Origin of Li+ Solvation Ability of Electrolyte Solvent: Ring Strain
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
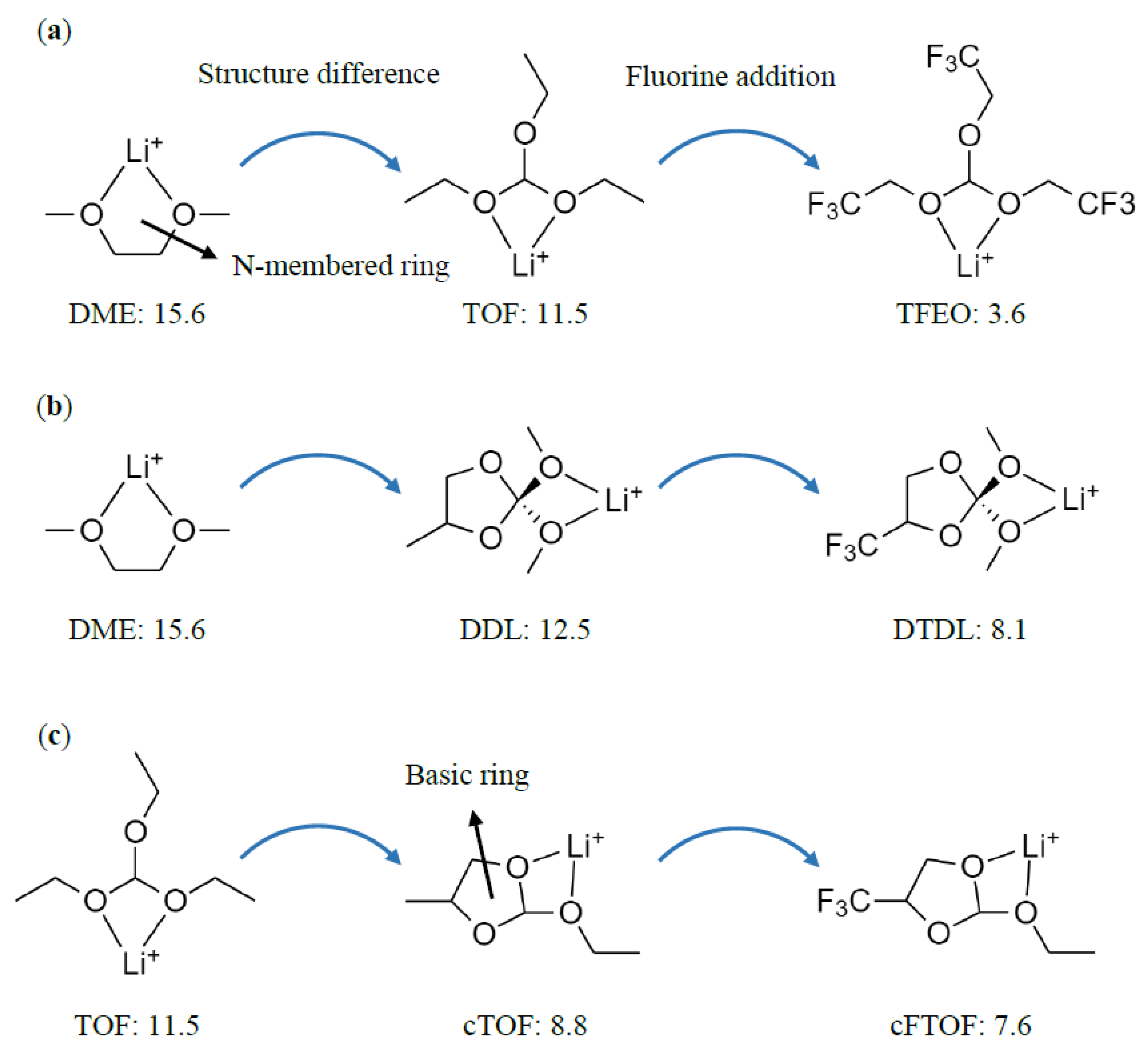
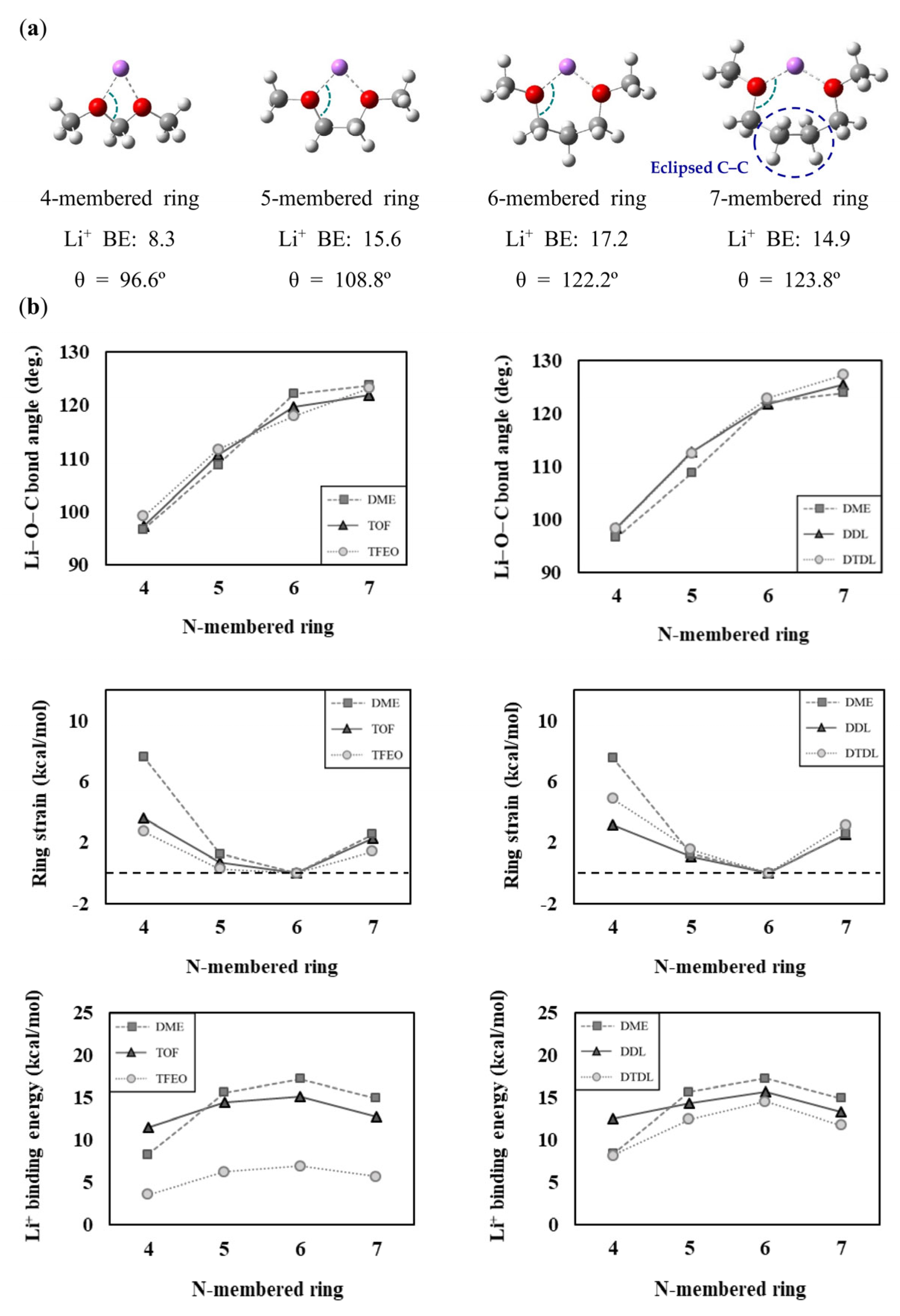
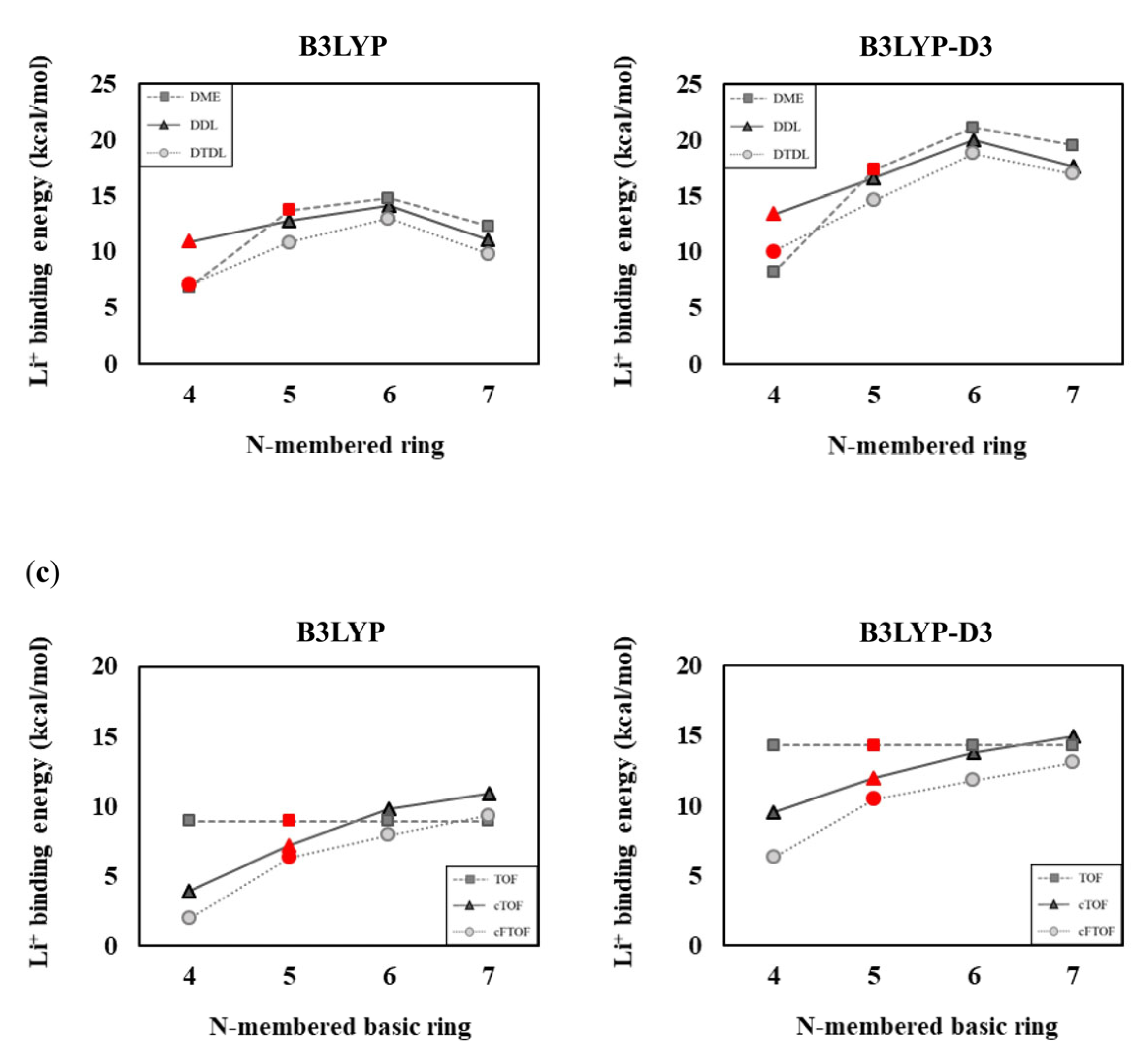
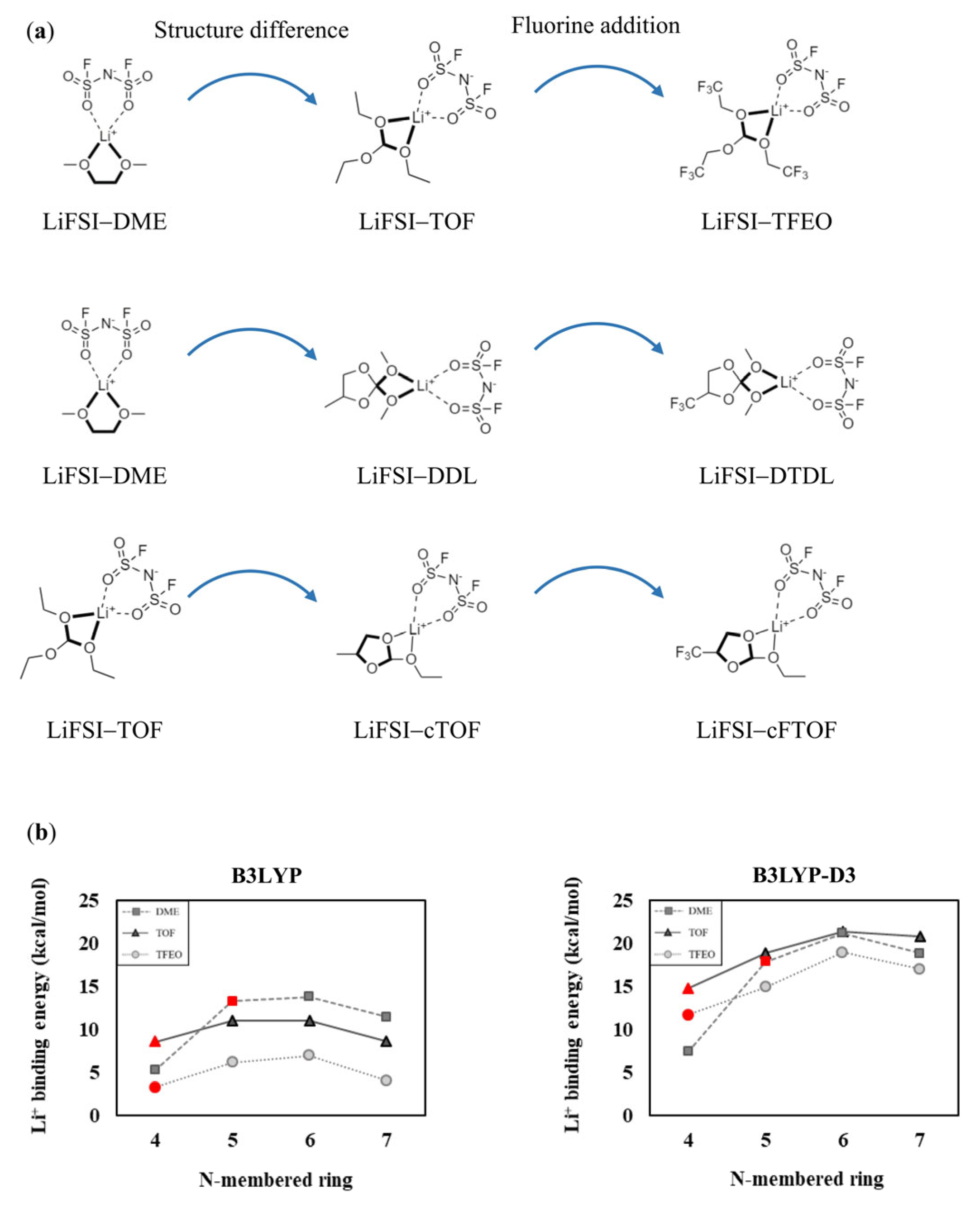
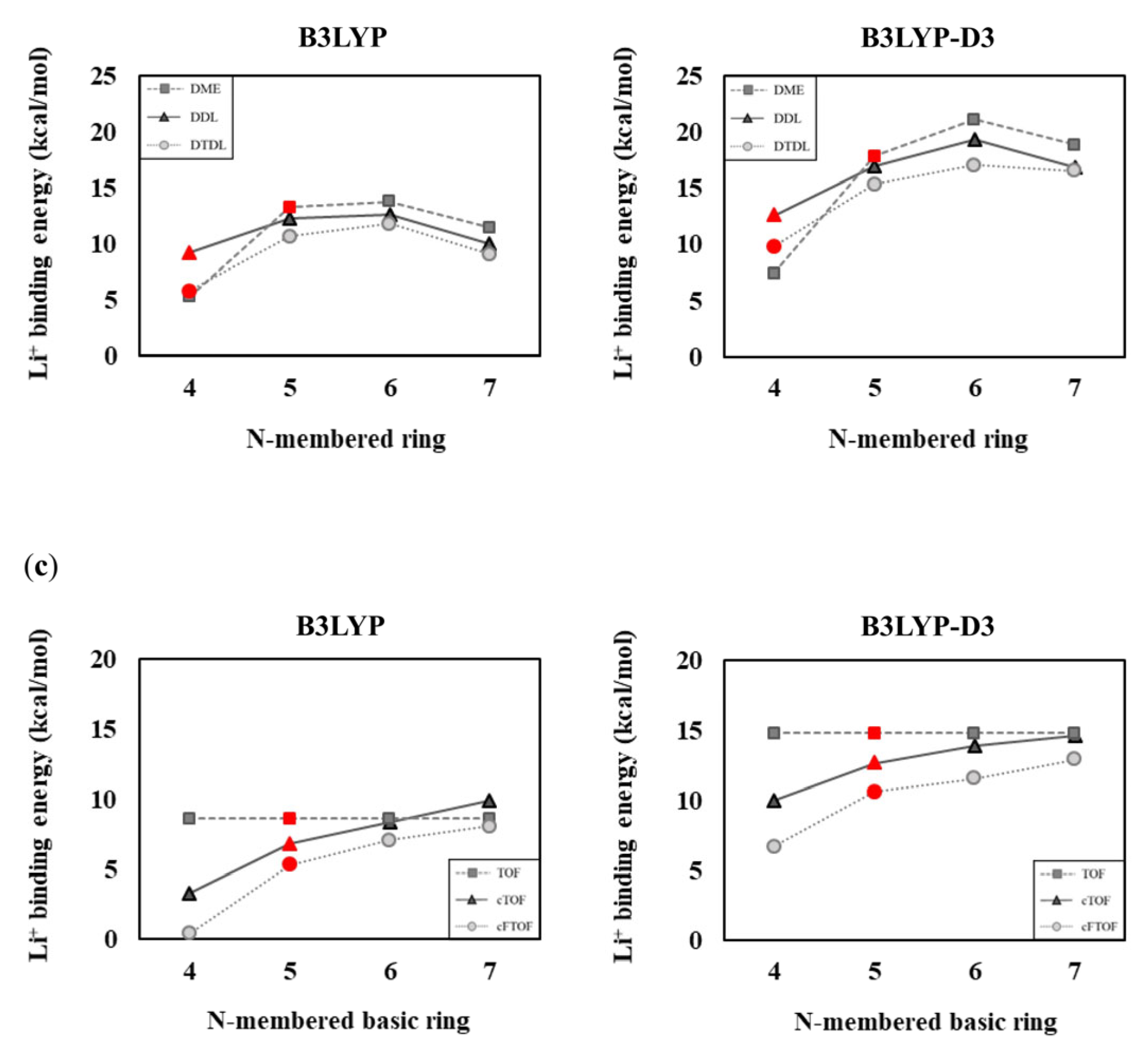
3.1. Structural Effects of N-Membered Rings on Solvation Ability
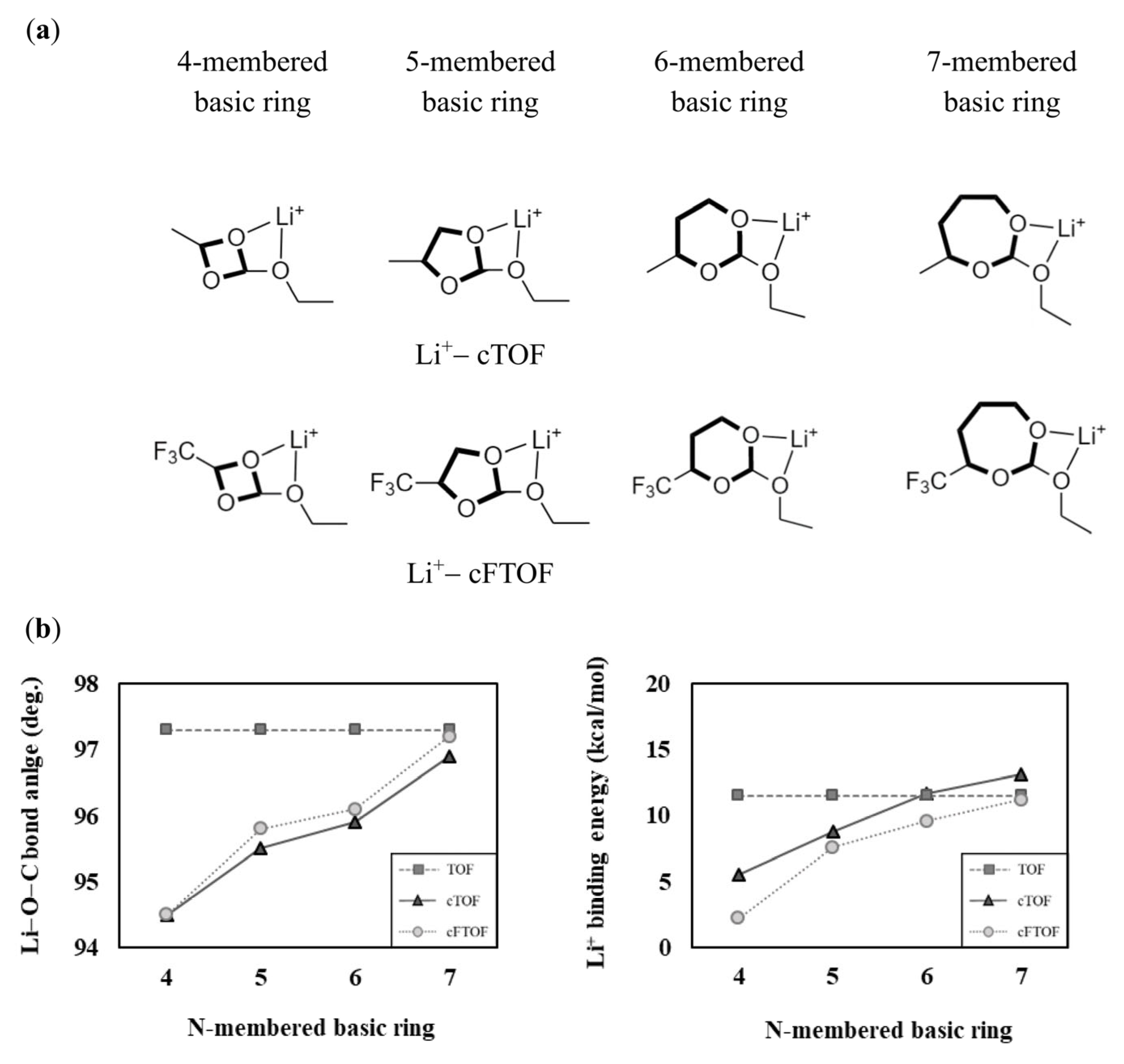
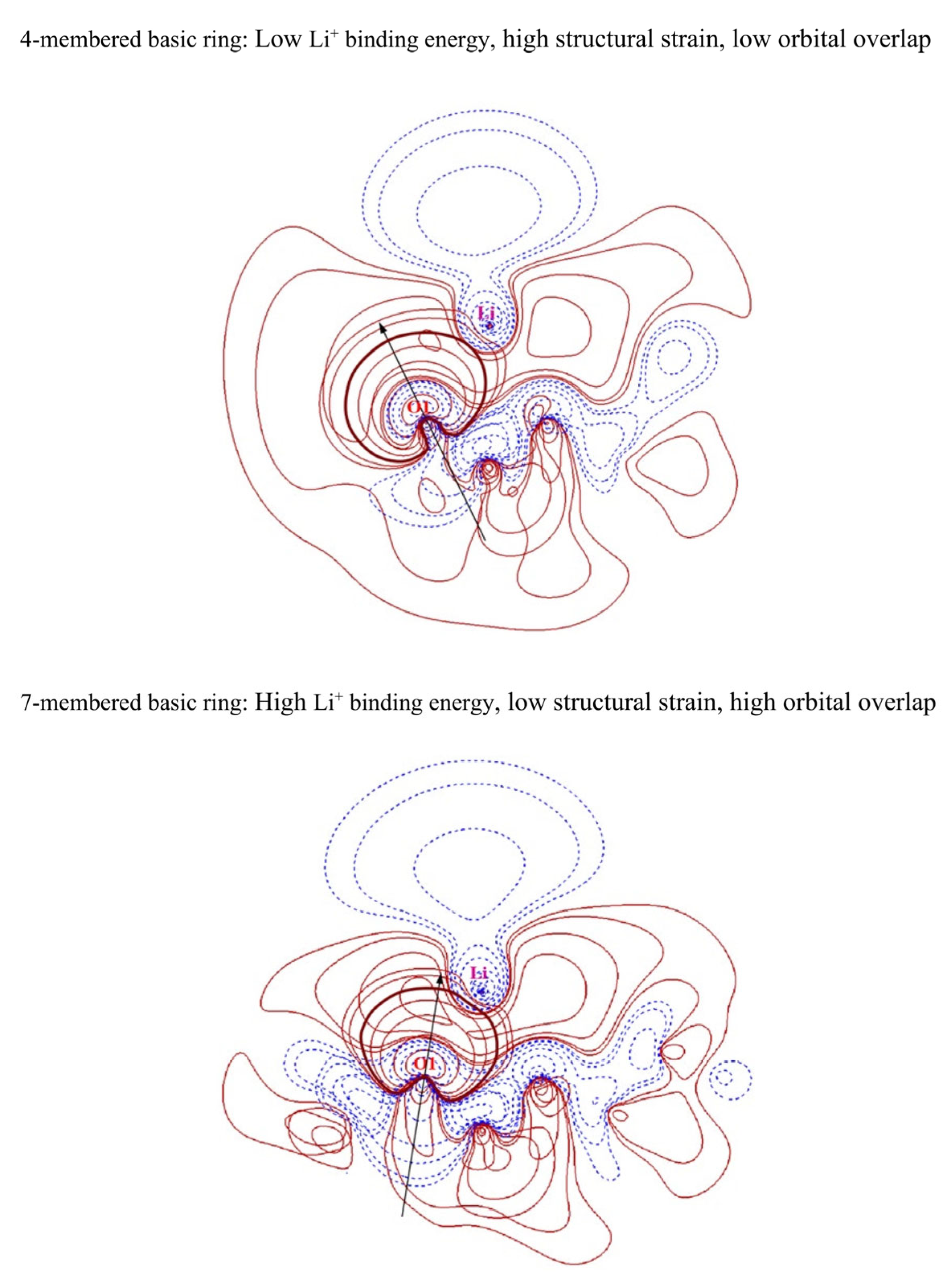
3.2. Structural Effects of Basic Rings on Solvation Ability
3.3. DFT Functional Dependence and Consideration of Solvent−Solvent and Solvent−Salt Interactions within the Solvation Sheath
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niu, C.; Lee, H.; Chen, S.; Li, Q.; Du, J.; Xu, W.; Zhang, J.G.; Whittingham, M.S.; Xiao, J.; Liu, J. High-energy lithium metal pouch cells with limited anode swelling and long stable cycles. Nat. Energy 2019, 4, 551–559. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, T.K.; Kim, S.; Lee, J.; Ahn, Y.; Kim, K.; Ma, H.; Park, G.; Lee, S.M.; Kwak, S.K.; et al. Fluorine-incorporated interface enhances cycling stability of lithium metal batteries with Ni-rich NCM cathodes. Nano Energy 2020, 67, 104309. [Google Scholar] [CrossRef]
- Kim, K.J.; Leem, H.J.; Yu, J.; Kim, H. Spontaneous Lithiophilic and Lithium-Ion Conductive Functional Layer Formation Enabled by Solution-Casted Zinc Nitride for Highly Stable Lithium Metal Electrode in Carbonate Electrolyte. Int. J. Energy Res. 2023, 2023, 9526791. [Google Scholar] [CrossRef]
- Yan, C.; Cheng, X.B.; Tian, Y.; Chen, X.; Zhang, X.Q.; Li, W.J.; Huang, J.Q.; Zhang, Q. Dual-Layered Film Protected Lithium Metal Anode to Enable Dendrite-Free Lithium Deposition. Adv. Mater. 2018, 30, 1707629. [Google Scholar] [CrossRef]
- Zhang, J.G.; Xu, W.; Xiao, J.; Cao, X.; Liu, J. Lithium Metal Anodes with Nonaqueous Electrolytes. Chem. Rev. 2020, 120, 13312–13348. [Google Scholar] [CrossRef]
- Tan, J.; Ryan, E.M. Structured electrolytes to suppress dendrite growth in high energy density batteries. Int. J. Energy Res. 2016, 40, 1800–1810. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, L.; Xie, B.; Zhang, S.; Jiang, Z.; Xu, G.; Li, J.; Cui, G. Deciphering the Thermal Failure Mechanism of Anode-Free Lithium Metal Pouch Batteries. Adv. Energy Mater. 2023, 13, 2203648. [Google Scholar] [CrossRef]
- Jiang, F.N.; Cheng, X.B.; Yang, S.J.; Xie, J.; Yuan, H.; Liu, L.; Huang, J.Q.; Zhang, Q. Thermoresponsive Electrolytes for Safe Lithium-Metal Batteries. Adv. Mater. 2023, 35, 2209114. [Google Scholar] [CrossRef]
