Tellurium Corrosion of Type 304/304L Stainless Steel, Iron, Chromium, and Nickel in High-Temperature Liquid Sodium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental
2.3. Microstructure Characterization
3. Results and Discussion
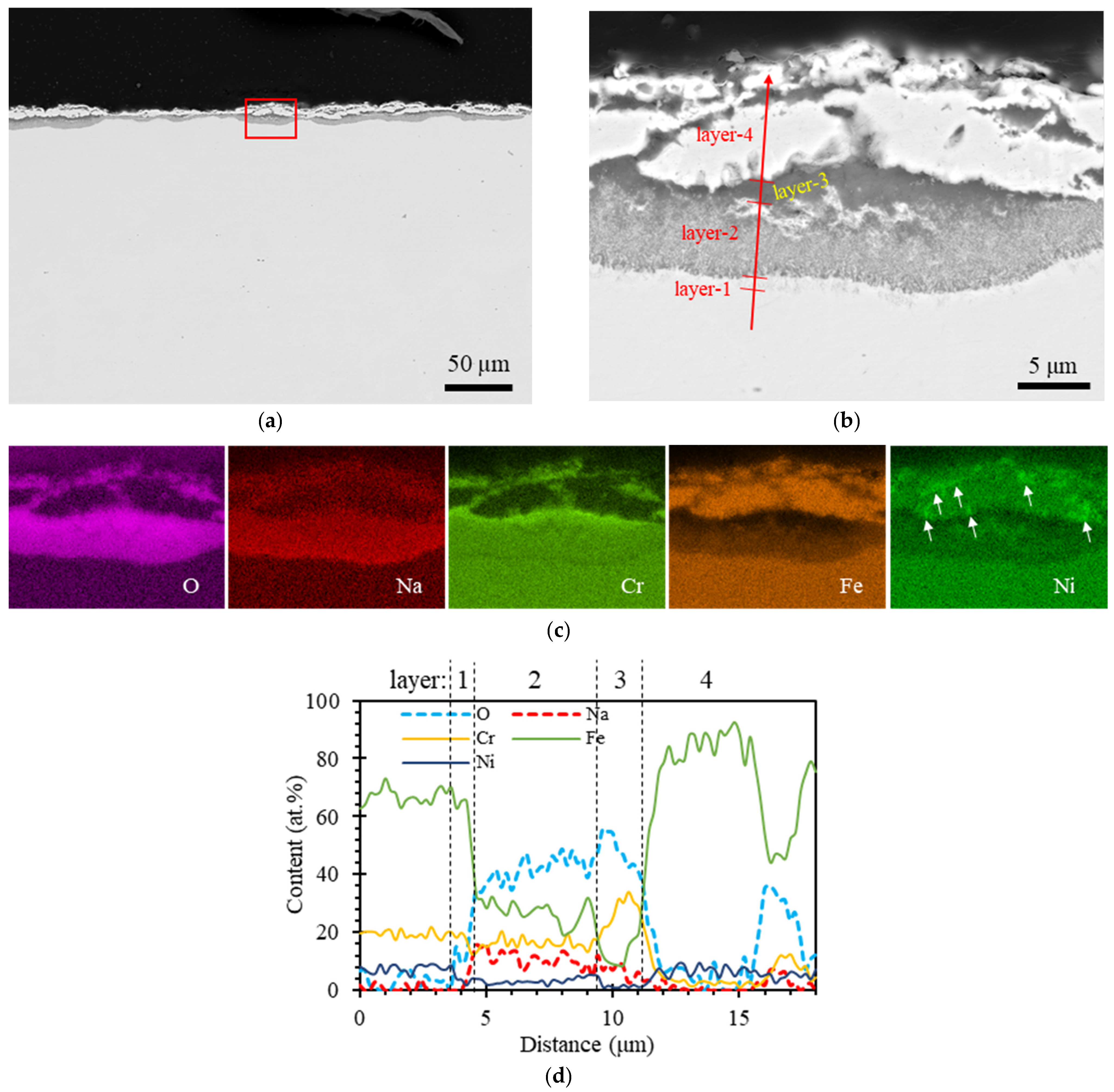
3.1. SS304
3.2. Chromium
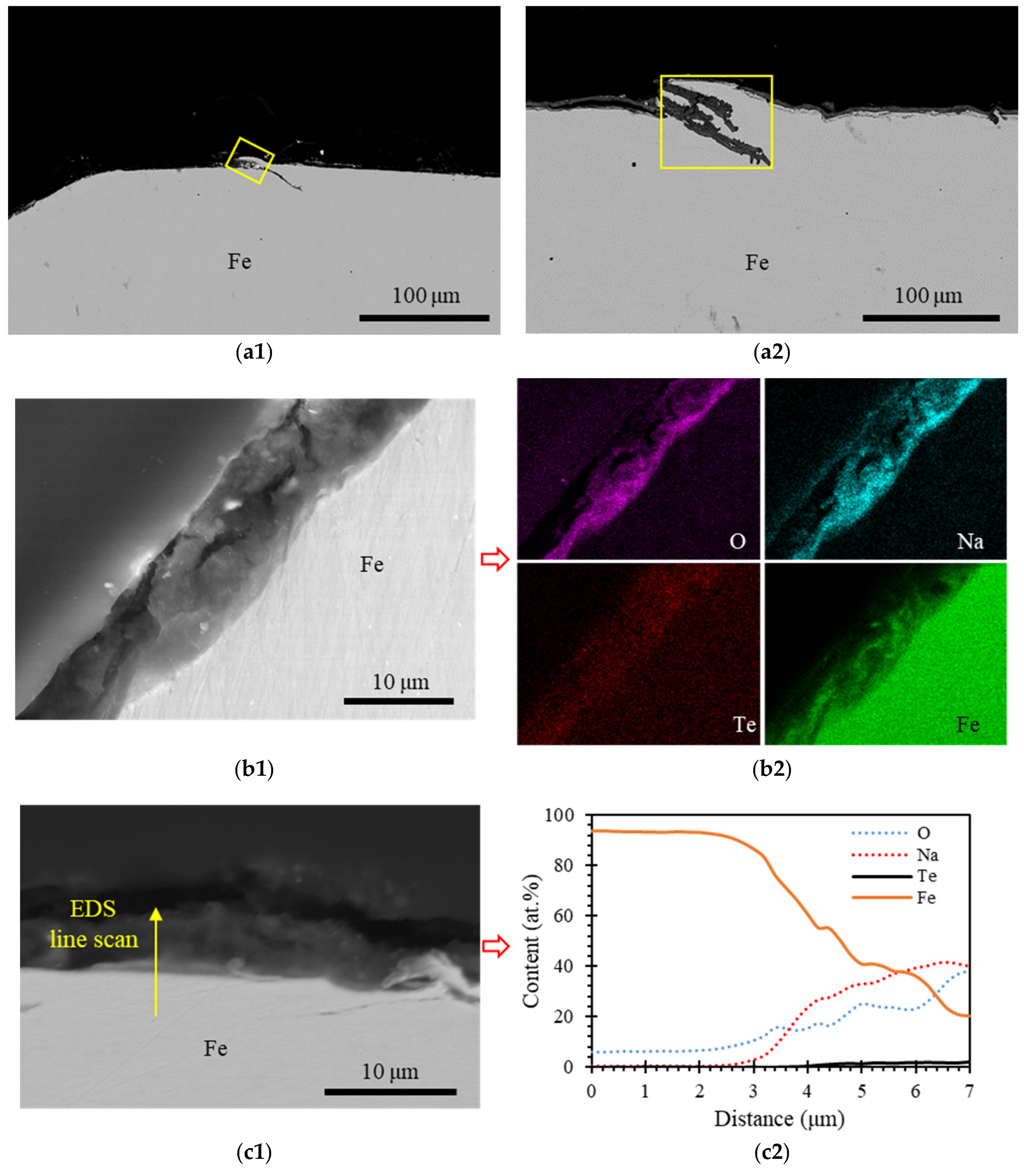
3.3. Iron
3.4. Nickel
4. Discussion
4.1. Oxide Layers
4.2. Tellurium Penetration
5. Conclusions
- The oxygen level emerges as a critical factor in the formation of stable oxide compound layers, which play a crucial role in mitigating Te-induced pitting to some extent. When the oxygen level was maintained at 10 ppm, multiple oxide layers formed on the surface of SS304. These layers consisted of a compact NaCrO2 thin interlayer and porous outer layers comprising Na-Fe-Ni-O. Notably, this oxygen level exceeded the threshold for NaCrO2 formation but remained below the levels required for Na4FeO3 and Na2NiO2. The compact NaCrO2 thin interlayer was effective in hindering Te penetration, unlike porous oxide layers. At an oxygen level of 0.01 ppm, stable ternary oxide compounds failed to form.
