Lignin-Based Mesoporous Hollow Carbon@MnO2 Nanosphere Composite as an Anodic Material for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of the Hollow L-C-NSs@MnO2 Nanosphere Composite
2.3. Characterizations
2.4. Electrochemical Measurements
3. Results and Discussion
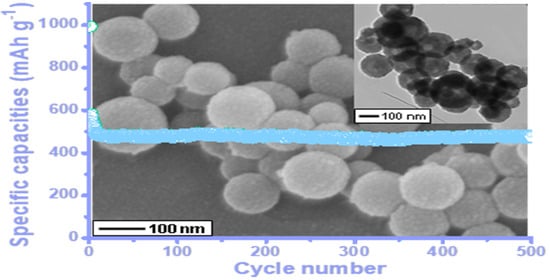
3.1. Characterizations of the Hollow L-C-NSs@MnO2 Nanosphere Composite
3.2. Electrochemical Properties of the Porous Hollow L-C-NSs@MnO2 Nanosphere Composite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, X.; Fang, S.; Li, Z.; Wu, Z.; Liu, Y.; Liu, Y.; Gao, M.; Du, W.; Yang, Y. Constructing a conductive and buffer network on microscale silicon based anodes for high-performance lithium-ion batteries. J. Alloys Compd. 2023, 949, 169846. [Google Scholar] [CrossRef]
- Sui, D.; Yao, M.; Si, L.; Yan, K.; Shi, J.; Wang, J.; Xu, C.C.; Zhang, Y. Biomass-derived carbon coated SiO2 nanotubes as superior anode for lithium-ion batteries. Carbon 2023, 205, 510–518. [Google Scholar] [CrossRef]
- Lin, Z.; Li, S.; Huang, J. Natural cellulose derived nanocomposites as anodic materials for lithium-ion batteries. Chem. Rec. 2020, 20, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Tenhaeff, W.E.; Rios, O.; More, K.; McGuire, M.A. Highly robust lithium ion battery anodes from lignin: An abundant, renewable, and low-cost material. Adv. Funct. Mater. 2014, 24, 86–94. [Google Scholar] [CrossRef]
- Chu, H.; Wu, Q.; Huang, J. Rice husk derived silicon/carbon and silica/carbon nanocomposites as anodic materials for lithium-ion batteries. Colloids Surf. A 2018, 558, 495–503. [Google Scholar] [CrossRef]
- Chang, Z.; Yu, B.; Wang, C. Influence of H2 reduction on lignin-based hard carbon performance in lithium ion batteries. Electrochim. Acta 2015, 176, 1352–1357. [Google Scholar] [CrossRef]
- Zhang, X.; Han, S.; Xiao, P.; Fan, C.; Zhang, W. Thermal reduction of graphene oxide mixed with hard carbon and their high performance as lithium ion battery anode. Carbon 2016, 100, 600–607. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Chen, L.; Huang, X. Monodispersed hard carbon spherules with uniform nanopores. Carbon 2001, 39, 2211–2214. [Google Scholar] [CrossRef]
- Han, Y.-J.; Chung, D.; Nakabayashi, K.; Chung, J.-D.; Jin, M.; Yoon, S.-H. Effect of heat pre-treatment conditions on the electrochemical properties of mangrove wood-derived hard carbon as an effective anode material for lithium-ion batteries. Electrochim. Acta 2016, 213, 432–438. [Google Scholar] [CrossRef]
- Chen, F.; Wu, L.; Zhou, Z.; Ju, J.; Zhao, Z.; Zhong, M.; Kuang, T. MoS2 decorated lignin-derived hierarchical mesoporous carbon hybrid nanospheres with exceptional Li-ion battery cycle stability. Chin. Chem. Lett. 2019, 30, 197–202. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Z.; Liu, A.; Lu, J.; Du, J.; Tao, Y.; Cheng, Y.; Wang, H. A porous carbon based on the surface and structural regulation of wasted lignin for long-cycle lithium-ion battery. Int. J. Biol. Macromol. 2022, 222, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Culebras, M.; Geaney, H.; Beaucamp, A.; Upadhyaya, P.; Dalton, E.; Ryan, K.M.; Collins, M.N. Bio-derived carbon nanofibres from lignin as high-performance Li-ion anode materials. Adv. Sustain. Syst. 2019, 12, 4516–4521. [Google Scholar] [CrossRef] [PubMed]
