A Review: Design from Beta Titanium Alloys to Medium-Entropy Alloys for Biomedical Applications
Abstract
:1. Introduction
2. Metastable β-Phase Biomedical Ti Alloys
2.1. Phase Stability Calculations
2.2. Advantages of Metastable β-Ti Alloys
3. HEAs
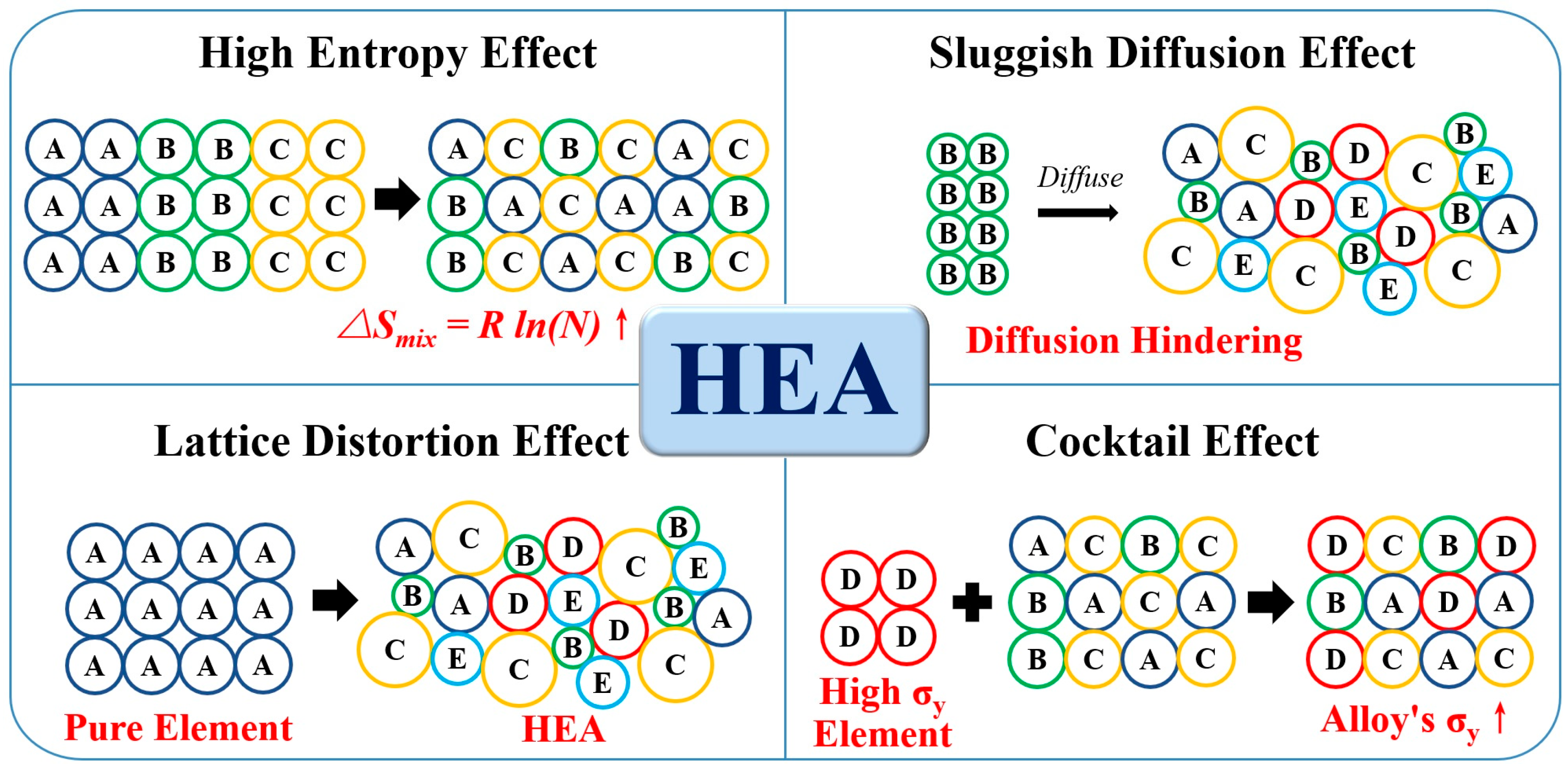
3.1. Four Core Effects
3.1.1. High Entropy Effect
3.1.2. Sluggish Diffusion Effect
3.1.3. Lattice Distortion Effect
3.1.4. Cocktail Effect
3.2. Thermodynamic Parameters
4. Evolution of Biomedical High-Entropy Alloys (Bio-HEAs)
4.1. Equiatomic Bio-HEAs
4.2. Advantages of Non-Equiatomic Ti-Rich HEAs
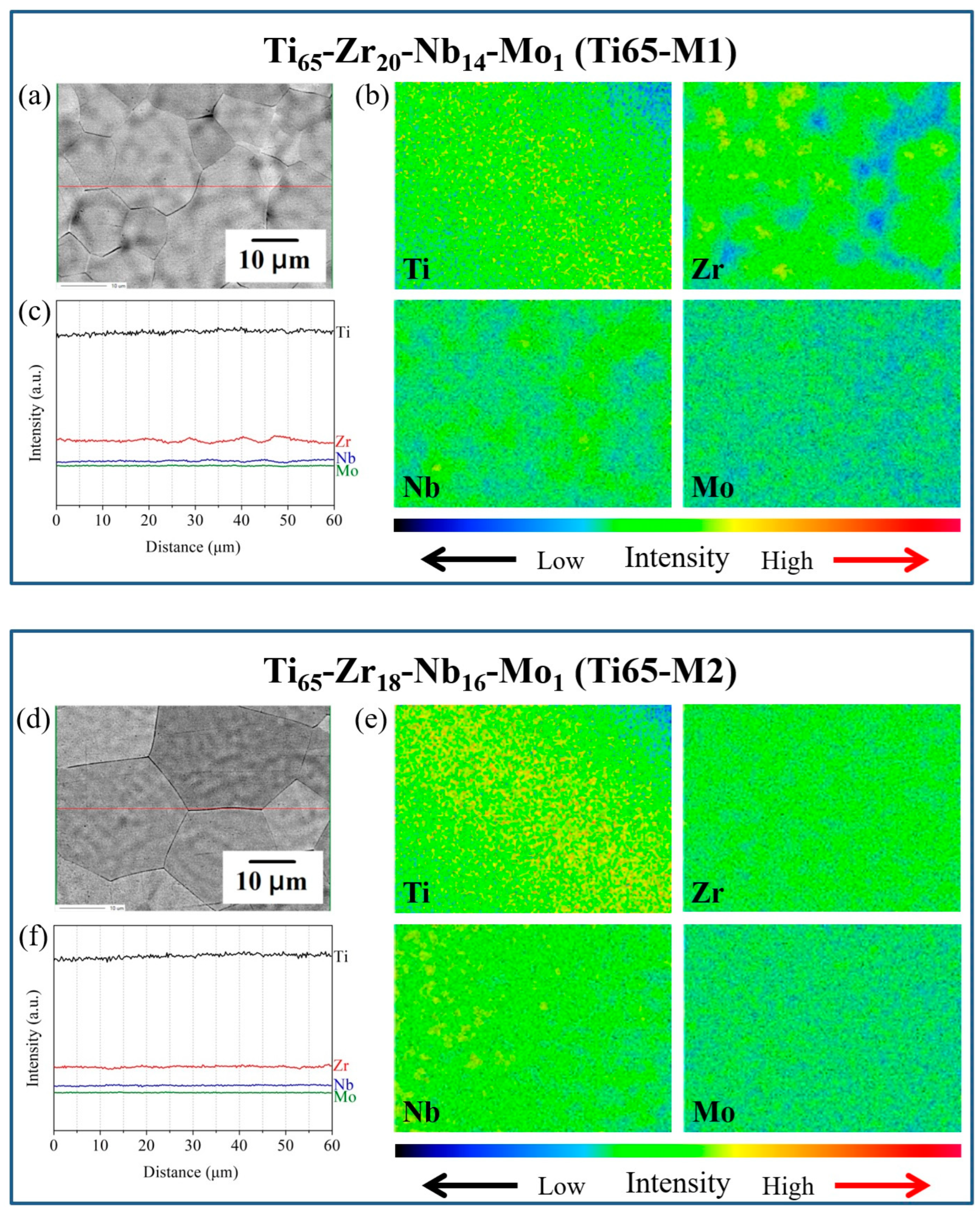
4.2.1. Improvement of Compositional Segregation
4.2.2. Enhancing Mechanical Strength
4.2.3. Improving Ductility
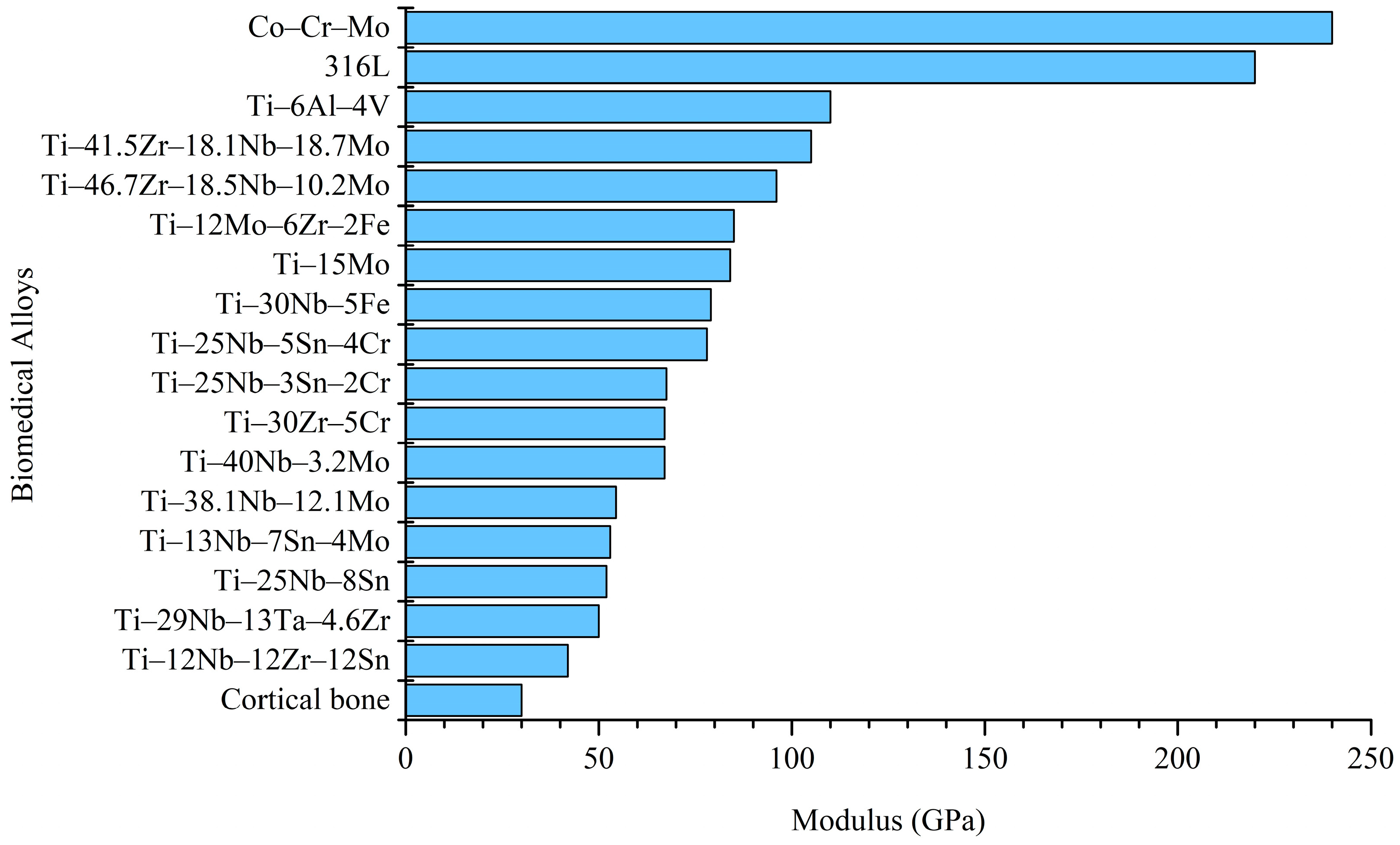
4.2.4. Decrease in Modulus
4.2.5. Enhancing Corrosion Resistance
4.2.6. Enhancing Biocompatibility
5. Biomedical MEAs (Bio-MEAs)
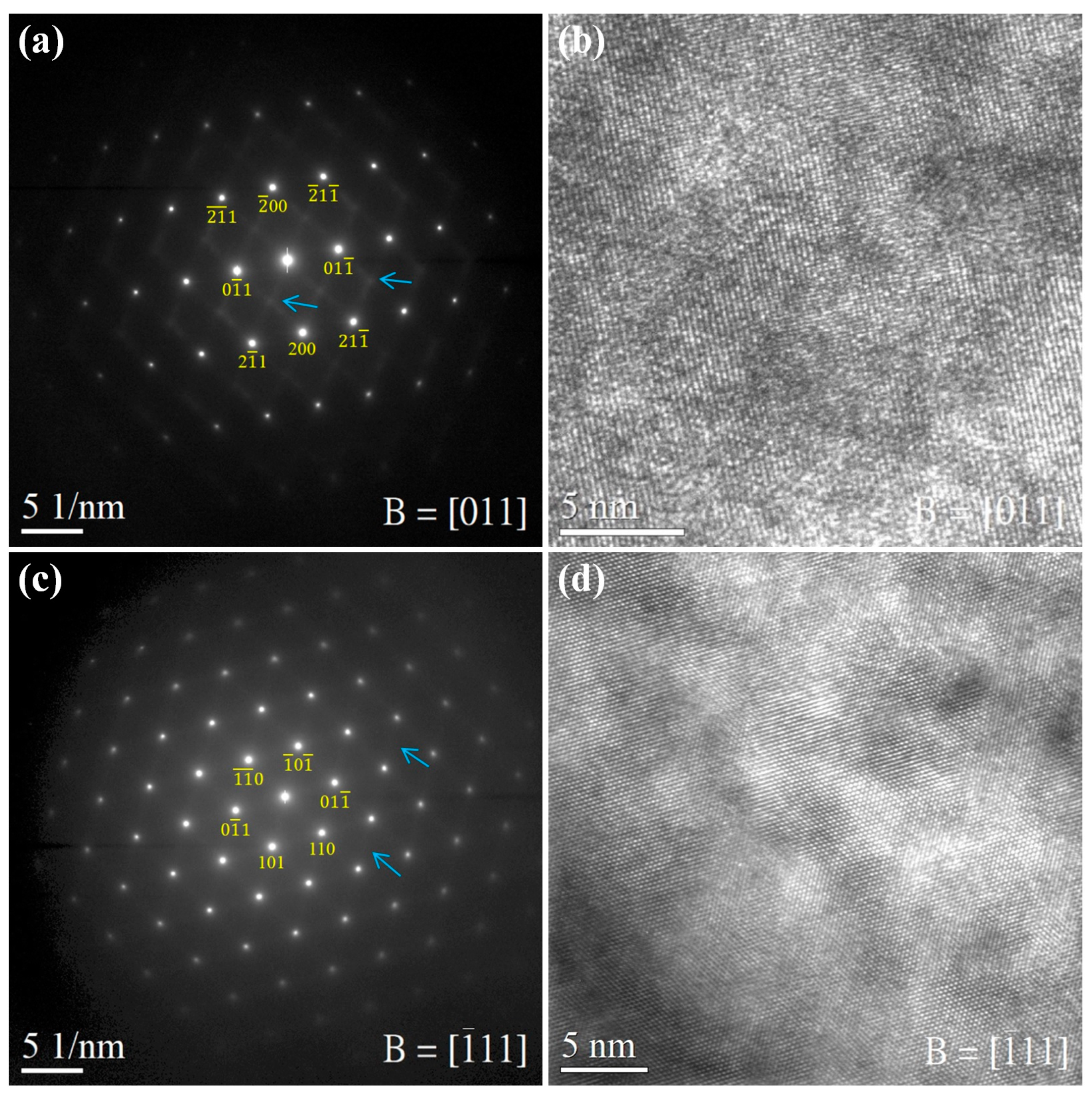
6. Metastable β Ti-Rich HEAs/MEAs
Relationships between Thermodynamic Parameters and Modulus
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.C.; Chen, L.Y. A review on biomedical titanium alloys: Recent progress and prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, D.; Shahzad, M.B.; Kang, Q.; Sun, Y.; Sun, Z.; Zhang, S.; Ren, L.; Yang, C.; Yang, K. Effect of surface passivation on corrosion resistance and antibacterial properties of Cu-bearing 316L stainless steel. Appl. Surf. Sci. 2016, 386, 371–380. [Google Scholar] [CrossRef]
