A Polytetrafluoroethylene-Based Solvent-Free Procedure for the Manufacturing of Lithium-Ion Batteries
Abstract
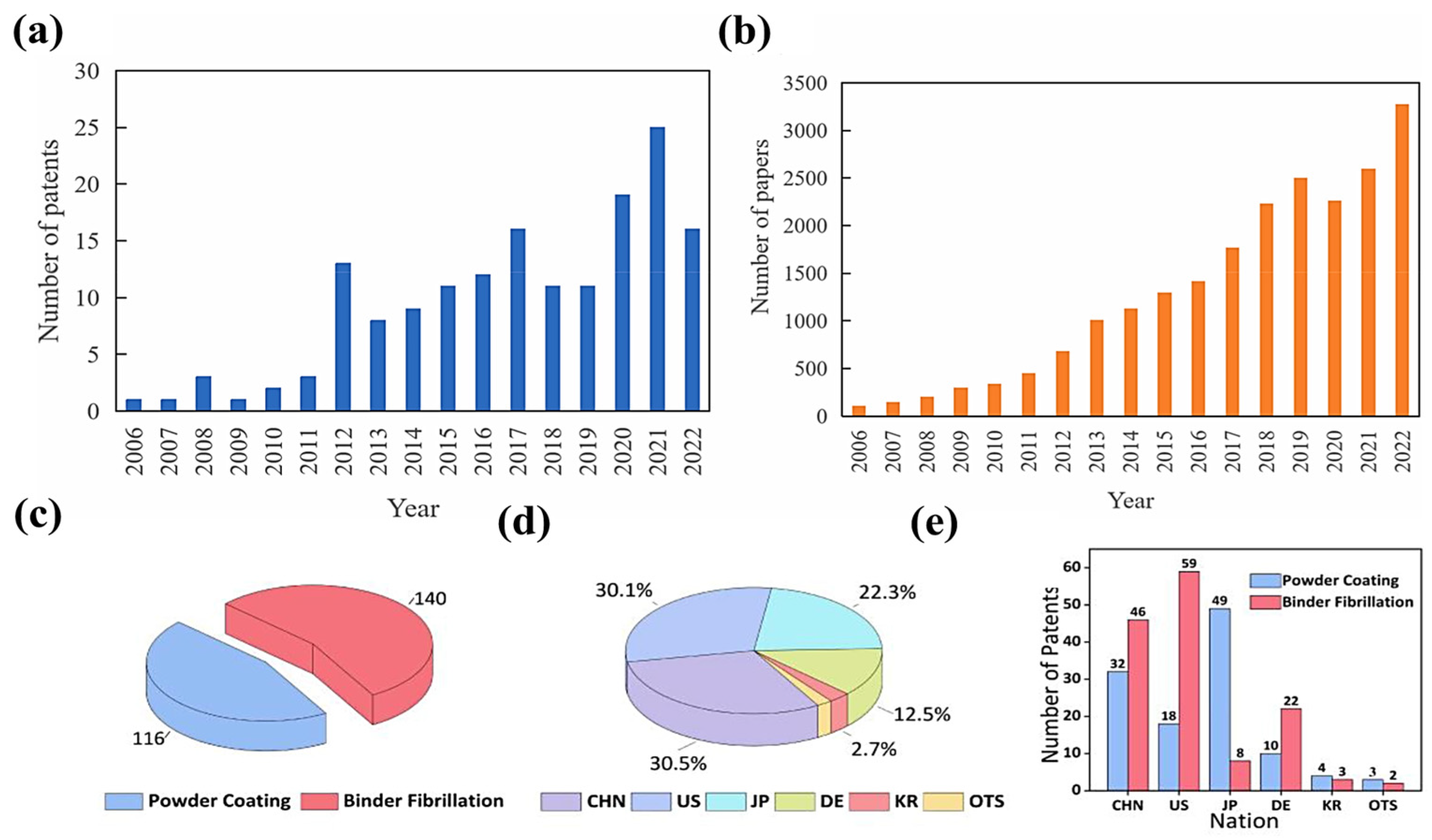
:1. Introduction
2. Developments in SF Processes and Binders
2.1. SF Processes
- I.
- Dry spray deposition
- II.
- Vapor deposition
- III.
- Melting and extrusion
- IV.
- 3D printing
- V.
- Direct pressing
- VI.
- Polymer fibrillation
2.2. Binders
3. Binders of PTFE
3.1. Aqueous Binders of PTFE
3.2. SF Binders of PTFE
3.2.1. Molecular Structure
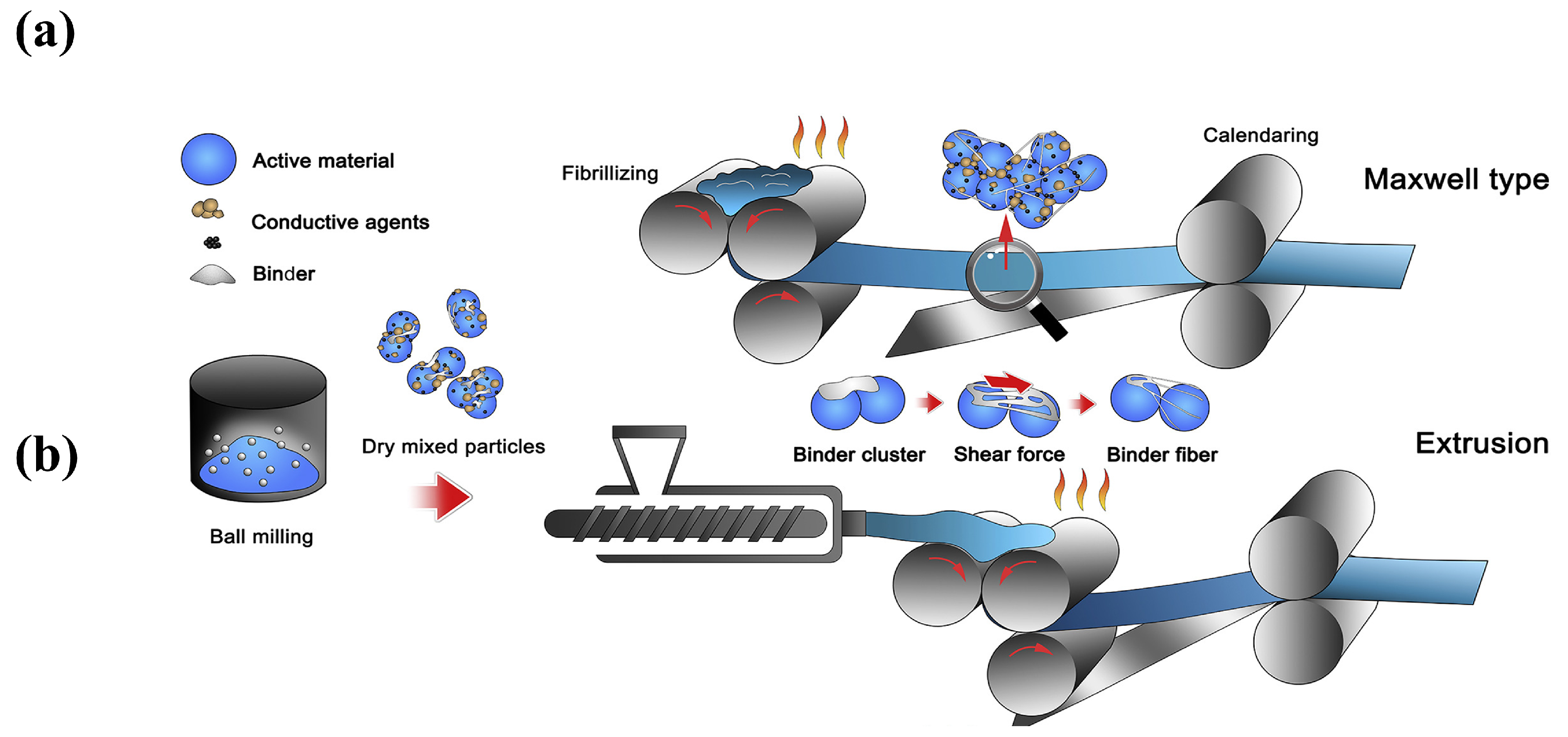
3.2.2. The Principle of Polymer Fibrillation

3.2.3. Factors Affecting PTFE Fibrillation
4. SF Process with PTFE Binder
4.1. Positive Characteristics
- I.
- It is environmentally friendly and suitable for large-scale production.
- II.
- It exhibits a flatter electrode shape than that from the wet process.
- III.
- It offers greater compaction density.
- IV.
- It improves the performance of the battery
- V.
- It allows for the possibility of prepare solid-state batteries.
4.2. Development Status of SF Process with PTFE Binder
4.2.1. Effect of PTFE on SF Batteries
- I.
- Side reactions of PTFE binders
- II.
- Crystallinity
- III.
- Modified materials
4.2.2. Influence of Components Other Than Binders
- I.
- Conductive additives
- II.
- Electrode materials
- III.
- Collectors
4.2.3. Innovative Technology and System
- I.
- High-speed airflow technology
- II.
- Lithium-sulfur (Li-S) batteries
- III.
- High-voltage batteries
- IV.
- High-load batteries
- V.
- Solid-state batteries
5. Challenges and Recommendations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dang, C.C.; Mu, Q.; Xie, X.B.; Sun, X.Q.; Yang, X.Y.; Zhang, Y.P.; Maganti, S.; Huang, M.N.; Jiang, Q.L.; Seok, I.; et al. Recent Progress in Cathode Catalyst for Nonaqueous Lithium Oxygen Batteries: A review. Adv. Compos. Hybrid Mater. 2022, 5, 606–626. [Google Scholar] [CrossRef]
- Eng, A.Y.S.; Soni, C.B.; Lum, Y.; Khoo, E.; Yao, Z.; Vineeth, S.K.; Kumar, V.; Lu, J.; Johnson, C.S.; Wolverton, C.; et al. Theory-Guided Experimental Design in Battery Materials Research. Sci. Adv. 2022, 8, eabm2422. [Google Scholar] [CrossRef]
- Jiang, M.; Danilov, D.L.; Eichel, R.A.; Notten, P.H.L. A Review of Degradation Mechanisms and Recent Achievements for Ni-Rich Cathode-Based Li-Ion Batteries. Adv. Energy Mater. 2021, 11, 2103005. [Google Scholar] [CrossRef]
- Kalnaus, S.; Dudney, N.J.; Westover, A.S.; Herbert, E.; Hackney, S. Solid-State Batteries: The Critical Role of Mechanics. Science 2023, 381, eabg5998. [Google Scholar] [CrossRef]
- Li, J.L.; Fleetwood, J.; Hawley, W.B.; Kays, W. From Materials to Cell: State-of-the-Art and Prospective Technologies for Lithium-Ion Battery Electrode Processing. Chem. Rev. 2022, 122, 903–956. [Google Scholar] [CrossRef]
- Liu, W.; Placke, T.; Chau, K.T. Overview of Batteries and Battery Management for Electric Vehicles. Energy Rep. 2022, 8, 4058–4084. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, B.Q.; Sun, Q.; Wennersten, R. Application of Energy Storage in Integrated Energy Systems-A Solution to Fluctuation and Uncertainty of Renewable Energy. J. Energy Storage 2022, 52, 104812. [Google Scholar] [CrossRef]
- Viswanathan, V.; Epstein, A.H.; Chiang, Y.M.; Takeuchi, E.; Bradley, M.; Langford, J.; Winter, M. The Challenges and Opportunities of Battery-Powered Flight. Nature 2022, 601, 519–525. [Google Scholar] [CrossRef]
- Xing, C.W.; Li, M.C.; Liu, L.Y.; Lu, R.; Liu, N.; Wu, W.J.; Yuan, D.D. A Comprehensive Review on the Blending Condition Between Virgin and RAP Asphalt Binders in Hot Recycled Asphalt Mixtures: Mechanisms, Evaluation Methods, and Influencing Factors. J. Clean. Prod. 2023, 398, 136515. [Google Scholar] [CrossRef]
