Corrosion Resistance of Titanium Dental Implant Abutments: Comparative Analysis and Surface Characterization
Abstract
:1. Introduction
2. Materials and Methods
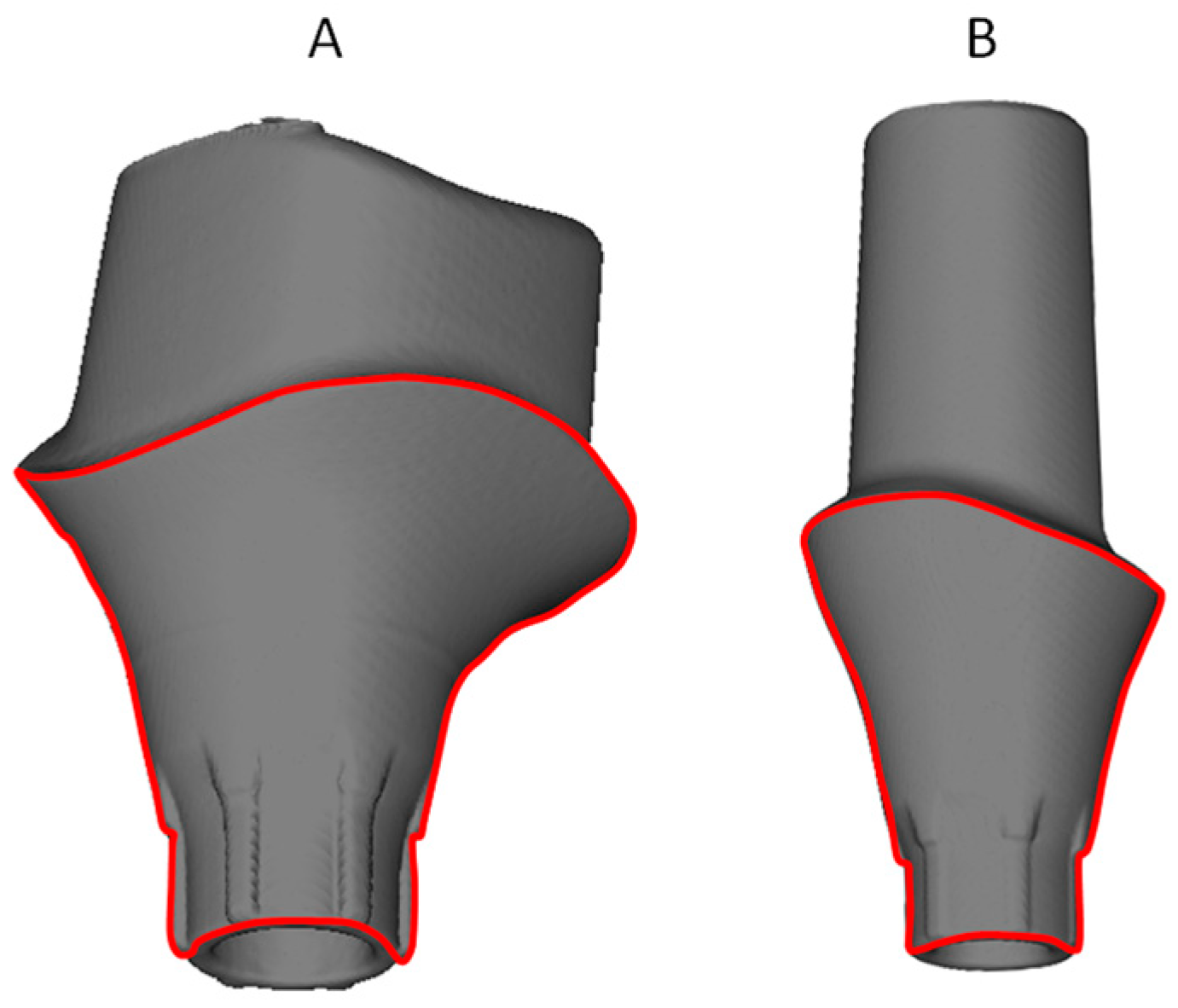