- Liu, Y.P.; Gilbert, J.L. The effect of simulated inflammatory conditions and pH on fretting corrosion of Co–Cr–Mo alloy surfaces. Wear 2017, 390–391, 302–311. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, B. In vivo corrosion of Co–Cr–Mo alloy and biological responses: A review. Mater. Technol. 2017, 33, 127–134. [Google Scholar] [CrossRef]
- Bocchetta, P.; Chen, L.Y.; Tardelli, J.D.C.; Reis, A.C.; Almeraya-Calderón, F.; Leo, P. Passive layers and corrosion resistance of biomedical Ti–6Al–4V and β-Ti alloys. Coatings 2021, 11, 487. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Kolli, R.P.; Devaraj, A. A review of metastable beta titanium alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef]
- de Oliveira, T.G.; Fagundes, D.V.; Capellato, P.; Sachs, D.; da Silva, A.A.A.P. A Review of Biomaterials Based on High-Entropy Alloys. Metals 2022, 12, 1940. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Abdel-Hady, M.; Fuwa, H.; Hinoshita, K.; Kimura, H.; Shinzato, Y.; Morinaga, M. Phase stability change with Zr content in β-type Ti–Nb alloys. Scr. Mater. 2007, 57, 1000–1003. [Google Scholar] [CrossRef]
- Moraes, P.E.L.; Contieri, R.J.; Lopes, E.S.N.; Robin, A.; Caram, R. Effects of Sn addition on the microstructure, mechanical properties and corrosion behavior of Ti–Nb–Sn alloys. Mater. Charact. 2014, 96, 273–281. [Google Scholar] [CrossRef]
