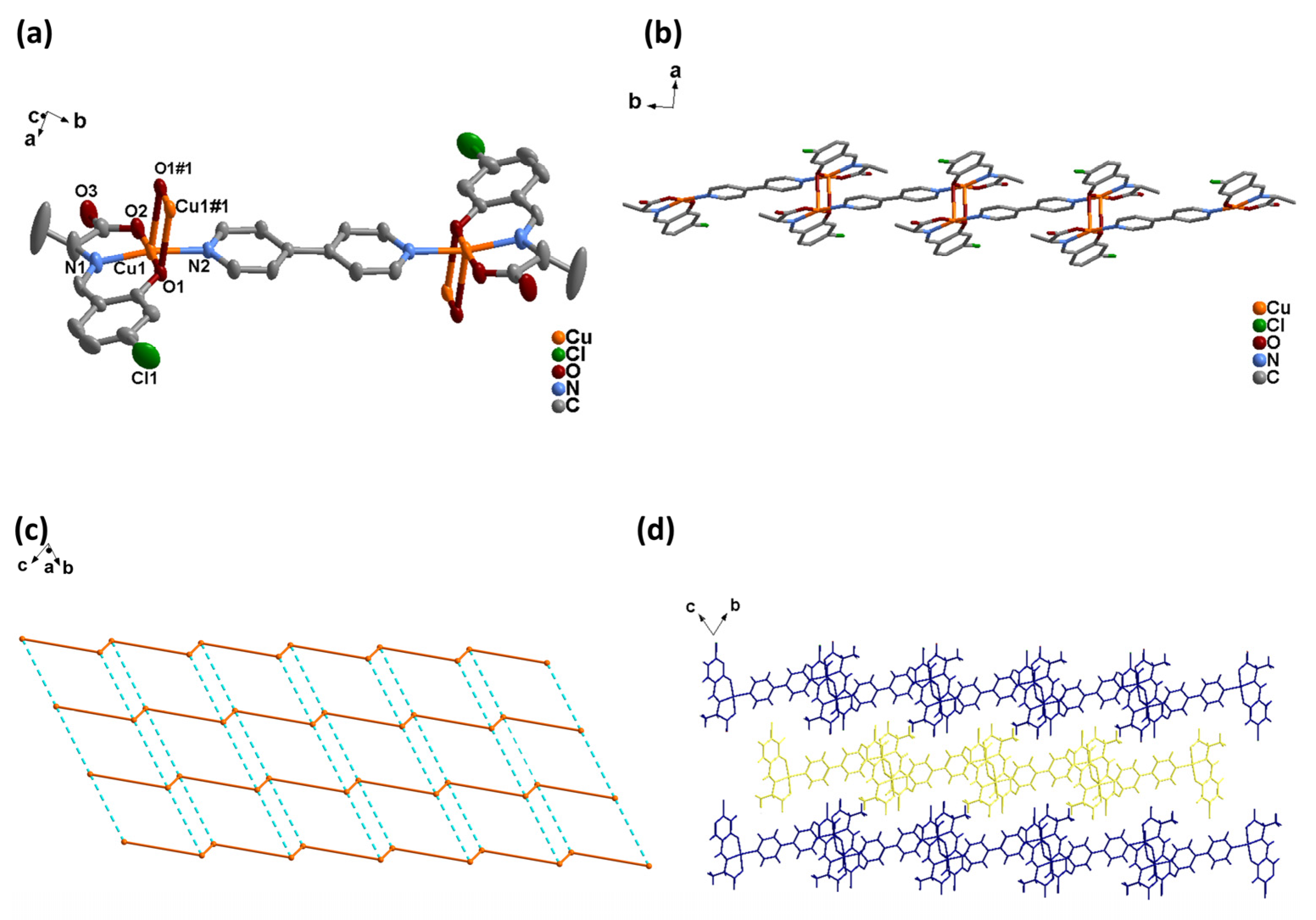
2.2.3. Synthesis of [Cu2(L-S)2 (4,4′-bipy)] (2-S)
2.2.4. Synthesis of [Cu2(L-R)2 (4,4′-bipy)] (2-R)
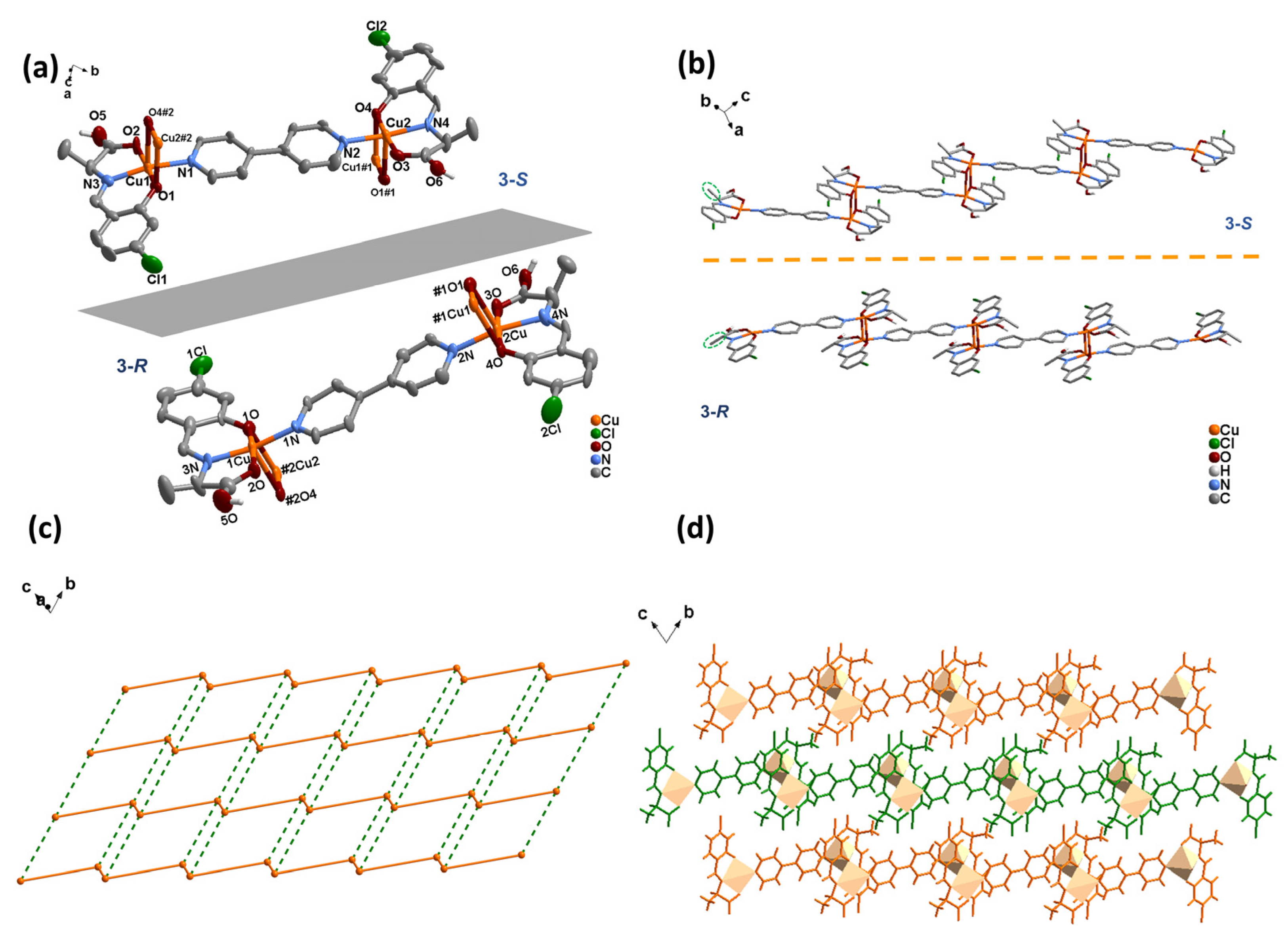
2.2.5. Synthesis of [Cu2(L-S)2(4,4′-bipy)]n (3-S)
2.2.6. Synthesis of [Cu2(L-R)2(4,4′-bipy)]n (3-R)
2.3. Methods
3. Results and Discussion
3.1. Crystal Structures
3.1.1. Crystal Structures of 1-S and 1-R
3.1.2. Crystal Structures of 2-S and 2-R
3.1.3. Crystal Structures of 3-S and 3-R
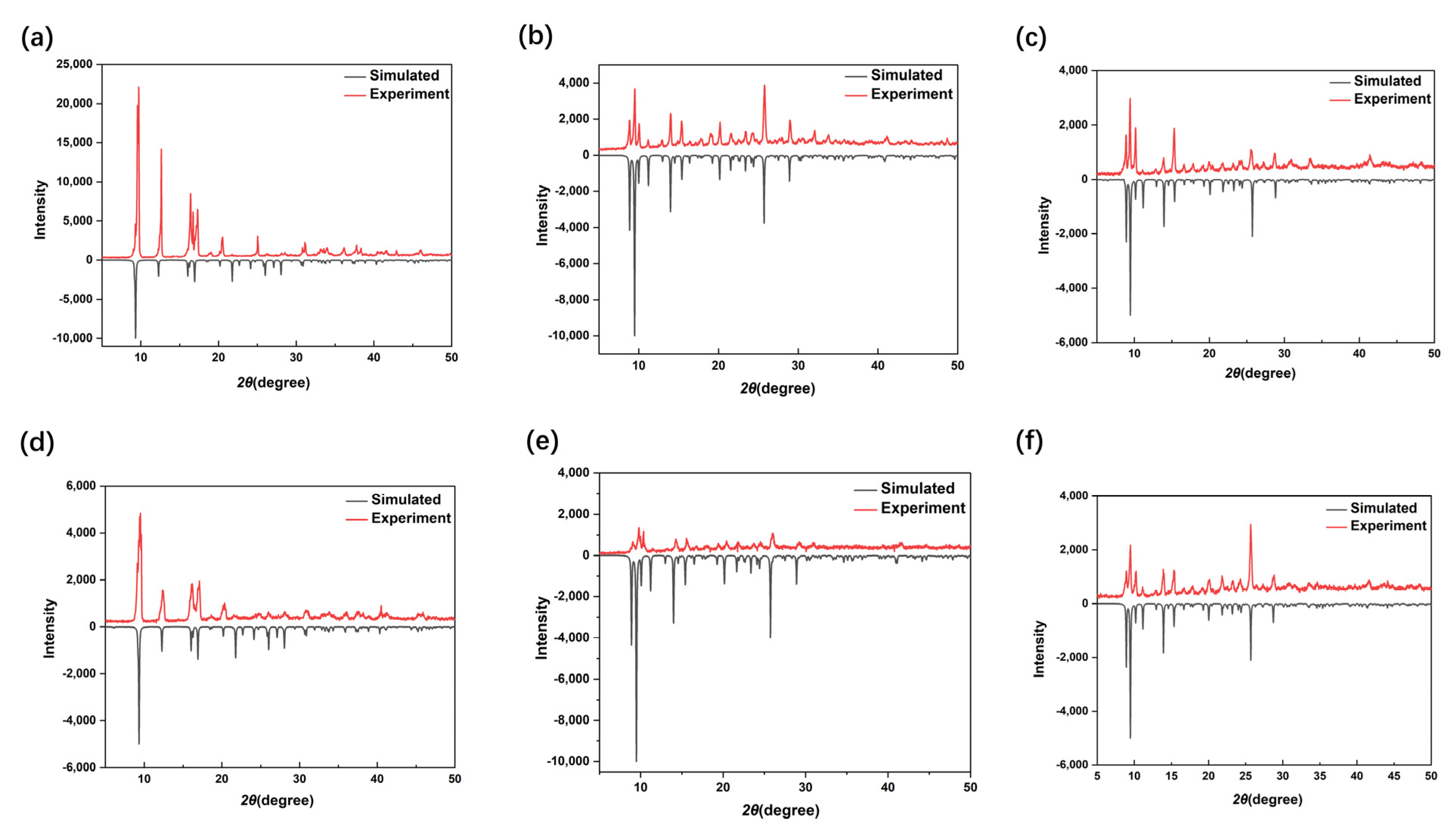
3.2. Thermal Stability and Powder X-ray Diffraction
3.3. UV-Vis Spectra of NaHL, and 1-S/R, 2-S/R and 3-S/R
3.4. Chirality and Chiral Recognition
3.5. ESI mass Spectrometry of 1-S/R, 2-S/R and 3-S/R
3.6. X-ray Photoelectron Spectroscopy (XPS) of 1-S/R, 2-S/R and 3-S/R
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, G.; Gou, F.; Cheng, J.; Zhang, X.; Zhou, X.; Xiang, H. Chiral and non-conjugated fluorescent salen ligands: AIE, anion probes, chiral recognition of unprotected amino acids, and cell imaging applications. RSC Adv. 2017, 7, 40640–40649. [Google Scholar] [CrossRef] [Green Version]
- Milos, N.; Helena, S. CZE and EKCC are increasingly complementing and competing with other chiral separation methods. Anal. Chem. 1994, 66, 646A–655A. [Google Scholar]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular Chirality in Self-Assembled Systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef]
- Clegg, M.L.; Morales de la Garza, L.; Karakatsani, S.; King, D.A.; Driver, S.M. Chirality in Amino Acid Overlayers on Cu Surfaces. Top. Catal. 2011, 54, 1429–1444. [Google Scholar] [CrossRef]
- Khatua, S.; Kang, J.; Kim, K.; Huh, J.O.; Lee, J.; Hong, C.S.; Churchill, D.G. Crystal Structures and Magnetic Properties of Newly Synthesized Mono- and Dinuclear Cu II Schiff-Base Complexes. Eur. J. Inorg. Chem. 2010, 2010, 5018–5026. [Google Scholar] [CrossRef]