- Chromium had greater resistance to Te-induced pitting when compared to other alloying elements, Fe and Ni. The depth of Te-induced pitting was greater in Fe and Ni compared to Cr. This difference resulted from the lower thermodynamic stability of Fe- and Ni-tellurides when compared to Cr-tellurides. Consequently, Fe- and Ni-tellurides were more prone to dissolution in the liquid sodium, while Cr-tellurides acted as a barrier against further tellurium-induced pitting.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Middleton, B.D.; Parma, E.J.; Olivier, T.J.; Phillips, J.; Lachance, J.L. The Development of a Realistic Source Term for Sodium-Cooled Fast Reactors: Assessment of Current Status and Future Needs; SAND2011-3404; Sandia National Laboratories (SNL): Albuquerque, NM, USA, 2011. [Google Scholar]
- Fission and Corrosion Product Behaviour in Liquid Metal Fast Breeder Reactors (LMFBRs); IAEA-TECDOC-687; International Atomic Energy Agency: Vienna, Austria, 1993.
- Grabaskas, D.; Brunett, A.; Bucknor, M.; Sienicki, J.; Sofu, T. Regulatory Technology Development Plan Sodium Fast Reactor Mechanistic Source Term Development; ANL-ART-3; Argonne National Laboratory (ANL): Argonne, IL, USA, 2015. [Google Scholar]
- Feurestein, H.; Hooper, A.; Johnson, F. Mechanisms of Release of Radioactive Products into Liquid-Metal Coolants, their Transport within the Circuits and Removal from LMFBRs. At. Energy Rev. 1979, 17, 697–761. [Google Scholar]
- Chellew, N.R.; Bennett, G.A. The Melt Refining of Irradiated Uranium: Application to EBR-II Fast Reactor Fuel. XII. The Behavior of Ruthenium, Molybdenum, Palladium, Rhodium, Technetium, Antimony, Cadmium, and Tellurium. Nucl. Sci. Eng. 1961, 9, 87–90. [Google Scholar] [CrossRef]
- Zhang, Z.-D.; Ren, C.-L.; Tan, M.-L.; Yang, Y.-Q.; Yin, Y.-R.; Wang, C.-Y.; Han, H.; Huai, P. Migration behavior of tellurium in bcc iron against typical alloying elements: A first-principles study. Comput. Mater. Sci. 2020, 181, 109571. [Google Scholar] [CrossRef]
- Rosenthal, M.W.; Kasten, P.R.; Briggs, R.B. Molten-Salt Reactors—History, Status, and Potential. Nucl. Appl. Technol. 2017, 8, 107–117. [Google Scholar] [CrossRef]
- Jia, Y.; Cheng, H.; Qiu, J.; Han, F.; Zou, Y.; Li, Z.; Zhou, X.; Xu, H. Effect of temperature on diffusion behavior of Te into nickel. J. Nucl. Mater. 2013, 441, 372–379. [Google Scholar] [CrossRef]
- Wu, B.H.; Jiang, L.; Ye, X.X.; Li, C.W.; Liang, J.P.; Liu, F.; Li, Z.J. On the origin of tellurium corrosion resistance of hot-rolled GH3535 alloy. Corros. Sci. 2020, 170, 108644. [Google Scholar] [CrossRef]
- Barker, M.G.; Wood, D.J. The corrosion of chromium, iron, and stainless steel in liquid sodium. J. Less Common Met. 1974, 35, 315–323. [Google Scholar] [CrossRef]