- Ikehata, H.; Nagasako, N.; Furuta, T.; Fukumoto, A.; Miwa, K.; Saito, T. First-principles calculations for development of low elastic modulus Ti alloys. Phys. Rev. B 2004, 70, 174113. [Google Scholar] [CrossRef]
- Zhou, Y.B. An Introduction to Titanium Alloy Casting; Aviation Industry Press: Beijing, China, 2000. [Google Scholar]
- Ho, W.F.; Ju, C.P.; Chern Lin, J.H. Structure and properties of cast binary Ti–Mo alloys. Biomaterials 1999, 20, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Paul, M.J.; Simson, C.; Niedermayer, J.; Gludovatz, B. Phase decomposition upon heat-treatment of a eutectoid Ti–Fe alloy processed by dual-wire-arc additive manufacturing. Mater. Lett. 2022, 319, 132305. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Gepreel, M.A.H.; Niinomi, M. Biocompatibility of Ti-alloys for long-term implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.F. A comparison of tensile properties and corrosion behavior of cast Ti–7.5Mo with c.p. Ti, Ti–15Mo and Ti–6Al–4V alloys. J. Alloys Compd. 2008, 464, 580–583. [Google Scholar] [CrossRef]
- Chui, P.; Jing, R.; Zhang, F.; Li, J.; Feng, T. Mechanical properties and corrosion behavior of β-type Ti–Zr–Nb–Mo alloys for biomedical application. J. Alloys Compd. 2020, 842, 155693. [Google Scholar] [CrossRef]
- ASTM F1813-13; Standard Specification for Wrought Titanium–12 Molybdenum–6 Zirconium–2 Iron Alloy for Surgical Implant (UNS R58120). ASTM International: West Conshohocken, PA, USA, 2013.