2.1. Samples Groups
2.2. Corrosion Examination
2.3. SEM-EDS Study
3. Results
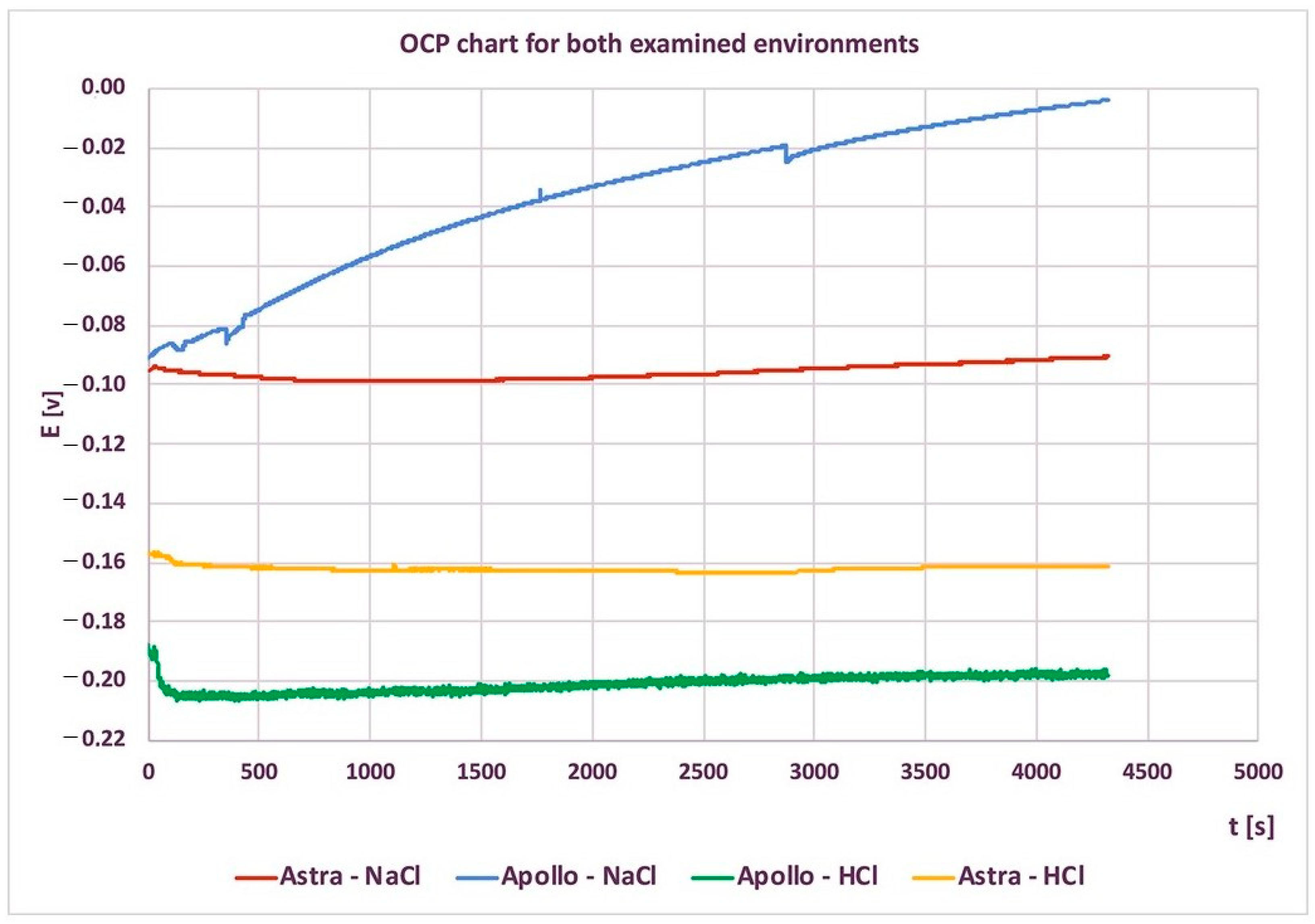
3.1. Corrosion Resistance
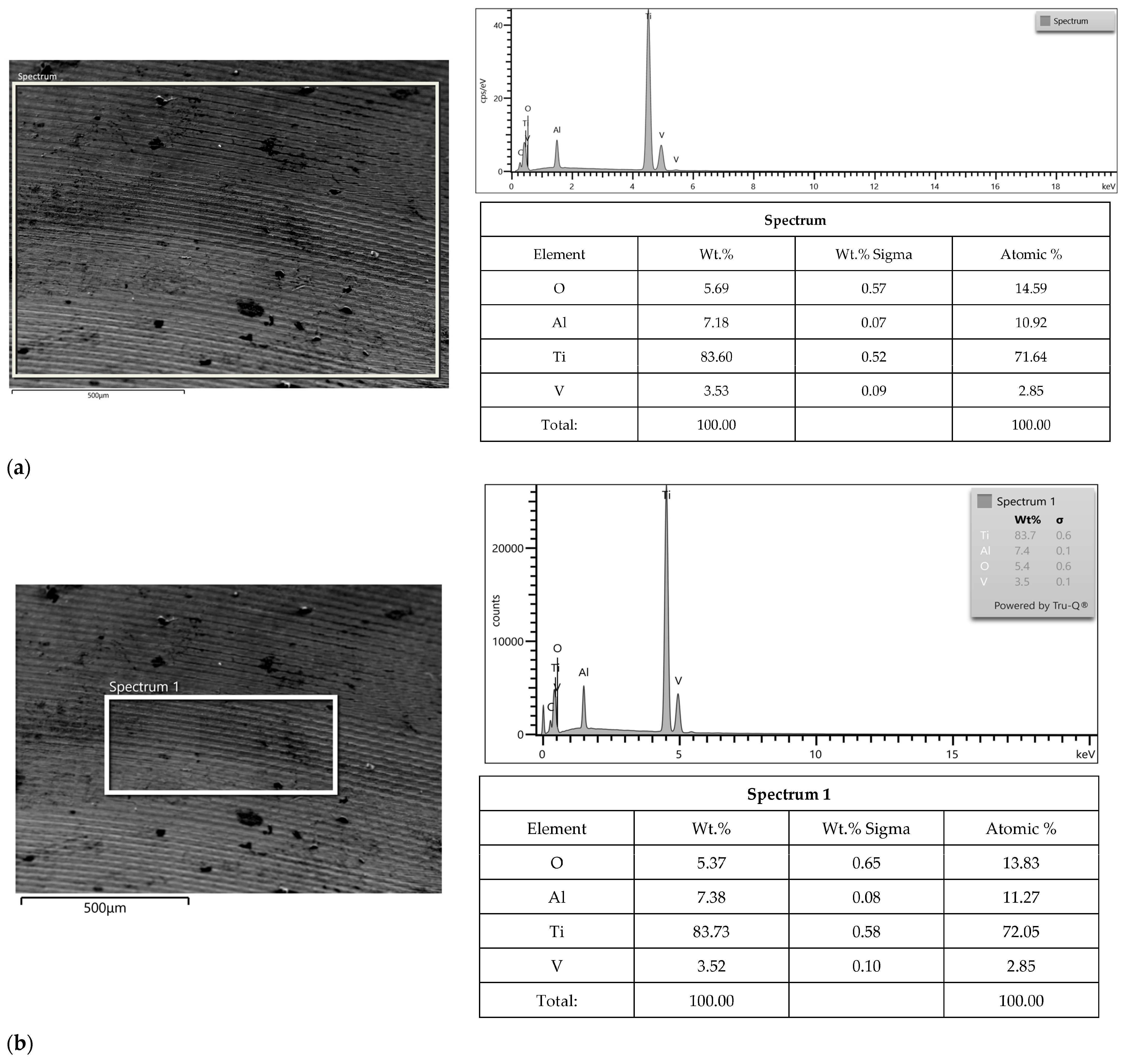
