Comprehensive Analysis of Geopolymer Materials: Properties, Environmental Impacts, and Applications
Abstract
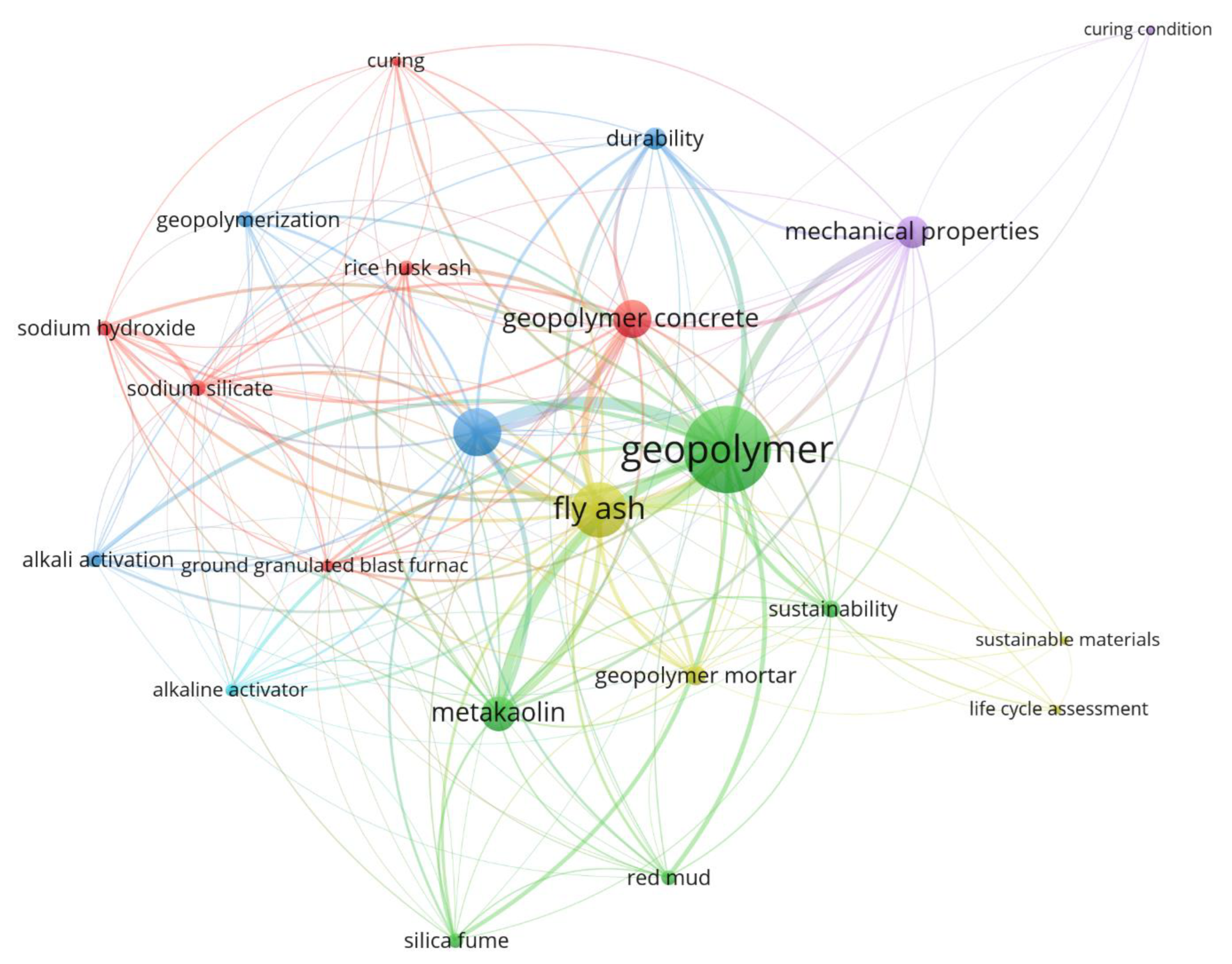
:1. Introduction and Background
Aim and Research Significance
2. Geopolymers Composition
3. Preparation of Geopolymer Concrete
3.1. Aluminosilicate Precursors
3.1.1. Fly Ash (FA)
3.1.2. Blast Furnace Slag (BFS)
3.1.3. Silica Fume (SF)
3.1.4. Metakaolin (MK)
3.1.5. Rice Husk Ash (RHA)
3.1.6. Red Mud (RM)
3.2. Activator
4. Properties of Geopolymer Materials