- Lopes, É.S.N.; Salvador, C.A.F.; Andrade, D.R.; Cremasco, A.; Campo, K.N.; Caram, R. Microstructure, Mechanical Properties, and Electrochemical Behavior of Ti–Nb–Fe Alloys Applied as Biomaterials. Metall. Mater. Trans. A 2016, 47, 3213–3226. [Google Scholar] [CrossRef]
- Jawed, S.F.; Rabadia, C.D.; Liu, Y.J.; Wang, L.Q.; Li, Y.H.; Zhang, X.H.; Zhang, L.C. Mechanical characterization and deformation behavior of β-stabilized Ti–Nb–Sn–Cr alloys. J. Alloys Compd. 2019, 792, 684–693. [Google Scholar] [CrossRef]
- Zhao, X.; Niinomi, M.; Nakai, M.; Miyamoto, G.; Furuhara, T. Microstructures and mechanical properties of metastable Ti–30Zr–(Cr, Mo) alloys with changeable Young’s modulus for spinal fixation applications. Acta Biomater. 2011, 7, 3230–3236. [Google Scholar] [CrossRef]
- Li, P.; Ma, X.; Tong, T.; Wang, Y. Microstructural and mechanical properties of β-type Ti–Mo–Nb biomedical alloys with low elastic modulus. J. Alloys Compd. 2020, 815, 152412. [Google Scholar] [CrossRef]
- Hsu, H.C.; Wong, K.K.; Wu, S.C.; Jheng, C.Y.; Ho, W.F. Structure and properties of metastable Ti–Nb–Sn–Mo alloys. MRS Commun. 2021, 11, 669–674. [Google Scholar] [CrossRef]
- Kuroda, D.; Niinomi, M.; Morinaga, M.; Kato, Y.; Yashiro, T. Design and mechanical properties of new β type titanium alloys for implant materials. Mater. Sci. Eng. A 1998, 243, 244–249. [Google Scholar] [CrossRef]
- Wu, C.T.; Chang, H.T.; Wu, C.Y.; Chen, S.W.; Huang, S.Y.; Huang, M.; Pan, Y.T.; Bradbury, P.; Chou, J.; Yen, H.W. Machine learning recommends affordable new Ti alloy with bone-like modulus. Mater. Today 2020, 34, 41–50. [Google Scholar] [CrossRef]
- ASTM F2066-18; Standard Specification for Wrought Titanium-15 Molybdenum Alloy for Surgical Implant Applications (UNS R58150). ASTM International: West Conshohocken, PA, USA, 2018.
- Hao, Y.L.; Li, S.J.; Sun, S.Y.; Zheng, C.Y.; Hu, Q.M.; Yang, R. Super-elastic titanium alloy with unstable plastic deformation. Appl. Phys. Lett. 2005, 87, 091906. [Google Scholar] [CrossRef]
- Hao, Y.L.; Li, S.J.; Sun, S.Y.; Zheng, C.Y.; Yang, R. Elastic deformation behaviour of Ti–24Nb–4Zr–7.9Sn for biomedical applications. Acta Biomater. 2007, 3, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.F.; Li, B.; Chen, B.H.; Wang, F.; Ma, W.; Zhang, X.Y.; Ma, M.Z.; Liu, R.P. Effect of Nb addition on the stability and biological corrosion resistance of Ti–Zr alloy passivation films. Corros. Sci. 2020, 170, 108696. [Google Scholar] [CrossRef]
- Hori, T.; Nagase, T.; Todai, M.; Matsugaki, A.; Nakano, T. Development of non-equiatomic Ti–Nb–Ta–Zr–Mo high-entropy alloys for metallic biomaterials. Scr. Mater. 2019, 172, 83–87. [Google Scholar] [CrossRef]
- Ho, W.F.; Wong, K.K.; Lee, M.H.; Thomas, J.L.; Chang, Y.C.; Wu, S.C.; Hsu, H.C.; Lin, H.Y. Biocompatibility of a Ti-Rich Medium-Entropy Alloy with Glioblastoma Astrocytoma Cells. Int. J. Mol. Sci. 2022, 23, 14552. [Google Scholar] [CrossRef]
- Chen, L.Y.; Cui, Y.W.; Zhang, L.C. Recent Development in Beta Titanium Alloys for Biomedical Applications. Metals 2020, 10, 1139. [Google Scholar] [CrossRef]
- Pang, J.C.; Li, S.X.; Wang, Z.G.; Zhang, Z.F. General relation between tensile strength and fatigue strength of metallic materials. Mater. Sci. Eng. A 2013, 564, 331–341. [Google Scholar] [CrossRef]
- Parida, J.; Mishra, S.C.; Behera, A. Synthesis and Characterization of Ti50Ni(50−x)Fex Alloy Produced by Mechanical Alloying and Pressure-Less Sintering. Met. Mater. Int. 2023, 29, 1145–1164. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Yeh, J.W.; Chen, S.K.; Shun, T.T. Wear resistance and high-temperature compression strength of Fcc Cu–Co–Ni–Cr–Al0.5–Fe alloy with boron addition. Metall. Mater. Trans. A 2004, 35, 1465–1469. [Google Scholar] [CrossRef]
- Chen, T.K.; Shun, T.T.; Yeh, J.W.; Wong, M.S. Nanostructured nitride films of multi-element high-entropy alloys by reactive DC sputtering. Surf. Coat. Technol. 2004, 188, 193–200. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Woodward, C.; Miracle, D.B. Low-density, refractory multi-principal element alloys of the Cr–Nb–Ti–V–Zr system: Microstructure and phase analysis. Acta. Mater. 2013, 61, 1545–1557. [Google Scholar] [CrossRef]