- Huang, L.; Lu, T.; Xu, G.; Zhang, X.; Jiang, Z.; Zhang, Z.; Wang, Y.; Han, P.; Cui, G.; Chen, L. Thermal runaway routes of large-format lithium-sulfur pouch cell batteries. Joule 2022, 6, 906–922. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Bai, P.; Li, J.; Brushett, F.R.; Bazant, M.Z. Transition of lithium growth mechanisms in liquid electrolytes. Energy Environ. Sci. 2016, 9, 3221–3229. [Google Scholar] [CrossRef]
- Worku, B.E.; Zheng, S.; Wang, B. Review of low-temperature lithium-ion battery progress: New battery system design imperative. Int. J. Energy Res. 2022, 46, 14609–14626. [Google Scholar] [CrossRef]
- Tran, N.; Ta, Q.T.H.; Tran, V.V. Review on the application of green and environmentally benign biowaste and natural substances in the synthesis of lithium-ion batteries. Int. J. Energy Res. 2022, 46, 13251–13275. [Google Scholar] [CrossRef]
- Li, G.; Gao, Y.; He, X.; Huang, Q.; Chen, S.; Kim, S.H.; Wang, D. Organosulfide-plasticized solid-electrolyte interphase layer enables stable lithium metal anodes for long-cycle lithium-sulfur batteries. Nat. Commun. 2017, 8, 850. [Google Scholar] [CrossRef]
- Zheng, J.; Engelhard, M.H.; Mei, D.; Jiao, S.; Polzin, B.J.; Zhang, J.G.; Xu, W. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy 2017, 2, 17012. [Google Scholar] [CrossRef]
- Qian, J.; Henderson, W.A.; Xu, W.; Bhattacharya, P.; Engelhard, M.; Borodin, O.; Zhang, J.G. High rate and stable cycling of lithium metal anode. Nat. Commun. 2015, 6, 6362. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Guo, F.; Li, H.; Cao, Y.; Ai, X.; Qian, J.; Li, Y.; Yang, H. Suppression of Dendritic Lithium Growth by in Situ Formation of a Chemically Stable and Mechanically Strong Solid Electrolyte Interphase. ACS Appl. Mater. Interfaces 2018, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Markevich, E.; Salitra, G.; Chesneau, F.; Schmidt, M.; Aurbach, D. Very Stable Lithium Metal Stripping-Plating at a High Rate and High Areal Capacity in Fluoroethylene Carbonate-Based Organic Electrolyte Solution. ACS Energy Lett. 2017, 2, 1321–1326. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Cheng, X.B.; Chen, X.; Yan, C.; Zhang, Q. Fluoroethylene Carbonate Additives to Render Uniform Li Deposits in Lithium Metal Batteries. Adv. Funct. Mater. 2017, 27, 1605989. [Google Scholar] [CrossRef]
- Xia, L.; Miao, H.; Wang, F.; Zhang, C.; Wang, J.; Zhao, J.; Yuan, J. Investigation of fluorinated ether-containing electrolytes for high energy-density nickel-rich LiNi0.8Co0.1Mn0.1O2 electrodes. Int. J. Energy Res. 2021, 45, 9936–9947. [Google Scholar] [CrossRef]
