Study on the Thermophysical Properties of 80% 10B Enrichment of B4C
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
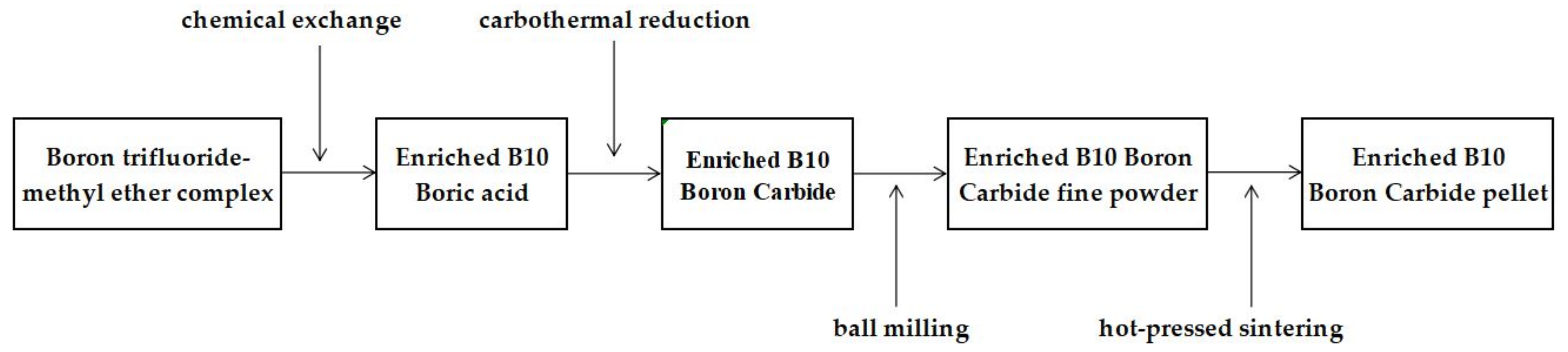
2.2. Methods
- Q: Radiant energy emitted from the surface of an object, W;
- σ: Stefan–Boltzmann constant, 5.67 × 10−8 W/m2·K4;
- ε: the whole emissivity of the object;
- A: Surface area of the object;
- T: Surface temperature, K.
3. Results
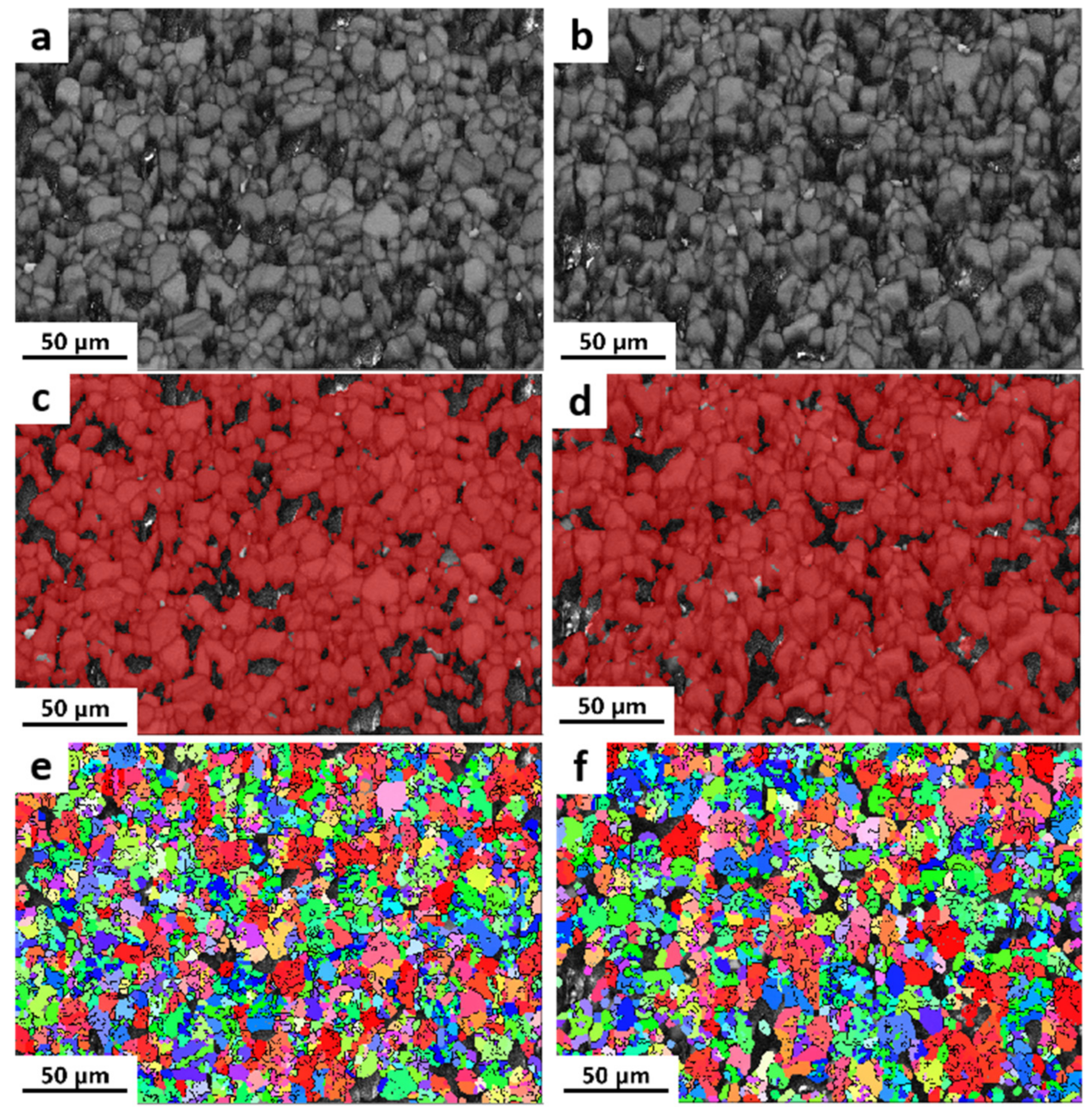
3.1. Microscopic Analysis
3.2. Thermal Expansion Coefficient
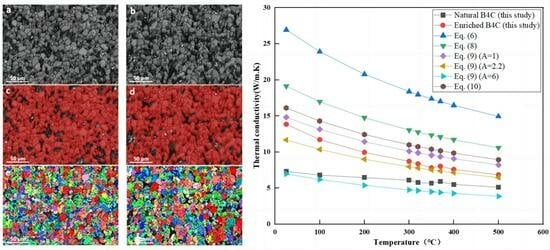
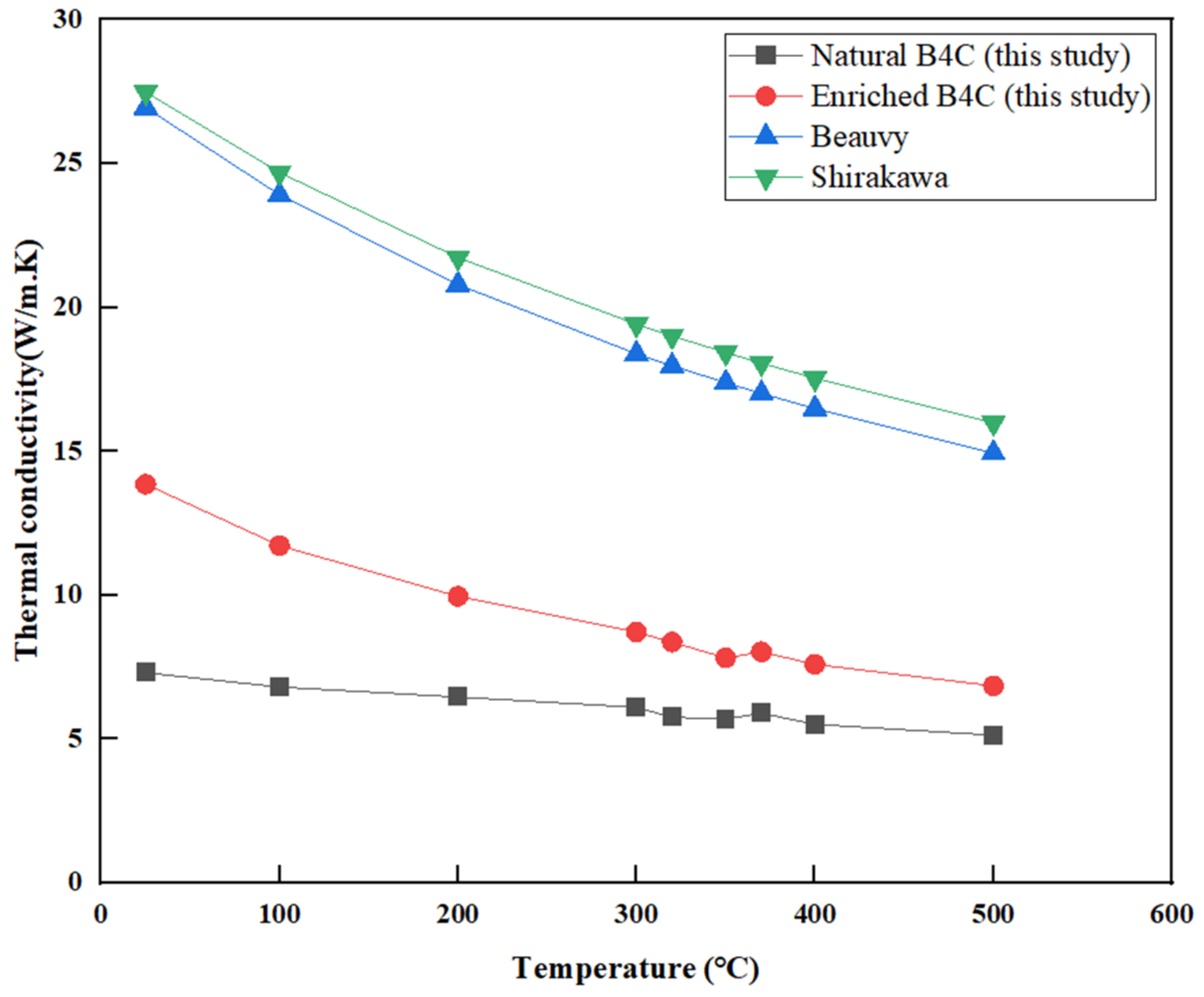
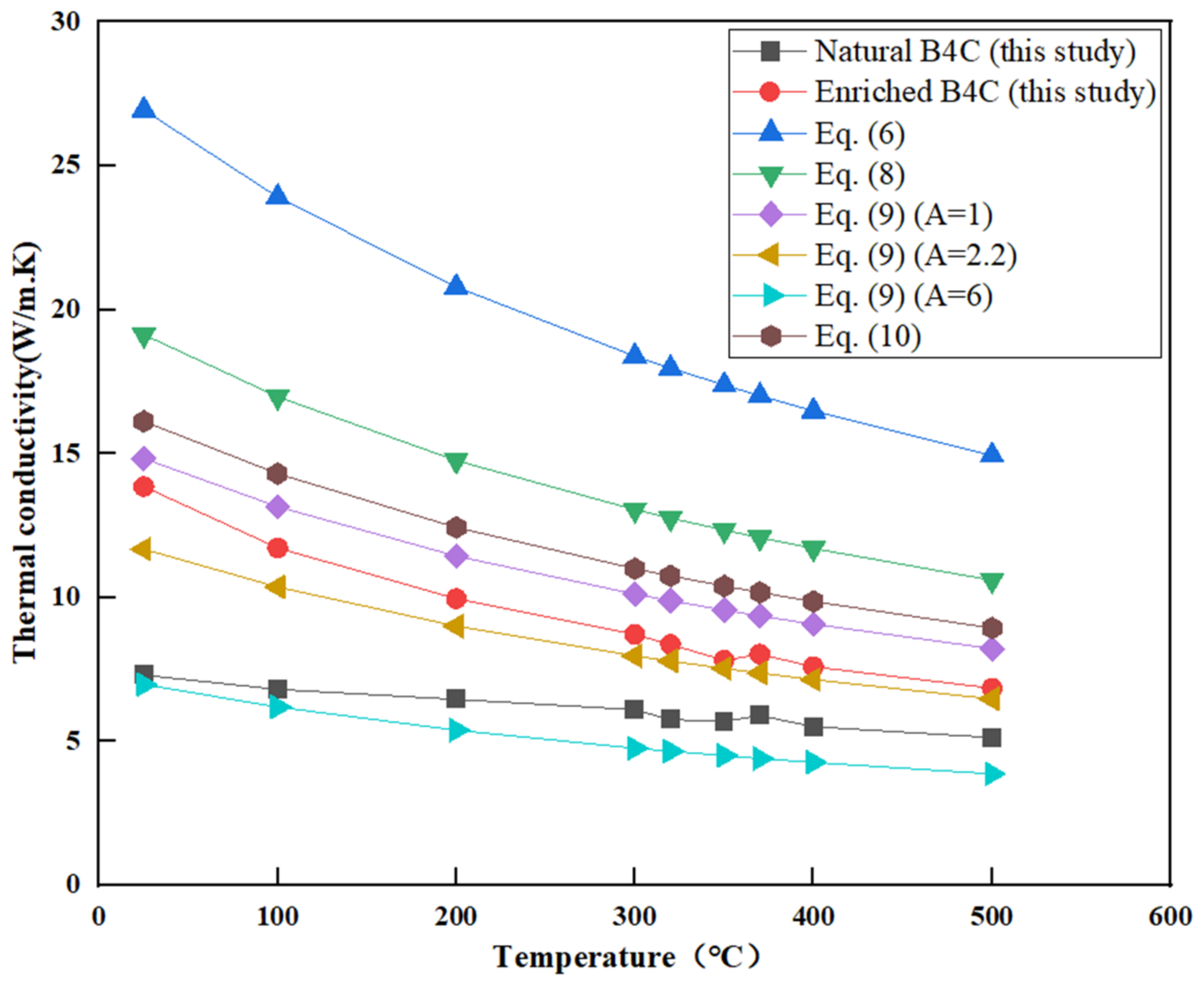
3.3. Thermal Conductivity
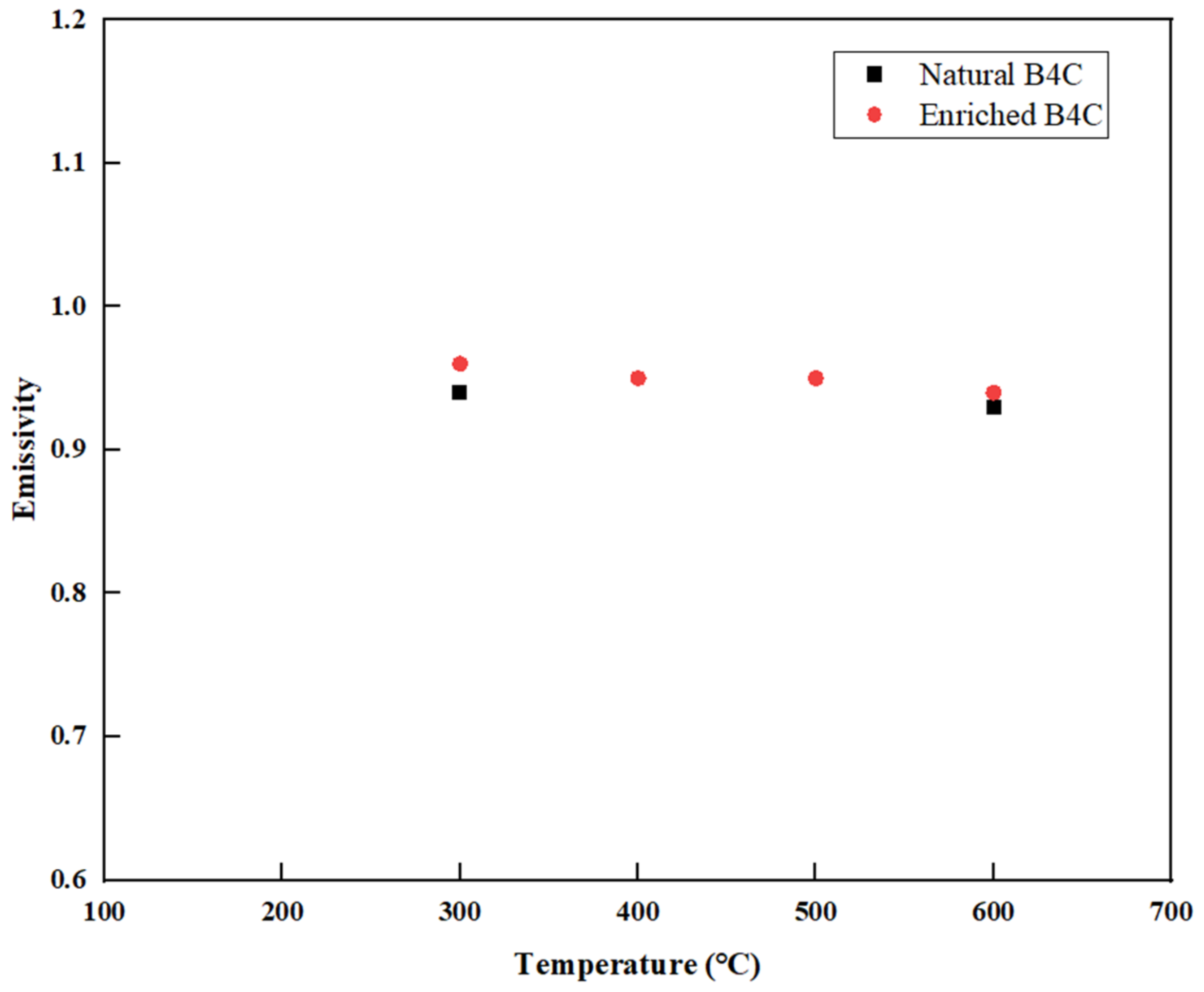
3.4. Emissivity
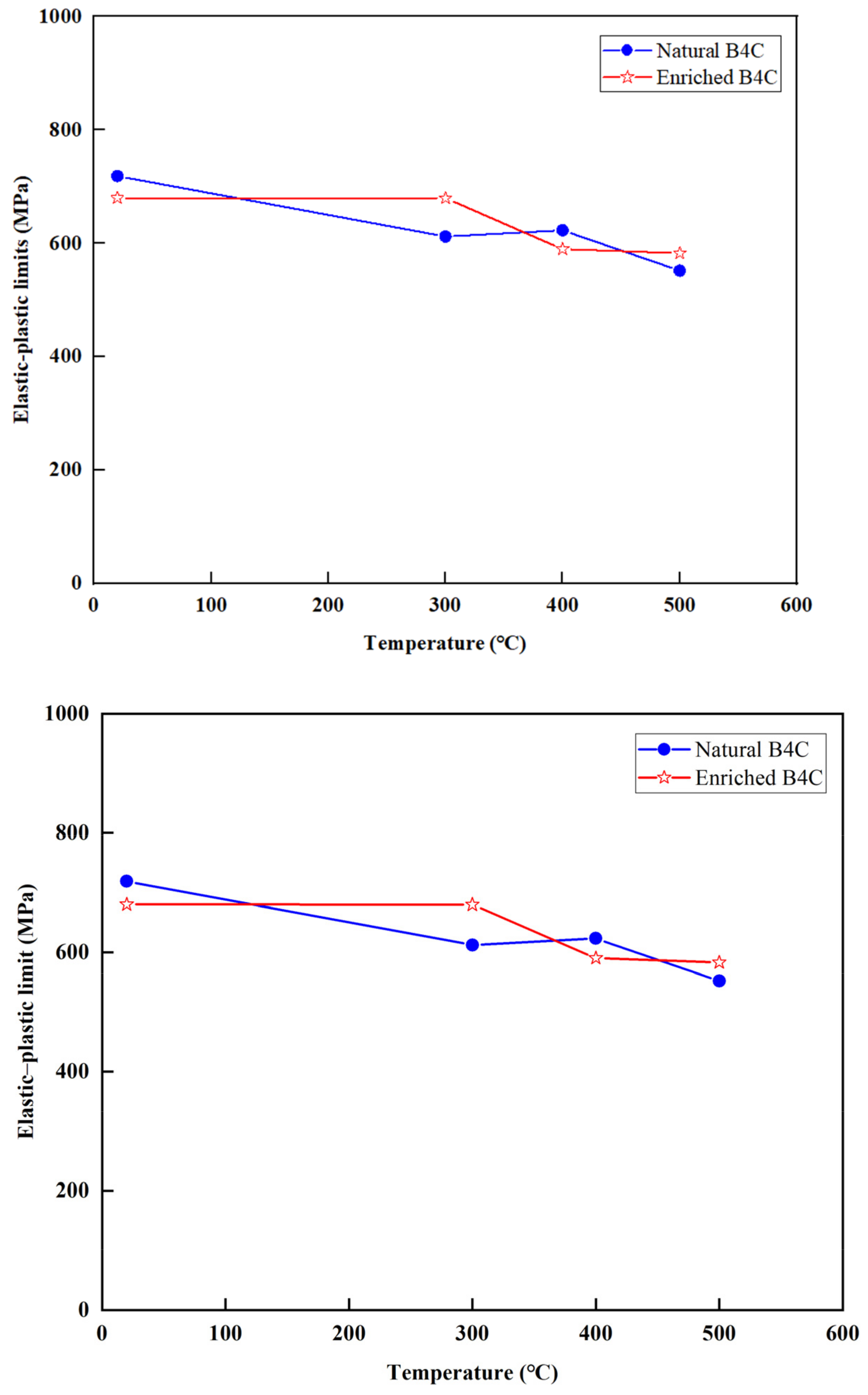
3.5. Elastic–Plastic Limit
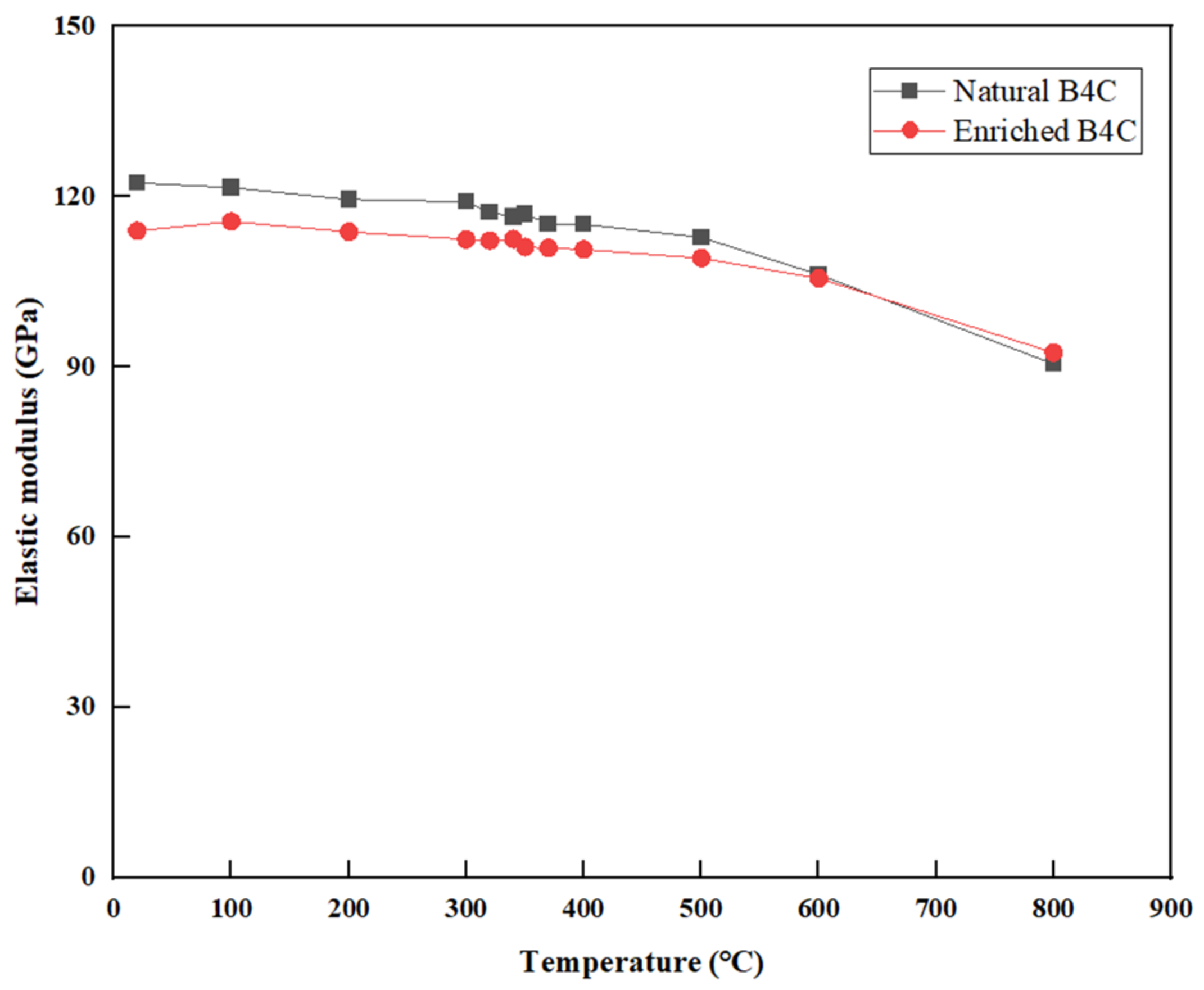
3.6. Elastic Modulus
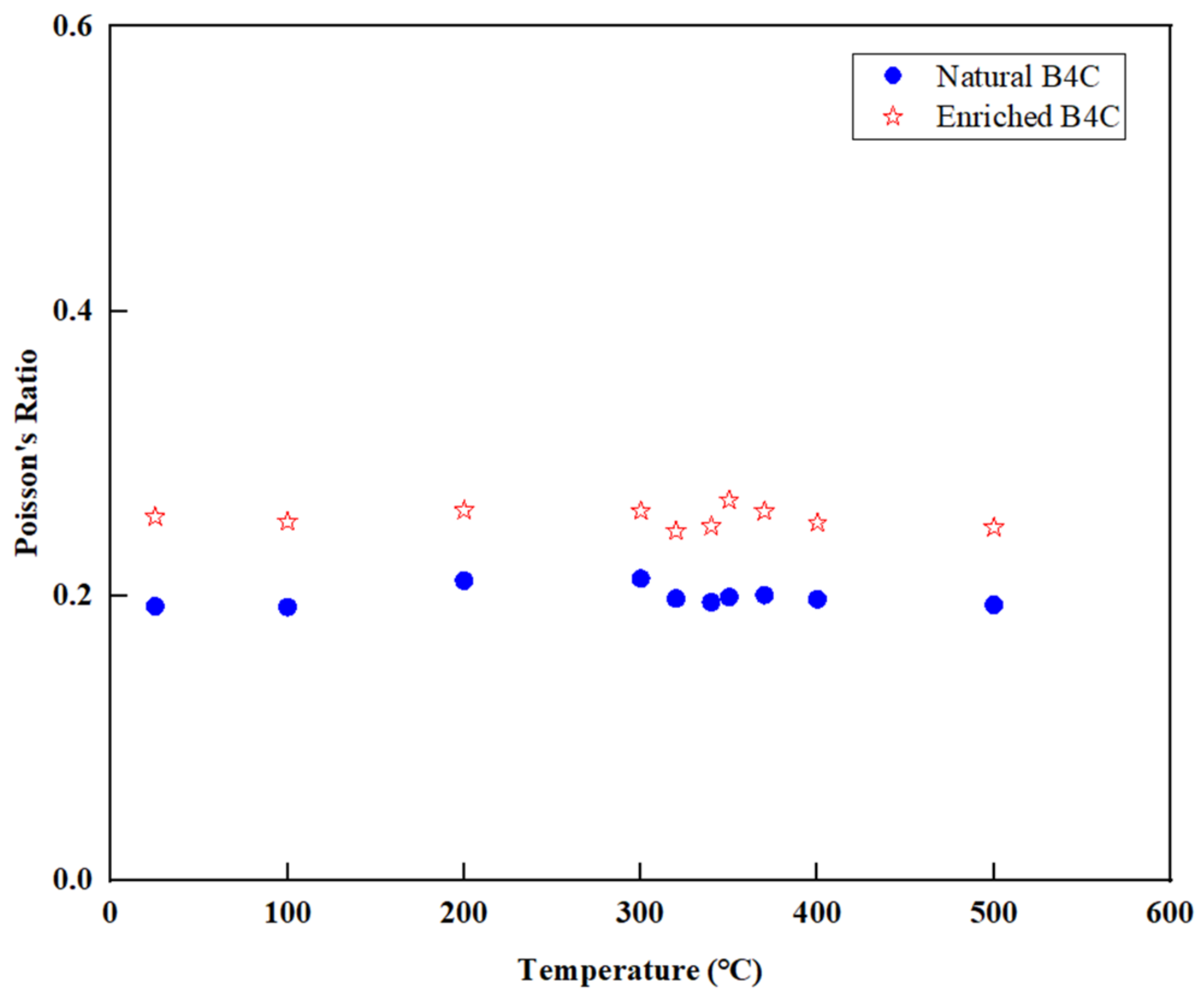
3.7. Poisson’s Ratio
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Domnich, V.; Reynaud, S.; Haber, R.A.; Chhowalla, M. Boron carbide: Structure, properties, and stability under stress. J. Am. Ceram. Soc. 2011, 94, 3605–3628. [Google Scholar] [CrossRef]