4.1. Compressive Strength (CS)
4.2. Geopolymer Materials Curing
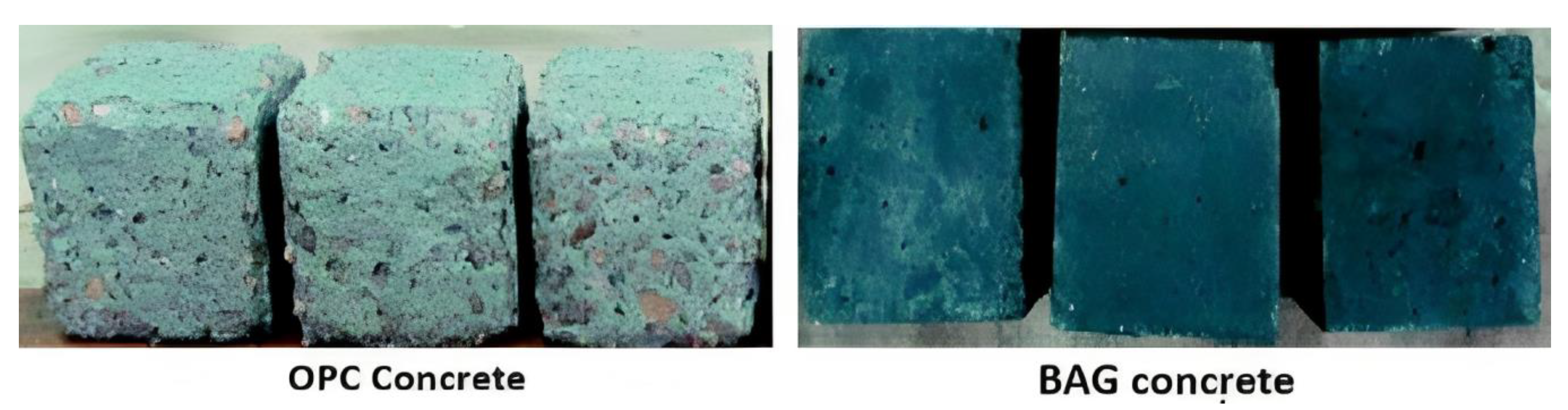
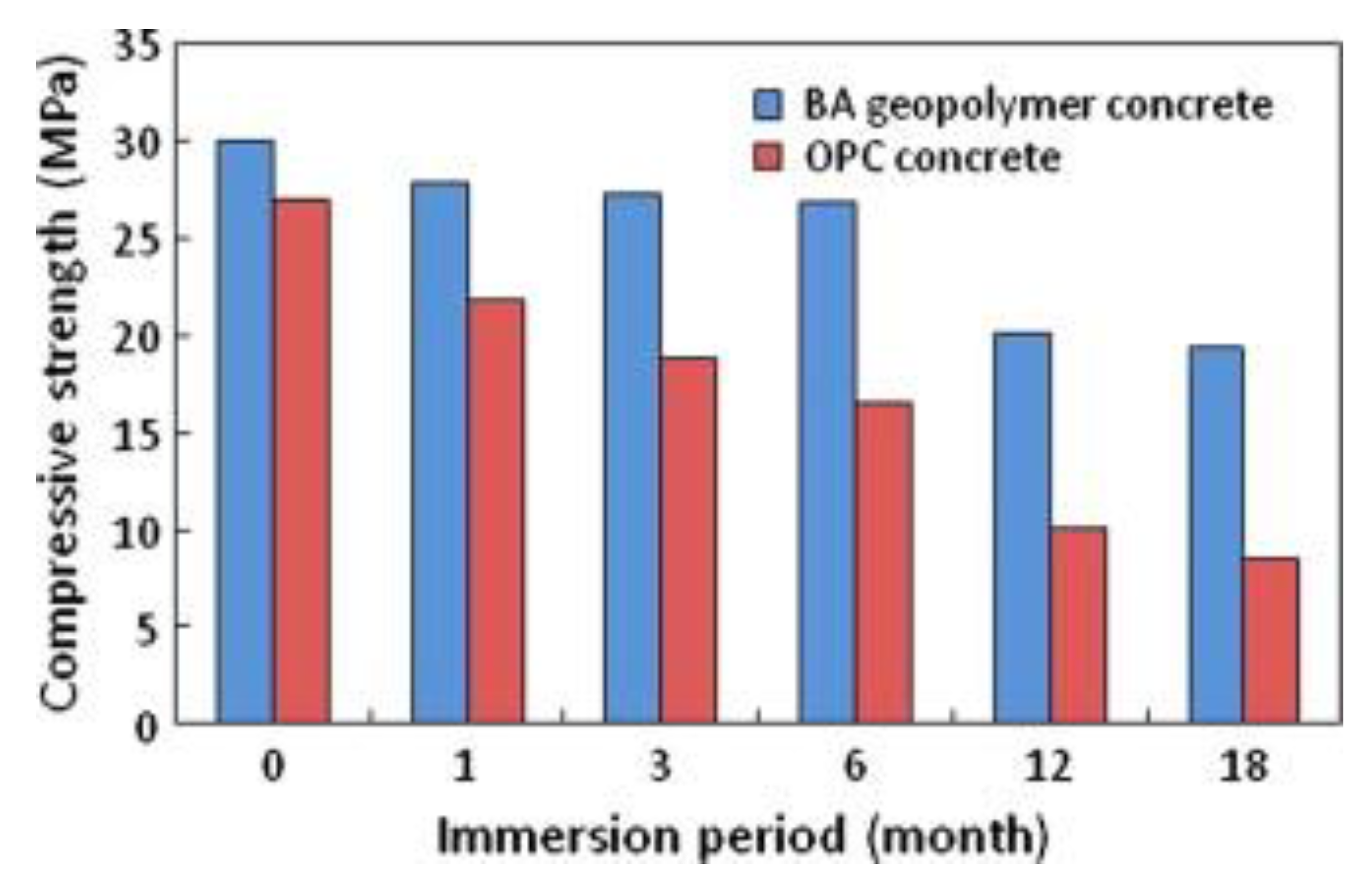
4.3. Durability of Geopolymer Materials
5. Applications of Geopolymers
6. The Environmental Impacts of Geopolymers
Global Warming Potential of Geopolymers
7. Conclusions
- Geopolymers derive their strength from abundant sources of active silicon and aluminum. The raw constituents of geopolymers typically encompass BFS, FA, MK, RHA, and others.
