Mechanical Properties and Hydration Degree of Magnesium Potassium Phosphate Cement Modified by Sintered Silt Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Treatment Process of Dredged Silt
2.3. Specimen Preparation
2.4. Experiment Methods
2.4.1. Compressive Strength
2.4.2. Setting Time
2.4.3. XRD
2.4.4. SEM and EDS
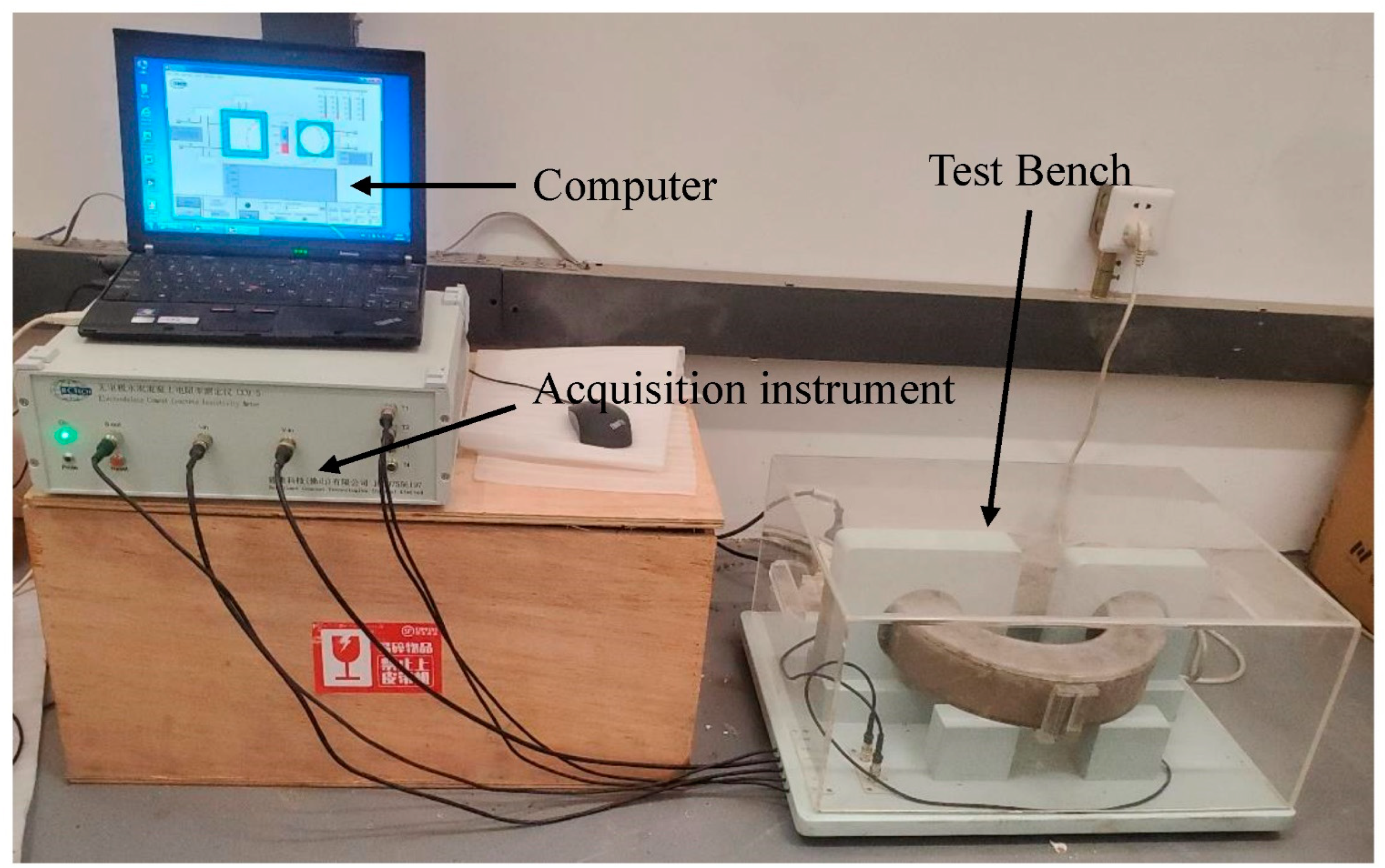
2.4.5. NC-ERM
3. Results and Discussion
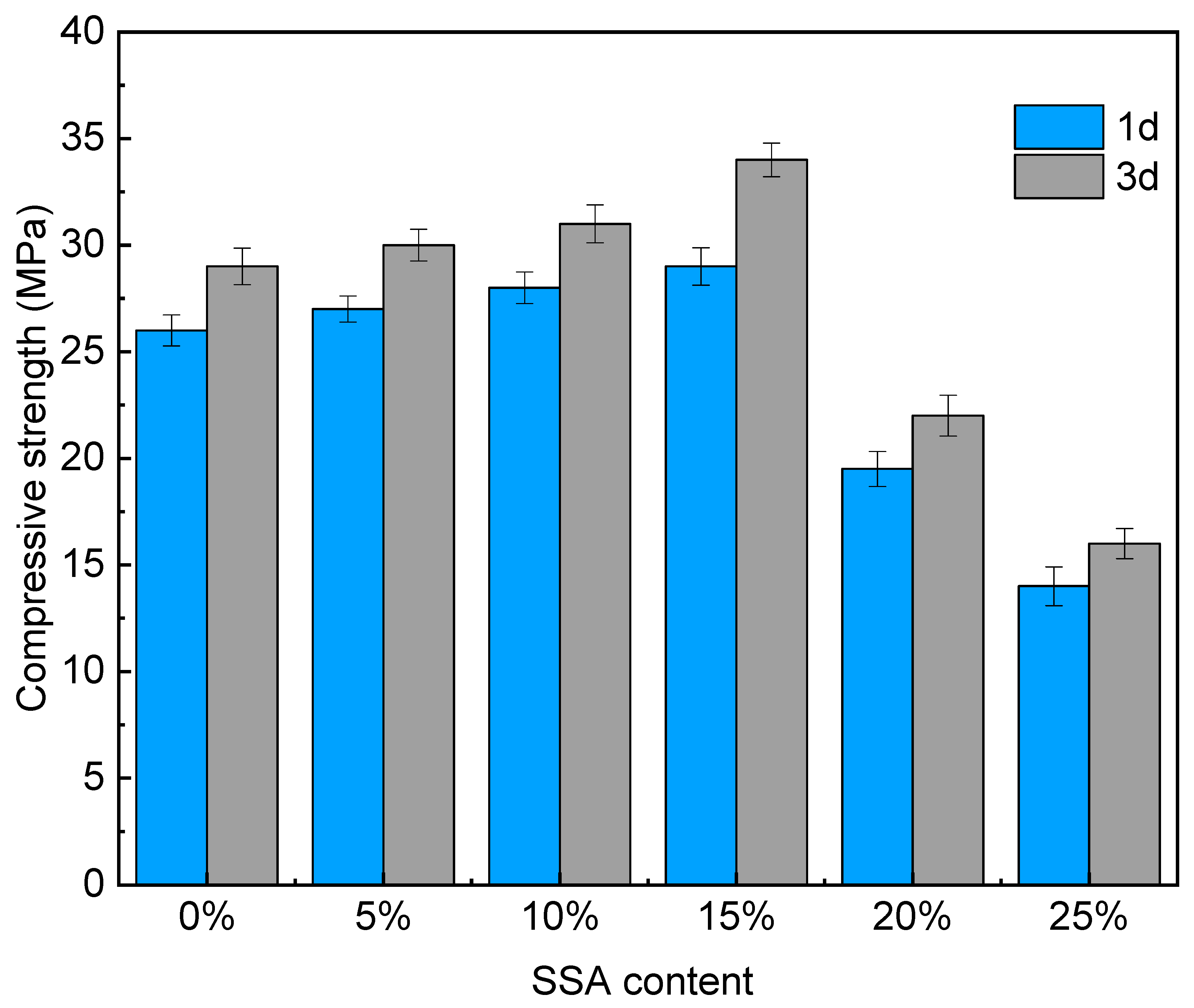
3.1. Mechanical Properties
3.2. Setting Time
3.3. Hydration Products
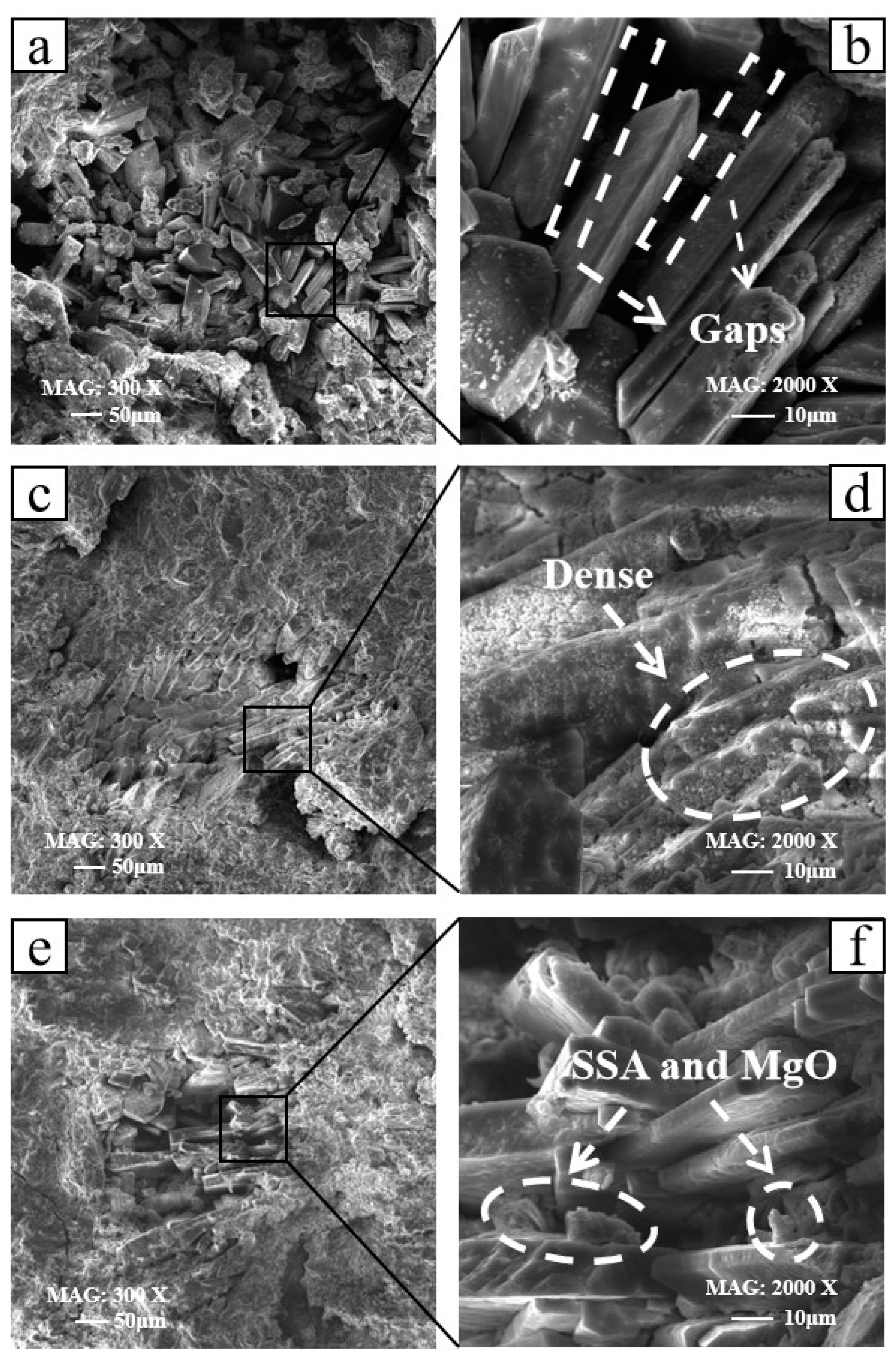
3.4. Microscopic Morphology
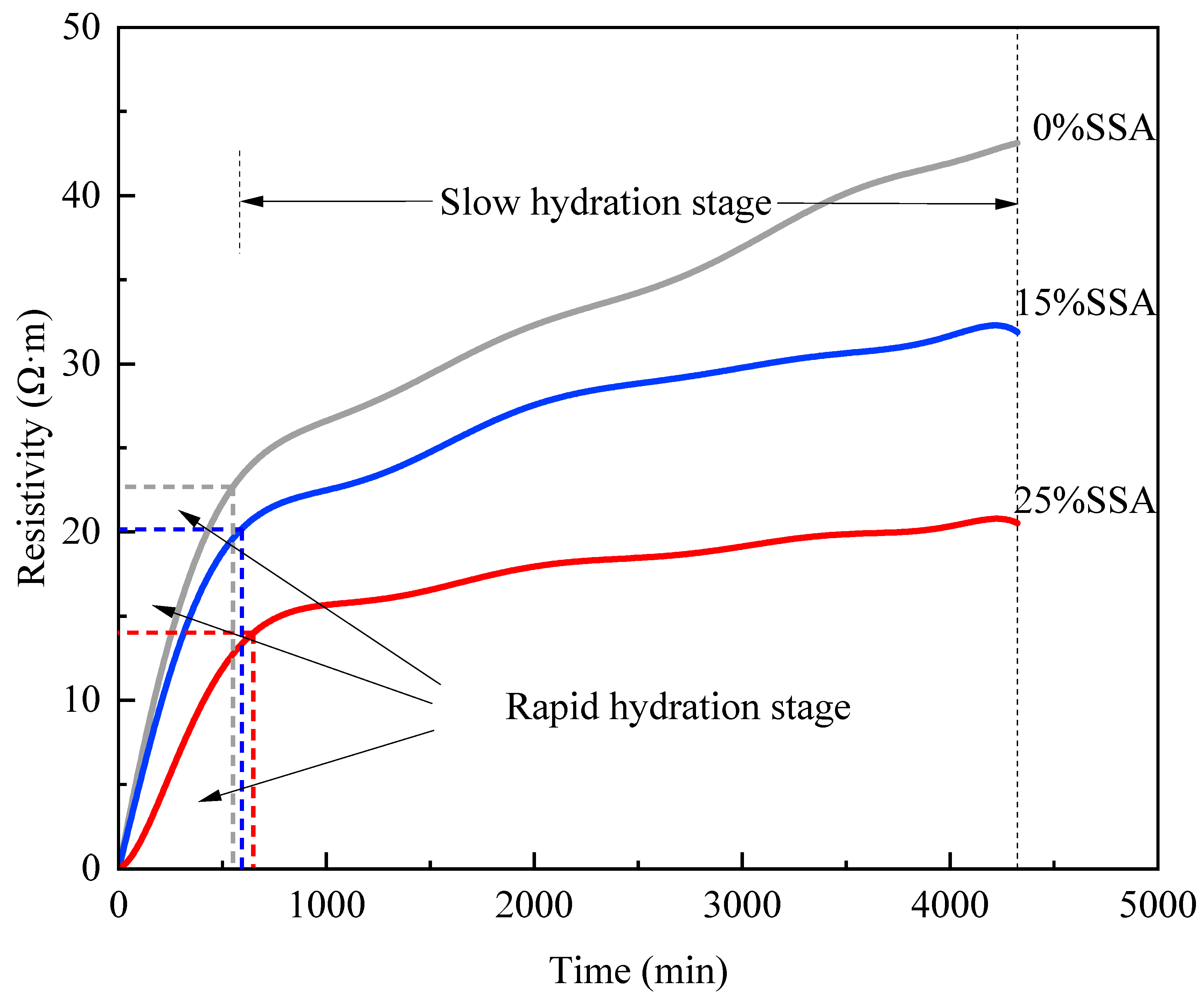
3.5. Hydration Process
4. Conclusions
- (1)
- Under different SSA contents, the compressive strength of MKPC was significantly improved with a 15% content of SSA, which was 11.5% and 17.2% higher than 0% SSA at 1d and 3d ages, respectively. The 20% and 25% SSA content made the compressive strength of MKPC drop sharply. Compared with 0% SSA, the 1d compressive strength of 20% and 25% SSA content decreased by 25% and 46.2%, respectively. The 3d compressive strength decreased by 24.2% and 44.8%, respectively. SSA had an obvious delaying effect on the hydration rate of MKPC, and the delaying effect on the growth rate of setting time was the most obvious when the SSA content was 10–15%.