- Miao, R.; Yang, J.; Xu, Z.; Wang, J.; Nuli, Y.; Sun, L. A new ether-based electrolyte for dendrite-free lithium-metal based rechargeable batteries. Sci. Rep. 2016, 6, 21771. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, T.; Ashirov, T.; El Kazzi, M.; Cancellieri, C.; Jeurgens, L.P.H.; Choi, J.W.; Coskun, A. Fluorinated ether electrolyte with controlled solvation structure for high voltage lithium metal batteries. Nat. Commun. 2022, 13, 2575. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Xu, W.; Chen, X.; Zhang, J.; Engelhard, M.H.; Zhang, Y.; Johnson, B.R.; Crum, J.V.; Blake, T.A.; Liu, X.; et al. Effects of Carbonate Solvents and Lithium Salts on Morphology and Coulombic Efficiency of Lithium Electrode. J. Electrochem. Soc. 2013, 160, A1894–A1901. [Google Scholar] [CrossRef]
- Leung, K.; Soto, F.; Hankins, K.; Balbuena, P.B.; Harrison, K.L. Stability of Solid Electrolyte Interphase Components on Lithium Metal and Reactive Anode Material Surfaces. J. Phys. Chem. C 2016, 120, 6302–6313. [Google Scholar] [CrossRef]
- Yoo, D.J.; Yang, S.; Kim, K.J.; Choi, J.W. Fluorinated Aromatic Diluent for High-Performance Lithium Metal Batteries. Angew. Chem. Int. Ed. 2020, 59, 14869–14876. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, W.; Li, Y.; Pei, A.; Boyle, D.T.; Cui, Y. Correlating Structure and Function of Battery Interphases at Atomic Resolution Using Cryoelectron Microscopy. Joule 2018, 2, 2167–2177. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef]
- Aurbach, D. Review of selected electrode-solution interactions which determine the performance of Li and Li ion batteries. J. Power Sources 2000, 89, 206–218. [Google Scholar] [CrossRef]
- Amanchukwu, C.V.; Yu, Z.; Kong, X.; Qin, J.; Cui, Y.; Bao, Z. A New Class of Ionically Conducting Fluorinated Ether Electrolytes with High Electrochemical Stability. J. Am. Chem. Soc. 2020, 142, 7393–7403. [Google Scholar] [CrossRef]
- Jiao, S.; Ren, X.; Cao, R.; Engelhard, M.H.; Liu, Y.; Hu, D.; Mei, D.; Zheng, J.; Zhao, W.; Li, Q.; et al. Stable cycling of high-voltage lithium metal batteries in ether electrolytes. Nat. Energy 2018, 3, 739–746. [Google Scholar] [CrossRef]
- Yoshida, K.; Nakamura, M.; Kazue, Y.; Tachikawa, N.; Tsuzuki, S.; Seki, S.; Dokko, K.; Watanabe, M. Oxidative-stability enhancement and charge transport mechanism in glyme-lithium salt equimolar complexes. J. Am. Chem. Soc. 2011, 133, 13121–13129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhao, Y.; El Kazzi, M.; Choi, J.W.; Coskun, A. Integrated Ring-Chain Design of a New Fluorinated Ether Solvent for High-Voltage Lithium-Metal Batteries. Angew. Chem. Int. Ed. 2022, 61, e202115884. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, H.; Kong, X.; Huang, W.; Tsao, Y.; Mackanic, D.G.; Wang, K.; Wang, X.; Huang, W.; Choudhury, S.; et al. Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries. Nat. Energy 2020, 5, 526–533. [Google Scholar] [CrossRef]