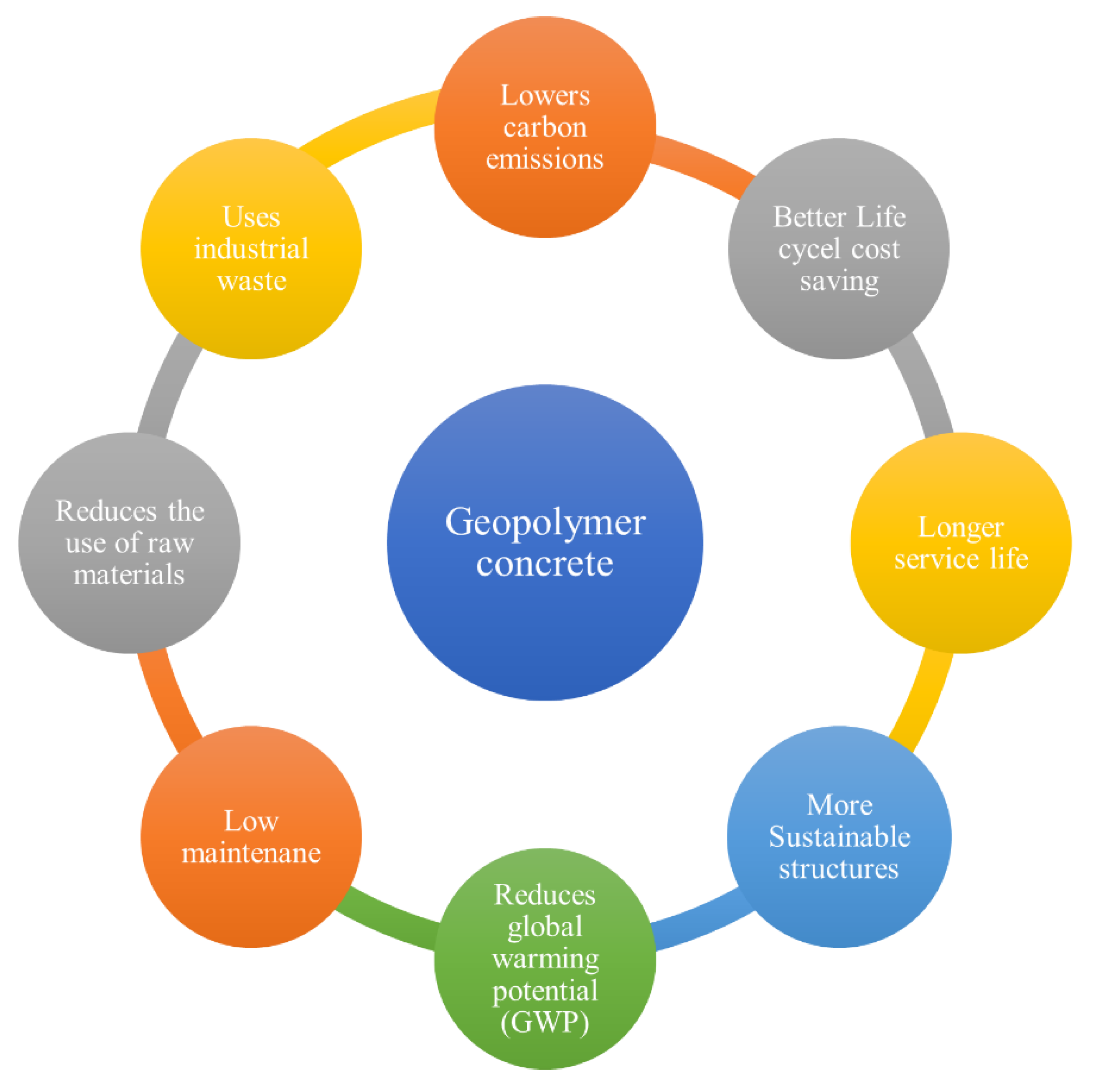
- GPC is an environmentally friendly building material with outstanding mechanical characteristics. It is seen as an attractive alternative for OPC concrete, which would be achievable if sufficient industrial and agricultural waste materials were available. Adopting geopolymer concrete instead of traditional OPC concrete could cause an 80% decrease in carbon dioxide emissions related to concrete manufacturing.