- Reddy, K.M.; Liu, P.; Hirata, A.; Fujita, T.; Chen, M.W. Atomic structure of amorphous shear bands in boron carbide. Nat. Commun. 2013, 4, 2483. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Schaefer, M.C.; Haber, R.A.; Hemker, K.J. Experimental observations of amorphization in stoichiometric and boron-rich boron carbide. Acta Mater. 2019, 181, 207–215. [Google Scholar] [CrossRef]
- Qu, Z.; Yu, C.; Wei, Y.; Su, X.; Du, A. Thermal Conductivity of boron carbide under fast neutron irradiation. J. Adv. Ceram. 2022, 11, 482–494. [Google Scholar] [CrossRef]
- Weaver, E.A.; Stegman, B.T.; Trice, R.W.; Youngblood, J.P. Mechanical properties of room-temperature injection molded, pressurelessly sintered boron carbide. Ceram. Int. 2022, 48, 11588–11596. [Google Scholar] [CrossRef]
- Wang, L.S. Preparation of boron carbide pellets and testing in fast breeder reactor. Chin. J. Nonferrous Met. 2006, 16, 1481–1485. [Google Scholar]
- Song, S.; Mu, Y.J.; Li, X.F.; Bai, P. Advance in boron-10 isotope separation by chemical exchange distillation. Ann. Nucl. Energy 2010, 37, 1–4. [Google Scholar] [CrossRef]