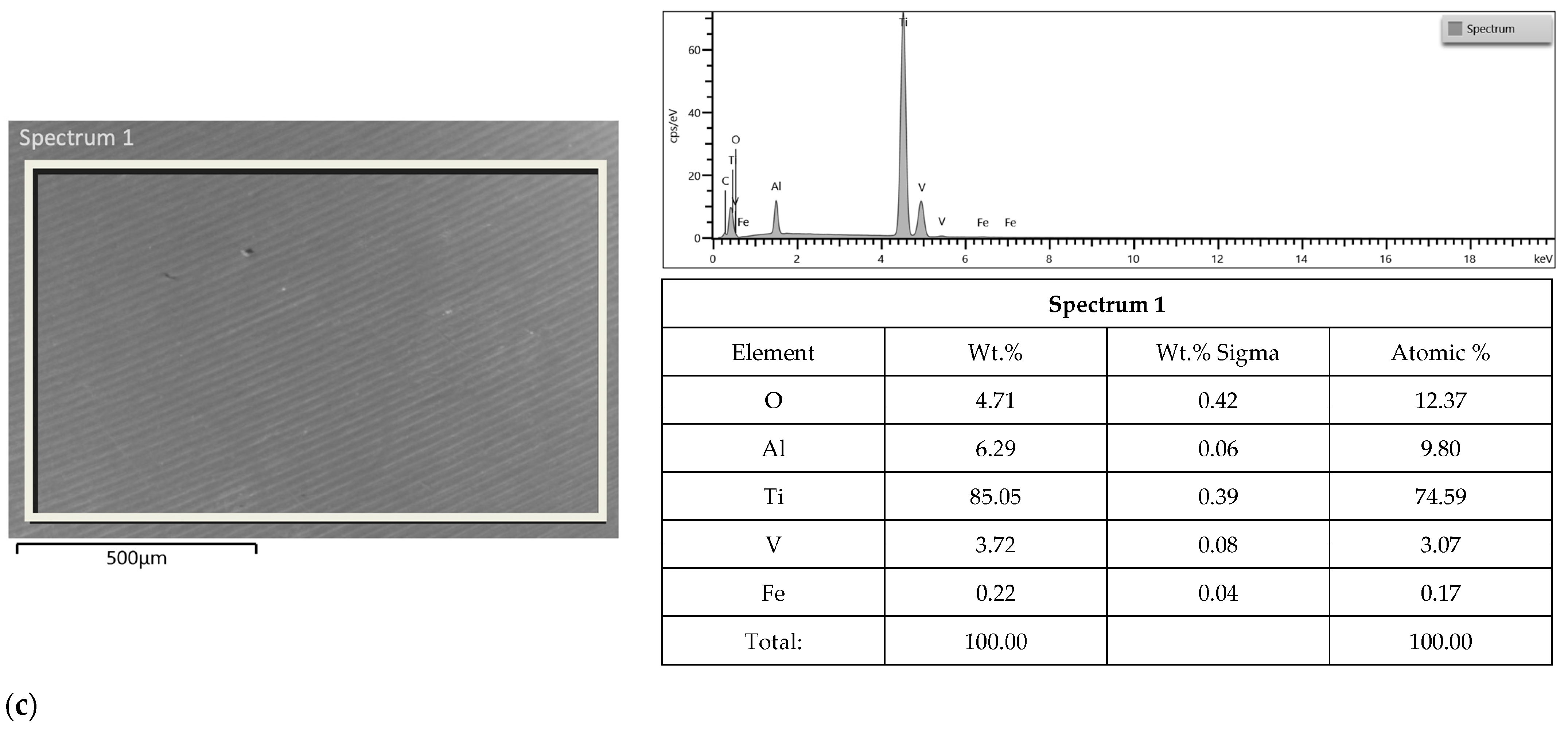
3.2. SEM-EDS Study
4. Discussion
5. Conclusions
- Astra and Apollo implant abutments revealed good corrosion resistance and a passivation layer on the surface.
