Ab Initio Molecular Dynamics Study of Electron Excitation Effects on UO2 and U3Si
Abstract
:1. Introduction
2. Computational Methods
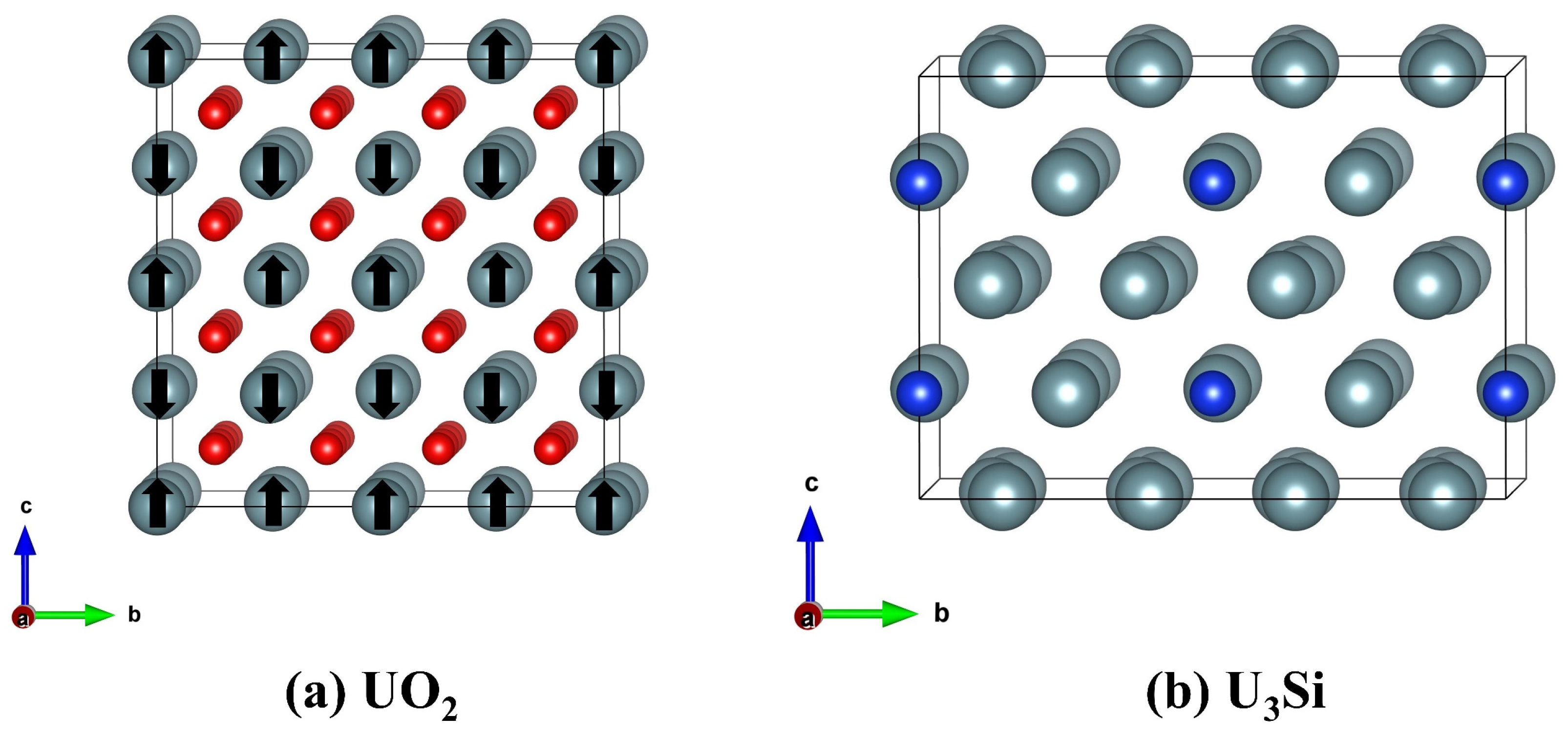
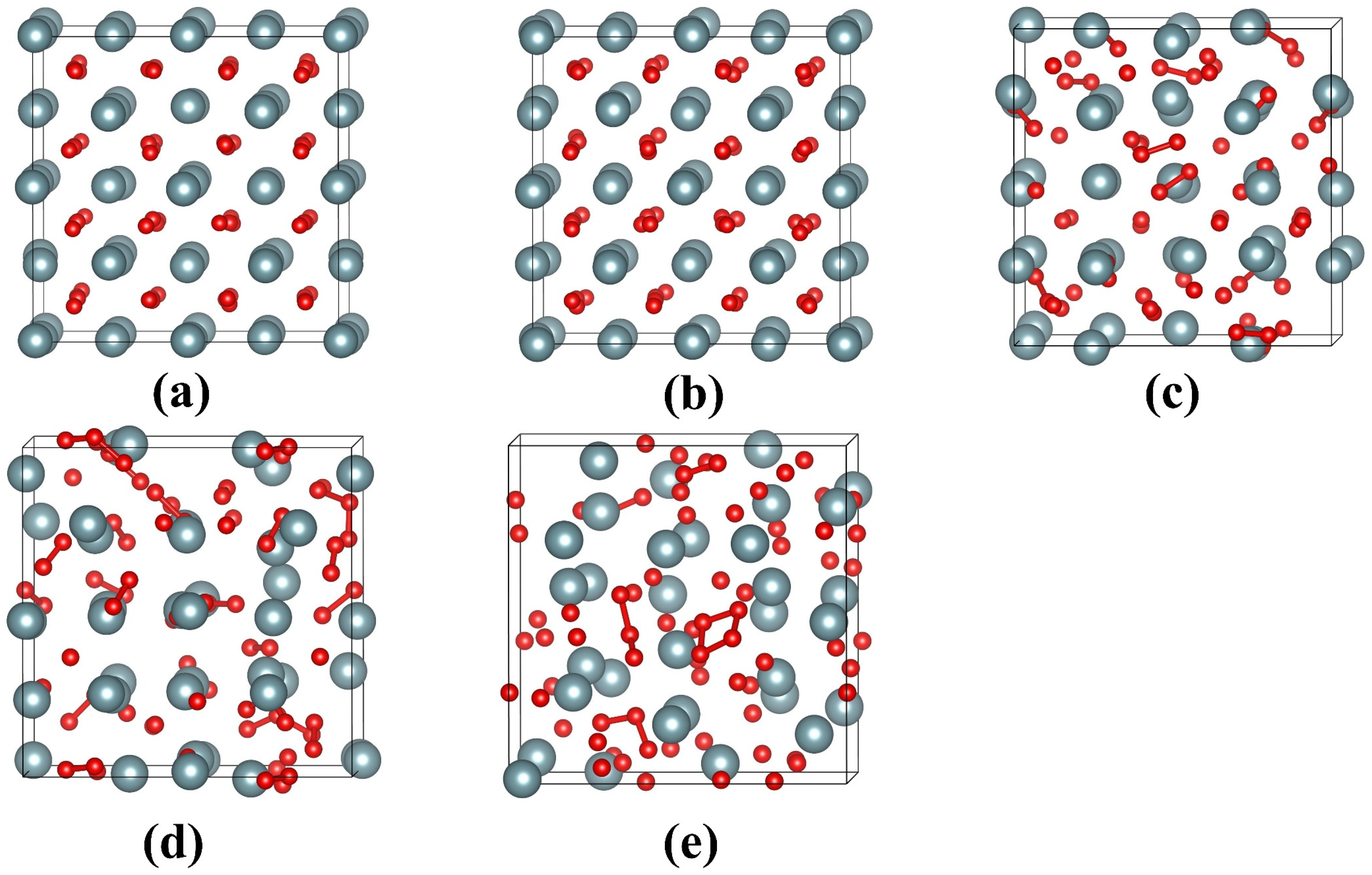
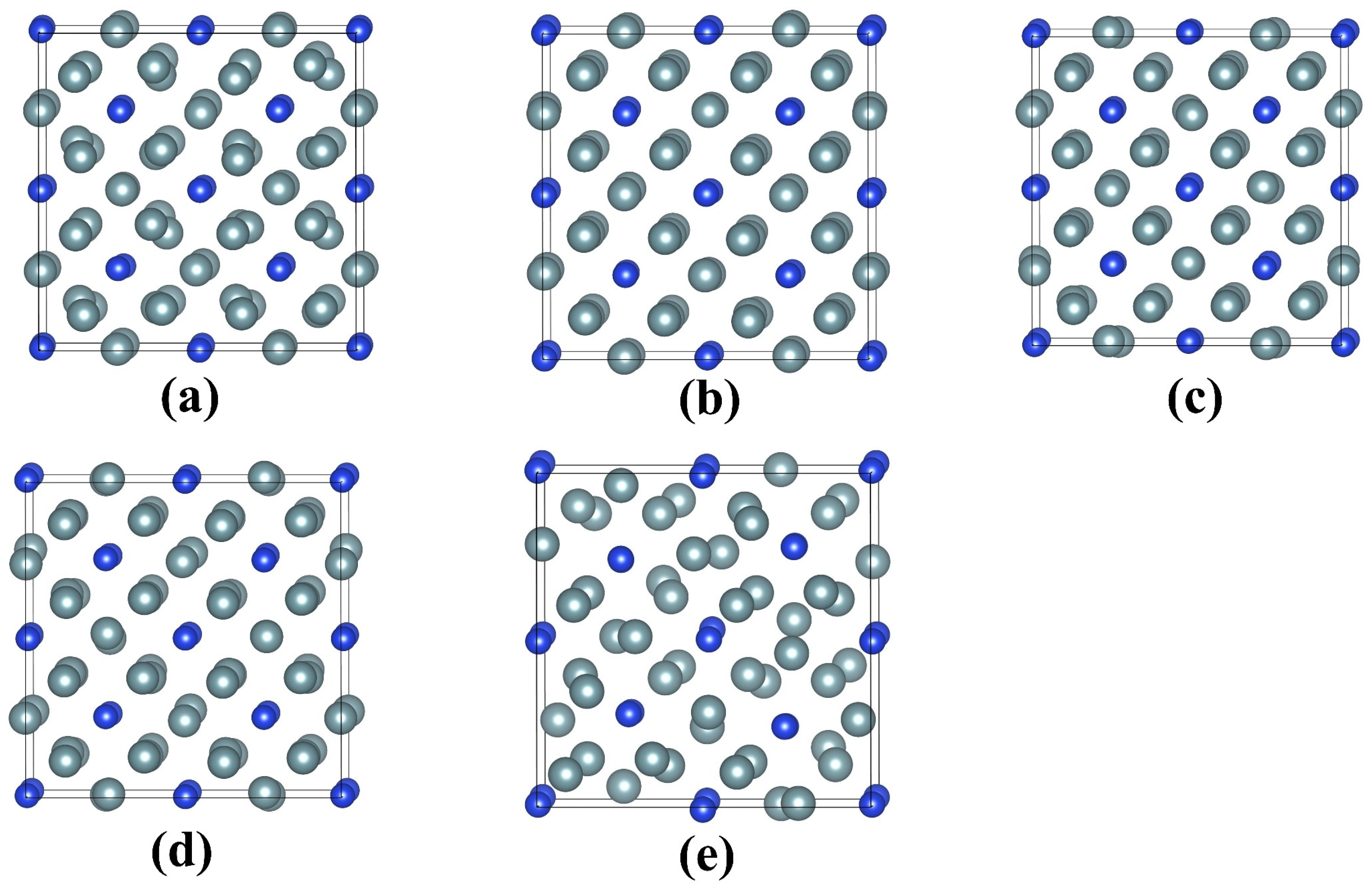
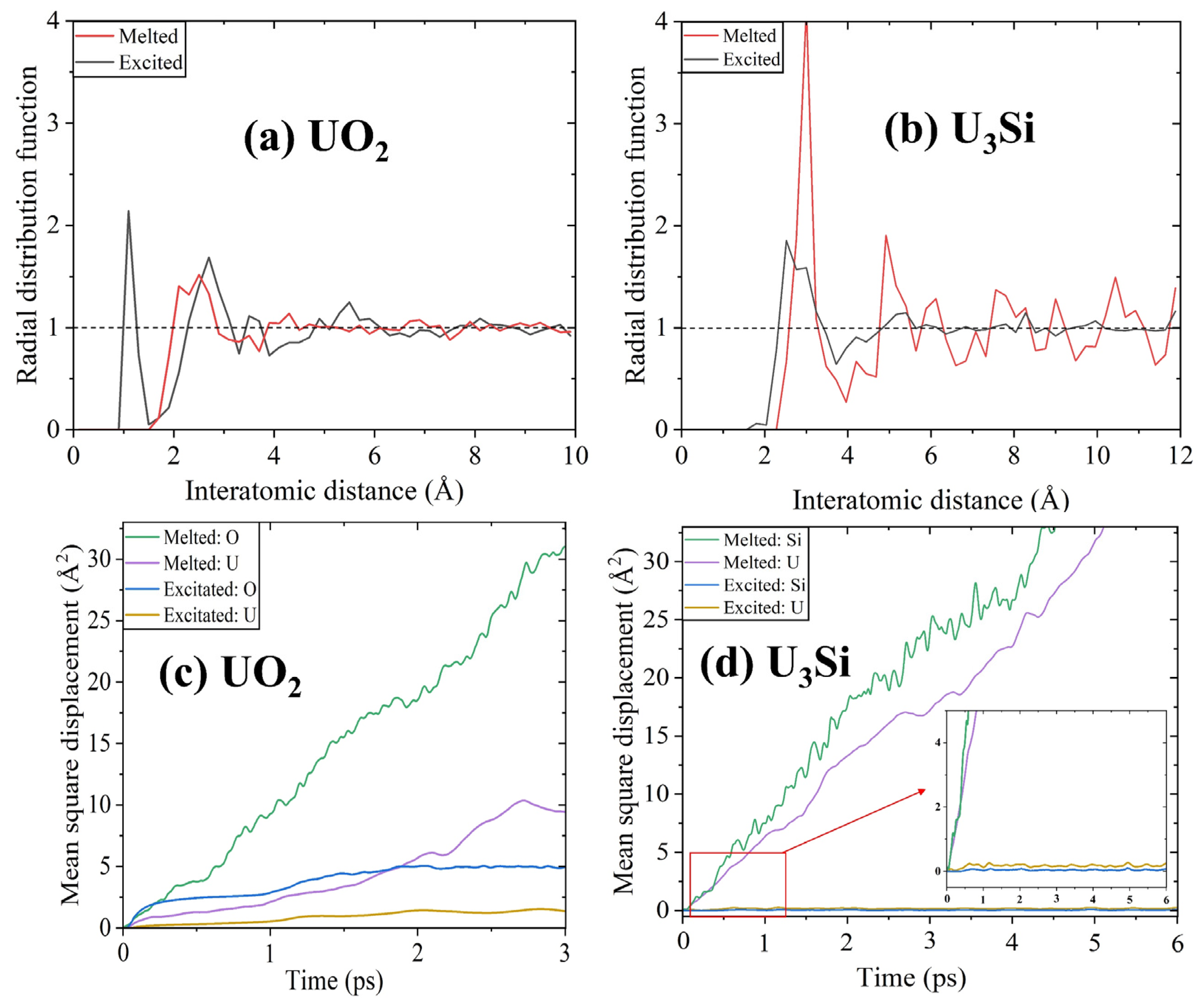
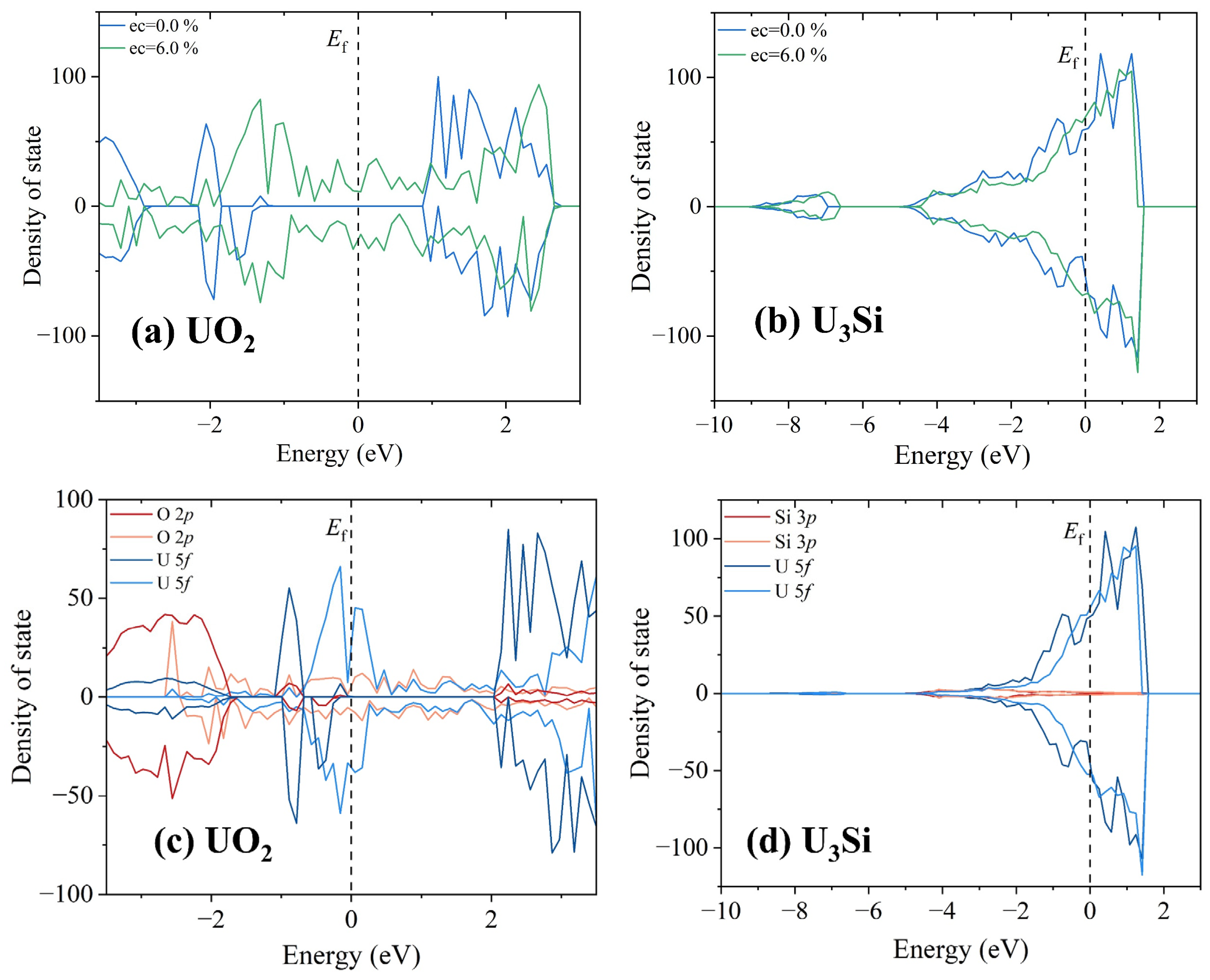
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, R. Multiphysics Coupled Modeling of Light Water Reactor Fuel Performance. Prog. Nucl. Energy 2016, 91, 38–48. [Google Scholar]
- Mirzaei, M. Structural variation within uraniumVI heterocyclic carboxylates: Solid and solution states studies. Inorganica Chim. Acta 2011, 370, 141–149. [Google Scholar] [CrossRef]
- Mirzaei, M. A new solvated complex of the uranyl ion (UO22+) with 8-hydroxyquinoline. Inorganica Chim. Acta 2015, 426, 136–141. [Google Scholar] [CrossRef]
- Manara, D. Melting of Stoichiometric and Hyperstoichiometric Uranium Dioxide. J. Nucl. Mat. 2005, 342, 148–163. [Google Scholar] [CrossRef]
- Yakub, E. Thermodynamic model of solid non-stoichiometric uranium dioxide. J. Phys. Condens. Matter 2006, 18, 1227–1248. [Google Scholar] [CrossRef]
- Owen, W.M. Diffusion in undoped and Cr-doped amorphous UO2. J. Nucl. Mat. 2023, 576, 154270. [Google Scholar] [CrossRef]