- (2)
- Compared with 0% SSA MKPC, no obvious new crystal hydration products were detected in SSA-blended MKPC. When the SSA content was 15%, the micro-filling effect made the K-struvite crystal stack more compact.
- (3)
- The addition of SSA delayed the early hydration process of MKPC. Compared with 0% SSA, 15% SSA content made the hydration rate of MKPC slower, but the hydration degree was deepened. The hydration degree of 25% SSA was lower than that of 0% SSA and 15% SSA.
- (4)
- SSA can be used as a mineral admixture to modify MKPC. SSA-blended MKPC showed better mechanical properties and hydration degree at 15% SSA content.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sikder, P.; Ren, Y.; Bhaduri, S.B. Microwave processing of calcium phosphate and magnesium phosphate based orthopedic bioceramics: A state-of-the-art review. Acta Biomater. 2020, 111, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huan, Z.; Wu, C.; Zhou, Z.; Chang, J. High-strength calcium silicate-incorporated magnesium phosphate bone cement with osteogenic potential for orthopedic application. Compos. Part B Eng. 2022, 247, 110324. [Google Scholar] [CrossRef]
- Gelli, R.; Bernardini, G.; Ridi, F. Strontium-loaded magnesium phosphate bone cements and effect of polymeric additives. Ceram. Int. 2023, 49, 31466–31476. [Google Scholar] [CrossRef]
- Mahjoory, M.; Shahgholi, M.; Karimipour, A. Investigation on the size and percentage effects of magnesium nanoparticles on thermophysical properties of reinforced calcium phosphate bone cement by molecular dynamic simulation. Heliyon 2023, 9, e18835. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhu, B.; Zhang, S.; Wu, X. Properties and applications of magnesia–phosphate cement mortar for rapid repair of concrete. Cem. Concr. Res. 2000, 30, 1807–1813. [Google Scholar] [CrossRef]
- Popovics, S.; Rajendran, N.; Penko, M. Rapid Hardening Cements for Repair of Concrete. ACI Mater. J. 1987, 84, 64–73. [Google Scholar]
- Seehra, S.S.; Gupta, S.; Kumar, S. Rapid setting magnesium phosphate cement for quick repair of concrete pavements—Characterisation and durability aspects. Cem. Concr. Res. 1993, 23, 254–266. [Google Scholar] [CrossRef]
- Vinokurov, S.E.; Kulyako, Y.M.; Slyuntchev, O.M.; Rovny, S.I.; Myasoedov, B.F. Low-temperature immobilization of actinides and other components of high-level waste in magnesium potassium phosphate matrices. J. Nucl. Mater. 2009, 385, 189–192. [Google Scholar] [CrossRef]
- Cao, X.; Ma, R.; Zhang, Q.; Wang, W.; Liao, Q.; Sun, S.; Zhang, P.; Liu, X. The factors influencing sludge incineration residue (SIR)-based magnesium potassium phosphate cement and the solidification/stabilization characteristics and mechanisms of heavy metals. Chemosphere 2020, 261, 127789. [Google Scholar] [CrossRef]
- Cirstea, N.F.; Badanoiu, A.I.; Voicu, G.; Nicoara, A.I. Waste glass recycling in magnesium phosphate coatings for the fire protection of steel structures. J. Build. Eng. 2023, 76, 107345. [Google Scholar] [CrossRef]
- Yi, J.; Wang, L. Effect of magnesium phosphate cement mortar coating on strand bond behaviour. J. Build. Eng. 2023, 64, 105613. [Google Scholar] [CrossRef]
- Ma, G.; Hu, T.; Wang, F.; Liu, X.; Li, Z. Magnesium phosphate cement for powder-based 3D concrete printing: Systematic evaluation and optimization of printability and printing quality. Cem. Concr. Compos. 2023, 139, 105000. [Google Scholar] [CrossRef]