- Zhu, C.; Usiskin, R.E.; Yu, Y.; Maier, J. The Nanoscale Circuitry of Battery Electrodes. Science 2017, 358, eaao2808. [Google Scholar] [CrossRef]
- Ludwig, A.; Wu, M.; Kharicha, A. On The Importance of Modeling 3D Shrinkage Cavities for the Prediction of Macrosegregation in Steel Ingots. CFD Model. Simu. Mat. Pro. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Pillai, A.M.; Salini, P.S.; John, B.; Devassy, M.T. Aqueous Binders for Cathodes: A Lodestar for Greener Lithium Ion Cells. Energy Fuels 2022, 36, 5063–5087. [Google Scholar] [CrossRef]
- Guo, R.N.; Han, W.Q. Effects of Structure and Properties of Polar Polymeric Binders on Lithium-ion Batteries. Inorg. Mater. 2019, 34, 1021–1029. [Google Scholar] [CrossRef]
- Wang, Y.B.; Yang, Q.; Guo, X.; Yang, S.; Chen, A.; Liang, G.J.; Zhi, C.Y. Strategies of Binder Design for High-Performance Lithium-Ion Batteries: A Mini Review. Rare Metals 2022, 41, 745–761. [Google Scholar] [CrossRef]
- Pettinger, K.-H.; Dong, W. When Does the Operation of a Battery Become Environmentally Positive? J. Electrochem. Soc. 2017, 164, A6274. [Google Scholar] [CrossRef]
- Yuan, C.; Deng, Y.; Li, T.; Yang, F. Manufacturing Energy Analysis of Lithium Ion Battery Pack for Electric Vehicles. CIRP Annals 2017, 66, 53–56. [Google Scholar] [CrossRef]
- Ahmed, S.; Nelson, P.A.; Gallagher, K.G.; Dees, D.W. Energy impact of cathode drying and solvent recovery during lithium-ion battery manufacturing. J. Power Sources 2016, 322, 169–178. [Google Scholar] [CrossRef]
- Schmitt, M.; Baunach, M.; Wengeler, L.; Peters, K.; Junges, P.; Scharfer, P.; Schabel, W. Slot-Die Processing of Lithium-Ion Battery Electrodes—Coating Window Characterization. Chem. Eng. Process 2013, 68, 32–37. [Google Scholar] [CrossRef]
- Stein, M.; Mistry, A.; Mukherjee, P.P. Mechanistic Understanding of the Role of Evaporation in Electrode Processing. J. Electrochem. Soc. 2017, 164, A1616. [Google Scholar] [CrossRef]
- Rollag, K.; Juarez-Robles, D.; Du, Z.; Wood, D.L., III; Mukherjee, P.P. Drying Temperature and Capillarity-Driven Crack Formation in Aqueous Processing of Li-Ion Battery Electrodes. ACS Appl. Energy Mater. 2019, 2, 4464–4476. [Google Scholar] [CrossRef]
- Flick, B.; Talsness, C.E.; Jäckh, R.; Buesen, R.; Klug, S. Embryotoxic Potential of N-methyl-pyrrolidone (NMP) and Three of Its Metabolites Using The Rat Whole Embryo Culture System. Toxicol. Appl. Pharmacol. 2009, 237, 154–167. [Google Scholar] [CrossRef]
- Lingappan, N.; Kong, L.X.; Pecht, M. The Significance of Aqueous Binders in Lithium-Ion Batteries. Renew. Sust. Energ. Rev. 2021, 147, 111227. [Google Scholar] [CrossRef]
- Wood, D.L.; Li, J.; Daniel, C. Prospects for Reducing the Processing Cost of Lithium Ion Batteries. J. Power Sources 2015, 275, 234–242. [Google Scholar] [CrossRef]
- Kang, Y.Q.; Deng, C.J.; Chen, Y.Q.; Liu, X.Y.; Liang, Z.; Li, T.; Hu, Q.; Zhao, Y. Binder-Free Electrodes and Their Application for Li-Ion Batteries. Nanoscale Res. Lett. 2020, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Qin, X.Y.; Zhao, S.Q.; Wang, A.; Luo, J.; Wang, Z.L.; Kang, F.Y.; Lin, Z.Q.; Li, B.H. Advanced Matrixes for Binder-Free Nanostructured Electrodes in Lithium-Ion Batteries. Adv. Mater. 2020, 32, 1908445. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Wang, Z.; Volkov, V.; Lou, F.; Yu, Z. Recent Technology Development in Solvent-Free Electrode Fabrication for Lithium-Ion Batteries. Renew. Sust. Energ. Rev. 2023, 183, 113515. [Google Scholar] [CrossRef]
- Al-Shroofy, M.; Zhang, Q.; Xu, J.; Chen, T.; Kaur, A.P.; Cheng, Y.-T. Solvent-Free Dry Powder Coating Process for Low-Cost Manufacturing of LiNi1/3Mn1/3Co1/3O2 Cathodes in Lithium-Ion Batteries. J. Power Sources 2017, 352, 187–193. [Google Scholar] [CrossRef]
- Wecker, S.M.; Davidson, T.; Baker, D.W. Preferred Orientation of Crystallites in Uniaxially Deformed Polytetrafluoroethylene. J. Appl. Phys. 1972, 43, 4344–4348. [Google Scholar] [CrossRef]