- Ševeček, M. Development of high thermal conductivity UO2–Th heterogeneous fuel. Prog. Nucl. Energy 2018, 108, 489–496. [Google Scholar] [CrossRef]
- White, J.T. Thermophysical Properties of U3Si2 to 1773 K. J. Nucl. Mat. 2015, 464, 275–280. [Google Scholar] [CrossRef]
- Idiri, M. Behavior of actinide dioxides under pressure: UO2 and ThO2. Phys. Rev. B 2004, 70, 014113. [Google Scholar] [CrossRef]
- Gorton, J.P. Impact of Uranium Oxide (UO2) Fuel with Molybdenum (Mo) Inserts on Pressurized Water Reactor Performance and Safety. J. Nucl. Mat. 2020, 542, 152492. [Google Scholar]
- Balboa, H. Damage characterization of (U,Pu)O2 under irradiation by molecular dynamics simulations. J. Nucl. Mat. 2018, 512, 440–449. [Google Scholar]
- Kandan, R. Calorimetric measurements on (U,Th)O2 solid solutions. J. Nucl. Mat. 2009, 384, 231–235. [Google Scholar] [CrossRef]
- Meyer, M.K. 7—Research reactor fuels. Adv. Nucl. Fuel Chem. 2020, 273–312. [Google Scholar] [CrossRef]
- Yin, Q. Electronic correlation and transport properties of nuclear fuel materials. Phys. Rev. B 2011, 84, 195111. [Google Scholar] [CrossRef]
- Matzke, H. Diffusion Processes in Nuclear Fuels. J. Less-Common Met. 1986, 121, 537–564. [Google Scholar]
- White, J. Thermophysical properties of U3Si to 1150 K. J. Nucl. Mat. 2014, 452, 304–310. [Google Scholar] [CrossRef]