- Bhatt, N.P.; Borgstedt, H.U. Corrosion behaviour of structural materials in sodium influenced by formation of ternary oxides. Mater. Corros. 1988, 39, 115–123. [Google Scholar] [CrossRef]
- Walker, R.A.; Pratt, J.N. The solubilities of bismuth and tellurium in liquid sodium. J. Nucl. Mater. 1970, 34, 165–173. [Google Scholar] [CrossRef]
- Borgstedt, H.U.; Guminski, C. Solubilities and Solution Chemistry in Liquid Alkali Metals. Monatshefte Für Chem./Chem. Mon. 2000, 131, 0917–0930. [Google Scholar] [CrossRef]
- Noden, J.D. A general equation for the solubility of oxygen in liquid sodium. J. Br. Nucl. Energy Soc. 1973, 12, 57–62. [Google Scholar]
- Grønvold, F.; Westrum, E.F. Thermodynamic aspects of the magnetic transitions in the chromium tellurides Heat Capacities of Cr5Te6, Cr3Te4 and Cr2Te3 from 5 to 350 K. Z. Für Anorg. Und Allg. Chem. 1964, 328, 272–282. [Google Scholar] [CrossRef]
- Arvhult, C.M.; Guéneau, C.; Gossé, S.; Selleby, M. Thermodynamic assessment of the Fe-Te system. Part II: Thermodynamic modeling. J. Alloys Compd. 2018, 767, 883–893. [Google Scholar] [CrossRef]






| Test Material | Test Condition | ||
|---|---|---|---|
| Test Time (d) | Glovebox Oxygen Level (ppm) | Liquid Condition | |
| SS304 | 30 | 10 | 1 mol.% Te in liquid sodium at 773 K |
| Cr | 30 | 0.01 | |
| Fe | 30 | 0.01 | |
| Ni | 30 | 0.01 | |
| Test Material | Glovebox Oxygen Level (ppm) | Oxide Layer Characteristics | Te-Induced Pitting Characteristics |
|---|---|---|---|
| SS304 | 10 | 10–20 µm thick, no Te composition, multiple layers with various compositions | Densified pits of 10 µm depth |
| Cr | 0.01 | No visible oxide layer | A few pits of 5–10 µm depth |
| Fe | 0.01 | 10 µm thick with a little Te composition, single layer | A few pits of 50 µm depth |
| Ni | 0.01 | 10 µm thick with about 30 at.% Te, single layer | Densified pits of 10–30 µm depth |
| Metal | ||||
|---|---|---|---|---|
| Na → Na2O | 10 | −647,900 | 0.01 | −852,336 |
| Cr → Cr2O3 | −463,673 | −668,109 | ||
| Fe → FeO | −392,432 | −596,867 | ||
| Ni → NiO | −339,780 | −544,215 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y. Tellurium Corrosion of Type 304/304L Stainless Steel, Iron, Chromium, and Nickel in High-Temperature Liquid Sodium. Materials 2023, 16, 6798. https://doi.org/10.3390/ma16206798
Xie Y. Tellurium Corrosion of Type 304/304L Stainless Steel, Iron, Chromium, and Nickel in High-Temperature Liquid Sodium. Materials. 2023; 16(20):6798. https://doi.org/10.3390/ma16206798
Chicago/Turabian StyleXie, Yi. 2023. "Tellurium Corrosion of Type 304/304L Stainless Steel, Iron, Chromium, and Nickel in High-Temperature Liquid Sodium" Materials 16, no. 20: 6798. https://doi.org/10.3390/ma16206798