- It was observed that the ultimate properties of the geopolymer are contingent on its chemical composition, with the elements Al, Na, H2O, and Si being pivotal in the formation of the dominant N-A-S-H gel and, consequently, influencing the chemical attributes of the geopolymer.
- The findings from this study suggest that geopolymer concrete shows substantial promise and feasibility as an eco-friendly construction material. It holds potential as a possible substitute for conventional concrete in future applications.
- The study found that curing temperature, silicate content, and the alkaline solution-to-binder proportion are the primary factors significantly impacting the compressive strength of geopolymer concrete.
- The utilization of an alkaline activator plays a significant role in environmental impact, particularly in the case of GPC. Therefore, it is crucial to carefully choose the suitable source of alkaline activators for the GPC mixture.
- Employing waste materials in producing activated alkali substances offers economic advantages and significant environmental benefits by reducing reliance on Portland cement. Additionally, this approach addresses the challenges linked with the disposal of substantial quantities of waste, including ash from coal-fired thermoelectric plants and slag from metal production, mitigating potential environmental hazards.
- The reactivity of geopolymers is notably affected by both curing duration and temperature. Furthermore, factors such as particle size and water content play crucial roles in altering the durability of these materials.
- Geopolymers are not strictly an alternative aiming to rival the established Ordinary Portland Cement (OPC) industry on a worldwide scale. Instead, they can be seen as a technological advancement that cement manufacturers can adopt to diversify their portfolio of cement-based products for the market.
8. Future Directions
- Only a limited number of researchers have undertaken experiments concerning the structural applications of GPC. Therefore, there is a pressing need for more extensive research in this field to facilitate the widespread adoption of GPC applications within the construction industry. The utilization of GPC appears to hold significant promise in advancing sustainable construction practices in this sector.
- Analyzing the environmental and economic implications of GPC usage is crucial. Conducting a thorough evaluation of its impacts, both in terms of costs and sustainability, can raise awareness and promote its wider adoption. Furthermore, such research attempts can provide valuable insights into innovative approaches for further mitigating the environmental footprint and expenses associated with GPC.
- One of the main challenges confronting the widespread acceptance of geopolymerization is the entrenched dominance of OPC within the industry. Additionally, the industry tends to be cautious and conservative when it comes to adopting new technologies and products that could potentially replace established ones. Overcoming these obstacles will necessitate sustained and intensified efforts from the research community.
- Despite the multitude of field applications in the construction industry, there is a pressing requirement for a practical code of practice specifically tailored for geopolymers. The formulation of these materials should be grounded in extensive research and field data to facilitate widespread adoption by consumers.
- The rheological characteristics of alkali-activated specimens derived from different source materials remain unexplored territory and necessitate further investigation.
- Whereas GPC has been in existence for some time, there remains a necessity for conducting more extensive, long-term studies. Unlike OPC concrete, there is a restricted comprehension of GPC’s durability, particularly concerning formulations using unconventional precursors. Therefore, alongside short-term investigations, there should be an increased emphasis on studying its long-term performance. Employing various accelerated testing methods could prove beneficial in thoroughly evaluating the extended performance of GPC.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, B.; Zhao, Y.; Bai, H.; Kang, S.; Zhang, T.; Song, S. Eco-Friendly Geopolymer Prepared from Solid Wastes: A Critical Review. Chemosphere 2021, 267, 128900. [Google Scholar] [CrossRef]