- Wang, H.; Yu, Z.; Kong, X.; Huang, W.; Zhang, Z.; Mackanic, D.G.; Huang, X.; Qin, J.; Bao, Z.; Cui, Y. Dual-Solvent Li-Ion Solvation Enables High-Performance Li-Metal Batteries. Adv. Mater. 2021, 33, 2008619. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qi, F.; Yin, L.; Shi, Y.; Sun, C.; An, B.; Cheng, H.M.; Li, F. An Anion-Tuned Solid Electrolyte Interphase with Fast Ion Transfer Kinetics for Stable Lithium Anodes. Adv. Energy Mater. 2020, 10, 1903843. [Google Scholar] [CrossRef]
- Zhu, Z.; Tang, Y.; Lv, Z.; Wei, J.; Zhang, Y.; Wang, R.; Zhang, W.; Xia, H.; Ge, M.; Chen, X. Fluoroethylene Carbonate Enabling a Robust LiF-rich Solid Electrolyte Interphase to Enhance the Stability of the MoS2 Anode for Lithium-Ion Storage. Angew. Chem. 2018, 130, 3718–3722. [Google Scholar] [CrossRef]
- Cao, X.; Ren, X.; Zou, L.; Engelhard, M.H.; Huang, W.; Wang, H.; Matthews, B.E.; Lee, H.; Niu, C.; Arey, B.W.; et al. Monolithic solid–electrolyte interphases formed in fluorinated orthoformate-based electrolytes minimize Li depletion and pulverization. Nat. Energy 2019, 4, 796–805. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Chen, X.; Hou, L.P.; Li, B.Q.; Cheng, X.B.; Huang, J.Q.; Zhang, Q. Regulating Anions in the Solvation Sheath of Lithium Ions for Stable Lithium Metal Batteries. ACS Energy Lett. 2019, 4, 411–416. [Google Scholar] [CrossRef]
- Abe, T.; Fukuda, H.; Iriyama, Y.; Ogumi, Z. Solvated Li-Ion Transfer at Interface Between Graphite and Electrolyte. J. Electrochem. Soc. 2004, 151, A1120. [Google Scholar] [CrossRef]
- Xu, K.; Lam, Y.; Zhang, S.S.; Jow, T.R.; Curtis, T.B. Solvation sheath of Li+ in nonaqueous electrolytes and its implication of graphite/electrolyte interface chemistry. J. Phys. Chem. C. 2007, 111, 7411–7421. [Google Scholar] [CrossRef]
- Meng, J.; Chu, F.; Hu, J.; Li, C. Liquid Polydimethylsiloxane Grafting to Enable Dendrite-Free Li Plating for Highly Reversible Li-Metal Batteries. Adv. Funct. Mater. 2019, 29, 1902220. [Google Scholar] [CrossRef]