- Culebras, M.; Ren, G.; Connell, S.; Vilatela, J.J.; Collins, M.N. Lignin doped carbon nanotube yarns for improved thermoelectric efficiency. Adv. Sustain. Syst. 2020, 4, 2000147. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, H.; Li, F.; Li, X.; Wang, Z.; Cui, L.; Wang, J. Cooperation of nitrogen-doping and catalysis to improve the Li-ion storage performance of lignin-based hard carbon. J. Energy Chem. 2018, 27, 1390–1396. [Google Scholar] [CrossRef]
- Choi, D.I.; Lee, J.-N.; Song, J.; Kang, P.-H.; Park, J.-K.; Lee, Y.M. Fabrication of polyacrylonitrile/lignin-based carbon nanofibers for high-power lithium ion battery anodes. J. Solid State Electrochem. 2013, 17, 2471–2475. [Google Scholar] [CrossRef]
- Wang, S.-X.; Yang, L.; Stubbs, L.P.; Li, X.; He, C. Lignin-derived fused electrospun carbon fibrous mats as high performance anode materials for lithium ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 12275–12282. [Google Scholar] [CrossRef]
- Ma, X.; Smirnova, A.L.; Fong, H. Flexible lignin-derived carbon nanofiber substrates functionalized with iron (III) oxide nanoparticles as lithium-ion battery anodes. Mater. Sci. Eng. B 2019, 241, 100–104. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, F.; Kuang, T.; Chang, L.; Yang, J.; Fan, P.; Zhao, Z.; Zhong, M. Lignin-derived hierarchical mesoporous carbon and NiO hybrid nanospheres with exceptional Li-ion battery and pseudocapacitive properties. Electrochim. Acta 2018, 274, 288–297. [Google Scholar] [CrossRef]
- Du, J.; Li, Q.; Chai, J.; Jiang, L.; Zhang, Q.; Han, N.; Zhang, W.; Tang, B. Review of metal oxides as anode materials for lithium-ion batteries. Dalton Trans. 2022, 51, 9584–9590. [Google Scholar] [CrossRef]
- Liao, W.; Chen, H.; Zeng, Y.; Liu, L. Recent progress in the fabrication of nanostructured zinc-based ternary metal oxides for high-performance lithium-ion batteries. J. Appl. Electrochem. 2023, 53, 1077–1107. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, E.; Kim, S.; Byon, H.R. Layered transition metal oxides (LTMO) for oxygen evolution reactions and aqueous Li-ion batteries. Chem. Sci. 2023, 14, 10644–10663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Kitchaev, D.A.; Lebens-Higgins, Z.; Vinckeviciute, J.; Zuba, M.; Reeves, P.J.; Grey, C.P.; Whittingham, M.S.; Piper, L.F.J.; Ven, A.V.; et al. Pushing the limit of 3d transition metal-based layered oxides that use both cation and anion redox for energy storage. Nat. Rev. Mater. 2022, 7, 522–540. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, S.; Li, J.; Li, S.; Zhang, G.; Li, Z.; Liu, H. Pre-lithiation optimized voltage ranges and MnO2/rGO negative electrodes with oxygen vacancies for enhanced performance of lithium-ion capacitors. Electrochim. Acta 2022, 421, 140406. [Google Scholar] [CrossRef]
- Vishwanathan, S.; Moolayadukkam, S.; Gangaiah, V.K.; Matte, H.S.S.R. Amorphous MnO2-modified FeOOH ternary composite with high pseudocapacitance as anode for lithium-ion batteries. ACS Appl. Energy Mater. 2023, 6, 2022–2030. [Google Scholar] [CrossRef]
- Liu, J.; Wang, M.; Wang, Q.; Zhao, X.; Song, Y.; Zhao, T.; Sun, J. Sea Urchin-like Si@MnO2@rGO as anodes for high-performance lithium-ion batteries. Nanomaterials 2022, 12, 285. [Google Scholar] [CrossRef]