- Huang, T.; Song, D.; Zhou, L.; Pan, L.; Zhang, S. Self-Alkali-Activated Self-Cementation Achievement and Mechanism Exploration for the Synergistic Treatment of the Municipal Solid Waste Incineration Fly Ashes and the Arsenic-Contaminated Soils. Chemosphere 2023, 325, 138397. [Google Scholar] [CrossRef]
- Amjath, M.; Kerbache, L.; Smith, J.M.; Elomri, A. Optimisation of Buffer Allocations in Manufacturing Systems: A Study on Intra and Outbound Logistics Systems Using Finite Queueing Networks. Appl. Sci. 2023, 13, 9525. [Google Scholar] [CrossRef]
- Bui, T.-D.; Tseng, J.-W.; Tseng, M.-L.; Lim, M.K. Opportunities and Challenges for Solid Waste Reuse and Recycling in Emerging Economies: A Hybrid Analysis. Resour. Conserv. Recycl. 2022, 177, 105968. [Google Scholar] [CrossRef]
- Tayeh, B.A.; Zeyad, A.M.; Agwa, I.S.; Amin, M. Effect of Elevated Temperatures on Mechanical Properties of Lightweight Geopolymer Concrete. Case Stud. Constr. Mater. 2021, 15, e00673. [Google Scholar] [CrossRef]
- De Oliveira, L.B.; De Azevedo, A.R.G.; Marvila, M.T.; Pereira, E.C.; Fediuk, R.; Vieira, C.M.F. Durability of Geopolymers with Industrial Waste. Case Stud. Constr. Mater. 2022, 16, e00839. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A Review and Prospects for the Minerals Industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-Activated Binders: A Review. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef]
- Hong, S.; Kim, H. Effects of Microwave Energy on Fast Compressive Strength Development of Coal Bottom Ash-Based Geopolymers. Sci. Rep. 2019, 9, 15694. [Google Scholar] [CrossRef]
- Davidovits, J.; Cordi, S. Synthesis of New High Temperature Geo-Polymers for Reinforced Plastics/Composites. Spe. Pactec. 1979, 79, 151–154. [Google Scholar]
- Wan, Q.; Rao, F.; Song, S.; García, R.E.; Estrella, R.M.; Patiño, C.L.; Zhang, Y. Geopolymerization Reaction, Microstructure and Simulation of Metakaolin-Based Geopolymers at Extended Si/Al Ratios. Cem. Concr. Compos. 2017, 79, 45–52. [Google Scholar] [CrossRef]
- Aiken, T.A.; Kwasny, J.; Sha, W.; Soutsos, M.N. Effect of Slag Content and Activator Dosage on the Resistance of Fly Ash Geopolymer Binders to Sulfuric Acid Attack. Cem. Concr. Res. 2018, 111, 23–40. [Google Scholar] [CrossRef]
- Aliques-Granero, J.; Tognonvi, M.T.; Tagnit-Hamou, A. Durability Study of AAMs: Sulfate Attack Resistance. Constr. Build. Mater. 2019, 229, 117100. [Google Scholar] [CrossRef]
- Kurda, R.; Silva, R.V.; De Brito, J. Incorporation of Alkali-Activated Municipal Solid Waste Incinerator Bottom Ash in Mortar and Concrete: A Critical Review. Materials 2020, 13, 3428. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined Clay Limestone Cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Degefa, A.B.; Park, S.; Yang, B.; Park, S. Predicting the Degree of Reaction of Supplementary Cementitious Materials in Hydrated Portland Cement. Sustainability 2023, 15, 15471. [Google Scholar] [CrossRef]
- Tariku, D.; Degefa, A.B.; Son, H.M.; Park, S. Exploring the Applicability of Thermodynamic Modeling to Predict the Degree of Reaction of Supplementary Cementitious Materials in Portland Cements. Dev. Built Environ. 2023, 16, 100256. [Google Scholar] [CrossRef]
- Ramanathan, S.; Moon, H.; Croly, M.; Chung, C.-W.; Suraneni, P. Predicting the Degree of Reaction of Supplementary Cementitious Materials in Cementitious Pastes Using a Pozzolanic Test. Constr. Build. Mater. 2019, 204, 621–630. [Google Scholar] [CrossRef]
- Salas, D.A.; Ramirez, A.D.; Ulloa, N.; Baykara, H.; Boero, A.J. Life Cycle Assessment of Geopolymer Concrete. Constr. Build. Mater. 2018, 190, 170–177. [Google Scholar] [CrossRef]
- Sbahieh, S.; Zaher Serdar, M.; Al-Ghamdi, S.G. Decarbonization Strategies of Building Materials Used in the Construction Industry. Mater. Today Proc. 2023, 40, S2214785323046163. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Xiao, R.; Polaczyk, P.; Zhang, M.; Hu, W.; Bai, Y.; Huang, B. A Comparative Study on Geopolymers Synthesized by Different Classes of Fly Ash after Exposure to Elevated Temperatures. J. Clean. Prod. 2020, 270, 122500. [Google Scholar] [CrossRef]
- Kalombe, R.M.; Ojumu, V.T.; Eze, C.P.; Nyale, S.M.; Kevern, J.; Petrik, L.F. Fly Ash-Based Geopolymer Building Materials for Green and Sustainable Development. Materials 2020, 13, 5699. [Google Scholar] [CrossRef] [PubMed]