- Hu, J.; Chen, K.; Li, C. Nanostructured Li-Rich Fluoride Coated by Ionic Liquid as High Ion-Conductivity Solid Electrolyte Additive to Suppress Dendrite Growth at Li Metal Anode. ACS Appl. Mater. Interfaces 2018, 10, 34322–34331. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Wang, R.L.; Buhrmester, C.; Dahn, J.R. Calculations of Oxidation Potentials of Redox Shuttle Additives for Li-Ion Cells. J. Electrochem. Soc. 2006, 153, A445. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, L.; Li, W.; Bedrov, D. Why do sulfone-based electrolytes show stability at high voltages? insight from density functional theory. J. Phys. Chem. Lett. 2013, 4, 3992–3999. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Parr, R.G.; Weitao, Y. Density-Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, MI, USA, 1994. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Abbotto, A.; Streitwieser, A.; Schleyer, P.V.R. Ab initio and semiempirical study of the effect of ethereal solvent on aggregation of a lithium enolate. J. Am. Chem. Soc. 1997, 119, 11255–11268. [Google Scholar] [CrossRef]
- Wang, Y.; Balbuena, P.B. Associations of alkyl carbonates: Intermolecular C−H⋯O interactions. J. Phys. Chem. A. 2001, 105, 9972–9982. [Google Scholar] [CrossRef]
- Leggesse, E.G.; Lin, R.T.; Teng, T.F.; Chen, C.L.; Jiang, J.C. Oxidative decomposition of propylene carbonate in lithium ion batteries: A DFT study. J. Phys. Chem. A. 2013, 117, 7959–7969. [Google Scholar] [CrossRef]
- Shakourian-Fard, M.; Kamath, G.; Taimoory, S.M.; Trant, J.F. Calcium-Ion Batteries: Identifying Ideal Electrolytes for Next-Generation Energy Storage Using Computational Analysis. J. Phys. Chem. C. 2019, 123, 15885–15896. [Google Scholar] [CrossRef]
- Shakourian-Fard, M.; Kamath, G.; Smith, K.; Xiong, H.; Sankaranarayanan, S.K.R.S. Trends in Na-Ion Solvation with Alkyl-Carbonate Electrolytes for Sodium-Ion Batteries: Insights from First-Principles Calculations. J. Phys. Chem. C 2015, 119, 22747–22759. [Google Scholar] [CrossRef]
- Shakourian-Fard, M.; Kamath, G.; Sankaranarayanan, S.K.R.S. Electronic Structure Insights into the Solvation of Magnesium Ions with Cyclic and Acyclic Carbonates. ChemPhysChem 2015, 16, 3607–3617. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to Isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Cheviri, M.; Lakshmipathi, S. DFT study of chemical reactivity parameters of lithium polysulfide molecules Li2Sn(1≤n≤8) in gas and solvent phase. Comput. Theor. Chem. 2021, 1202, 113323. [Google Scholar] [CrossRef]
- Du, G.Y.; Liu, C.Y.; Li, E.Y. A DFT Investigation on the Origins of Solvent-Dependent Polysulfide Reduction Mechanism in Rechargeable Li-S Batteries. Catalysts 2020, 10, 911. [Google Scholar] [CrossRef]
- Kamphaus, E.P.; Hight, K.; Dermott, M.; Balbuena, P.B. Model systems for screening and investigation of lithium metal electrode chemistry and dendrite formation. Phys. Chem. Chem. Phys. 2020, 22, 575–588. [Google Scholar] [CrossRef]