- Wang, T. First-principles investigations on the electronic structures of U3Si2. J. Nucl. Mat. 2016, 469, 194–199. [Google Scholar] [CrossRef]
- Metzger, K.E. Determination of mechanical behavior of U3Si2 nuclear fuel by microindentation method. Prog. Nucl. Energy 2017, 99, 147–154. [Google Scholar] [CrossRef]
- Albarhoum, M. Thermohydraulic features of the use of U3Si LEU fuel in low-power research reactors. Prog. Nucl. Energy 2010, 52, 819. [Google Scholar]
- Mei, Z.-G. First-principles study of surface properties of uranium silicides. J. Nucl. Mat. 2019, 513, 192–197. [Google Scholar] [CrossRef]
- Mohamad, A. Thermal and Mechanical Properties of U3Si and USi3. Ann. Nucl. Energy 2019, 133, 186–193. [Google Scholar] [CrossRef]
- Middleburgh, S.C. Structural Stability and Fission Product Behaviour in U3Si. J. Nucl. Mat. 2015, 466, 739–744. [Google Scholar] [CrossRef]
- Rest, J. A Generalized Model for Radiation-Induced Amorphization and Crystallization of U3Si and U3Si2 and Recrystallization of UO2. J. Nucl. Mater. 1997, 240, 205–214. [Google Scholar] [CrossRef]
- Onofri, C. Full characterization of dislocations in ion-irradiated polycrystalline UO2. J. Nucl. Mater. 2017, 494, 252–259. [Google Scholar] [CrossRef]