- Golewski, G.L. The Effect of the Addition of Coal Fly Ash (CFA) on the Control of Water Movement within the Structure of the Concrete. Materials 2023, 16, 5218. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, R.; Gunathilake, C.; Dassanayake, R. Suitability of Reusing the Spent Diatomaceous Earth in Brick Production: A Review. Adv. Technol. 2022, 2, 151–166. [Google Scholar] [CrossRef]
- Kipsanai, J.J.; Wambua, P.M.; Namango, S.S.; Amziane, S. A Review on the Incorporation of Diatomaceous Earth as a Geopolymer-Based Concrete Building Resource. Materials 2022, 15, 7130. [Google Scholar] [CrossRef] [PubMed]
- Kipsanai, J.J.; Amziane, S.; Wambua, P.M.; Namango, S.S. An Evaluation of the Mechanical and Physical Properties Of Sisal Fiber-Reinforced Alkaline Activated Diatomaceous Earth-Based Geopolymer Concrete. Int. J. Acad. Eng. Res. 2023, 7, 15–23. [Google Scholar]
- Fennis, S.; Walraven, J.C. Using Particle Packing Technology for Sustainable Concrete Mixture Design. Heron 2012, 57, 73–101. [Google Scholar]
- Abushama, W.J.; Tamimi, A.K.; Tabsh, S.W.; El-Emam, M.M.; Ibrahim, A.; Mohammed Ali, T.K. Influence of Optimum Particle Packing on the Macro and Micro Properties of Sustainable Concrete. Sustainability 2023, 15, 14331. [Google Scholar] [CrossRef]
- Niyazuddin; Umesh, B. Mechanical and Durability Properties of Standard and High Strength Geopolymer Concrete Using Particle Packing Theory. Constr. Build. Mater. 2023, 400, 132722. [Google Scholar] [CrossRef]
- Sbahieh, S.; Rabie, M.; Ebead, U.; Al-Ghamdi, S.G. The Mechanical and Environmental Performance of Fiber-Reinforced Polymers in Concrete Structures: Opportunities, Challenges and Future Directions. Buildings 2022, 12, 1417. [Google Scholar] [CrossRef]
- Sbahieh, S.; Tahir, F.; Al-Ghamdi, S.G. Environmental and Mechanical Performance of Different Fiber Reinforced Polymers in Beams. Mater. Today Proc. 2022, 62, 3548–3552. [Google Scholar] [CrossRef]
- Sbahieh, S.; Mckay, G.; Al-Ghamdi, S.G. A Comparative Life Cycle Assessment of Fiber-Reinforced Polymers as a Sustainable Reinforcement Option in Concrete Beams. Front. Built Environ. 2023, 9, 1194121. [Google Scholar] [CrossRef]
- Alsabri, A.; Tahir, F.; Al-Ghamdi, S.G. Life-Cycle Assessment of Polypropylene Production in the Gulf Cooperation Council (GCC) Region. Polymers 2021, 13, 3793. [Google Scholar] [CrossRef] [PubMed]
- Habert, G.; d’Espinose de Lacaillerie, J.B.; Roussel, N. An Environmental Evaluation of Geopolymer Based Concrete Production: Reviewing Current Research Trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and Carbon Emissions for Geopolymer Pastes in Comparison to Ordinary Portland Cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.; Mejía-Arcila, J.; Mejía de Gutiérrez, R.; Martínez, E. Life Cycle Assessment (LCA) of an Alkali-Activated Binary Concrete Based on Natural Volcanic Pozzolan: A Comparative Analysis to OPC Concrete. Constr. Build. Mater. 2018, 176, 103–111. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon Dioxide Equivalent (CO2-e) Emissions: A Comparison between Geopolymer and OPC Cement Concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Heah, C.Y.; Kamarudin, H.; Mustafa Al Bakri, A.M.; Bnhussain, M.; Luqman, M.; Khairul Nizar, I.; Ruzaidi, C.M.; Liew, Y.M. Study on Solids-to-Liquid and Alkaline Activator Ratios on Kaolin-Based Geopolymers. Constr. Build. Mater. 2012, 35, 912–922. [Google Scholar] [CrossRef]
- Mallikarjuna Rao, G.; Gunneswara Rao, T.D. A Quantitative Method of Approach in Designing the Mix Proportions of Fly Ash and GGBS-Based Geopolymer Concrete. Aust. J. Civ. Eng. 2018, 16, 53–63. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of Curing Temperature on the Development of Hard Structure of Metakaolin-Based Geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Patankar, S.V.; Jamkar, S.S.; Ghugal, Y.M. Effect of water-to-geopolymer binder ratio on the production of fly ash based geopolymer concrete. Int. J. Adv. Technol. Civ. Eng. 2012, 1, 296–300. [Google Scholar] [CrossRef]
- Castillo, H.; Collado, H.; Droguett, T.; Sánchez, S.; Vesely, M.; Garrido, P.; Palma, S. Factors Affecting the Compressive Strength of Geopolymers: A Review. Minerals 2021, 11, 1317. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 1-84569-638-7. [Google Scholar]