- Kamphaus, E.P.; Balbuena, P.B. Polysulfide reduction and Li2S phase formation in the presence of lithium metal and solid electrolyte interphase layer. J. Power Sources 2021, 485, 229289. [Google Scholar] [CrossRef]
- Han, J.; Balbuena, P.B. First-principles calculations of oxidation potentials of electrolytes in lithium-sulfur batteries and their variations with changes in environment. Phys. Chem. Chem. Phys. 2018, 20, 18811–18827. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from a Natural Bond Orbital, Donor—Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F. Natural localized molecular orbitals. J. Chem. Phys. 1985, 83, 1736–1740. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Pollard, T.P.; Li, Q.; Tan, S.; Hou, S.; Wan, H.; Chen, F.; He, E.; Hu, K.; et al. Electrolyte design for Li-ion batteries under extreme operating conditions. Nature 2023, 614, 694–700. [Google Scholar] [CrossRef]
- Peng, C.; Ning, G.H.; Su, J.; Zhong, G.; Tang, W.; Tian, B.; Su, C.; Yu, D.; Zu, L.; Yang, J.; et al. Reversible multi-electron redox chemistry of π-conjugated N-containing heteroaromatic molecule-based organic cathodes. Nat. Energy 2017, 2, 17074. [Google Scholar] [CrossRef]
- Wen, B.; Deng, Z.; Tsai, P.C.; Lebens-Higgins, Z.W.; Piper, L.F.J.; Ong, S.P.; Chiang, Y.M. Ultrafast ion transport at a cathode–electrolyte interface and its strong dependence on salt solvation. Nat. Energy 2020, 5, 578–586. [Google Scholar] [CrossRef]
- Holoubek, J.; Liu, H.; Wu, Z.; Yin, Y.; Xing, X.; Cai, G.; Yu, S.; Zhou, H.; Pascal, T.A.; Chen, Z.; et al. Tailoring electrolyte solvation for Li metal batteries cycled at ultra-low temperature. Nat. Energy 2021, 6, 303–313. [Google Scholar] [CrossRef]
- Kim, M.S.; Zhang, Z.; Rudnicki, P.E.; Yu, Z.; Wang, J.; Wang, H.; Oyakhire, S.T.; Chen, Y.; Kim, S.C.; Zhang, W.; et al. Suspension electrolyte with modified Li+ solvation environment for lithium metal batteries. Nat. Mater. 2022, 21, 445–454. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys. 2006, 125, 194101. [Google Scholar] [CrossRef] [PubMed]
- Hlil, A.R.; Balogh, J.; Moncho, S.; Su, H.L.; Tuba, R.; Brothers, E.N.; Al-Hashimi, M.; Bazzi, H.S. Ring opening metathesis polymerization (ROMP) of five- to eight-membered cyclic olefins: Computational, thermodynamic, and experimental approach. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3137–3145. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Ring strain energies from ab initio calculations. J. Am. Chem. Soc. 1998, 120, 4450–4458. [Google Scholar] [CrossRef]