- Belokon, Y.N.; Zel’tser, I.E.; Bakhmutov, V.I.; Saporovskaya, M.B.; Ryzhov, M.G.; Yanovskii, A.I.; Struchkov, Y.T.; Belikov, V.M. Asymmetric synthesis of threonine and partial resolution and retroracemization of.alpha.-amino acids via copper(II) complexes of their Schiff bases with (S)-2-N-(N′-benzylprolyl)aminobenzaldehyde and (S)-2-N-(N′-benzylprolyl)aminoacetophenone. Crystal and molecular structure of a copper(II) complex of glycine Schiff base with (S)-2-N-(N′-benzylprolyl)aminoacetophenone. J. Am. Chem. Soc. 2002, 105, 2010–2017. [Google Scholar]
- Abdel-Rahman, L.H.; El-Khatib, R.M.; Nassr, L.A.E.; Abu-Dief, A.M. Synthesis, physicochemical studies, embryos toxicity and DNA interaction of some new Iron(II) Schiff base amino acid complexes. J. Mol. Struct. 2013, 1040, 9–18. [Google Scholar] [CrossRef]
- Aceña, J.L.; Sorochinsky, A.E.; Moriwaki, H.; Sato, T.; Soloshonok, V.A. Synthesis of fluorine-containing α-amino acids in enantiomerically pure form via homologation of Ni(II) complexes of glycine and alanine Schiff bases. J. Fluor. Chem. 2013, 155, 21–38. [Google Scholar] [CrossRef]
- Şendil, K.; Tekin, T.; Göksu, H.; Oğuz, M.; Anıl, B.; Gültekin, M.S. A Novel Method for the Synthesis of Newfangled Asymmetric Schiff Bases from α-amino Acids under Ultrasonic Conditions and in Aqueous Medium. J. Chin. Chem. Soc. 2016, 63, 808–817. [Google Scholar] [CrossRef]
- Yan, Z.-H.; Li, D.; Yin, X.-B. Review for chiral-at-metal complexes and metal-organic framework enantiomorphs. Sci. Bull. 2017, 62, 1344–1354. [Google Scholar] [CrossRef] [Green Version]
- Noyori, R. Asymmetric Catalysis: Science and Opportunities (Nobel Lecture 2001). Adv. Synth. Catal. 2003, 345, 15–32. [Google Scholar] [CrossRef]
- Uchida, Y.; Iwama, S.; Coquerel, G.; Tamura, R. A Kinetic/Thermodynamic Origin of Regular Chiral Fluctuation or Symmetry Breaking Unique to Preferential Enrichment. Chemistry 2016, 22, 11660–11666. [Google Scholar] [CrossRef]
- Horiguchi, M.; Okuhara, S.; Shimano, E.; Fujimoto, D.; Takahashi, H.; Tsue, H.; Tamura, R. Control of the Mode of Polymorphic Transition Inducing Preferential Enrichment by Modifying the Molecular Structure or Adding Seed Crystals: Significant Influence of CH/F Hydrogen Bonds. Cryst. Growth Des. 2007, 8, 540–548. [Google Scholar] [CrossRef]