- Abdollahi, M.; Ahmadi, S.J. Application of ideal temperature gradient technology to optimize the chemical exchange and distillation process of boron isotopes separation by (CH3)2O-BF3 complex. Chem. Eng. Process. Process Intensif. 2014, 76, 26–32. [Google Scholar] [CrossRef]
- Zou, N.Z.; Zhu, Y.M.; Zhao, G.F. A discussion on IR Emissivity dependence on Temperature. Infrared Technol. 1996, 19, 1–4. [Google Scholar]
- Murgatroyd, R.A.; Kelly, B. Technology and assessment of neutron absorbing materials. At. Energy Rev. 1977, 15, 3–74. [Google Scholar]
- Beauvy, B.; Guery, M. Physical Properties of Boron Carbide; No. CEA-CONF-6652; CEA Centre d’Etudes Nucleaires de Saclay: Saclay, France, 1983. [Google Scholar]
- Wang, L.S.; Fan, Y.C.; Wu, F.; Ying, B.Y. The position of boron carbide in neutron absorbing materials and its properties relation to nuclear applications. Mater. Sci. Eng. Powder Metall. 2000, 5, 113–120. [Google Scholar]
- Franel, J.; Kingery, W.D. Thermal Conductivity: IX, Experimental Investigation of Effect of Porosity on Thermal Conductivity. J. Am. Ceram. Soc. 1954, 37, 99–107. [Google Scholar] [CrossRef]