- Tsai, M.H. Physical properties of high entropy alloys. Entropy 2013, 15, 5338–5345. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Iijima, Y.; Nagase, T.; Matsugaki, A.; Wang, P.; Ameyama, K.; Nakano, T. Design and development of Ti–Zr–Hf–Nb–Ta–Mo high-entropy alloys for metallic biomaterials. Mater. Des. 2021, 202, 109548. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Chen, L.; Li, F.; Zhao, H.; Wang, X.; Zhou, G. Microstructure and Texture Evolution of a Dynamic Compressed Medium-Entropy Co–Cr0.4–Ni–Si0.3 Alloy. Crystals 2023, 13, 1390. [Google Scholar] [CrossRef]
- Mustafa, F.; Egilmez, M.; Abuzaid, W.; El-Khatib, S.; Nawaz, T.; Ahmad, S.; Alagoz, S. Strange Metallicity and Magnetic Order in the Co–Ni–(Cr/V) Medium-Entropy Alloy System. Materials 2023, 16, 1044. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, Y.; Duan, S.; Dong, Y.; Li, C.; Zhang, Z. Rapid Design, Microstructures, and Properties of Low-Cost Co-Free Al–Cr–Fe–Ni Eutectic Medium Entropy Alloys. Materials 2023, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, Y. A body-centered cubic Zr50–Ti35–Nb15 medium-entropy alloy with unique properties. Scr. Mater. 2020, 178, 329–333. [Google Scholar] [CrossRef]
- Yurchenko, N.; Panina, E.; Kapustin, D.; Novikov, V.; Volosevich, D.; Klimova-Korsmik, O.; Salishchev, G.; Zherebtsov, S.; Stepanov, N. A lightweight intermetallic Al–Cr–Ti medium-entropy alloy with good mechanical properties and oxidation resistance. J. Alloys Compd. 2023, 967, 171865. [Google Scholar] [CrossRef]
- Bała, P.; Górecki, K.; Dziurka, R.; Kozieł, T. The Effect of Al and Ti Additions on Solid Solution Strengthening and Precipitation Hardening in Co–Ni–Fe Medium-Entropy Alloys. Materials 2023, 16, 6297. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Xu, J. Ti–Zr–Nb–Ta–Mo high-entropy alloy designed for orthopedic implants: As-cast microstructure and mechanical properties. Mater. Sci. Eng. C 2017, 73, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Senkov, O.N.; Scott, J.M.; Senkova, S.V.; Meisenkothen, F.; Miracle, D.B.; Woodward, C.F.J. Microstructure and elevated temperature properties of a refractory Ta–Nb–Hf–Zr–Ti alloy. Mater. Sci. 2012, 47, 4062–4074. [Google Scholar] [CrossRef]
- Sin, J.R.; Neville, A.; Emami, N. Corrosion and tribocorrosion of hafnium in simulated body fluids. J. Biomed. Mater. Res. 2014, 102, 1157–1164. [Google Scholar]
- Fellah, M.; Hezil, N.; Abdul Samad, M.; Djellabi, R.; Montagne, A.; Mejias, A.; Kossman, S.; Iost, A.; Purnama, A.; Obrosov, A.; et al. Effect of Molybdenum Content on Structural, Mechanical, and Tribological Properties of Hot Isostatically Pressed β-Type Titanium Alloys for Orthopedic Applications. J. Mater. Eng. Perform. 2019, 28, 5988–5999. [Google Scholar] [CrossRef]
- Nagase, T.; Todai, M.; Hori, T.; Matsugaki, A.; Sekita, A.; Nakano, T. Novel Ti–Nb–Ta–Zr–Mo high-entropy alloys for metallic biomaterials. Scr. Mater. 2017, 129, 65–68. [Google Scholar]
- Nagase, T.; Todai, M.; Hori, T.; Nakano, T. Microstructure of equiatomic and non-equiatomic Ti–Nb–Ta–Zr–Mo high-entropy alloys for metallic biomaterials. J. Alloys Compd. 2018, 753, 412–421. [Google Scholar] [CrossRef]
- Nagase, T.; Iijima, Y.; Matsugaki, A.; Ameyama, K.; Nakano, T. Design and fabrication of Ti–Zr–Hf–Cr–Mo and Ti–Zr–Hf–Co–Cr–Mo high-entropy alloys as metallic biomaterials. Mater. Sci. Eng. C 2020, 107, 110322. [Google Scholar] [CrossRef]
- Stein, F.; Leineweber, A. Laves phases: A review of their functional and structural applications and an improved fundamental understanding of stability and properties. J. Mater. Sci. 2021, 56, 5321–5427. [Google Scholar] [CrossRef]
- Han, Z.D.; Luan, H.W.; Liu, X.; Chen, N.; Li, X.Y.; Shao, Y.; Yao, K.F. Microstructures and mechanical properties of Tix–Nb–Mo–Ta–W refractory high-entropy alloys. Mater. Sci. Eng. A 2018, 712, 380–385. [Google Scholar] [CrossRef]