- Takahashi, H.; Iwama, S.; Clevers, S.; Veesler, S.; Coquerel, G.; Tsue, H.; Tamura, R. In Situ Observation of Polymorphic Transition during Crystallization of Organic Compounds Showing Preferential Enrichment By Means Of Temperature-Controlled Video-Microscopy and Time-Resolved X-ray Powder Diffraction. Cryst. Growth Des. 2016, 17, 671–676. [Google Scholar] [CrossRef]
- Gonnade, R.G.; Iwama, S.; Sugiwake, R.; Manoj, K.; Takahashi, H.; Tsue, H.; Tamura, R. Occurrence of spontaneous resolution of ketoprofen with a racemic crystal structure by simple crystallization under nonequilibrium preferential enrichment conditions. Chem. Commun. 2012, 48, 2791–2793. [Google Scholar] [CrossRef] [Green Version]
- Iwama, S.; Takahashi, H.; Tsue, H.; Tamura, R. Case Study on the Interpretation of Crystal Structures Inducing Preferential Enrichment Based on the Graph Set Analysis of Hydrogen Bond Motifs. Cryst. Growth Des. 2015, 15, 3052–3062. [Google Scholar] [CrossRef]
- Tamura, R.; Fujimoto, D.; Lepp, Z.; Misaki, K.; Miura, H.; Takahashi, H.; Ushio, T.; Nakai, T.; Hirotsu, K. Mechanism of preferential enrichment, an unusual enantiomeric resolution phenomenon caused by polymorphic transition during crystallization of mixed crystals composed of two enantiomers. J. Am. Chem. Soc. 2002, 124, 13139–13153. [Google Scholar] [CrossRef] [PubMed]
- Gonnade, R.G.; Iwama, S.; Mori, Y.; Takahashi, H.; Tsue, H.; Tamura, R. Observation of Efficient Preferential Enrichment Phenomenon for a Cocrystal of (dl)-Phenylalanine and Fumaric Acid under Nonequilibrium Crystallization Conditions. Cryst. Growth Des. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Brewer, G.; Brewer, C.; Butcher, R.J.; Zemba, M. Structural evidence of a ketimine as the product of an amino acid with an aldehyde. An intermediate in the racemization and transamination of amino acids. Inorg. Chem. Commun. 2016, 64, 35–38. [Google Scholar] [CrossRef]
- Rajesh, C.M.; Ray, M. Characterization of a meso-chiral isomer of a hexanuclear Cu(II) cage from racemization of the L-alanine Schiff base. Dalton Trans. 2014, 43, 12952–12960. [Google Scholar] [CrossRef]
- Smith, G.G.; Sivakua, T. Mechanism of the racemization of amino acids. Kinetics of racemization of arylglycines. J. Org. Chem. 2002, 48, 627–634. [Google Scholar] [CrossRef]