- Amjath, M.; Kerbache, L.; Smith, J.M.; Elomri, A. Fleet Sizing of Trucks for an Inter-Facility Material Handling System Using Closed Queueing Networks. Oper. Res. Perspect. 2022, 9, 100245. [Google Scholar] [CrossRef]
- Burduhos Nergis, D.D.; Abdullah, M.M.A.B.; Vizureanu, P.; Tahir, M.F.M. Geopolymers and Their Uses: Review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012019. [Google Scholar] [CrossRef]
- Keyte, L.M. Fly Ash Glass Chemistry and Inorganic Polymer Cements. In Geopolymers; Elsevier: Amsterdam, The Netherlands, 2009; pp. 15–36. ISBN 978-1-84569-449-4. [Google Scholar]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; Van Deventer, J.S.J. Understanding the Relationship between Geopolymer Composition, Microstructure and Mechanical Properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Nanostructure/Microstructure of Fly Ash Geopolymers. In Geopolymers; Elsevier: Amsterdam, The Netherlands, 2009; pp. 89–117. ISBN 978-1-84569-449-4. [Google Scholar]
- Castillo, H.; Collado, H.; Droguett, T.; Vesely, M.; Garrido, P.; Palma, S. State of the Art of Geopolymers: A Review. e-Polymers 2022, 22, 108–124. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Geopolymers as an Alternative to Portland Cement: An Overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean Production and Properties of Geopolymer Concrete; A Review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Nawaz, M.; Heitor, A.; Sivakumar, M. Geopolymers in Construction—Recent Developments. Constr. Build. Mater. 2020, 260, 120472. [Google Scholar] [CrossRef]
- Imtiaz, L.; Rehman, S.K.U.; Ali Memon, S.; Khizar Khan, M.; Faisal Javed, M. A Review of Recent Developments and Advances in Eco-Friendly Geopolymer Concrete. Appl. Sci. 2020, 10, 7838. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-Part Alkali-Activated Materials: A Review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Payá, J.; Monzó, J.; Borrachero, M.V.; Tashima, M.M. Reuse of Aluminosilicate Industrial Waste Materials in the Production of Alkali-Activated Concrete Binders. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Amsterdam, The Netherlands, 2015; pp. 487–518. ISBN 978-1-78242-276-1. [Google Scholar]
- Yao, Z.T.; Xia, M.S.; Sarker, P.K.; Chen, T. A Review of the Alumina Recovery from Coal Fly Ash, with a Focus in China. Fuel 2014, 120, 74–85. [Google Scholar] [CrossRef]
- Ge, J.; Yoon, S.; Choi, N. Application of Fly Ash as an Adsorbent for Removal of Air and Water Pollutants. Appl. Sci. 2018, 8, 1116. [Google Scholar] [CrossRef]
- Alterary, S.S.; Marei, N.H. Fly Ash Properties, Characterization, and Applications: A Review. J. King Saud Univ. Sci. 2021, 33, 101536. [Google Scholar] [CrossRef]
- Abdalla, A.; Salih, A. Microstructure and Chemical Characterizations with Soft Computing Models to Evaluate the Influence of Calcium Oxide and Silicon Dioxide in the Fly Ash and Cement Kiln Dust on the Compressive Strength of Cement Mortar. Resour. Conserv. Recycl. Adv. 2022, 15, 200090. [Google Scholar] [CrossRef]
- ASTM Committee C-09; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PN, USA, 2022.
- Suraneni, P.; Burris, L.; Shearer, C.R.; Hooton, D. ASTM C618 Fly Ash Specification: Comparison with Other Specifications, Shortcomings, and Solutions. ACI Mater. J. 2021, 118. [Google Scholar] [CrossRef]
- Kelechi, S.E.; Adamu, M.; Uche, O.A.U.; Okokpujie, I.P.; Ibrahim, Y.E.; Obianyo, I.I. A Comprehensive Review on Coal Fly Ash and Its Application in the Construction Industry. Cogent Eng. 2022, 9, 2114201. [Google Scholar] [CrossRef]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A Comprehensive Review on the Applications of Coal Fly Ash. Earth Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Cwirzen, A. Properties of SCC with Industrial By-Products as Aggregates. In Self-Compacting Concrete: Materials, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 249–281. ISBN 978-0-12-817369-5. [Google Scholar]
- Awoyera, P.O.; Babalola, O.E.; Aluko, O.G. The Use of Slags in Recycled Aggregate Concrete. In The Structural Integrity of Recycled Aggregate Concrete Produced with Fillers and Pozzolans; Elsevier: Amsterdam, The Netherlands, 2022; pp. 145–170. ISBN 978-0-12-824105-9. [Google Scholar]
- Talling, B.; Krivenko, P. Blast Furnace Slag-the Ultimate Binder. In Waste Materials Used in Concrete Manufacturing; Elsevier: Amsterdam, The Netherlands, 1996; pp. 235–289. ISBN 978-0-8155-1393-3. [Google Scholar]