- Puts, G.J.; Crouse, P.; Ameduri, B.M. Polytetrafluoroethylene: Synthesis and Characterization of the Original Extreme Polymer. Chem. Rev. 2019, 119, 1763–1805. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Nam, J.S.; Linh Nguyen, C.T.; Jo, Y.; Lee, K.; Hwang, S.M.; Kim, Y.J. Design of Densified Nickel-Rich Layered Composite Cathode via The Dry-Film Process for Sulfide-Based Solid-State Batteries. J. Mater. Chem. A 2022, 10, 23222–23231. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Wang, Z.; Xu, J.; Ma, T.; Chen, L.; Li, H.; Wu, F. Progress in Solvent-Free Dry-Film Technology for Batteries and Supercapacitors. Mater. Today 2022, 55, 92–109. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.; Wang, J.; Wang, Y. Current and Future Lithium-Ion Battery Manufacturing. iScience 2021, 24, 102332. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, B.; Liu, J.; Chen, I.M.; Liu, Y.; Shou, W.; Wang, Y.; Pan, H. Understanding Interfacial-Energy-Driven Dry Powder Mixing for Solvent-Free Additive Manufacturing of Li-Ion Battery Electrodes. Ad. Mater. Interfaces 2017, 4, 1700570. [Google Scholar] [CrossRef]
- Shiraki, S.; Oki, H.; Takagi, Y.; Suzuki, T.; Kumatani, A.; Shimizu, R.; Haruta, M.; Ohsawa, T.; Sato, Y.; Ikuhara, Y.; et al. Fabrication of All-Solid-State Battery Using Epitaxial LiCoO2 Thin Films. J. Power Sources 2014, 267, 881–887. [Google Scholar] [CrossRef]
- Subramanyam, G.; Cole, M.W.; Sun, N.X.; Kalkur, T.S.; Sbrockey, N.M.; Tompa, G.S.; Guo, X.; Chen, C.; Alpay, S.P.; Rossetti, G.A.; et al. Challenges and Opportunities for Multi-Functional Oxide Thin Films for Voltage Tunable Radio Frequency/Microwave Components. J. Appl. Phys. 2013, 114, 191301. [Google Scholar] [CrossRef]
- Sotomayor, M.E.; Torre-Gamarra, C.d.l.; Levenfeld, B.; Sanchez, J.-Y.; Varez, A.; Kim, G.-T.; Varzi, A.; Passerini, S. Ultra-Thick Battery Electrodes for High Gravimetric and Volumetric Energy Density Li-Ion Batteries. J. Power Sources 2019, 437, 226923. [Google Scholar] [CrossRef]
- Trembacki, B.; Duoss, E.; Oxberry, G.; Stadermann, M.; Murthy, J. Mesoscale Electrochemical Performance Simulation of 3D Interpenetrating Lithium-Ion Battery Electrodes. J. Electrochem. Soc. 2019, 166, A923. [Google Scholar] [CrossRef]
- Carneiro, O.S.; Silva, A.F.; Gomes, R. Fused Deposition Modeling with Polypropylene. Mater. Des. 2015, 83, 768–776. [Google Scholar] [CrossRef]
- Kirsch, D.J.; Lacey, S.D.; Kuang, Y.; Pastel, G.; Xie, H.; Connell, J.W.; Lin, Y.; Hu, L. Scalable Dry Processing of Binder-Free Lithium-Ion Battery Electrodes Enabled by Holey Graphene. ACS Appl. Energy Mater. 2019, 2, 2990–2997. [Google Scholar] [CrossRef]
- Han, X.; Funk, M.R.; Shen, F.; Chen, Y.-C.; Li, Y.; Campbell, C.J.; Dai, J.; Yang, X.; Kim, J.W.; Liao, Y.; et al. Scalable Holey Graphene Synthesis and Dense Electrode Fabrication toward High-Performance Ultracapacitors. ACS Nano 2014, 8, 8255–8265. [Google Scholar] [CrossRef]
- Lee, D.J.; Jang, J.; Lee, J.-P.; Wu, J.; Chen, Y.-T.; Holoubek, J.; Yu, K.; Ham, S.-Y.; Jeon, Y.; Kim, T.-H.; et al. Physio-Electrochemically Durable Dry-Processed Solid-State Electrolyte Films for All-Solid-State Batteries. Adv. Funct. Mater. 2023, 33, 2301341. [Google Scholar] [CrossRef]
- Wood, D.L.; Wood, M.; Li, J.; Du, Z.; Ruther, R.E.; Hays, K.A.; Muralidharan, N.; Geng, L.; Mao, C.; Belharouak, I. Perspectives on The Relationship Between Materials Chemistry and Roll-to-Roll Electrode Manufacturing for High-Energy Lithium-Ion Batteries. Energy Stor. Mater. 2020, 29, 254–265. [Google Scholar] [CrossRef]
- Ludwig, B.; Zheng, Z.; Shou, W.; Wang, Y.; Pan, H. Solvent-Free Manufacturing of Electrodes for Lithium-ion Batteries. Sci. Rep. 2016, 6, 23150. [Google Scholar] [CrossRef]