- Luo, C.; Chen, Y.; Tian, Q.; Zhang, W.; Sui, Z. Ultrathin porous MnO2@C nanosheets for high-performance lithium-ion battery anodes. J. Electroanal. Chem. 2023, 930, 117173. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Jia, M.; Nan, T.; Zhang, H.; Zhao, S.; Shen, Q. Elaborate construction and electrochemical properties of lignin derived macro-/micro-porous carbon-sulfur composites for rechargeable lithium-sulfur batteries: The effect of sulfur-loading time. J. Alloys Compd. 2017, 709, 677–685. [Google Scholar] [CrossRef]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Olmedo-Martínez, J.L.; Vega-Rios, A.; Flores-Gallardo, S.; Zaragoza-Contreras, E.A. Lignin in storage and renewable energy applications: A review. J. Energy Chem. 2018, 27, 1422–1438. [Google Scholar] [CrossRef]
- Li, X.-S.; Xu, M.-M.; Yang, Y.; Huang, Q.-B.; Wang, X.-Y.; Ren, J.-L.; Wang, X.-H. MnO2@corncob carbon composite electrode and all-solid-state supercapacitor with improved electrochemical performance. Materials 2019, 12, 2379. [Google Scholar] [CrossRef]
- Li, S.; Yang, M.; He, G.; Qi, D.; Huang, J. A cellulose-derived nanofibrous MnO2-TiO2-carbon composite as anodic material for lithium-ion batteries. Materials 2021, 14, 3411. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, J.S.; Lu, Q.; Lou, X.W. Porous spheres assembled from polythiophene (PTh)-coated ultrathin MnO2 nanosheets with enhanced lithium storage capabilities. J. Phys. Chem. C 2010, 114, 12048–12051. [Google Scholar] [CrossRef]
- Xia, H.; Lai, M.O.; Lu, L. Nanoflaky MnO2/carbon nanotube nanocomposites as anode materials for lithium-ion batteries. J. Mater. Chem. 2010, 20, 6896–6902. [Google Scholar] [CrossRef]
- Xu, G.; Ding, B.; Shen, L.; Nie, P.; Han, J.; Zhang, X. Sulfur embedded in metal organic framework-derived hierarchically porous carbon nanoplates for high performance lithium-sulfur battery. J. Mater. Chem. A 2013, 1, 4490–4496. [Google Scholar] [CrossRef]
- Liao, J.-Y.; Higgins, D.; Lui, G.; Chabot, V.; Xiao, X.; Che, Z. Multifunctional TiO2-C/MnO2 core-double-shell nanowire arrays as high-performance 3D electrodes for lithium ion batteries. Nano Lett. 2013, 13, 5467–5473. [Google Scholar] [CrossRef]
- He, C.; Wu, S.; Zhao, N.; Shi, C.; Liu, E.; Li, J. Carbon encapsulated Fe3O4 nanoparticles as a high-rate lithium ion battery anode material. ACS Nano 2013, 7, 4459–4469. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, T. Reduced graphene oxide anchored with MnO2 nanorods as anode for high rate and long cycle Lithium ion batteries. Electrochim. Acta 2016, 201, 165–171. [Google Scholar]
- Yan, D.; Yan, P.; Cheng, S.; Chen, J.; Zhuo, R.; Feng, J.; Zhang, G. Fabrication, in-depth characterization, and formation mechanism of crystalline porous birnessite MnO2 film with amorphous bottom layers by hydrothermal method. Cryst. Growth Des. 2009, 9, 218–222. [Google Scholar] [CrossRef]
- Yang, W.; Su, Z.; Xu, Z.; Yang, W.; Peng, Y.; Li, J. Comparative study of α-, β-, γ- and δ-MnO2 on toluene oxidation: Oxygen vacancies and reaction intermediates. Appl. Catal. B 2020, 260, 118150. [Google Scholar] [CrossRef]
- Liu, L.; Shen, Z.; Zhang, X.; Ma, S. Facile controlled synthesis of MnO2 nanostructures for high performance anodes in lithium-ion batteries. J. Mater. Sci-Mater. Electron. 2019, 30, 1480–1486. [Google Scholar] [CrossRef]