- Yan, L.; Li, Z.; Xiong, Y.; Zhong, X.; Peng, S.; Li, H. Zinc(ii) Schiff base complexes as dual probes for the detection of NH4+ and HPO42− ions. New J. Chem. 2022, 46, 12910–12917. [Google Scholar] [CrossRef]
- Li, Z.; Su, H.; Zhu, Y.; Yan, L.; Li, H. Structural transformation of copper coordination complexes accompanied with chiral transformation. CrystEngComm 2022, 24, 2402–2409. [Google Scholar] [CrossRef]
- Zhong, X.; Li, Z.; Shi, R.; Yan, L.; Zhu, Y.; Li, H. Schiff Base-Modified Nanomaterials for Ion Detection: A Review. ACS Appl. Nano Mater. 2022, 5, 13998–14020. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Zehra, S.; Roisnel, T.; Arjmand, F. Enantiomeric Amino Acid Schiff Base Copper(II) Complexes as a New Class of RNA-Targeted Metallo-Intercalators: Single X-ray Crystal Structural Details, Comparative in Vitro DNA/RNA Binding Profile, Cleavage, and Cytotoxicity. ACS Omega 2019, 4, 7691–7705. [Google Scholar] [CrossRef]
- Barma, A.; Bhattacharjee, A.; Roy, P. Dinuclear Copper(II) Complexes with N,O Donor Ligands: Partial Ligand Hydrolysis and Alcohol Oxidation Catalysis. Eur. J. Inorg. Chem. 2021, 2021, 2284–2292. [Google Scholar] [CrossRef]
- Koh, L.L.; Ranford, J.O.; Robinson, W.T.; Svensson, J.O.; Tan, A.L.; Wu, D. Model for the Reduced Schiff Base Intermediate between Amino Acids and Pyridoxal: Copper(II) Complexes of N-(2-Hydroxybenzyl)amino Acids with Nonpolar Side Chains and the Crystal Structures of [Cu(N-(2-hydroxybenzyl)-D,L-alanine)(phen)].H(2)O and [Cu(N-(2-hydroxybenzyl)-D,L-alanine)(imidazole)]. Inorg. Chem. 1996, 35, 6466–6472. [Google Scholar]
- Awasthi, S.; NT, S. Crystal structure of Alanine-Copper(II) complex to understand the mechanism of salt induced prebiotic oligomerization of amino acids. Cryst. Res. Technol. 2015, 50, 304–311. [Google Scholar] [CrossRef]
- Ishihara, K.; Nishimura, K.; Yamakawa, K. Enantio- and Site-Selective alpha-Fluorination of N-Acyl 3,5-Dimethylpyrazoles Catalyzed by Chiral pi-Cu(II) Complexes. Angew. Chem. 2020, 59, 17641–17647. [Google Scholar] [CrossRef] [PubMed]