- Hieu, D.; Joon, S.; Yudi, Y. Dry Electrode Coating Technology; Maxwell Technologies: San Diego, CA, USA, 2018; Available online: https://api.semanticscholar.org/CorpusID:201928996 (accessed on 19 October 2023).
- Lu, Y.; Zhao, C.-Z.; Yuan, H.; Hu, J.-K.; Huang, J.-Q.; Zhang, Q. Dry Electrode Technology, The Rising Star in Solid-state Battery Industrialization. Matter 2022, 5, 876–898. [Google Scholar] [CrossRef]
- Zou, F.; Manthiram, A. A Review of the Design of Advanced Binders for High-Performance Batteries. Adv. Energy Mater. 2020, 10, 2002508. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and Prospects of PVDF Based Polymer Electrolytes. J. Energy Chem. 2022, 64, 62–84. [Google Scholar] [CrossRef]
- Zhu, T.; Sternlicht, H.; Ha, Y.; Fang, C.; Liu, D.; Savitzky, B.H.; Zhao, X.; Lu, Y.; Fu, Y.; Ophus, C.; et al. Formation of Hierarchically Ordered Structures in Conductive Polymers to Enhance the Performances of Lithium-Ion Batteries. Nat. Energy 2023, 8, 129–137. [Google Scholar] [CrossRef]
- Oh, J.; Choi, S.H.; Chang, B.; Lee, J.; Lee, T.; Lee, N.; Kim, H.; Kim, Y.; Im, G.; Lee, S.; et al. Elastic Binder for High-Performance Sulfide-Based All-Solid-State Batteries. ACS Energy Lett. 2022, 7, 1374–1382. [Google Scholar] [CrossRef]
- Mu, P.; Zhang, H.; Jiang, H.; Dong, T.; Zhang, S.; Wang, C.; Li, J.; Ma, Y.; Dong, S.; Cui, G. Bioinspired Antiaging Binder Additive Addressing the Challenge of Chemical Degradation of Electrolyte at Cathode/Electrolyte Interphase. J. Am. Chem. Soc. 2021, 143, 18041–18051. [Google Scholar] [CrossRef]
- Maleki, H.; Deng, G.; Kerzhner-Haller, I.; Anani, A.; Howard, J.N. Thermal Stability Studies of Binder Materials in Anodes for Lithium-Ion Batteries. J. Electrochem. Soc 2000, 147, 4470. [Google Scholar] [CrossRef]
- Yonaga, A.; Kawauchi, S.; Mori, Y.; Xuanchen, L.; Ishikawa, S.; Nunoshita, K.; Inoue, G.; Matsunaga, T. Effects of Dry Powder Mixing on Electrochemical Performance of Lithium-ion Battery Electrode Using Solvent-Free Dry Forming Process. J. Power Sources 2023, 581, 233466. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, D.; Xiao, M.; Wang, S.; Feng, Y.; Huang, S.; Meng, Y. New Potential Substitute of PVDF Binder: Poly(propylene carbonate) for Solvent-Free Manufacturing High-Loading Cathodes of LiFePO4|Li Batteries. Ionics 2023, 29, 3895–3906. [Google Scholar] [CrossRef]
- Abdel-Hakim, A.; El-Basheer, T.M.; Abdelkhalik, A. Mechanical, Acoustical and Flammability Properties of SBR and SBR-PU Foam Layered Structure. Poly. Test. 2020, 88, 106536. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Ma, T.; Wang, Z.; Gao, Q.; Xu, J.; Chen, L.; Li, H.; Wu, F. Long-Life Sulfide All-Solid-State Battery Enabled by Substrate-Modulated Dry-Process Binder. Adv. Energy Mater. 2022, 12, 2201732. [Google Scholar] [CrossRef]
- Park, J.; Willenbacher, N.; Ahn, K.H. How the Interaction Between Styrene-Butadiene-Rubber (SBR) Binder and a Secondary Fluid Affects the Rheology, Microstructure and Adhesive Properties of Capillary-Suspension-Type Graphite Slurries Used for Li-ion Battery Anodes. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123692. [Google Scholar] [CrossRef]
- Dueramae, I.; Okhawilai, M.; Kasemsiri, P.; Uyama, H.; Kita, R. Properties Enhancement of Carboxymethyl Cellulose with Thermo-Responsive Polymer as Solid Polymer Electrolyte for Zinc Ion Battery. Sci. Rep. 2020, 10, 12587. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, J.; Park, K.; Kim, S.; Nam, K.W.; Char, K.; Choi, J.W. Host–Guest Interlocked Complex Binder for Silicon–Graphite Composite Electrodes in Lithium Ion Batteries. Adv. Energy Mater. 2022, 12, 2103718. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; El Salmawi, K.M. Preparation and Properties of Carboxymethyl Cellulose (CMC)/Sodium alginate (SA) Blends Induced by Gamma Irradiation. J. Polym. Environ. 2013, 21, 520–527. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, J.; Zhang, T.; Nuli, Y.; Wang, J.; Hirano, S.-i. Silicon Microparticle Anodes with Self-Healing Multiple Network Binder. Joule 2018, 2, 950–961. [Google Scholar] [CrossRef]