- Lai, H.; Li, J.; Chen, Z.; Huang, Z. Carbon nanohorns as a high-performance carrier for MnO2 anode in lithium-ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 2325–2328. [Google Scholar] [CrossRef]
- Yue, J.; Gu, X.; Chen, L.; Wang, N.; Jiang, X.; Xu, H.; Yang, J.; Qian, Y. General synthesis of hollow MnO2, Mn3O4 and MnO nanospheres as superior anode materials for lithium ion batteries. J. Mater. Chem. A 2014, 2, 17421–17426. [Google Scholar] [CrossRef]
- Li, J.; Zou, M.; Zhao, Y.; Lin, Y.; Lai, H.; Guan, L.; Huang, Z. Coaxial MWNTs@MnO2 confined in conducting PPy for kinetically efficient and long-term lithium ion storage. Electrochim. Acta 2013, 111, 165–171. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Z.; Su, Y.; Ruan, H.; Hu, R.; Zhang, L. MnO2 nanorods/3D-rGO composite as high performance anode materials for Li-ion batteries. Appl. Surf. Sci. 2017, 392, 777–784. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Shaijumon, M.M.; Gowda, S.R.; Ajayan, P.M. Coaxial MnO2/carbon nanotube array electrodes for high performance lithium batteries. Nano Lett. 2009, 9, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, D.; Liu, W.; Shi, B.; Guo, R.; Pei, H.; Xie, J. A three-dimensional core-shell nanostructured composite of polypyrrole wrapped MnO2/reduced graphene oxide/carbon nanotube for high performance lithium ion batteries. J. Colloid Interface Sci. 2017, 493, 241–248. [Google Scholar] [CrossRef]
- Cai, Z.; Lin, X.; Yan, M.; Han, C.; Han, L.; Hercule, K.M.; Niu, C.; Yuan, Z.; Xu, W.; Qu, L.; et al. Manganese oxide/carbon yolk-shell nanorod anodes for high capacity lithium batteries. Nano Lett. 2015, 15, 738–744. [Google Scholar] [CrossRef]
- Jiang, Z.; Pei, B.; Manthiram, A. Randomly stacked holey graphene anodes for lithium ion batteries with enhanced electrochemical performance. J. Mater. Chem. A 2013, 1, 7775–7781. [Google Scholar] [CrossRef]









| Materials | Current Density (mA g−1) | Cycle Number | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|
| Lignin carbon fibers | 15 | 70 | 193 | [4] |
| Lignin-based porous carbon nanospheres | 100 | 50 | 300 | [10] |
| MWNTS@MnO2 | 100 | 100 | ~200 | [42] |
| MnO2 nanorods/3D-rGO | 100 | 60 | 595 | [43] |
| Coaxial MnO2/carbon nanotube | 50 | 15 | 500 | [44] |
| MnO2-rGO-CNTs | 100 | 200 | 380.9 | [45] |
| Hollow L-C-NSs@MnO2 nanospheres | 100 | 500 | 478 | This work |
| Samples | Cycle | Rs (Ω) | Rct (Ω) |
|---|---|---|---|
| L-C-NSs@MnO2 | 20th | 7.3 | 29.6 |
| 50th | 8.6 | 32.3 | |
| 100th | 8.7 | 40.5 | |
| 20th | 8.8 | 41.3 | |
| lignin-carbon@MnO2 | 50th | 9.6 | 50.9 |
| 100th | 11.9 | 65.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Huang, J.; He, G. Lignin-Based Mesoporous Hollow Carbon@MnO2 Nanosphere Composite as an Anodic Material for Lithium-Ion Batteries. Materials 2023, 16, 7283. https://doi.org/10.3390/ma16237283
Li S, Huang J, He G. Lignin-Based Mesoporous Hollow Carbon@MnO2 Nanosphere Composite as an Anodic Material for Lithium-Ion Batteries. Materials. 2023; 16(23):7283. https://doi.org/10.3390/ma16237283
Chicago/Turabian StyleLi, Shun, Jianguo Huang, and Guijin He. 2023. "Lignin-Based Mesoporous Hollow Carbon@MnO2 Nanosphere Composite as an Anodic Material for Lithium-Ion Batteries" Materials 16, no. 23: 7283. https://doi.org/10.3390/ma16237283