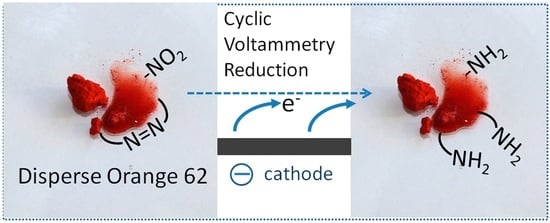
Cyclic Voltammetry of C.I. Disperse Orange 62 in an Aqueous Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cyclic Voltammetry
3. Results and Discussion
3.1. Cyclic Voltammetry of DO62 in Dissolved State
3.2. Cyclic Voltammetry of Dispersed Dyes in Aqueous Electrolyte
 | (1) |
| (2) |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bechtold, T.; Pham, T. Textile Chemistry, 2nd ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2023. [Google Scholar]
- Zhou, X.; Zhou, Y.; Liu, J.; Song, S.; Sun, J.; Zhu, G.; Gong, H.; Wang, L.; Wu, C.; Li, M. Study on the pollution characteristics and emission factors of PCDD/Fs from disperse dye production in China. Chemosphere 2019, 228, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huda, S. Comparison of disperse dye exhaustion, color yield, and colorfastness between polylactide and poly(ethylene terephthalate). J. Appl. Polym. Sci. 2003, 90, 3285–3290. [Google Scholar] [CrossRef]
- Gupta, K.V.; Khamparia, S.; Tyagi, I.; Jaspal, D.; Malviya, A. Decolorization of mixture of dyes: A critical review. Glob. J. Environ. Sci. Manag. 2015, 1, 71–94. [Google Scholar] [CrossRef]
- Ajam, N.H.; Jaeel, A.J. Decolorization of Disperse Dyes: A Review. AIP Conf. Proc. 2023, 2787, 060007. [Google Scholar] [CrossRef]
- Wang, B.; Chen, Y.; Guan, J.; Ding, Y.; He, Y.; Zhang, X.; Shukurov, N.; Ferreira, L.F.R.; Liu, J.; Zhu, M. Biodecolorization and Ecotoxicity Abatement of Disperse Dye-Production Wastewater Treatment with Pycnoporus Laccase. Int. J. Environ. Res. Public Health 2022, 19, 7983. [Google Scholar] [CrossRef]
- El Aggadi, S.; El Abbassi, Z.; El Hourch, A. Color removal from dye-containing aqueous solutions by electrooxidation. Desalination Water Treat. 2021, 215, 232–236. [Google Scholar] [CrossRef]
- El Aggadi, S.; Loudiyi, N.; Chadil, A.; Cherkaoui, O.; El Hourch, A. Electrochemical oxidation of textile azo dye reactive orange 16 on the Platinum electrode. Mediterr. J. Chem. 2020, 10, 82–89. [Google Scholar] [CrossRef]
- Zollinger, H. Color Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Joudi, M.; Mouldar, J.; Hafdi, H.; Nasrellah, H.; Hatimi, B.; El Mhammedi, M.A.; Bakasse, M. Factorial experimental design for the removal of disperse dyes using hydroxyapatite prepared from Moroccan phosphogypsum. Mediterr. J. Chem. 2019, 8, 1–9. [Google Scholar] [CrossRef]
- Mwashighadi, N.M. Electrochemical Treatment of Textile Dye Wastewater by Aluminium and Stainless-Steel Electrodes; Kenyatta University: Nairobi, Kenya, 2020. [Google Scholar]
- Turhan, K. Determination of optimal conditions in decolorization of disperse dyes in aqueous solution by ozonation. Glob. Nest J. 2021, 23, 143–151. [Google Scholar]
- Lou, J.; Ren, L.; Yuan, J.; Xu, J.; Gu, Z.; Fan, X. Mechanism and application of ozone fading: Oxidative decolorisation of disperse dyes and waste-dyed polyester fabrics. Color. Technol. 2023, 139, 338–349. [Google Scholar] [CrossRef]
- Aquino, J.M.; Rocha-Filho, R.C.; Bocchi, N.; Biaggio, S.R. Electrochemical degradation of the Disperse Orange 29 dye on a β-PbO2 anode assessed by the response surface methodology. J. Environ. Chem. Eng. 2013, 1, 954–961. [Google Scholar] [CrossRef]
- Bechtold, T.; Turcanu, A.; Schrott, W. Electrochemical decolourisation of dispersed indigo on boron-doped diamond anodes. Diam. Relat. Mater. 2006, 15, 1513–1519. [Google Scholar] [CrossRef]
- Hamed, H.H.; Alabdraba, W.M.S.; Mohammed, A.E. Degradation of Disperse Blue 79 Dye in Aqueous solution using Fenton (H2O2/Fe2+) Process. Sci. Int. 2018, 30, 605–611. [Google Scholar]
- Szpyrkowicz, L.; Juzzolino, C.; Kaul, S.N.; Daniele, S.; De Faveri, M.D. Electrochemical Oxidation of Dyeing Baths Bearing Disperse Dyes. Ind. Eng. Chem. Res. 2000, 39, 3241–3248. [Google Scholar] [CrossRef]