- Das, U.; Zhang, G.; Hu, B.; Hock, A.S.; Redfern, P.C.; Miller, J.T.; Curtiss, L.A. Effect of Siloxane Ring Strain and Cation Charge Density on the Formation of Coordinately Unsaturated Metal Sites on Silica: Insights from Density Functional Theory (DFT) Studies. ACS Catal. 2015, 5, 7177–7185. [Google Scholar] [CrossRef]
- Hanson, P.; Hendrickx, R.A.A.J.; Lindsay Smith, J.R.; Miller, J.T. A kinetic investigation, supported by theoretical calculations, of steric and ring strain effects on the oxidation of sulfides and sulfoxides by dimethyldioxirane in acetone. New J. Chem. 2010, 34, 65–84. [Google Scholar] [CrossRef]
- Bauzá, A.; Quiñonero, D.; Deyà, P.M.; Frontera, A. Estimating ring strain energies in small carbocycles by means of the Bader’s theory of ‘atoms-in-molecules’. Chem. Phys. Lett. 2012, 536, 165–169. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Yang, S.; Xue, X.S.; Wu, X.; Zhu, C. Radical-mediated C−C cleavage of unstrained cycloketones and DFT study for unusual regioselectivity. Nat. Commun. 2020, 11, 672. [Google Scholar] [CrossRef]
- Kimura, A.; Kawauchi, S.; Yamamoto, T.; Tezuka, Y. SN2 regioselectivity in the esterification of 5- and 7-membered azacycloalkane quaternary salts: A DFT study to reveal the transition state ring conformation prevailing over the ground state ring strain. Org. Biomol. Chem. 2014, 12, 6717–6724. [Google Scholar] [CrossRef]
- Yasarawan, N.; Thipyapong, K.; Ruangpornvisuti, V. Substituent effect on the proton-related phenomena and chelation behavior of hydroxypicolinic compounds: A DFT investigation. Struct. Chem. 2016, 27, 505–524. [Google Scholar] [CrossRef]
- Callsen, M.; Sodeyama, K.; Futera, Z.; Tateyama, Y.; Hamada, I. The solvation structure of lithium ions in an ether-based electrolyte solution from first-principles molecular dynamics. J. Phys. Chem. B. 2017, 121, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Wu, P.; Cai, C. Mechanism of Methanol Decomposition on the Pt3Ni(111) Surface: DFT Study. J. Phys. Chem. C. 2017, 121, 9348–9360. [Google Scholar] [CrossRef]
- Nguyen, L.H.B.; Picard, T.; Sergent, N.; Raynaud, C.; Filhol, J.S.; Doublet, M.L. Investigation of alkali and alkaline earth solvation structures in tetraglyme solvent. Phys. Chem. Chem. Phys. 2021, 23, 26120–26129. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; He, M.; Amine, R.; Chen, Z.; Yu, Z.; Rojas, T.; Cheng, A.T.; Ngo, K.; Amine, K. Unveiling decaying mechanism through quantitative structure-activity relationship in electrolytes for lithium-ion batteries. Nano Energy 2021, 83, 105843. [Google Scholar] [CrossRef]