- Willie, K.D.A. Analysis of Burnup effects and Its Integrity Assessment in the Interim of Irradiation with Molecular Dynamics. MRS Adv. 2020, 5, 1799–1810. [Google Scholar] [CrossRef]
- Yun, D. Current state and prospect on the development of advanced nuclear fuel system materials: A review. Mater. Rep. Energy 2021, 1, 100007. [Google Scholar] [CrossRef]
- Barthe, M.F. Positron annihilation characteristics in UO2: For lattice and vacancy defects induced by electron irradiation. Phys. Status Solidi 2007, 4, 3627–3632. [Google Scholar] [CrossRef]
- Miao, Y. Nano-crystallization induced by high-energy heavy ion irradiation in UO2. Scr. Mater. 2018, 155, 169–174. [Google Scholar] [CrossRef]
- Miao, Y. Microstructure investigations of U3Si2 implanted by high-energy Xe ions at 600 °C. J. Nucl. Mater. 2018, 503, 314–322. [Google Scholar] [CrossRef]
- Jiang, M. An AIMD+U simulation of low-energy displacement events in UO2. J. Nucl. Mater. 2020, 540, 152379. [Google Scholar] [CrossRef]
- Xiao, H.Y. Ab initio molecular dynamics simulations of low-energy recoil events in ThO2, CeO2, and ZrO2. Phys. Rev. B 2012, 86, 054109. [Google Scholar] [CrossRef]
- Itoh, N. Material modification by electronic excitation. Radiat. Eff. Defects Solids 2006, 146, 1–10. [Google Scholar] [CrossRef]