- Ding, Z.; Dong, B.; Xing, F.; Han, N.; Li, Z. Cementing mechanism of potassium phosphate based magnesium phosphate cement. Ceram. Int. 2012, 38, 6281–6288. [Google Scholar] [CrossRef]
- Haque, M.A.; Chen, B.; Li, S. Water-resisting performances and mechanisms of magnesium phosphate cement mortars comprising with fly-ash and silica fume. J. Clean Prod. 2022, 369, 133347. [Google Scholar] [CrossRef]
- Feng, H.; Liang, J.; Guo, A.; Lv, L.; Sun, Z.; Sheikh, M.N.; Liu, F. Development and design of ultra-high ductile magnesium phosphate cement-based composite using fly ash and silica fume. Cem. Concr. Compos. 2023, 137, 104923. [Google Scholar] [CrossRef]
- Dong, D.; Huang, Y.; Pei, Y.; Zhang, X.; Cui, N.; Zhao, P.; Hou, P.; Lu, L. Effect of spherical silica fume and fly ash on the rheological property, fluidity, setting time, compressive strength, water resistance and drying shrinkage of magnesium ammonium phosphate cement. J. Build. Eng. 2023, 63, 105484. [Google Scholar] [CrossRef]
- Feng, H.; Nie, S.; Guo, A.; Lv, L.; Yu, J. Evaluation on the performance of magnesium phosphate cement-based engineered cementitious composites (MPC-ECC) with blended fly ash/silica fume. Constr. Build. Mater. 2022, 341, 127861. [Google Scholar] [CrossRef]
- Feng, H.; Nie, S.; Guo, A.; Lv, L.; Chu, L.; Yu, J. Fresh properties and compressive strength of MPC-based materials with blended mineral admixtures. Case Stud. Constr. Mater. 2022, 17, e01201. [Google Scholar] [CrossRef]
- Qin, Z.; Zhou, S.; Ma, C.; Long, G.; Xie, Y.; Chen, B. Roles of metakaolin in magnesium phosphate cement: Effect of the replacement ratio of magnesia by metakaolin with different particle sizes. Constr. Build. Mater. 2019, 227, 116675. [Google Scholar] [CrossRef]
- Runqing, L.; Wei, W.; Dingwen, Q.; Yuanquan, Y. Static and dynamic mechanical properties of magnesium phosphate cement modified by metakaolin after high-temperature treatment. Constr. Build. Mater. 2023, 392, 131933. [Google Scholar] [CrossRef]
- Mo, L.; Lv, L.; Deng, M.; Qian, J. Influence of fly ash and metakaolin on the microstructure and compressive strength of magnesium potassium phosphate cement paste. Cem. Concr. Res. 2018, 111, 116–129. [Google Scholar] [CrossRef]
- Lu, K.; Wang, B.; Han, Z.; Ji, R. Experimental study of magnesium ammonium phosphate cements modified by fly ash and metakaolin. J. Build. Eng. 2022, 51, 104137. [Google Scholar] [CrossRef]
- Ruan, W.; Ma, Y.; Liao, J.; Ma, T.; Zhu, Y.; Zhou, A. Effects of steel slag on the microstructure and mechanical properties of magnesium phosphate cement. J. Build. Eng. 2022, 49, 104120. [Google Scholar] [CrossRef]
- Lang, L.; Duan, H.; Chen, B. Properties of pervious concrete made from steel slag and magnesium phosphate cement. Constr. Build. Mater. 2019, 209, 95–104. [Google Scholar] [CrossRef]
- Jing, Y.; Jiang, Y.; Chen, B.; Wang, L. Influence of steel slag powder on the characteristics of magnesium phosphate cement. J. Build. Eng. 2023, 77, 107454. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, D.; Wang, X.; Han, Z.; Tao, R.; Zhang, G.; Hou, D.; Wu, D.; Ding, Q. Impact of polyethylene fiber on the ductility and durability of magnesium phosphate cement. J. Build. Eng. 2023, 68, 106123. [Google Scholar] [CrossRef]
- Maldonado-Alameda, A.; Alfocea-Roig, A.; Huete-Hernández, S.; Giro-Paloma, J.; Chimenos, J.M.; Formosa, J. Magnesium phosphate cement incorporating sheep wool fibre for thermal insulation applications. J. Build. Eng. 2023, 76, 107043. [Google Scholar] [CrossRef]