- Hu, Y.; Shao, D.; Chen, Y.; Peng, J.; Dai, S.; Huang, M.; Guo, Z.-H.; Luo, X.; Yue, K. A Physically Cross-Linked Hydrogen-Bonded Polymeric Composite Binder for High-Performance Silicon Anodes. ACS Appl. Energy Mater. 2021, 4, 10886–10895. [Google Scholar] [CrossRef]
- Senthil, C.; Kim, S.-S.; Jung, H.Y. Flame Retardant High-Power Li-S Flexible Batteries Enabled by Bio-macromolecular Binder Integrating Conformal Fractions. Nat. Commun. 2022, 13, 145. [Google Scholar] [CrossRef]
- Mackanic, D.G.; Yan, X.; Zhang, Q.; Matsuhisa, N.; Yu, Z.; Jiang, Y.; Manika, T.; Lopez, J.; Yan, H.; Liu, K.; et al. Decoupling of Mechanical Properties and Ionic Conductivity in Supramolecular Lithium Ion Conductors. Nat. Commun. 2019, 10, 5384. [Google Scholar] [CrossRef]
- Dong, T.; Zhang, H.; Hu, R.; Mu, P.; Liu, Z.; Du, X.; Lu, C.; Lu, G.; Liu, W.; Cui, G. A Rigid-Flexible Coupling Poly (vinylene carbonate) Based Cross-Linked Network: A versatile Polymer Platform for Solid-state Polymer Lithium Batteries. Energy Stor. Mater. 2022, 50, 525–532. [Google Scholar] [CrossRef]
- Xia, J.; Wang, Z.; Rodrig, N.D.; Nan, B.; Zhang, J.; Zhang, W.; Lucht, B.L.; Yang, C.; Wang, C. Super-Reversible CuF2 Cathodes Enabled by Cu2+-Coordinated Alginate. Adv. Mater. 2022, 34, 2205229. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.K.; Kwon, T.-w.; Lee, I.; Kim, T.-S.; Coskun, A.; Choi, J.W. Millipede-inspired structural design principle for high performance polysaccharide binders in silicon anodes. Energy Environ. Sci. 2015, 8, 1224–1230. [Google Scholar] [CrossRef]
- Strand, A.; Kouko, J.; Oksanen, A.; Salminen, K.; Ketola, A.; Retulainen, E.; Sundberg, A. Enhanced Strength, Stiffness and Elongation Potential of Paper by Spray Addition of Polysaccharides. Cellulose 2019, 26, 3473–3487. [Google Scholar] [CrossRef]
- Qin, T.; Yang, H.; Li, Q.; Yu, X.; Li, H. Design of Functional Binders for High-Specific-Energy Lithium-Ion Batteries: From Molecular Structure to Electrode Properties. Ind. Eng. Chem. Res. 2023. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Zhang, Y.; Wang, H.; Aboalhassan, A.A.; Li, G.; Xu, G.; Xue, C.; Yu, J.; Yan, J.; et al. Highly Elastic Block Copolymer Binders for Silicon Anodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 38132–38139. [Google Scholar] [CrossRef]
- Ignatieva, L.N.; Mashchenko, V.A.; Zverev, G.A.; Ustinov, A.Y.; Slobodyuk, A.B.; Bouznik, V.M. Study of The Manufactured Copolymers of Ethylene with Tetrafluoroethylene. J. Fluor. Chem. 2020, 231, 109460. [Google Scholar] [CrossRef]
- Jiang, Z.; Guo, Z.; Jia, Z.; Xiao, C.; An, S. Chemical Stability of Novel Melt Spinning FEP Fibers. e-Polymers 2016, 16, 171–176. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, X.; Shen, Z.; Ma, H.; Wang, J.; Wang, S.; Liu, L.; Liu, B.; Liu, L.; Zhao, Y. Green water-based binders for LiFePO4/C cathodes in Li-ion batteries: A comparative study. New J. Chem. 2021, 45, 9846–9855. [Google Scholar] [CrossRef]
- Huang, S.; Chen, H.; Chen, M.; Huang, Y.; He, X.; Zhuo, H.; Chen, S. Design of Conductive Binders for LiFePO4 Cathodes with Long-Term Cycle Life. ACS Sustain. Chem. Eng. 2021, 9, 13277–13286. [Google Scholar] [CrossRef]
- Huang, S.; Huang, X.T.; Huang, Y.Y.; He, X.Q.; Zhuo, H.T.; Chen, S.J. Rational Design of Effective Binders for LiFePO4 Cathodes. Polymers 2021, 13, 3146. [Google Scholar] [CrossRef]