- Zhao, S. Ab Initio Study of Electronic Excitation Effects on SrTiO3. J. Phys. Chem. C 2017, 121, 26622–26628. [Google Scholar] [CrossRef]
- Ullah, A. Continuous Stiffness Measurements and Nanoindentation Studies of bcc Mo: An Atomistic Approach. Indian Inst. Met. 2022, 75, 1555–1561. [Google Scholar] [CrossRef]
- Li, S.M. Contribution of F-Electron Excitation to Electronic Stopping Power of Platinum for Protons. Phys. Rev. B 2022, 106, 014103. [Google Scholar] [CrossRef]
- Li, X.B. Role of electronic excitation in the amorphization of Ge-Sb-Te alloys. Phys. Rev. Lett. 2011, 107, 015501. [Google Scholar] [CrossRef]
- Kabler, M.N. Vacancy-interstitial pair production via electron-hole recombination in halide crystals. Phys. Rev. B 1978, 18, 4. [Google Scholar] [CrossRef]
- Jiang, N. Electron beam damage in oxides: A review. Rep. Prog. Phys. 2016, 79, 016501. [Google Scholar] [CrossRef]
- Hobbs, L.W. The role of topology and geometry in the irradiation-induced amorphization of network structures. J. Non-Cryst. Solids 1995, 182, 27–39. [Google Scholar] [CrossRef]
- Xiao, H.Y. Electronic excitation induced amorphization in titanate pyrochlores: An ab initio molecular dynamics study. Sci. Rep. 2015, 5, 8265. [Google Scholar] [CrossRef]