- Apollo abutments exhibited better corrosion resistance in a neutral environment, hence Astra abutments were found to be more resistant to corrosion in an acidic medium.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuffellato, N.; Massariol, M.; Gandini, A.; Dovigo, S. Intraorally welded wing abutments supporting full-arch prostheses: A retrospective clinical study. Dent. Med. Probl. 2022, 59, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Tamrakar, S.K.; Mishra, S.K.; Chowdhary, R.; Rao, S. Comparative analysis of stress distribution around CFR-PEEK implants and titanium implants with different prosthetic crowns: A finite element analysis. Dent. Med. Probl. 2021, 58, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, J.; Lapinska, B.; Nissan, J.; Lukomska-Szymanska, M. Factors influencing marginal bone loss around dental implants: A narrative review. Coatings 2021, 11, 865. [Google Scholar] [CrossRef]
- Silva, R.C.S.; Agrelli, A.; Andrade, A.N.; Mendes-Marques, C.L.; Arruda, I.R.S.; Santos, L.R.L.; Vasconcelos, N.F.; Machado, G. Titanium Dental Implants: An Overview of Applied Nanobiotechnology to Improve Biocompatibility and Prevent Infections. Materials 2022, 15, 3150. [Google Scholar] [CrossRef] [PubMed]
- Atapour, M.; Pilchak, A.L.; Shamanian, M.; Fathi, M.H. Corrosion behavior of Ti–8Al–1Mo–1V alloy compared to Ti–6Al–4V. Mater. Des. 2011, 32, 1692–1696. [Google Scholar] [CrossRef]
- Lutjering, G.; Wiliams, J.G. Titanium; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Mandil, G.; Le, V.T.; Paris, H.; Suard, M. Building new entities from existing titanium part by electron beam melting: Microstructures and mechanical properties. Int. J. Adv. Manuf. Technol. 2016, 85, 1835–1846. [Google Scholar] [CrossRef]
- Arlucea, N.; Brizuela-Velasco, A.; Dieguez-Pereira, M.; Punset, M.; Molmeneu, M.; Lasheras, F.S.; Dellanos-Lanchares, H.; Álvarez-Arenal, Á. Zirconia vs. Titanium Dental Implants: Primary Stability In-Vitro Analysis. Materials 2021, 14, 7886. [Google Scholar] [CrossRef] [PubMed]
- Veiga, C.; Loureiro, A.J.R.; Davim, J.P. Properties and applications of titanium alloys. Rev. Adv. Mater. Sci. 2012, 32, 133–148. [Google Scholar]
- Ramadan, R.; Elsherbeeny, Y.; Thabet, Y.; Kandil, B.; Ghali, R. Retention of a telescopic overdenture on customized abutments after the simulation of 1 year in function. Dent. Med. Probl. 2021, 58, 201–206. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Tsoi, J.K.H.; Matinlinna, J.P. Binary titanium alloys as dental implant materials—A review. Regen. Biomater. 2017, 4, 315. [Google Scholar] [CrossRef]
- Goutam, M.; Giriyapura, C.; Mishra, S.; Gupta, S. Titanium Allergy: A Literature Review. Indian J. Dermatol. 2014, 59, 630. [Google Scholar] [CrossRef] [PubMed]
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clin. Oral Implant. Res. 2008, 19, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Insua, A.; Monje, A.; Wang, H.L.; Miron, R.J. Basis of bone metabolism around dental implants during osseointegration and peri-implant bone loss. J. Biomed. Mater. Res. Part A 2017, 105, 2075–2089. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A Review on the Wettability of Dental Implant Surfaces: Theoretical and Experimental Aspects. Acta Biomater. 2014, 10, 2894. [Google Scholar] [CrossRef] [PubMed]