- Gao, S.; Su, Y.; Bao, L.; Li, N.; Chen, L.; Zheng, Y.; Tian, J.; Li, J.; Chen, S.; Wu, F. High-Performance LiFePO4/C Electrode with Polytetrafluoroethylene as An Aqueous-Based Binder. J. Power Sources 2015, 298, 292–298. [Google Scholar] [CrossRef]
- Priyono, S.; Lubis, B.M.; Humaidi, S.; Prihandoko, B. Heating Effect on Manufacturing Li4Ti5O12 Electrode Sheet with PTFE Binder on Battery Cell Performance. IOP Conf. Ser.: Mater. Sci. Eng. 2018, 367, 012007. [Google Scholar] [CrossRef]
- Li, W.; Mays, S.; Lam, D. Material and Finite Element Analysis of Poly(tetrafluoroethylene) otary Seals. Plast. Rubber Compos. 2002, 31, 359–363. [Google Scholar] [CrossRef]
- Brown, E.N.; Rae, P.J.; Bruce Orler, E.; Gray, G.T.; Dattelbaum, D.M. The Effect of Crystallinity on the Fracture of Polytetrafluoroethylene (PTFE). Mater. Sci. Eng. C 2006, 26, 1338–1343. [Google Scholar] [CrossRef]
- Brown, E.N.; Dattelbaum, D.M. The Role of Crystalline Phase on Fracture and Microstructure Evolution of Polytetrafluoroethylene (PTFE). Polymer 2005, 46, 3056–3068. [Google Scholar] [CrossRef]
- Joyce, J.A. Fracture Toughness Evaluation of Polytetrafluoroethylene. Polym. Eng. Sci. 2003, 43, 1702–1714. [Google Scholar] [CrossRef]
- Rae, P.J.; Brown, E.N. The Properties of Poly(tetrafluoroethylene) (PTFE) in Tension. Polymer 2005, 46, 8128–8140. [Google Scholar] [CrossRef]
- Pruitt, L.A. Deformation, Yielding, Fracture and Fatigue Behavior of Conventional and Highly Cross-Linked Ultra High Molecular Weight Polyethylene. Biomaterials 2005, 26, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.N.; Trujillo, C.P.; Gray, G.T.; Rae, P.J.; Bourne, N.K. Soft Recovery of Polytetrafluoroethylene Shocked Through The Crystalline Phase II-III Transition. J. Appl. Phys. 2007, 101, 024916. [Google Scholar] [CrossRef]
- Millett, J.C.F.; Brown, E.N.; Gray, G.T.; Bourne, N.K.; Wood, D.C.; Appleby-Thomas, G. The Effects of Changing Chemistry on the Shock Response of Basic Polymers. J. Dyn. Behav. Mater. 2016, 2, 326–336. [Google Scholar] [CrossRef]
- Brown, E.N.; Rae, P.J.; Dattelbaum, D.M.; Clausen, B.; Brown, D.W. In-situ Measurement of Crystalline Lattice Strains in Polytetrafluoroethylene. Exp. Mech. 2008, 48, 119–131. [Google Scholar] [CrossRef]
- Lee, D.; Manthiram, A. Stable Cycling with Intimate Contacts Enabled by Crystallinity-Controlled PTFE-Based Solvent-Free Cathodes in All-Solid-State Batteries. Small Methods 2023, 7, 2201680. [Google Scholar] [CrossRef]
- Kitamura, T.; Okabe, S.; Tanigaki, M.; Kurumada, K.; Ohshima, M.; Kanazawa, S. Morphology Change in Polytetrafluoroethylene (PTFE) Porous Membrane Caused by Heat Treatment. Polym. Eng. Sci. 2000, 40, 809–817. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Feng, B.; Li, Y.; Wu, S.; Yin, Q.; Yu, Z.; Huang, J. The Effect of Temperature-Induced Phase Transition of PTFE on The Dynamic Mechanical Behavior and Impact-Induced Initiation Characteristics of Al/PTFE. Polym. Test. 2020, 91, 106835. [Google Scholar] [CrossRef]
- Sato, K.; Tominaga, Y.; Imai, Y.; Yoshiyama, T.; Aburatani, Y. Deformation Capability of Poly(tetrafluoroethylene) Materials: Estimation with X-ray Diffraction Measurements. Polym. Test 2022, 113, 107690. [Google Scholar] [CrossRef]
- Zhang, A.; Chai, J.; Yang, C.; Zhao, J.; Zhao, G.; Wang, G. Fibrosis Mechanism, Crystallization Behavior and Mechanical Properties of In-Situ Fibrillary PTFE Reinforced PP Composites. Mater. Des. 2021, 211, 110157. [Google Scholar] [CrossRef]
- Jordan, J.L.; Siviour, C.R.; Foley, J.R.; Brown, E.N. Compressive Properties of Extruded Polytetrafluoroethylene. Polymer 2007, 48, 4184–4195. [Google Scholar] [CrossRef]
- Zerilli, F.J. Dislocation Mechanics-Based Constitutive Equations. Metall. Mater. Trans. A 2004, 35, 2547–2555. [Google Scholar] [CrossRef]