- Homan, F.J. Performance Modeling of Neutron Absorbing. Nucl. Technol. 1972, 16, 216–225. [Google Scholar] [CrossRef]
- Bouchacourt, M.; Thevenot, F. The correlation between the thermoelectric properties and stoichiometry in the boron carbide phase B4C-B10.5C. J. Mater. Sci. 1985, 20, 1237–1247. [Google Scholar] [CrossRef]
- Garnier, J.E.; Begej, S. Ex-reactor Determination of Thermal Gap and Contact Conductance between Uranium Dioxyde and Zircaloy-4 interfaces. In Stage II: High Gas Pressure; US Nuclear Regulatory Commission: Rockville, MD, USA, 1979. [Google Scholar]
- Kuliiev, R.; Orlovskaya, N.; Hyer, H.; Sohn, Y.; Lugovy, M.; Ha, D.; Radovic, M.; Castle, E.G.; Reece, M.J.; Sasikumar, P.V.W.; et al. Spark Plasma Sintered B4C-Structure, Thermal, Electrical and Mechanical Properties. Materials 2020, 13, 1612. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Vanmeensel, K.; van der Biest, O.; Vleugels, J. In Situ Synthesis and Sensification of Submicrometer-Grained B4C–TiB2 Composites by Pulsed Electric Current Sintering. J. Eur. Ceram. Soc. 2011, 31, 637–644. [Google Scholar] [CrossRef]