- Kohn, W. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Dudarev, S.L. Electron-Energy-Loss Spectra and the Structural Stability of Nickel Oxide: An Lsda+U Study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Kresse, G. Ab Initio Molecular Dynamics for Liquid Metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.Y. Structural behavior of uranium dioxide under pressure by LSDA+Ucalculations. Phys. Rev. B 2007, 75, 054111. [Google Scholar] [CrossRef]
- Noordhoek, M.J. Phase equilibria in the U-Si system from first-principles calculations. J. Nucl. Mater. 2016, 479, 216–223. [Google Scholar] [CrossRef]
- Kresse, G. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Ceperley, D.M. Ground State of the Electron Gas by a Stochastic Method. Phys. Rev. Lett. 1980, 45, 566–569. [Google Scholar] [CrossRef]
- Blöchl, P.E. Improved Tetrahedron Method for Brillouin-Zone Integrations. Phys. Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef]
- Feng, S. The effects of Xe/Cs occupation on the thermal transport properties of U3Si: A first-principles study. J. Nucl. Mater. 2023, 586, 154657. [Google Scholar] [CrossRef]
- Dudarev, S.L. Electronic Structure and Elastic Properties of Strongly Correlated Metal Oxides from First Principles: LSDA + U, SiC-LSDA and Eels Study of UO2 and NiO. Phys. Status Solidi 1998, 166, 429–443. [Google Scholar] [CrossRef]