- Liu, X.X.; Li, T.S.; Liu, X.J.; Lv, R.G.; Cong, P.H. An Investigation on The Friction of Oriented Polytetrafluoroethylene (PTFE). Wear 2007, 262, 1414–1418. [Google Scholar] [CrossRef]
- Liu, X.X.; Li, T.S.; Liu, X.J.; Lv, R.G.; Cong, P.H. Effect of Structure on The Tribological Properties of Polytetrafluoroethylene Drawn Uniaxially at The Melting Point. J. Appl. Polym. Sci. 2007, 106, 1332–1336. [Google Scholar] [CrossRef]
- Barry, P.R.; Chiu, P.Y.; Perry, S.S.; Sawyer, W.G.; Phillpot, S.R.; Sinnott, S.B. The Effect of Normal Load on Polytetrafluoroethylene Tribology. J. Phys. Condens. Matter Inst. Phys. J. 2009, 21, 144201. [Google Scholar] [CrossRef]
- Jang, I.; Burris, D.L.; Dickrell, P.L.; Barry, P.R.; Santos, C.; Perry, S.S.; Phillpot, S.R.; Sinnott, S.B.; Sawyer, W.G. Sliding Orientation Effects on The Tribological Properties of Polytetrafluoroethylene. J. Appl. Phys. 2007, 102, 123509. [Google Scholar] [CrossRef]
- Ma, J.; Chen, B.B.; Wang, L.L.; Cui, G.L. Progress and Prospect on Failure Mechanisms of Solid-State Lithium Batteries. J. Power Sources 2018, 392, 94–115. [Google Scholar] [CrossRef]
- Rae, P.J.; Dattelbaum, D.M. The properties of poly (tetrafluoroethylene) (PTFE) in compression. Polymer 2004, 45, 7615–7625. [Google Scholar] [CrossRef]
- Xie, Q.; Cui, Z.H.; Manthiram, A. Unveiling the Stabilities of Nickel-Based Layered Oxide Cathodes at an Identical Degree of Delithiation in Lithium-Based Batteries. Adv. Mater. 2021, 33, 2100804. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.T.; Walther, F.; Ahmed, S.; Ruess, R.; Hertle, J.; Mogwitz, B.; Volz, K.; Janek, J. Formation of an Artificial Cathode-Electrolyte Interphase to Suppress Interfacial Degradation of Ni-Rich Cathode Active Material with Sulfide Electrolytes for Solid- State Batteries. ACS Energy Lett. 2023, 8, 1322–1329. [Google Scholar] [CrossRef]
- Yao, W.; Chouchane, M.; Li, W.; Bai, S.; Liu, Z.; Li, L.; Chen, A.X.; Sayahpour, B.; Shimizu, R.; Raghavendran, G.; et al. A 5 V-Class Cobalt-Free Battery Cathode with High Loading Enabled by Dry Coating. Energy Environ. Sci. 2023, 16, 1620–1630. [Google Scholar] [CrossRef]
- Wu, Q.; Zheng, J.P.; Hendrickson, M.; Plichta, E.J. Dry Process for Fabricating Low Cost and High Performance Electrode for Energy Storage Devices. MRS Adv. 2019, 4, 857–863. [Google Scholar] [CrossRef]
- Li, G. The Influence of Polytetrafluorethylene Reduction on the Capacity Loss of the Carbon Anode for Lithium Ion Batteries. Solid State Ion. 1996, 90, 221–225. [Google Scholar] [CrossRef]
- Salini, P.S.; Gopinadh, S.V.; Kalpakasseri, A.; John, B.; Devassy, M.T. Toward Greener and Sustainable Li-Ion Cells: An Overview of Aqueous-Based Binder Systems. ACS Sustain. Chem. Eng. 2020, 8, 4003–4025. [Google Scholar] [CrossRef]
- Tao, R.; Tan, S.; Meyer Iii, H.M.; Sun, X.-G.; Steinhoff, B.; Sardo, K.; Bishtawi, A.; Gibbs, T.; Li, J. Insights into the Chemistry of the Cathodic Electrolyte Interphase for PTFE-Based Dry-Processed Cathodes. ACS Appl. Mater. Interfaces 2023, 15, 40488–40495. [Google Scholar] [CrossRef] [PubMed]
- Hippauf, F.; Schumm, B.; Doerfler, S.; Althues, H.; Fujiki, S.; Shiratsuchi, T.; Tsujimura, T.; Aihara, Y.; Kaskel, S. Overcoming Binder Limitations of Sheet-Type Solid-State Cathodes Using a Solvent-Free Dry-Film Approach. Energy Stor. Mater. 2019, 21, 390–398. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Lou, F.; Yu, Z. Leveraging Synergies by Combining Polytetrafluorethylene with Polyvinylidene Fluoride for Solvent-Free Graphite Anode Fabrication. Energy Technol. 2022, 10, 2200732. [Google Scholar] [CrossRef]
- Hong, S.B.; Lee, Y.J.; Kim, U.H.; Bak, C.; Lee, Y.M.; Cho, W.; Hah, H.J.; Sun, Y.K.; Kim, D.W. All-Solid-State Lithium Batteries: Li+-Conducting Ionomer Binder for Dry-Processed Composite Cathodes. ACS Energy Letter. 2022, 7, 1092–1100. [Google Scholar] [CrossRef]