- Dub, S.; Brazhkin, V.; Belous, V.; Tolmacheva, G.; Kononevskii, P. Comparative Nanoindentation of Single Crystals of Hard and Superhard Oxidess. J. Superhard Mater. 2014, 36, 217–230. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, R.X.; Xie, G.S.; Wang, X.R. Performance analysis of B4C used as shielding material in China experiment fast reactor. J. Power Energy Syst. 2008, 2, 864–873. [Google Scholar]
- Thevenot, F. A review on Boron Carbide. Key Eng. Mater. 1991, 56, 59–88. [Google Scholar] [CrossRef]
- Sairam, K.; Sonber, J.; Murthy, T.; Subramanian, C.; Fotedar, R.; Nanekar, P.; Hunbli, R. Influence of Spark Plasma Sintering Parameters on Densification and Mechanical Properties of Boron Carbide. Int. J. Refract. Met. Hard Mater. 2014, 42, 185–192. [Google Scholar] [CrossRef]
- Schwetz, K.A.; Grellner, W. The influence of carbon on the microstructure and mechanical properties of sintered boron carbide. Less Common Met. 1981, 82, 37–47. [Google Scholar] [CrossRef]
- Lee, H.; Speyer, R.F. Hardness and Fracture Toughness of Pressureless-Sintered Boron Carbide (B4C). J. Am. Ceram. Soc. 2002, 85, 1921–1923. [Google Scholar] [CrossRef]
- Hashin, Z.; Rosen, B.W. The elastic moduli of fiber-reinforced materials. J. Appl. Mech. 1964, 31, 223–232. [Google Scholar] [CrossRef]
- Hollenberg, G.W. Thermal induced stresses and fractures in boron carbide pellets. Ceram. Bull. 1980, 59, 538–548. [Google Scholar]
- Emin, D. Theory of electronic and thermal transport in boron carbides. In The Physics and Chemistry of Carbides, Nitrides and Borides; Springer: Dordrecht, The Netherlands, 1990; Volume 185, pp. 691–704. [Google Scholar]
- Savinnich, A.; Garkushkin, G.; Razorenov, S.; Rumiancev, V. Dynamic strength of reaction-sintered boron carbide ceramics. Tech. Phys. 2015, 60, 863–868. [Google Scholar] [CrossRef]
- Grady, D. Shock-Compression Properties of Ceramics; No. SAND-91-1474C; CONF-9110228-4; Sandia National Labs: Albuquerque, NM, USA, 1991. [Google Scholar]
- Dandekar, D.P. Shock Response of Boron Carbide; No. ARL-TR-2456; Army Research Laboratory: Adelphi, MD, USA, 2001. [Google Scholar]