- Satheesh, C.E.; Raghavendra Kumar, P.; Sharma, P.; Lingaraju, K.; Palakshamurthy, B.S.; Raja Naika, H. Synthesis, characterisation and antimicrobial activity of new palladium and nickel complexes containing Schiff bases. Inorg. Chim. Acta 2016, 442, 1–9. [Google Scholar] [CrossRef]
- Dhahagani, K.; Kesavan, M.P.; Gujuluva Gangatharan Vinoth, K.; Ravi, L.; Rajagopal, G.; Rajesh, J. Crystal structure, optical properties, DFT analysis of new morpholine based Schiff base ligands and their copper(II) complexes: DNA, protein docking analyses, antibacterial study and anticancer evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 119–130. [Google Scholar] [CrossRef]
- Satheesh, C.E.; Raghavendra Kumar, P.; Shivakumar, N.; Lingaraju, K.; Murali Krishna, P.; Rajanaika, H.; Hosamani, A. Synthesis, structural characterization, antimicrobial and DNA binding studies of homoleptic zinc and copper complexes of NO Schiff bases derived from homoveratrylamine. Inorg. Chim. Acta 2019, 495, 118929. [Google Scholar] [CrossRef]
- Yusuf, T.L.; Oladipo, S.D.; Zamisa, S.; Kumalo, H.M.; Lawal, I.A.; Lawal, M.M.; Mabuba, N. Design of New Schiff-Base Copper(II) Complexes: Synthesis, Crystal Structures, DFT Study, and Binding Potency toward Cytochrome P450 3A4. ACS Omega 2021, 6, 13704–13718. [Google Scholar] [CrossRef]
- Bania, K.K.; Karunakar, G.V.; Goutham, K.; Deka, R.C. Enantioselective Henry reaction catalyzed by "ship in a bottle" complexes. Inorg. Chem. 2013, 52, 8017–8029. [Google Scholar] [CrossRef]
- Kuhne, I.A.; Ozarowski, A.; Sultan, A.; Esien, K.; Carter, A.B.; Wix, P.; Casey, A.; Heerah-Booluck, M.; Keene, T.D.; Muller-Bunz, H.; et al. Homochiral Mn(3+) Spin-Crossover Complexes: A Structural and Spectroscopic Study. Inorg. Chem. 2022, 61, 3458–3471. [Google Scholar] [CrossRef] [PubMed]
- Barwiolek, M.; Szlyk, E.; Surdykowski, A.; Wojtczak, A. New nickel(II) and copper(II) complexes with unsymmetrical Schiff bases derived from (1R,2R)(-)cyclohexanediamine and the application of Cu(II) complexes for hybrid thin layers deposition. Dalton Trans. 2013, 42, 11476–11487. [Google Scholar] [CrossRef] [PubMed]
- Ganczar, E.; Gawryszewska, P.; Kinzhybalo, V.; Białońska, A. Photoreactive Crystal of a Copper(I) Coordination Compound with a Cinnamaldehyde Derivative. Cryst. Growth Des. 2021, 21, 7023–7033. [Google Scholar] [CrossRef]