- Sokolowski, K. Ultrafast laser-induced order-disorder transitions in semiconductors. Phys. Rev. B 1995, 51, 14186. [Google Scholar] [CrossRef]
- Chen, Q.Y. First-principles study of the electronic structure and optical properties of UO2. J. Nucl. Mater. 2010, 401, 118–123. [Google Scholar] [CrossRef]
- Chen, Y. Multi-layered cement-hydrogel composite with high toughness, low thermal conductivity, and self-healing capability. Nat. Commun. 2023, 14, 3438. [Google Scholar] [CrossRef] [PubMed]
- Sha, W. Electroless Copper and Nickel–Phosphorus Plating; Woodhead Publishing: Cambridge, UK, 2011; pp. 82–103. [Google Scholar]
- Weber, W.J. Radiation Effects in Crystalline Ceramics for the Immobilization of High-Level Nuclear Waste and Plutonium. J. Mater. Res. 1998, 13, 1434. [Google Scholar] [CrossRef]
- Gupta, A. Laser Irradiation Induced Atomic Structure Modifications in Strontium Titanate. JOM 2022, 74, 143. [Google Scholar] [CrossRef]
- Takeda, S. Amorphization in Silicon by Electron Irradiation. Phys. Rev. Lett. 1999, 83, 320. [Google Scholar] [CrossRef]
- Konishi, M. Ultrafast amorphization in Ge10Sb2Te13 thin film induced by single femtosecond laser pulse. Appl. Opt. 2010, 49, 3470. [Google Scholar] [CrossRef]