- Grady, D.E. Hugoniot equation of state and dynamic strength of boron carbide. J. Appl. Phys. 2015, 117, 165904. [Google Scholar] [CrossRef]
- Hollenberg, G.W.; Walther, G. The elastic modulus and fracture of boron carbide. J. Am. Ceram. Soc. 1980, 63, 610–613. [Google Scholar] [CrossRef]
- Champagne, B.; Angers, R. Mechanical properties of hot-pressed B-B4C materials. J. Am. Ceram. Soc. 1979, 62, 149–153. [Google Scholar] [CrossRef]
- Sha, W.; Liu, Y.; Zhou, Y.; Huang, Y.; Huang, Z. Effect of Carbon Content on Mechanical Properties of Boron Carbide Ceramics Composites Prepared by Reaction Sintering. Materials 2022, 15, 6028. [Google Scholar] [CrossRef]
- Cheng, C.; Reddy, K.M.; Hirata, A.; Fujita, T.; Chen, M. Structure and mechanical properties of boron-rich boron carbides. J. Eur. Ceram. Soc. 2017, 37, 4514–4523. [Google Scholar] [CrossRef]


| Bt + Ct | Free B | Free C | F | Cl | Ca | Fe | Si | B/C | |
|---|---|---|---|---|---|---|---|---|---|
| Requirements | ≥98.0 | ≤0.50 | ≤0.70 | ≤0.0025 | ≤0.0075 | ≤0.30 | ≤1.0 | ≤0.30 | 4 ± 0.3 |