- Chen, S.H.; Zhang, J.S.; Guan, S.; Li, T.; Liu, J.Q.; Wu, F.F.; Wu, Y.C. Microstructure and mechanical properties of W–Nb–Mo–Ta–Zrx (x = 0.1, 0.3, 0.5, 1.0) refractory high entropy alloys. Mater. Sci. Eng. A 2022, 835, 142701. [Google Scholar] [CrossRef]
- Yang, W.; Pang, S.; Liu, Y.; Wang, Q.; Liaw, P.K.; Zhang, T. Design and properties of novel Ti–Zr–Hf–Nb–Ta high-entropy alloys for biomedical applications. Intermetallics 2022, 141, 107421. [Google Scholar] [CrossRef]
- Yu, S.Y.; Brodrick, C.W.; Ryan, M.P.; Scully, J.R. Effects of Nb and Zr alloying additions on the activation behavior of Ti in hydrochloric acid. J. Electrochem. Soc. 1999, 146, 4429–4438. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, Z.; Feng, Z.; Pan, B.; Zhang, X.; Ma, M.; Liu, R. Effect of zirconium content on the microstructure and corrosion behavior of Ti–6Al–4V–xZr alloys. Corros. Sci. 2016, 112, 687–695. [Google Scholar] [CrossRef]
- Hua, N.; Wang, W.; Wang, Q.; Ye, Y.; Lin, S.; Zhang, L.; Guo, Q.; Brechtl, J.; Liaw, P.K. Mechanical, corrosion, and wear properties of biomedical Ti–Zr–Nb–Ta–Mo high entropy alloys. J. Alloys Compd. 2021, 861, 157997. [Google Scholar] [CrossRef]
- Minagar, S.; Berndt, C.C.; Wang, J.; Ivanova, E.; Wen, C. A review of the application of anodization for the fabrication of nanotubes on metal implant surfaces. Acta Biomater. 2012, 8, 2875–2888. [Google Scholar] [CrossRef]
- Träger, D.; Słota, D.; Niziołek, K.; Florkiewicz, W.; Sobczak-Kupiec, A. Hybrid Polymer–Inorganic Coatings Enriched with Carbon Nanotubes on Ti–6Al–4V Alloy for Biomedical Applications. Coatings 2023, 13, 1813. [Google Scholar] [CrossRef]
- Rahmani, R.; Lopes, S.I.; Prashanth, K.G. Selective Laser Melting and Spark Plasma Sintering: A Perspective on Functional Biomaterials. J. Funct. Biomater. 2023, 14, 521. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.E.; Jorge, A.M., Jr.; Asato, G.H.; Roche, V. Formation of self-ordered oxide nanotubes layer on the equiatomic Ti–Nb–Zr–Hf–Ta high entropy alloy and bioactivation procedure. J. Alloys Compd. 2021, 865, 158837. [Google Scholar] [CrossRef]
- Shun, T.T.; Chang, L.Y.; Shiu, M.H. Microstructures and mechanical properties of multiprincipal component Co–Cr–Fe–Ni–Tix alloys. Mater. Sci. Eng. A 2012, 556, 170–174. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Qian, M.; Shi, Z.; Song, T.; Huang, L.; Zou, J. A novel quaternary equiatomic Ti–Zr–Nb–Ta medium entropy alloy (MEA). Intermetallics 2018, 101, 39–43. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Qian, M.; Shi, Z.; Song, T.; Huang, L.; Zou, J. Compositional design of strong and ductile (tensile) Ti–Zr–Nb–Ta medium entropy alloys (MEAs) using the atomic mismatch approach. Mater. Sci. Eng. A 2019, 742, 762–772. [Google Scholar] [CrossRef]
- Mustafi, L.; Nguyen, V.T.; Lu, S.L.; Song, T.; Murdoch, B.J.; Fabijanic, D.M.; Qian, M. Microstructure, tensile properties and deformation behaviour of a promising bio-applicable new Ti35–Zr15–Nb25–Ta25 medium entropy alloy (MEA). Mater. Sci. Eng. A 2021, 824, 141805. [Google Scholar] [CrossRef]
- Wong, K.K.; Hsu, H.C.; Wu, S.C.; Ho, W.F. Structure and properties of Ti-rich Ti–Zr–Nb–Mo medium-entropy alloys. J. Alloys Compd. 2021, 868, 159137. [Google Scholar] [CrossRef]
- Son, S.; Lee, D.; Kwon, H.; Moon, J.; Park, K.B.; Kim, A.; Choi, J.; Jeong, J.H.; Cho, S.; Kim, H.S. Microstructure and mechanical properties of equiatomic Ti-containing medium-entropy alloys. J. Alloys Compd. 2023, 935, 168089. [Google Scholar] [CrossRef]
- Lilensten, L.; Couzinié, J.; Bourgon, J.; Perrière, L.; Dirras, G.; Prima, F.; Guillot, I. Design and tensile properties of a bcc Ti-rich high-entropy alloy with transformation-induced plasticity. Mater. Res. Lett. 2017, 5, 110–116. [Google Scholar]