- El Khalloufi, M.; Drevelle, O.; Soucy, G.; Wang, S.; Wang, X.; Yang, J. Titanium: An Overview of Resources and Production Methods. Minerals 2021, 11, 1425. [Google Scholar] [CrossRef]
- Suito, H.; Iwawaki, Y.; Goto, T.; Tomotake, Y.; Ichikawa, T. Oral Factors Affecting Titanium Elution and Corrosion: An In Vitro Study Using Simulated Body Fluid. PLoS ONE 2013, 8, e66052. [Google Scholar] [CrossRef]
- Berbel, L.O.; Banczek, E.D.; Karousis, I.K.; Kotsakis, G.A.; Costa, I. Determinants of corrosion resistance of Ti-6Al-4V alloy dental implants in an In Vitro model of peri-implant inflammation. PLoS ONE 2019, 14, e0210530. [Google Scholar]
- Bodunrin, M.O.; Chown, L.H.; Van Der Merwe, J.W.; Alaneme, K.K.; Oganbule, C.; Klenam, D.E.P.; Mphasha, N.P. Corrosion behavior of titanium alloys in acidic and saline media: Role of alloy design, passivation integrity, and electrolyte modification. Corros. Rev. 2020, 38, 25–47. [Google Scholar] [CrossRef]
- Grigoriev, S.; Peretyagin, N.; Apelfeld, A.; Smirnov, A.; Yanushevich, O.; Krikheli, N.; Kramar, O.; Kramar, S.; Peretyagin, P. Investigation of MAO Coatings Characteristics on Titanium Products Obtained by EBM Method Using Additive Manufacturing. Materials 2022, 15, 4535. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Khan, A.; Qurashi, A.; Badeghaish, W.; Noui-Mehidi, M.N.; Aziz, M.A. Frontiers and Challenges in Electrochemical Corrosion Monitoring; Surface and Downhole Applications. Sensors 2020, 20, 6583. [Google Scholar] [CrossRef] [PubMed]
- Leban, M.B.; Kosec, T.; Finšgar, M. Corrosion characterization and ion release in SLM-manufactured and wrought Ti6Al4V alloy in an oral environment. Corros. Sci. 2022, 209, 110716. [Google Scholar] [CrossRef]
- Noumbissi, S.; Scarano, A.; Gupta, S. A literature review study on atomic ions dissolution of titanium and its alloys in implant dentistry. Materials 2019, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Hashim, D.; Cionca, N. What is the impact of titanium particles and biocorrosion on implant survival and complications? A critical review. Clin. Oral Implant. Res. 2018, 29, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shah, K.; Dong, S.; Peterson, L.; Callagon La Plante, E.; Sant, G. Elucidating the corrosion-related degradation mechanisms of a Ti-6Al-4V dental implant. Dent. Mater. 2020, 36, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Wang, L.; Chen, Y.; Li, L.; He, Y.; Ding, Z. Corrosion behavior of titanium in artificial saliva by lactic acid. Materials 2014, 7, 5528–5542. [Google Scholar] [CrossRef] [PubMed]
- Güleryüz, H.; Çimenoǧlu, H. Effect of thermal oxidation on corrosion and corrosion–wear behaviour of a Ti–6Al–4V alloy. Biomaterials 2004, 25, 3325–3333. [Google Scholar] [CrossRef]
- Wu, G.; Yu, M.; Liu, J.; Li, S.; Wu, L.; Zhang, Y. Surface characteristics of anodic oxide films fabricated in acid and neutral electrolytes on Ti–10V–2Fe–3Al alloy. Surf. Interface Anal. 2013, 45, 661–666. [Google Scholar] [CrossRef]
- Rauscher, Á.; Lukáacs, Z. Effect of hydrogen sulphide on The corrosion behaviour of titanium. Mater. Corros. 1987, 38, 326–329. [Google Scholar] [CrossRef]
- Pouilleau, J.; Devilliers, D.; Garrido, F.; Durand-Vidal, S.; Mahé, E. Structure and composition of passive titanium oxide films. Mater. Sci. Eng. B 1997, 47, 235–243. [Google Scholar] [CrossRef]