- Bertolini, S.; Jacob, T. Density Functional Theory Studies on Sulfur-Polyacrylonitrile as a Cathode Host Material for Lithium-Sulfur Batteries. ACS Omega 2021, 6, 9700–9708. [Google Scholar] [CrossRef]
- Shi, H.; Wang, F.; Chen, W.; Tang, S.; Zhang, W.; Li, W.; Sun, H.; Zhang, J.; Wang, R. Theoretical design and simulation of supramolecular polymer unit based on multiple hydrogen bonds. J. Mol. Graph. Model. 2015, 59, 31–39. [Google Scholar] [CrossRef]
- Pham, T.A.; Kweon, K.E.; Samanta, A.; Lordi, V.; Pask, J.E. Solvation and Dynamics of Sodium and Potassium in Ethylene Carbonate from ab Initio Molecular Dynamics Simulations. J. Phys. Chem. C 2017, 121, 21913–21920. [Google Scholar] [CrossRef]
- Shakourian-Fard, M.; Kamath, G.; Sankaranarayanan, S.K.R.S. Evaluating the Free Energies of Solvation and Electronic Structures of Lithium-Ion Battery Electrolytes. ChemPhysChem 2016, 17, 2916–2930. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C.R. Discovering Chemistry with Natural Bond Orbitals; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Zhao, Y.; Zhou, T.; Jeurgens, L.P.H.; Kong, X.; Choi, J.W.; Coskun, A. Electrolyte engineering for highly inorganic solid electrolyte interphase in high-performance lithium metal batteries. Chem 2023, 9, 682–697. [Google Scholar] [CrossRef]
- Ma, T.; Ni, Y.; Wang, Q.; Zhang, W.; Jin, S.; Zheng, S.; Yang, X.; Hou, Y.; Tao, Z.; Chen, J. Optimize Lithium Deposition at Low Temperature by Weakly Solvating Power Solvent. Angew. Chem. Int. Ed. 2022, 61, e202207927. [Google Scholar] [CrossRef]
- Ding, K.; Xu, C.; Peng, Z.; Long, X.; Shi, J.; Li, Z.; Zhang, Y.; Lai, J.; Chen, L.; Cai, Y.P.; et al. Tuning the Solvent Alkyl Chain to Tailor Electrolyte Solvation for Stable Li-Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 44470–44478. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fan, Z.; Cui, Z.; Tan, L.; Ruan, D.; Zhao, X.; Jiang, J.; Jiao, S.; Ren, X. Unveiling the Critical Role of Ion Coordination Configuration of Ether Electrolytes for High Voltage Lithium Metal Batteries. Angew. Chem. Int. Ed. 2023, 62, e202219310. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Shin, K.-H.; Han, Y.-K. Origin of Li+ Solvation Ability of Electrolyte Solvent: Ring Strain. Materials 2023, 16, 6995. https://doi.org/10.3390/ma16216995
Choi J, Shin K-H, Han Y-K. Origin of Li+ Solvation Ability of Electrolyte Solvent: Ring Strain. Materials. 2023; 16(21):6995. https://doi.org/10.3390/ma16216995
Chicago/Turabian StyleChoi, Jihoon, Kyoung-Hee Shin, and Young-Kyu Han. 2023. "Origin of Li+ Solvation Ability of Electrolyte Solvent: Ring Strain" Materials 16, no. 21: 6995. https://doi.org/10.3390/ma16216995