- Sabiah, S.; Varghese, B.; Murthy, N.N. Mononuclear [(BP)(2)MX](n+) (M = Cu(2+), Co(2+), Zn(2+); X = OH(2), Cl(-)) complexes with a new biphenyl appended N-bidentate ligand: Structural, spectroscopic, solution equilibrium and ligand dynamic studies. Dalton Trans. 2009, 9770–9780. [Google Scholar] [CrossRef]
- Sciortino, G.; Marechal, J.D.; Fabian, I.; Lihi, N.; Garribba, E. Quantitative prediction of electronic absorption spectra of copper(II)-bioligand systems: Validation and applications. J. Inorg. Biochem. 2020, 204, 110953. [Google Scholar] [CrossRef]
- Hazra, M.; Dolai, T.; Pandey, A.; Dey, S.K.; Patra, A. Synthesis and Characterisation of Copper(II) Complexes with Tridentate NNO Functionalized Ligand: Density Function Theory Study, DNA Binding Mechanism, Optical Properties, and Biological Application. Bioinorg. Chem. Appl. 2014, 2014, 104046. [Google Scholar] [CrossRef]
- Akitsu, T.; Yamaguchi, J.; Uchida, N.; Aritake, Y. The Studies of Conditions for Inducing Chirality to Cu(II) Complexes by Chiral Zn(II) and Ni(II) Complexes with Schiff Base. Res. Lett. Mater. Sci. 2009, 2009, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Sunaga, N.; Haraguchi, T.; Akitsu, T. Orientation of Chiral Schiff Base Metal Complexes Involving Azo-Groups for Induced CD on Gold Nanoparticles by Polarized UV Light Irradiation. Symmetry 2019, 11, 1094. [Google Scholar] [CrossRef] [Green Version]
- Colak, A.; Terzi, U.; Col, M.; Karaoglu, S.A.; Karabocek, S.; Kucukdumlu, A.; Ayaz, F.A. DNA binding, antioxidant and antimicrobial activities of homo- and heteronuclear copper(II) and nickel(II) complexes with new oxime-type ligands. Eur. J. Med. Chem. 2010, 45, 5169–5175. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, A.; Spielberg, E.T.; Gorls, H.; Plass, W. Chiral tetranuclear mu3-alkoxo-bridged copper(II) complex with 2 + 4 cubane-like Cu4O4 core framework and ferromagnetic ground state. Inorg. Chem. 2008, 47, 2485–2493. [Google Scholar] [CrossRef]