- Ugajin, M. Behavior of Neutron-Irradiated U3Si. J. Nucl. Mater. 1997, 248, 204–208. [Google Scholar] [CrossRef]
- Mafi, E. Electronically Driven Amorphization in Phase-Change In2Se3 Nanowires. J. Phys. Chem. C 2012, 116, 22539–22544. [Google Scholar] [CrossRef]
- Killeen, J.C. The Effect of Niobium Oxide Additions on the Electrical Conductivity of UO2. J. Nucl. Mater. 1980, 88, 185–192. [Google Scholar] [CrossRef]
- Luo, Y. Electron work function: An indicative parameter towards a novel material design methodology. Sci. Rep. 2021, 11, 11565. [Google Scholar] [CrossRef] [PubMed]
- Michaelson, H.B. The work function of the elements and its periodicity. J. Appl. Phys. 1977, 48, 4729–4733. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, R.; Zhao, S.; Xiao, H. Ab Initio Molecular Dynamics Study of Electron Excitation Effects on UO2 and U3Si. Materials 2023, 16, 6911. https://doi.org/10.3390/ma16216911
Jin R, Zhao S, Xiao H. Ab Initio Molecular Dynamics Study of Electron Excitation Effects on UO2 and U3Si. Materials. 2023; 16(21):6911. https://doi.org/10.3390/ma16216911
Chicago/Turabian StyleJin, Ruoyan, Siqin Zhao, and Haiyan Xiao. 2023. "Ab Initio Molecular Dynamics Study of Electron Excitation Effects on UO2 and U3Si" Materials 16, no. 21: 6911. https://doi.org/10.3390/ma16216911