| Natural B4C | 99.15 | 0.06 | 0.31 | 0.00001 | 0.00002 | 0.09 | 0.07 | 0.12 | 4.28 |
| Enrichment of B4C | 99.32 | 0.28 | 0.50 | 0.00001 | 0.00005 | 0.09 | 0.05 | 0.20 | 4.07 |
| Device Name | MTS 810 100kN Material Testing Machine |
|---|---|
| Origin of equipment | USA |
| Measuring range | 2 kN~100 kN |
| Uncertainty or Accuracy class or Maximum permissible error | 0.5%, k = 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Z.; Hu, H.; Cao, J.; Lin, S.; Li, C.; Nie, L.; Zhou, X.; Ren, Q.; Lv, Q.; Hu, J. Study on the Thermophysical Properties of 80% 10B Enrichment of B4C. Materials 2023, 16, 7212. https://doi.org/10.3390/ma16227212
Lv Z, Hu H, Cao J, Lin S, Li C, Nie L, Zhou X, Ren Q, Lv Q, Hu J. Study on the Thermophysical Properties of 80% 10B Enrichment of B4C. Materials. 2023; 16(22):7212. https://doi.org/10.3390/ma16227212
Chicago/Turabian StyleLv, Zhipeng, Haixiang Hu, Jin Cao, Shaofang Lin, Changzheng Li, Lihong Nie, Xuanpu Zhou, Qisen Ren, Qingyang Lv, and Jing Hu. 2023. "Study on the Thermophysical Properties of 80% 10B Enrichment of B4C" Materials 16, no. 22: 7212. https://doi.org/10.3390/ma16227212