- Ene, C.D.; Maxim, C.; Rouzieres, M.; Clerac, R.; Avarvari, N.; Andruh, M. Enantiopure versus Racemic Mixture in Reversible, Two-Step, Single-Crystal-to-Single-Crystal Transformations of Copper(II) Complexes. Chemistry 2018, 24, 8569–8576. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Moriwaki, H.; Miwa, T.; Hoang, B.; Wang, P.; Soloshonok, V.A. Large Scale Synthesis of Chiral (3Z,5Z)-2,7-Dihydro-1H-azepine-Derived Hamari Ligand for General Asymmetric Synthesis of Tailor-Made Amino Acids. Org. Process Res. Dev. 2019, 23, 619–628. [Google Scholar] [CrossRef]
- Song, W.J.; Su, H.; Zhou, P.; Zhu, Y.H.; Khan, M.A.; Song, J.B.; Li, H. Controllable synthesis of two adenosine 5′-monophosphate nucleotide coordination polymers via pH regulation: Crystal structure and chirality. Dalton Trans. 2021, 50, 4713–4719. [Google Scholar] [CrossRef]
- Schneider, J.D.; Smith, B.A.; Williams, G.A.; Powell, D.R.; Perez, F.; Rowe, G.T.; Yang, L. Synthesis and Characterization of Cu(II) and Mixed-Valence Cu(I)Cu(II) Clusters Supported by Pyridylamide Ligands. Inorg. Chem. 2020, 59, 5433–5446. [Google Scholar] [CrossRef]
- Park, S.J.; Yun, E.-J. Preparation of sputter-deposited CuOx thin film with p-type conductivity and application as thin film transistor. J. Korean Phys. Soc. 2022, 81, 867–875. [Google Scholar] [CrossRef]
- Sinapi, F.; Julien, S.; Auguste, D.; Hevesi, L.; Delhalle, J.; Mekhalif, Z. Monolayers and mixed-layers on copper towards corrosion protection. Electrochim. Acta 2008, 53, 4228–4238. [Google Scholar] [CrossRef]
- Gong, B.; Bai, E.; Feng, X.; Yi, L.; Wang, Y.; Chen, X.; Zhu, X.; Duan, Y.; Huang, Y. Characterization of Chalkophomycin, a Copper(II) Metallophore with an Unprecedented Molecular Architecture. J. Am. Chem. Soc. 2021, 143, 20579–20584. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.L.; Taylor, J.A. X-ray photoelectron and Auger spectroscopic studies of Cu2S and CuS. J. Mater. Sci. Lett. 1986, 5, 384–386. [Google Scholar] [CrossRef]
- Yue, S.; Ding, X.; Liu, X.; Guo, Y.; Wang, Y. High-efficient production of fatty alcohol via hydrogenation of fatty acid over Cu-NbOx/SBA-15 catalyst. Catal. Today 2022, 405–406, 221–226. [Google Scholar] [CrossRef]




| Complex | 1-S | 2-S | 3-S |
|---|---|---|---|