- Shoesmith, D.W.; Noël, J.J. 3.10: Corrosion of Titanium and its alloys. In Shreirs Corros; Stott, B.C., Ed.; Elsevier: Oxford, UK, 2010; pp. 2042–2052. [Google Scholar]
- Mogoda, A.S.; Ahmad, Y.H.; Badawy, W.A. Corrosion behaviour of Ti-6Al-4V alloy in concentrated hydrochloric and sulphuric acids. J. Appl. Electrochem. 2004, 34, 873–878. [Google Scholar] [CrossRef]
- Afzali, P.; Ghomashchi, R.; Oskouei, R.H. On the corrosion Behaviour of low modulus titanium alloys for medical implant applications: A review. Metals 2019, 9, 878. [Google Scholar] [CrossRef]
- Abd El Daym, D.A.; Gheith, M.E.; Abbas, N.A.; Rashed, L.A.; Abd El Aziz, Z.A. Electrochemical assessment of laser-treated titanium alloy used for dental applications at acidic pH condition (in vitro study). Dent. Res. J. 2019, 16, 304–309. [Google Scholar] [CrossRef]
- Han, M.K.; Kim, J.Y.; Hwang, M.J.; Song, H.J.; Park, Y.J. Effect of Nb on the Microstructure, Mechanical Properties, Corrosion Behavior, and Cytotoxicity of Ti-Nb Alloys. Materials 2015, 8, 5986–6003. [Google Scholar] [CrossRef] [PubMed]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Olguín-Coca, J.; López-Léon, L.D.; De Los Rios, J.P.F.; Almeraya-Calderón, F. Electrochemical Noise Analysis of the Corrosion of Titanium Alloys in NaCl and H2SO4 Solutions. Metals 2021, 11, 105. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Muresan, I. The influence of artificial body fluids on metallic corrosion. Key Eng. Mater. 2002, 230–232, 459–462. [Google Scholar] [CrossRef]
- Hedberg, Y.S. Role of proteins in the degradation of relatively inert alloys in the human body. NPJ Mater. Degrad. 2018, 2, 26. [Google Scholar] [CrossRef]
- Wang, M.L.; Tuli, R.; Manner, P.A.; Sharkey, P.F.; Hall, D.J.; Tuan, R.S. Direct and indirect induction of apoptosis in human mesenchymal stem cells in response to titanium particles. J. Orthop. Res. 2003, 21, 697–707. [Google Scholar] [CrossRef]
- Polyzois, I.; Nikolopoulos, D.; Michos, I.; Patsouris, E.; Theocharis, S. Local and systemic toxicity of nanoscale debris particles in total hip arthroplasty. J. Appl. Toxicol. 2012, 32, 255–269. [Google Scholar] [CrossRef]
- Hsiao, I.L.; Huang, Y.J. Titanium oxide shell coatings decrease the cytotoxicity of ZnO nanoparticles. Chem. Res. Toxicol. 2011, 24, 303–313. [Google Scholar] [CrossRef]
- Minhas, B.; Dino, S.; Zuo, Y.; Qian, H.; Zhao, X. Improvement of Corrosion Resistance of TiO2 Layers in Strong Acidic Solutions by Anodizing and Thermal Oxidation Treatment. Materials 2021, 14, 1188. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Sun, J.; Han, J.; Wu, G. Corrosion Behavior in Hydrochloric Acid of Pure Titanium after Ultrasonic Severe Surface Rolling. Metals 2022, 12, 1951. [Google Scholar] [CrossRef]
- Arya, S.B.; Bhattacharjee, A.; Roy, M. Electrochemical corrosion behavior of Ti-10V-2Fe-3Al in different corrosive media. Mater. Corros. 2018, 69, 1025–1038. [Google Scholar] [CrossRef]
- Yu, S.Y.; Scully, J.R. Corrosion and Passivity of Ti-13% Nb-13% Zr in Comparison to Other Biomedical Implant Alloys. Corrosion 1997, 53, 965–976. [Google Scholar] [CrossRef]
- Frayret, J.P.; Caprani, A.; Jaszay, T.; Priem, F. Influence of Aluminum and Vanadium on the Anodic Dissolution of Ti-Al and Ti-V Binary Alloys in Concentrated Hydrochloric Acid. Corrosion 1985, 41, 656–664. [Google Scholar] [CrossRef]