- Liu, Z.; Lai, Z.; Luo, X.; Xiao, R.; Chen, J.; Lu, Z. Properties of magnesium phosphate cement reinforced with natural brucite fiber. Constr. Build. Mater. 2023, 393, 132057. [Google Scholar] [CrossRef]
- Feng, H.; Li, L.; Wang, W.; Cheng, Z.; Gao, D. Mechanical properties of high ductility hybrid fibres reinforced magnesium phosphate cement-based composites. Compos. Struct. 2022, 284, 115219. [Google Scholar] [CrossRef]
- Zárybnická, L.; Machotová, J.; Mácová, P.; Machová, D.; Viani, A. Design of polymeric binders to improve the properties of magnesium phosphate cement. Constr. Build. Mater. 2021, 290, 123202. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, W.; Liu, Z.; Yang, Y.; Long, W.; Zhang, Y.; Dong, B. Hydration Behavior of Magnesium Potassium Phosphate Cement: Experimental Study and Thermodynamic Modeling. Materials 2022, 15, 8496. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Bagherzadeh, S.A.; Shahrajabian, H.; Karimipour, A.; Jadidi, H.; Bach, Q.-V. Controlled elitist multi-objective genetic algorithm joined with neural network to study the effects of nano-clay percentage on cell size and polymer foams density of PVC/clay nanocomposites. J. Therm. Anal. Calorim. 2020, 139, 2801–2810. [Google Scholar] [CrossRef]
- Xiaohong, D.; Huajiang, C.; Bagherzadeh, S.A.; Shayan, M.; Akbari, M. Statistical estimation the thermal conductivity of MWCNTs-SiO2/Water-EG nanofluid using the ridge regression method. Phys. A Stat. Mech. Its Appl. 2020, 537, 122782. [Google Scholar] [CrossRef]
- Mahjoory, M.; Shahgholi, M.; Karimipour, A. The effects of initial temperature and pressure on the mechanical properties of reinforced calcium phosphate cement with magnesium nanoparticles: A molecular dynamics approach. Int. Commun. Heat Mass Transf. 2022, 135, 106067. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, W.; Yang, Y.; Hou, D.; Dong, B. Research on the mechanical properties of polyvinyl alcohol-modified waste rubber-filled cement paste using digital image correlation technology. Compos. Struct. 2023, 320, 117164. [Google Scholar] [CrossRef]
- Andrade, A.; Schuiling, R.D. The chemistry of struvite crystallization. Miner. J. 2001, 23, 37–46. [Google Scholar]
- Wagh, A.S.; Jeong, S.Y. Chemically bonded phosphate ceramics: I, A dissolution model of formation. J. Am. Ceram. Soc. 2003, 86, 1838–1844. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Wan, Y.; Yu, X.; Zhao, J.; Shao, J. Recycling of dredged river silt reinforced by an eco-friendly technology as microbial induced calcium carbonate precipitation (MICP). Soils Found. 2022, 62, 101216. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, W.; Yang, Y.; Hou, D.; Dong, B. Machine learning prediction models for compressive strength of calcined sludge-cement composites. Constr. Build. Mater. 2022, 346, 128442. [Google Scholar] [CrossRef]
- Oliva, M.; Vargas, F.; Lopez, M. Designing the incineration process for improving the cementitious performance of sewage sludge ash in Portland and blended cement systems. J. Clean Prod. 2019, 223, 1029–1041. [Google Scholar] [CrossRef]
- Martins, J.V.; Garcia, D.C.S.; Aguilar, M.T.P.; dos Santos, W.J. Influence of replacing Portland cement with three different concrete sludge wastes. Constr. Build. Mater. 2021, 303, 124519. [Google Scholar] [CrossRef]