- Yuan, Y.; Wu, Y.; Yang, Z.; Liang, X.; Lei, Z.; Huang, H.; Wang, H.; Liu, X.; An, K.; Wu, W.; et al. Formation, structure and properties of biocompatible Ti–Zr–Hf–Nb–Ta high-entropy alloys. Mater. Res. Lett. 2019, 7, 225–231. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Yang, X.; Hou, M. New Ti–Ta–Nb–Zr–Mo high-entropy alloys for metallic biomaterials. Mater. Res. Express 2021, 8, 105403. [Google Scholar] [CrossRef]
- Wong, K.K.; Hsu, H.C.; Wu, S.C.; Ho, W.F. Novel Metastable Nonequiatomic Ti–Zr–Nb–Mo Medium-Entropy Alloys with High Yield-Strength-to-Elastic-Modulus Ratios. Met. Mater. Int. 2022, 868, 159137. [Google Scholar] [CrossRef]
- Motallebzadeh, A.; Peighambardoust, N.S.; Sheikh, S.; Murakami, H.; Guo, S.; Canadinc, D. Microstructural, mechanical and electrochemical characterization of Ti–Zr–Ta–Hf–Nb and Ti1.5–Zr–Ta0.5–Hf0.5–Nb0.5 refractory high-entropy alloys for biomedical applications. Intermetallics 2019, 113, 106572. [Google Scholar] [CrossRef]
- Lin, S.; Lai, W.; Vogel, F.; Tong, X.; You, D.; Li, W.; Wang, X. Mechanical and corrosion properties of biomedical (TiZr)90-x–Nbx–Ta5–Mo5 medium entropy alloys. Int. J. Refract. Hard Met. 2023, 116, 106361. [Google Scholar] [CrossRef]
- Yang, W.; Pang, S.J.; Wang, G.; Liu, Y.; Liaw, P.K.; Zhang, T. Ti–Zr–Hf–Nb–Ta–Sn high-entropy alloys with good properties as potential biomaterials. Rare Met. 2022, 41, 2305–2315. [Google Scholar] [CrossRef]
- Wong, K.K.; Hsu, H.C.; Wu, S.C.; Hung, T.L.; Ho, W.F. Structure, Properties, and Corrosion Behavior of Ti-Rich Ti–Zr–Nb–Ta Medium-Entropy Alloys with β + α″ + α′ for Biomedical Application. Materials 2022, 15, 7953. [Google Scholar] [CrossRef]
- Eldabah, N.M.; Shoukry, A.; Khair-Eldeen, W.; Kobayashi, S.; Gepreel, M.A.H. Design and characterization of low Young’s modulus Ti–Zr–Nb-based medium entropy alloys assisted by extreme learning machine for biomedical applications. J. Alloys Compd. 2023, 968, 171755. [Google Scholar] [CrossRef]
- Hu, S.; Li, T.; Su, Z.; Meng, S.; Jia, Z.; Liu, D. A novel Ti–Zr–Nb medium entropy alloy (MEA) with appropriate elastic modulus for biocompatible materials. Mater. Sci. Eng. B 2021, 270, 115226. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Yuan, Z. Structures and properties of biocompatible Ti–Zr–Nb–Fe–Mo medium entropy alloys. Mater. Today Commun. 2022, 32, 103808. [Google Scholar] [CrossRef]
- Hu, S.; Li, X.; Lin, Y.; Li, T.; Zhang, G.; Li, J.; Zhang, X.; Liu, D. Systematic study of (TiZr)x–Nby–(TaMo)z medium entropy alloys for biomedical implants. J. Mater. Res. Technol. 2023, 24, 7683–7703. [Google Scholar] [CrossRef]
- Li, T.; Hu, S.; Wang, L.; Jia, Z.; Li, Q.; Liu, D. Effect of Sn on microstructure and properties of Ti–Zr–Nb–Sn medium-entropy alloys (MEAs). J. Mater. Res. 2022, 37, 1762–1770. [Google Scholar] [CrossRef]




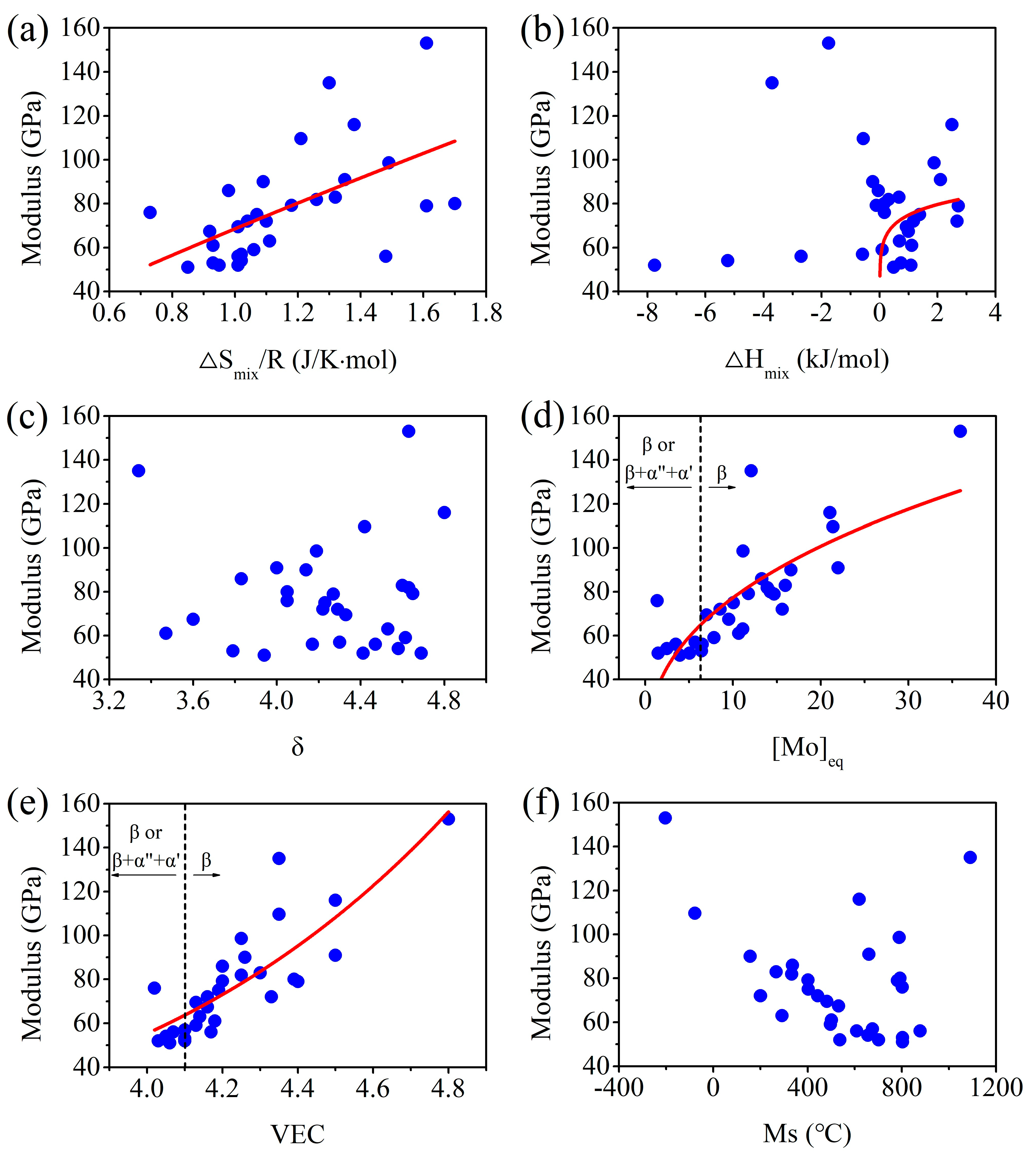

| Alloy | Phase | δ | ΔSmix/R (J/K·mol) | ΔHmix (kJ/mol) | Test Methods | σy (MPa) | E (GPa) | [Mo]eq | VEC | Bo | Md | Ms | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti33.33–V33.33–Mo33.33 | β | 3.97 | 1.1 | 0 | Compression | 1116 | N/A | 68.62 | 4.12 | 2.886 | 2.093 | –1258 | [74] |
| Ti30–Nb20–Ta20–Zr20–Mo10 | β1 + β2 | 4.11 | 1.55 | 0 | Compression | 1132 | N/A | 27.82 | 4.6 | 3.009 | 2.508 | 214 | [77] |
| Ti33.33–Zr16.67–Nb16.67–Ta16.67–Mo16.67 | β1 + β2 | 4.23 | 1.56 | –1.33 | Compression | 1600 | N/A | 32.84 | 4.67 | 2.996 | 2.458 | –87 | [64] |
| Ti45–Zr25–Nb25–Ta5 | β | 4.58 | 1.2 | 2.14 | Tensile | 790 | N/A | 15.13 | 4.3 | 2.959 | 2.567 | 432 | [70] |
| Ti30.5–Zr30.5–Nb13–Ta13–Mo13 | β | 4.87 | 1.52 | –0.74 | Compression | 1250 | N/A | 26.27 | 4.52 | 3.002 | 2.540 | 36 | [32] |
| Ti28.33–Zr28.33–Hf28.33–Nb6.74–Ta6.74–Mo1.55 | β | 5.17 | 1.5 | 0.99 | Tensile | 780 | N/A | 6.68 | 4.17 | 3.014 | 2.732 | 828 | [44] |
| Ti33.33–Zr33.33–Mo33.33 | β1 + β2 | 5.94 | 1.1 | –1.78 | Compression | 1598 | N/A | 40.82 | 4.67 | 2.979 | 2.447 | –1116 | [74] |
| Ti25–V25–Zr25–Mo25 | β1 + β2 + Laves | 6.77 | 1.39 | –2.5 | Compression | 1623 | N/A | 46.73 | 4.03 | 2.936 | 2.304 | –711 | [74] |
| Ti20–Zr20–Nb20–Ta20–Mo20 | β1 + β2 | 4.63 | 1.61 | –1.76 | Compression | 1390 | 153 | 35.93 | 4.8 | 3.036 | 2.459 | –204 | [51] |