- Yu, F.; Addison, O.; Davenport, A. Temperature-Dependence Corrosion Behavior of Ti6Al4V in the Presence of HCl. Front. Mater. 2022, 9, 880702. [Google Scholar] [CrossRef]
- Metikoš-Huković, M.; Kwokal, A.; Piljac, J. The influence of niobium and vanadium on passivity of titanium-based implants in physiological solution. Biomaterials 2003, 24, 3765–3775. [Google Scholar] [CrossRef]
- Zakir, M.; Laiho, T.; Granroth, S.; Kukk, E.; Chu, C.H.; Tsoi, J.K.H.; Matinlinna, J.P. The effect of different strong acids and silica-coating on resin Ti adhesion. Surf. Interface Anal. 2023, 55, 701–711. [Google Scholar] [CrossRef]
- Nabhani, M.; Shoja Razavi, R.; Barekat, M. Corrosion study of laser cladded Ti-6Al-4V alloy in different corrosive environments. Eng. Fail. Anal. 2019, 97, 234–241. [Google Scholar] [CrossRef]
- Zhang, H.; Man, C.; Wang, L.; Dong, C.; Wang, L.; Kong, D.; Wang, X. Different corrosion behaviors between α and β phases of Ti6Al4V in fluoride-containing solutions: Influence of alloying element Al. Corros. Sci. 2020, 169, 108605. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Xu, W.; Zhang, J.; Yang, L.; Peng, Z.; Hou, B. Microstructure Refinement on Crevice Corrosion of High-Speed Rail Steel U75V Visualized by an In Situ Monitoring System. Front. Mater. 2022, 8, 820721. [Google Scholar] [CrossRef]
- Xu, W.; Deng, Y.; Zhang, B.; Zhang, J.; Peng, Z.; Hou, B.; Duan, J. Crevice corrosion of U75V high-speed rail steel with varying crevice gap size by in-situ monitoring. J. Mater. Res. Technol. 2022, 16, 1856–1874. [Google Scholar] [CrossRef]
- Gilbert, J.L.; Mali, S.; Urban, R.M.; Silverton, C.D.; Jacobs, J.J. In vivo oxide-induced stress corrosion cracking of Ti-6Al-4V in a neck–stem modular taper: Emergent behavior in a new mechanism of in vivo corrosion. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Atapour, M.; Pilchak, A.; Frankel, G.S.; Williams, J.C. Corrosion behaviour of investment cast and friction stir processed Ti-6Al-4V. Corros. Sci. 2010, 52, 3062–3069. [Google Scholar] [CrossRef]
- Codaro, E.N.; Nakazato, R.Z.; Horovistiz, A.L.; Ribeiro, L.M.; Ribeiro, R.B.; Hein, L.D. An image analysis study of pit formation on Ti–6Al–4V. Mater. Sci. Eng. A 2003, 341, 202–210. [Google Scholar] [CrossRef]
- Ciszak, C.; Popa, I.; Brossard, J.M.; Monceau, D.; Chevalier, S. NaCl induced corrosion of Ti-6Al-4V alloy at high temperature. Corros. Sci. 2016, 110, 91–104. [Google Scholar] [CrossRef]
- Liu, J.; Wu, L.; Yu, M.; Li, S.; Wu, G.; Zhang, Y. EIS characterization of sealed anodic oxide films on titanium alloy Ti-10V-2Fe-3Al. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2016, 31, 599–605. [Google Scholar] [CrossRef]
- Yu, S.Y.; Brodrick, C.W.; Ryan, M.P.; Scully, J.R. Effects of Nb and Zr Alloying Additions on the Activation Behavior of Ti in Hydrochloric Acid. J. Electrochem. Soc. 1999, 146, 4429–4438. [Google Scholar] [CrossRef]
- Xiang, S.; Yuan, Y.; Zhang, C.; Chen, J. Effects of Process Parameters on the Corrosion Resistanceand Biocompatibility of Ti6Al4V Parts Fabricated by Selective LaserMelting. ACS Omega 2022, 7, 5954. [Google Scholar] [CrossRef]
- Chandar, S.; Kotian, R.; Madhyastha, P.; Kabekkodu, S.; Rao, P. In vitro evaluation of cytotoxicity and corrosion behavior of commercially pure titanium and Ti-6Al-4V alloy for dental implants. J. Indian Prosthodont. Soc. 2017, 17, 35–40. [Google Scholar]