- Kozai, N.; Sato, J.; Osugi, T.; Shimoyama, I.; Sekine, Y.; Sakamoto, F.; Ohnuki, T. Sewage sludge ash contaminated with radiocesium: Solidification with alkaline-reacted metakaolinite (geopolymer) and Portland cement. J. Hazard. Mater. 2021, 416, 125965. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.S.; Jhang, B.-J.; Hwang, C.-L.; Huynh, T.-P. Development and characterization of a controlled low-strength material produced using a ternary mixture of Portland cement, fly ash, and waste water treatment sludge. J. Clean Prod. 2022, 356, 131899. [Google Scholar] [CrossRef]
- Zhao, Q.; Lv, T.; Liang, H.; Zhang, J.; Zhang, J. Enhancement of sintered sludge ash-modified cement paste with CaSO4 and CaCl2. Constr. Build. Mater. 2023, 383, 131245. [Google Scholar] [CrossRef]
- Wang, E.; Wang, D.; Liu, X. Research on magnesium phosphate cement retarder. Concrete 2012, 86–88. [Google Scholar]
- Pei, H.; Shao, H.; Li, Z. A novel early-age shrinkage measurement method based on non-contact electrical resistivity and FBG sensing techniques. Constr. Build. Mater. 2017, 156, 1158–1162. [Google Scholar] [CrossRef]
- Li, X.; Fu, Q.; Lv, Y.; Leng, D.; Jiang, D.; He, C.; Wu, K.; Dan, J. Influence of curing conditions on hydration of magnesium silicate hydrate cement. Constr. Build. Mater. 2022, 361, 129648. [Google Scholar] [CrossRef]
- Pang, B.; Liu, J.; Wang, B.; Liu, R.; Yang, Y. Enhancement of magnesium phosphate cement solidification of Pb2+ by K-struvite whisker in lead-contaminated solution. J. Clean Prod. 2021, 320, 128848. [Google Scholar] [CrossRef]
- Leng, Y.; Soares, A. Microbial phosphorus removal and recovery by struvite biomineralisation in comparison to chemical struvite precipitation in municipal wastewater. J. Environ. Chem. Eng. 2023, 11, 109208. [Google Scholar] [CrossRef]
- Viani, A.; Zárybnická, L.; Ševčík, R.; Mácová, P.; Machotová, J.; Veltruská, K. Struvite-K crystal growth inhibition by citric acid: Formation of complexes in solution and surface adsorption effects. J. Cryst. Growth 2022, 598, 126858. [Google Scholar] [CrossRef]
- Pang, B.; Liu, J.; Wang, B.; Liu, R.; Yang, Y. Effects of K-struvite on hydration behavior of magnesium potassium phosphate cement. Constr. Build. Mater. 2021, 275, 121741. [Google Scholar] [CrossRef]
- Xiao, L.; Li, Z. Early-age hydration of fresh concrete monitored by non-contact electrical resistivity measurement. Cem. Concr. Res. 2008, 38, 312–319. [Google Scholar] [CrossRef]





| Composition | MgO | SiO2 | CaO | Al2O3 | Fe2O3 | LOI |
|---|---|---|---|---|---|---|
| Content | 91.04 | 4.73 | 2.09 | 0.86 | 0.94 | 1.01 |
| Composition | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | f-CaO | LOI |
|---|---|---|---|---|---|---|---|---|
| Content | 55.83 | 17.49 | 7.65 | 9.42 | 3.62 | 1.35 | - | 3.52 |
| MgO | SSA | KH2PO4/(MgO + SSA) | Water/Binder |
|---|---|---|---|
| 100% | 0% | 0.4 | 0.24 |
| 95% | 5% | ||
| 90% | 10% | ||
| 85% | 15% | ||
| 80% | 20% | ||
| 75% | 25% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Yang, W.; Luo, Q.; Long, W.-J. Mechanical Properties and Hydration Degree of Magnesium Potassium Phosphate Cement Modified by Sintered Silt Ash. Materials 2023, 16, 7010. https://doi.org/10.3390/ma16217010
Zhang H, Yang W, Luo Q, Long W-J. Mechanical Properties and Hydration Degree of Magnesium Potassium Phosphate Cement Modified by Sintered Silt Ash. Materials. 2023; 16(21):7010. https://doi.org/10.3390/ma16217010
Chicago/Turabian StyleZhang, Hongguang, Wenya Yang, Qiling Luo, and Wu-Jian Long. 2023. "Mechanical Properties and Hydration Degree of Magnesium Potassium Phosphate Cement Modified by Sintered Silt Ash" Materials 16, no. 21: 7010. https://doi.org/10.3390/ma16217010