| Formula | C10H8ClCuNO3 | C30H25Cl2Cu2N4O6 | C30H27Cl2Cu2N4O6 |
| M (mol−1) | 289.16 | 732.91 | 734.51 |
| T (K) | 296(2) | 296(2) | 296(2) |
| Crystal system | Orthorhombic | Triclinic | Triclinic |
| Space group | P212121 | P1 | P1 |
| a (Å) | 4.9518(9) | 8.0364(14) | 8.0695(8) |
| b (Å) | 10.8863(19) | 10.2226(19) | 10.1572(10) |
| a (Å) | 19.221(3) | 11.121(2) | 10.9716(10) |
| α (°) | 90 | 65.922(4) | 66.809(2) |
| β (°) | 90 | 79.246(4) | 78.713(3) |
| γ (°) | 90 | 86.931(4) | 86.389(3) |
| V (Å3) | 1036.2(3) | 819.2(3) | 810.52(14) |
| Z | 4 | 1 | 1 |
| ρ(calculate) (g·cm−3) | 1.854 | 1.489 | 1.505 |
| F(000) | 580 | 372 | 372 |
| 2θRange (°) | 4.238–56.55 | 4.078–49.912 | 4.11–51.994 |
| GOF on F2 | 1.001 | 0.889 | 1.203 |
| Rint | 0.0510 | 0.0707 | 0.1642 |
| Reflections collected | 12,869 | 6101 | 8536 |
| Independent reflections | 2581 | 5483 | 6467 |
| R1[I > 2σ(I)] | 0.0311 | 0.0526 | 0.0589 |
| wR2[I > 2σ(I)] | 0.0625 | 0.1144 | 0.1648 |
| R1 (all data) | 0.0414 | 0.0935 | 0.0689 |
| wR2 (all data) | 0.0669 | 0.124 | 0.1703 |
| Residuals (e Å−3) | 0.33/−0.27 | 0.41/−0.55 | 1.84/−1.36 |
| Flack parameter | 0.016(11) | 0.40(5) | 0.00(4) |
| 1-S | 1-R | 2-S | 2-R | 3-S | 3-R | |
|---|---|---|---|---|---|---|
| Before pH | 2.62 | 2.75 | 4.41 | 3.65 | 8.92 | 9.02 |
| After pH | 2.7 | 2.76 | 4.74 | 4.23 | 9.33 | 9.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, L.; Li, Z.; Zhong, X.; Du, J.; Xiong, Y.; Peng, S.; Li, H. Preferential Enrichment of Enantiomer from Amino Acid Schiff Bases by Coordination Interaction and Crystallization. Materials 2023, 16, 530. https://doi.org/10.3390/ma16020530
Yan L, Li Z, Zhong X, Du J, Xiong Y, Peng S, Li H. Preferential Enrichment of Enantiomer from Amino Acid Schiff Bases by Coordination Interaction and Crystallization. Materials. 2023; 16(2):530. https://doi.org/10.3390/ma16020530
Chicago/Turabian StyleYan, Li, Zhongkui Li, Xue Zhong, Jianxin Du, Yan Xiong, Shaochun Peng, and Hui Li. 2023. "Preferential Enrichment of Enantiomer from Amino Acid Schiff Bases by Coordination Interaction and Crystallization" Materials 16, no. 2: 530. https://doi.org/10.3390/ma16020530