- Ionescu, F.; Reclaru, L.; Ardelean, L.C.; Blatter, A. Comparative Analysis of the Corrosion Resistance of Titanium Alloys Intended to Come into Direct or Prolonged Contact with Live Tissues. Materials 2019, 12, 2841. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.I.; Lee, J.B. Localized Corrosion Resistance on Additively Manufactured Ti Alloys by Means of Electrochemical Critical Localized Corrosion Potential in Biomedical Solution Environments. Materials 2021, 14, 7481. [Google Scholar] [CrossRef]
- Mogoda, A.S.; Ahmad, Y.H.; Badawy, W.A. Corrosion inhibition of Ti-6Al-4V alloy in sulfuric and hydrochloric acid solutions using inorganic passivators. Mater. Corros. 2004, 55, 449–456. [Google Scholar] [CrossRef]
- Giron, R.G.P.; Chen, X.; La Plante, E.C.; Gussev, M.N.; Leonard, K.J.; Sant, G. Revealing How Alkali Cations Affect the Surface Reactivityof Stainless Steel in Alkaline Aqueous Environments. ACS Omega 2018, 3, 14680. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Chacón-Nava, J.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.M.; Delgado, A.D.; Flores-De Los Rios, J.P.; Bocchetta, P.; Almeraya-Calderón, F. Electrochemical Corrosion of Titanium and Titanium Alloys Anodized in H2SO4 and H3PO4 Solutions. Coatings 2022, 12, 325. [Google Scholar] [CrossRef]
- Soares, F.M.S.; Elias, C.N.; Monteiro, E.S.; Coimbra, M.E.R.; Santana, A.I.C. Galvanic Corrosion of Ti Dental Implants Coupled to CoCrMo Prosthetic Component. Int. J. Biomater. 2021, 2021, 1313343. [Google Scholar] [CrossRef] [PubMed]

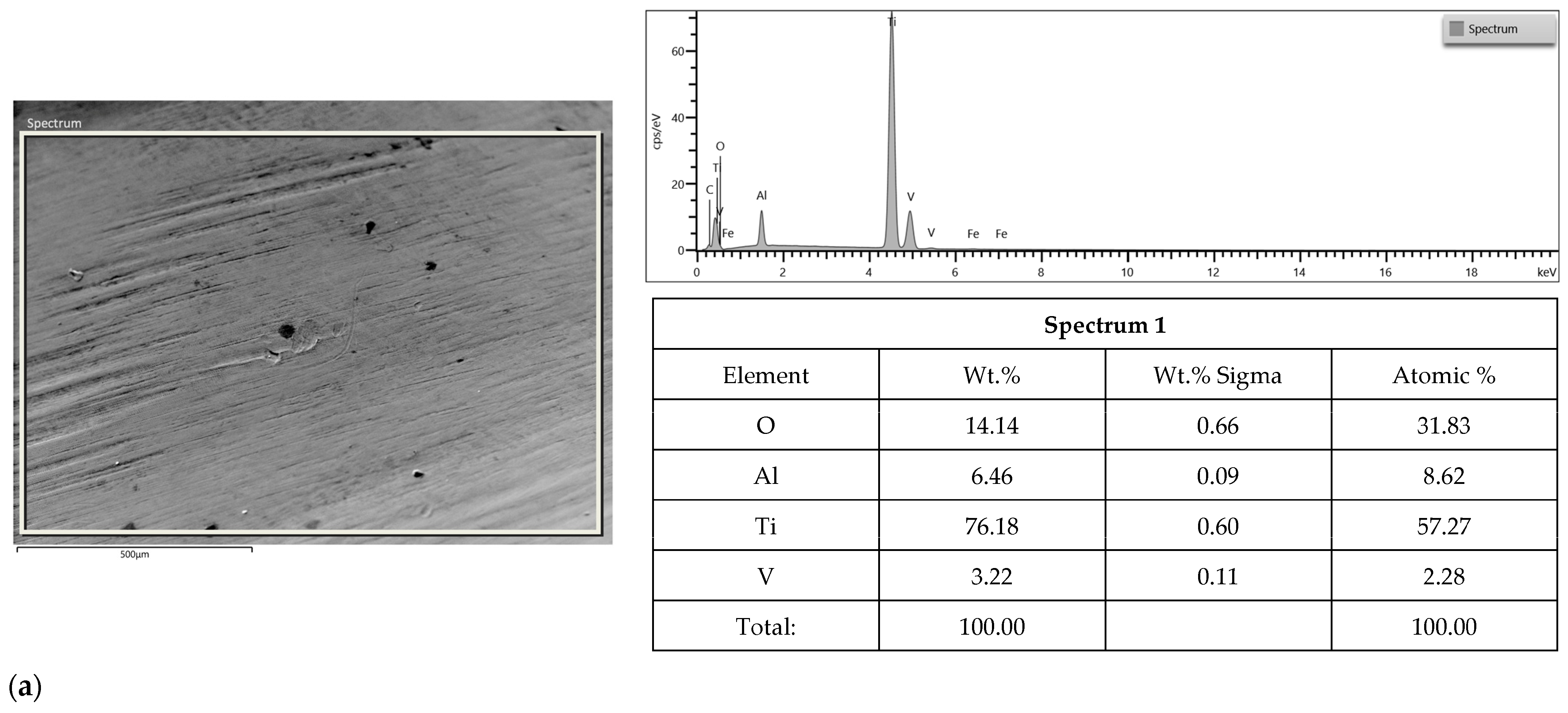


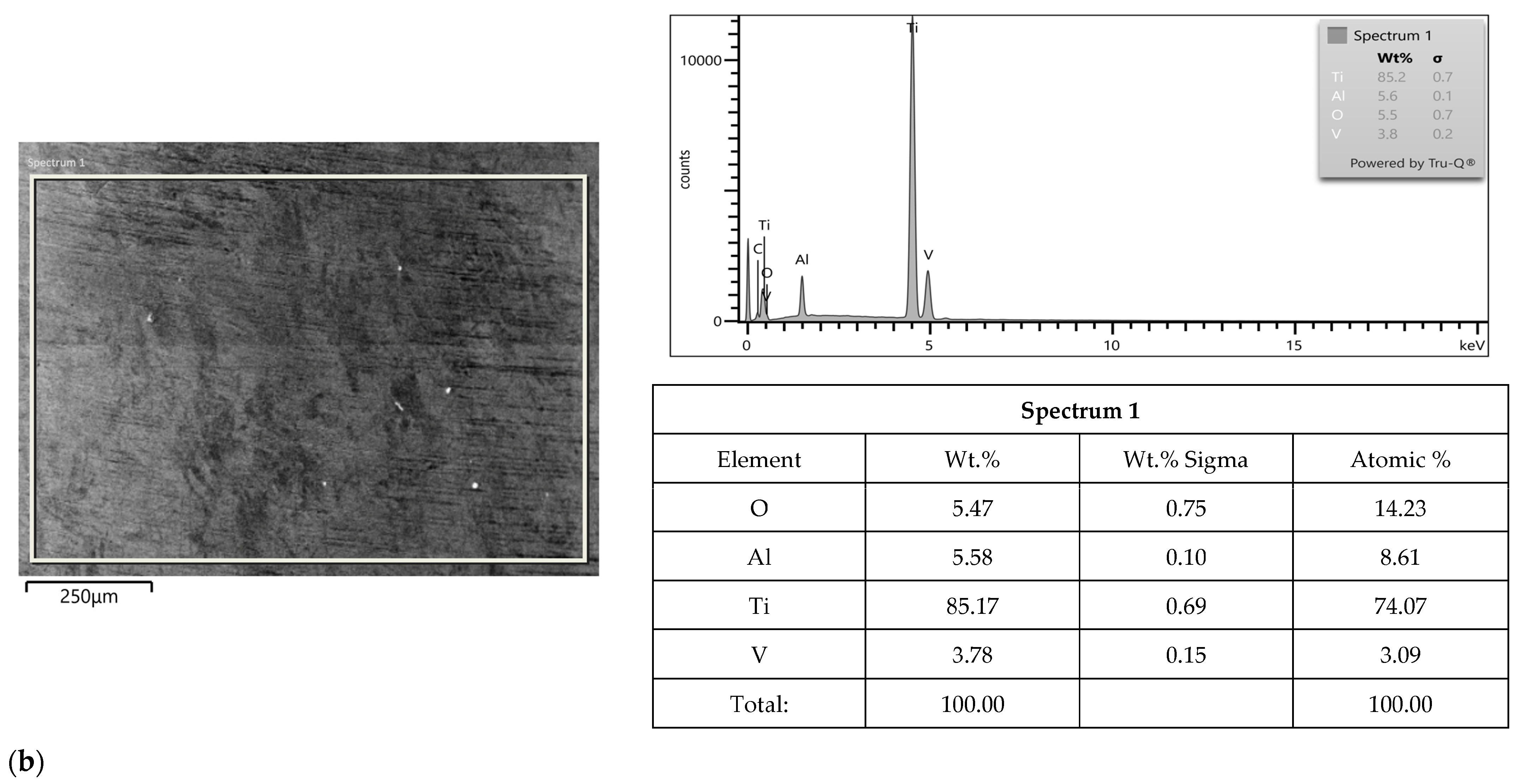
| Apollo Abutments | Astra Abutments | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| jcorr [A/cm2] | Ecorr [mV] | βa [V/dec] | βc [V/dec] | Rp [Ohm/cm2] | CR [mm/yr] | jcorr [A/cm2] | Ecorr [mV] | βa [V/dec] | βc [V/dec] | Rp [Ohm/cm2] | CR [mm/yr] |
| NaCl | |||||||||||
| 8.8 × 10−8 ± 2.5 × 10−8 | −174.5 ± 2.6 | 788.7 × 10−3 | 102.1 × 10−3 | 44.6 × 103 | 7.59 × 10−4 | 34.2 × 10−8 ± 2.5 × 10−8 | −141.6 ± 14.0 | 542.3 × 10−3 | 108.4 × 10−3 | 11.5 × 103 | 2.95 × 10−3 |
| HCl | |||||||||||
| 62.7 × 10−4 ± 9.3 × 10−4 | −261.6 ± 3.7 | 421.3 × 10−3 | 155.1 × 10−3 | 7.85 | 54.10 | 2.9 × 10−4 ± 0.8 × 10−4 | −272.6 ± 7.2 | 430.1 × 10−3 | 136.6 × 10−3 | 1.55 × 102 | 2.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalski, J.; Rylska, D.; Januszewicz, B.; Konieczny, B.; Cichomski, M.; Matinlinna, J.P.; Radwanski, M.; Sokolowski, J.; Lukomska-Szymanska, M. Corrosion Resistance of Titanium Dental Implant Abutments: Comparative Analysis and Surface Characterization. Materials 2023, 16, 6624. https://doi.org/10.3390/ma16206624
Kowalski J, Rylska D, Januszewicz B, Konieczny B, Cichomski M, Matinlinna JP, Radwanski M, Sokolowski J, Lukomska-Szymanska M. Corrosion Resistance of Titanium Dental Implant Abutments: Comparative Analysis and Surface Characterization. Materials. 2023; 16(20):6624. https://doi.org/10.3390/ma16206624
Chicago/Turabian StyleKowalski, Jakub, Dorota Rylska, Bartłomiej Januszewicz, Bartlomiej Konieczny, Michal Cichomski, Jukka P. Matinlinna, Mateusz Radwanski, Jerzy Sokolowski, and Monika Lukomska-Szymanska. 2023. "Corrosion Resistance of Titanium Dental Implant Abutments: Comparative Analysis and Surface Characterization" Materials 16, no. 20: 6624. https://doi.org/10.3390/ma16206624