| Ti15–Zr15–Nb35–Ta35 | β | 3.34 | 1.3 | –3.71 | Tensile | 970 | 135 | 12.09 | 4.35 | 3.066 | 2.541 | 1091 | [76] |
| Ti25–Zr25–Nb25–Ta25 | β | 4.8 | 1.38 | 2.5 | Compression | 1100 | 116 | 21.04 | 4.5 | 3.030 | 2.584 | 619 | [70] |
| Ti50–Zr25–Nb15–Mo10 | β | 4.42 | 1.21 | –0.56 | Bending | 1754 | 101 | 21.39 | 4.35 | 2.938 | 2.517 | –77 | [73] |
| Ti37.5–Zr25–Ta12.5–Hf12.5–Nb12.5 | β | 4.19 | 1.49 | 1.88 | Nano-indentation | N/A | 99 | 11.16 | 4.25 | 2.987 | 2.642 | 789 | [79] |
| Ti35–Zr15–Nb25–Ta25 | β | 4 | 1.35 | 2.1 | Tensile | 842 | 91 | 21.97 | 4.5 | 3.000 | 2.535 | 660 | [72] |
| Ti58–Zr23–Nb12–Mo7 | β | 4.14 | 1.09 | –0.24 | Bending | 1548 | 90 | 16.61 | 4.26 | 2.914 | 2.522 | 157 | [73] |
| Ti65–Zr20–Nb10–Mo5 | β | 3.83 | 0.98 | –0.04 | Bending | 1381 | 86 | 13.27 | 4.2 | 2.894 | 2.518 | 335 | [73] |
| Ti37.5–Zr37.5–Nb15–Mo5–Ta5 | β | 4.6 | 1.32 | 0.67 | Tensile | 925 | 83 | 15.97 | 4.3 | 2.979 | 2.606 | 266 | [80] |
| Ti40–Zr40–Nb10–Mo5–Ta5 | β | 4.63 | 1.26 | 0.31 | Tensile | 917 | 82 | 13.91 | 4.25 | 2.971 | 2.619 | 333 | [80] |
| Ti42.5–Zr42.5–Nb5–Mo5–Ta5 | β | 4.65 | 1.18 | –0.11 | Tensile | 911 | 79.2 | 11.78 | 4.2 | 2.963 | 2.633 | 402 | [80] |
| Ti35–Zr27.5–Hf27.5–Nb5–Ta5 | β | 4.13 | 1.38 | 0.6 | Tensile | 540 | 79 | 4.153 | 4.1 | 2.990 | 2.730 | 913 | [75] |
| Ti20–Zr20–Hf20–Nb20–Ta20 | β | 4.27 | 1.61 | 2.72 | Compression | 926 | 79 | 14.69 | 4.4 | 3.046 | 2.662 | 781 | [81] |
| Ti65–Zr33–Nb1–Ta1 | β + α″ + α′ | 4.05 | 0.73 | 0.17 | Bending | 1150 | 76 | 1.36 | 4.02 | 2.894 | 2.608 | 802 | [82] |
| Ti48–Zr34–Nb17–Mo1 | β | 4.23 | 1.07 | 1.38 | Compression | 666 | 75 | 10.06 | 4.19 | 2.946 | 2.604 | 403 | [83] |
| Ti33.33–Zr33.33–Nb33.33 | β | 4.22 | 1.1 | 2.67 | Tensile | 830 | 72 | 15.62 | 4.33 | 2.991 | 2.601 | 200 | [84] |
| Ti48–Zr40–Nb11–Mo1 | β | 4.33 | 1.01 | 0.93 | Compression | 629 | 69.5 | 7 | 4.13 | 2.945 | 2.634 | 482 | [83] |
| Ti65–Zr20–Nb14–Mo1 | β | 3.6 | 0.92 | 0.99 | Bending | 1188 | 67.4 | 9.53 | 4.16 | 2.896 | 2.542 | 531 | [78] |
| Ti45–Zr37–Nb16–Fe1–Mo1 | β | 4.53 | 1.11 | 0.69 | Tensile | 703 | 63 | 11.14 | 4.14 | 2.950 | 2.604 | 291 | [85] |
| Ti65–Zr18–Nb16–Mo1 | β | 3.47 | 0.93 | 1.11 | Bending | 1118 | 61 | 10.66 | 4.18 | 2.896 | 2.532 | 502 | [78] |
| Ti45–Zr45–Nb4–Ta3–Mo3 | β | 4.61 | 1.06 | 0.09 | Tensile | 821 | 59 | 7.84 | 4.13 | 2.954 | 2.653 | 497 | [86] |
| Ti45–Zr45–Nb5–Ta5 | β | 4.3 | 1.02 | –0.59 | Tensile | 690 | 57 | 5.7 | 4.1 | 2.956 | 2.669 | 676 | [76] |
| Ti27.78–Zr27.78–Hf27.78–Nb8.33–Ta8.33 | β | 4.17 | 1.48 | 0.93 | Compression | 834 | 56 | 6.52 | 4.17 | 3.016 | 2.734 | 878 | [81] |
| Ti45–Zr45–Nb7–Sn3 | β | 4.47 | 1.01 | –2.71 | Tensile | 720 | 56 | 3.49 | 4.07 | 2.930 | 2.654 | 608 | [87] |
| Ti45–Zr45–Nb5–Sn5 | β | 4.58 | 1.02 | –5.23 | Tensile | 750 | 54 | 2.48 | 4.05 | 2.913 | 2.648 | 655 | [87] |
| Ti65–Zr25–Nb5–Ta5 | β + α″ + α′ | 3.79 | 0.93 | 0.74 | Bending | 530 | 53 | 6.43 | 4.1 | 2.897 | 2.572 | 803 | [82] |
| Ti45–Zr45–Nb10 | β | 4.41 | 0.95 | 1.08 | Tensile | 678 | 52 | 5.04 | 4.1 | 2.954 | 2.664 | 537 | [86] |
| Ti45–Zr45–Nb3–Sn7 | β | 4.69 | 1.01 | –7.75 | Tensile | 790 | 52 | 1.48 | 4.03 | 2.897 | 2.641 | 702 | [87] |
| Ti65–Zr29–Nb3–Ta3 | β + α″ + α′ | 3.94 | 0.85 | 0.48 | Bending | 760 | 51 | 3.96 | 4.06 | 2.896 | 2.590 | 802 | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, K.-K.; Hsu, H.-C.; Wu, S.-C.; Ho, W.-F. A Review: Design from Beta Titanium Alloys to Medium-Entropy Alloys for Biomedical Applications. Materials 2023, 16, 7046. https://doi.org/10.3390/ma16217046
Wong K-K, Hsu H-C, Wu S-C, Ho W-F. A Review: Design from Beta Titanium Alloys to Medium-Entropy Alloys for Biomedical Applications. Materials. 2023; 16(21):7046. https://doi.org/10.3390/ma16217046
Chicago/Turabian StyleWong, Ka-Kin, Hsueh-Chuan Hsu, Shih-Ching Wu, and Wen-Fu Ho. 2023. "A Review: Design from Beta Titanium Alloys to Medium-Entropy Alloys for Biomedical Applications" Materials 16, no. 21: 7046. https://doi.org/10.3390/ma16217046