- Schmidt, F.; Kirchhoff, S.; Jägle, K.; De, A.; Ehrling, S.; Härtel, P.; Dörfler, S.; Abendroth, T.; Schumm, B.; Althues, H.; et al. Sustainable Protein-Based Binder for Lithium-Sulfur Cathodes Processed by a Solvent-Free Dry-Coating Method. ChemSusChem 2022, 15, e202201320. [Google Scholar] [CrossRef]
- Zhang, Y.; Huld, F.; Lu, S.; Jektvik, C.; Lou, F.; Yu, Z. Revisiting Polytetrafluorethylene Binder for Solvent-Free Lithium-Ion Battery Anode Fabrication. Batteries 2022, 8, 57. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Lou, F.; Yu, Z. Solvent-free Lithium Iron Phosphate Cathode Fabrication with Fibrillation of Polytetrafluoroethylene. Electrochim. Acta 2023, 456, 142469. [Google Scholar] [CrossRef]
- Park, G.; Kim, H.-S.; Lee, K.J. Solvent-Free Processed Cathode Slurry with Carbon Nanotube Conductors for Li-Ion Batteries. Nanomaterials 2023, 13, 324. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Liang, Y.; Gheytani, S.; Yao, Y. Cross-Conjugated Oligomeric Quinones for High Performance Organic Batteries. Nano Energy 2017, 37, 46–52. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, M.; Gao, H.; Hou, D.; Yu, C.; Liu, C.; Zhang, D.; Wu, J.-C.; Yang, J.; Chen, D. Dense Integration of Solvent-Free Electrodes for Li-Ion Supercabattery with Boosted Low Temperature Performance. J. Power Sources 2020, 473, 228553. [Google Scholar] [CrossRef]
- Fiedler, M.; Cangaz, S.; Hippauf, F.; Dörfler, S.; Abendroth, T.; Althues, H.; Kaskel, S. Mechanistic Insights into the Cycling Behavior of Sulfur Dry-Film Cathodes. Adv. Sustain. Syst. 2023, 7, 2200439. [Google Scholar] [CrossRef]
- Tao, R.; Steinhoff, B.; Sun, X.-G.; Sardo, K.; Skelly, B.; Meyer, H.M.; Sawicki, C.; Polizos, G.; Lyu, X.; Du, Z.; et al. High-Throughput and High-performance Lithium-ion Batteries via Dry Processing. Chem. Eng. J. 2023, 471, 144300. [Google Scholar] [CrossRef]
- Wang, C.; Yu, R.; Duan, H.; Lu, Q.; Li, Q.; Adair, K.R.; Bao, D.; Liu, Y.; Yang, R.; Wang, J.; et al. Solvent-Free Approach for Interweaving Freestanding and Ultrathin Inorganic Solid Electrolyte Membranes. ACS Energy Lett. 2022, 7, 410–416. [Google Scholar] [CrossRef]



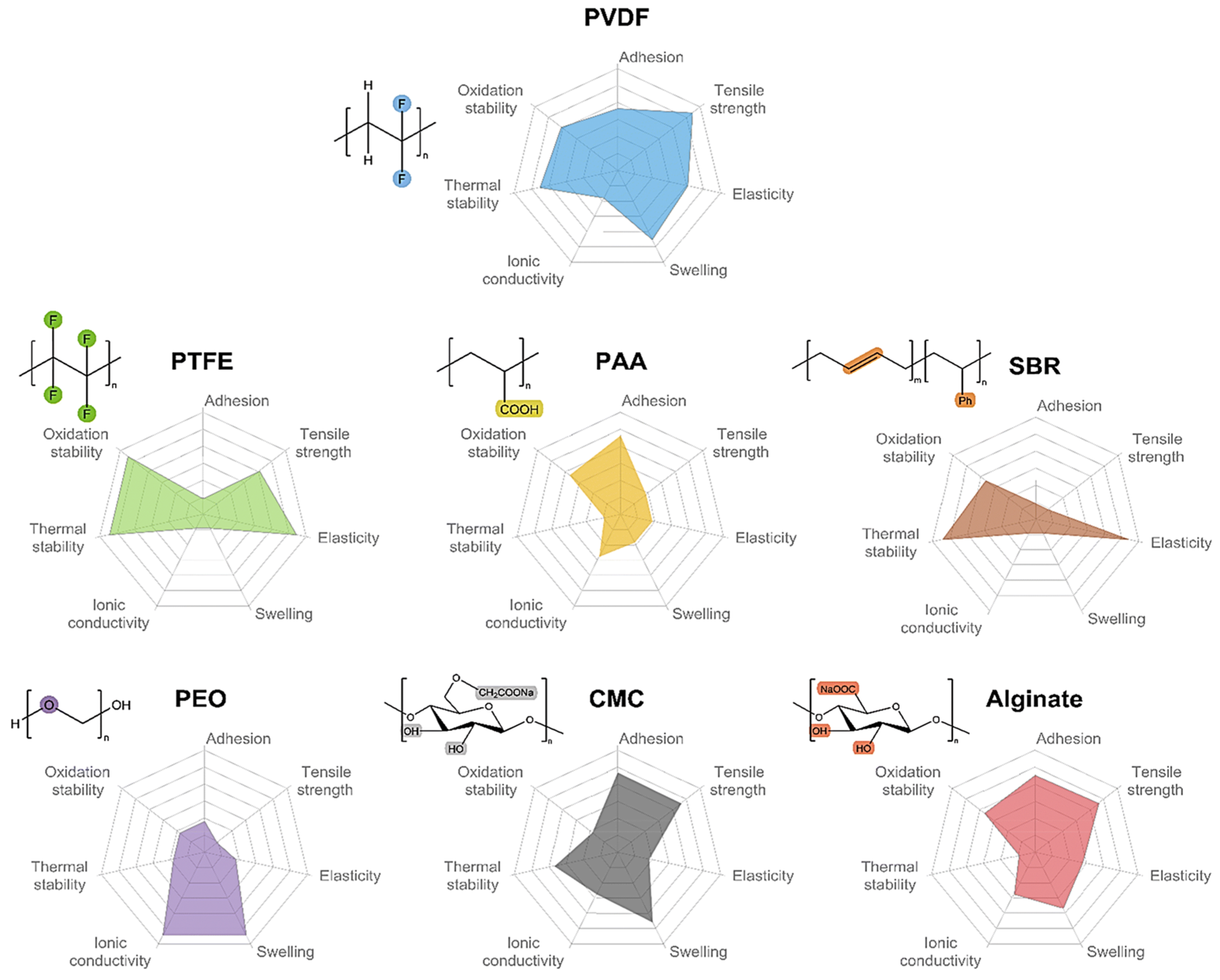






| Binder | Characteristic Functional Groups | Elastic Modulus (Mpa) | Break Elongation (%) | Ultimate Strength (MPa) | Adhesion Force (N cm−1) | Tg andd (°C) | References |
|---|---|---|---|---|---|---|---|
| PVDF | Fluorine | 1400 | 30 | 40 | 1 | −38,400 | [48,49,50,51,52,53] |
| PTFE | Fluorine | 400–1800 | 50–650 | 10–43 | – | −103,400 | [32] |
| SBR | Alkene, phenyl | 1.31 | 385 | 3.33 | 0.1–0.5 | −60,400 | [54,55,56] |
| CMC | Carboxyl ion | 1400 | <10 | 40 | 1.1–1.7 | 55,300 | [57,58,59] |
| PAA | Carboxyl | 450 | <5 | <10 | 1.5 | 115,150 | [60,61] |
| PEO | Ether | 700 | <10 | 15 | <0.5 | −50,350 | [62,63,64] |
| Alginate | Carboxyl ion | 1400 | 15 | 30 | 2 | 119, 200–500 | [65,66,67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chen, S.; Zhang, K.; Huang, L.; Shen, H.; Chen, Z.; Rong, C.; Wang, G.; Jiang, Z. A Polytetrafluoroethylene-Based Solvent-Free Procedure for the Manufacturing of Lithium-Ion Batteries. Materials 2023, 16, 7232. https://doi.org/10.3390/ma16227232
Wang X, Chen S, Zhang K, Huang L, Shen H, Chen Z, Rong C, Wang G, Jiang Z. A Polytetrafluoroethylene-Based Solvent-Free Procedure for the Manufacturing of Lithium-Ion Batteries. Materials. 2023; 16(22):7232. https://doi.org/10.3390/ma16227232
Chicago/Turabian StyleWang, Xuehan, Shuli Chen, Kaiqi Zhang, Licheng Huang, Huilin Shen, Zheng Chen, Changru Rong, Guibin Wang, and Zhenhua Jiang. 2023. "A Polytetrafluoroethylene-Based Solvent-Free Procedure for the Manufacturing of Lithium-Ion Batteries" Materials 16, no. 22: 7232. https://doi.org/10.3390/ma16227232