- Oguzie, K.; Oguzie, E.; Nwanonenyi, S.; Edoziem, J.; Vrsalovic, L. Electrochemical decolorization of Disperse Blue-1 dye in aqueous solution. Environ. Eng. Manag. J. 2021, 20, 1467–1476. [Google Scholar] [CrossRef]
- Nidheesh, P.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef]
- Li, Y.; Shen, J.; Liu, Q.; Zhu, Y.; Pang, Z.; Ge, M. Zinc-doped ferrite nanoparticles as bi-functional catalysts for decolorization of disperse dyes in glycolysis of colored poly(ethylene terephthalate) fabrics. J. Environ. Chem. Eng. 2023, 11, 109816. [Google Scholar] [CrossRef]
- Rafaqat, S.; Ali, N.; Torres, C.; Rittmann, B. Recent progress in treatment of dyes wastewater using microbial-electro-Fenton technology. RSC Adv. 2022, 12, 17104–17137. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Freeman, H.S.; Hinks, D. Toward closed loop recycling of polyester fabric: Step 1. decolorization using sodium formaldehyde sulfoxylate. J. Clean. Prod. 2020, 254, 120027. [Google Scholar] [CrossRef]
- Bechtold, T.; Burtscher, E.; Turcanu, A. Cathodic decolourisation of textile waste water containing reactive dyes using a multi-cathode electrolyser. J. Chem. Technol. Biotechnol. 2001, 76, 303–311. [Google Scholar] [CrossRef]
- Encinas-Yocupicio, A.; Razo-Flores, E.; Sánchez-Díaz, F.; dos Santos, A.; Field, J.; Cervantes, F. Catalytic effects of different redox mediators on the reductive decolorization of azo dyes. Water Sci. Technol. 2006, 54, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.J.; Isley, W.C.; Brennan, J.E.; Cramer, C.J.; Bühlmann, P. Electrochemical Reduction of 2,4-Dinitrotoluene in Aprotic and pH-Buffered Media. J. Phys. Chem. C 2015, 119, 13088–13097. [Google Scholar] [CrossRef]
- Squella, J.A.; Núñez-Vergara, L.J.; Campero, A.; Maraver, J.; Jara-Ulloa, P.; Carbajo, J. Electrochemical Reduction of 2-Nitroimidazole in Aqueous Mixed Medium. J. Electrochem. Soc. 2007, 154, F77–F81. [Google Scholar] [CrossRef]
- Rogers, J.W.; Watson, W.H. Electrochemical reduction of 2-Nitro- and 2,2’-Dinitrobiphenyl. Anal. Chim. Acta 1971, 54, 41–54. [Google Scholar] [CrossRef]
- Bergische Univerversität Wuppertal. Entwicklung und Erprobung einer kathodischen Nano- Filtrationsmembran für die Reduktive Behandlung und Filtration von Wasserunlöslichen Farbstoffen und Farbpigmenten zur Aufbereitung von Textilabwasser mit dem Ziel der Wasserkreislaufführung sowie dem Nac,” Wuppertal. 2008. Available online: https://www.lanuv.nrw.de/fileadmin/lanuv/wasser/abwasser/forschung/pdf/AbschlussberichtREDUCOL.pdf (accessed on 6 October 2023).
- Turcanu, A.; Bechtold, T. Indirect cathodic reduction of dispersed indigo by 1,2-dihydroxy-9,10-anthraquinone-3-sulphonate (Alizarin Red S). J. Solid State Electrochem. 2011, 15, 1875–1884. [Google Scholar] [CrossRef]










| DO62 Dissolution Variants | Electrode | Electrolyte | DO62 Concentrationg L−1 |
|---|---|---|---|
| 1—fully dissolved | Pt | LiCl/DMF | 0–10.25 |
| 2—dispersed, partially dissolved in aqueous electrolyte | HMDE | Na2SO4/NaOH | 0–2.33 |
| 3—dispersed, partially dissolved with the presence of surfactants | HMDE | Na2SO4/NaOH/CET | 0, 0.030 |
| 4—dispersed, partially dissolved with the presence of a mediator | HMDE | Na2SO4/NaOH/AQDS | 0, 1.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bechtold, T.; Aguiló-Aguayo, N.; Pham, T. Cyclic Voltammetry of C.I. Disperse Orange 62 in an Aqueous Electrolyte. Materials 2023, 16, 6901. https://doi.org/10.3390/ma16216901
Bechtold T, Aguiló-Aguayo N, Pham T. Cyclic Voltammetry of C.I. Disperse Orange 62 in an Aqueous Electrolyte. Materials. 2023; 16(21):6901. https://doi.org/10.3390/ma16216901
Chicago/Turabian StyleBechtold, Thomas, Noemí Aguiló-Aguayo, and Tung Pham. 2023. "Cyclic Voltammetry of C.I. Disperse Orange 62 in an Aqueous Electrolyte" Materials 16, no. 21: 6901. https://doi.org/10.3390/ma16216901