- Suresh, D.; Nagaraju, K. Ground Granulated Blast Slag (GGBS) in Concrete—A Review. IOSR J. Mech. Civ. Eng. 2015, 12, 76–82. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. Sustainable Geopolymer Concrete Using Ground Granulated Blast Furnace Slag and Rice Husk Ash: Strength and Permeability Properties. J. Clean. Prod. 2018, 205, 49–57. [Google Scholar] [CrossRef]
- Sakulich, A.R. Reinforced Geopolymer Composites for Enhanced Material Greenness and Durability. Sustain. Cities Soc. 2011, 1, 195–210. [Google Scholar] [CrossRef]
- Farooq, F.; Jin, X.; Faisal Javed, M.; Akbar, A.; Izhar Shah, M.; Aslam, F.; Alyousef, R. Geopolymer Concrete as Sustainable Material: A State of the Art Review. Constr. Build. Mater. 2021, 306, 124762. [Google Scholar] [CrossRef]
- Okoye, F.N.; Durgaprasad, J.; Singh, N.B. Effect of Silica Fume on the Mechanical Properties of Fly Ash Based-Geopolymer Concrete. Ceram. Int. 2016, 42, 3000–3006. [Google Scholar] [CrossRef]
- Xupeng, C.; Zhuowen, S.; Jianyong, P. Study on Metakaolin Impact on Concrete Performance of Resisting Complex Ions Corrosion. Front. Mater. 2021, 8, 788079. [Google Scholar] [CrossRef]
- Jindal, B.B.; Alomayri, T.; Hasan, A.; Kaze, C.R. Geopolymer Concrete with Metakaolin for Sustainability: A Comprehensive Review on Raw Material’s Properties, Synthesis, Performance, and Potential Application. Environ. Sci. Pollut. Res. 2022, 30, 25299–25324. [Google Scholar] [CrossRef] [PubMed]
- Albidah, A.; Alghannam, M.; Abbas, H.; Almusallam, T.; Al-Salloum, Y. Characteristics of Metakaolin-Based Geopolymer Concrete for Different Mix Design Parameters. J. Mater. Res. Technol. 2021, 10, 84–98. [Google Scholar] [CrossRef]
- Parathi, S.; Nagarajan, P.; Pallikkara, S.A. Ecofriendly Geopolymer Concrete: A Comprehensive Review. Clean Techn. Environ. Policy 2021, 23, 1701–1713. [Google Scholar] [CrossRef]
- Dai, S.; Wang, H.; An, S.; Yuan, L. Mechanical Properties and Microstructural Characterization of Metakaolin Geopolymers Based on Orthogonal Tests. Materials 2022, 15, 2957. [Google Scholar] [CrossRef]
- Gonçalves, M.R.F.; Bergmann, C.P. Thermal Insulators Made with Rice Husk Ashes: Production and Correlation between Properties and Microstructure. Constr. Build. Mater. 2007, 21, 2059–2065. [Google Scholar] [CrossRef]
- Prabu, B.; Shalini, A.; Kumar, J.K. Rice Husk Ash Based Geopolymer Concrete-a Review. Chem. Sci. Rev. Lett. 2014, 3, 288–294. [Google Scholar]
- Siddique, R.; Kunal; Mehta, A. Utilization of Industrial By-Products and Natural Ashes in Mortar and Concrete Development of Sustainable Construction Materials. In Nonconventional and Vernacular Construction Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 247–303. ISBN 978-0-08-102704-2. [Google Scholar]
- Hossain, S.S.; Roy, P.K.; Bae, C.-J. Utilization of Waste Rice Husk Ash for Sustainable Geopolymer: A Review. Constr. Build. Mater. 2021, 310, 125218. [Google Scholar] [CrossRef]
- Ai, T.; Zhong, D.; Zhang, Y.; Zong, J.; Yan, X.; Niu, Y. The Effect of Red Mud Content on the Compressive Strength of Geopolymers under Different Curing Systems. Buildings 2021, 11, 298. [Google Scholar] [CrossRef]
- Paramguru, R.K.; Rath, P.C.; Misra, V.N. Trends in red mud utilization—A review. Miner. Process. Extr. Metall. Rev. 2004, 26, 1–29. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Agatzini-Leonardou, S.; Oustadakis, P. Red Mud Addition in the Raw Meal for the Production of Portland Cement Clinker. J. Hazard. Mater. 2004, 116, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tang, Z.; Li, W.; Li, Y.; Tam, V.W.Y. Physical-Mechanical Properties of Fly Ash/GGBFS Geopolymer Composites with Recycled Aggregates. Constr. Build. Mater. 2019, 226, 139–151. [Google Scholar] [CrossRef]
- Bajpai, R.; Choudhary, K.; Srivastava, A.; Sangwan, K.S.; Singh, M. Environmental Impact Assessment of Fly Ash and Silica Fume Based Geopolymer Concrete. J. Clean. Prod. 2020, 254, 120147. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhao, R.; Yuan, Y.; Li, F.; Castel, A.; Xu, T. Ageing Coefficient for Early Age Tensile Creep of Blended Slag and Low Calcium Fly Ash Geopolymer Concrete. Constr. Build. Mater. 2020, 262, 119855. [Google Scholar] [CrossRef]
- Zannerni, G.M.; Fattah, K.P.; Al-Tamimi, A.K. Ambient-Cured Geopolymer Concrete with Single Alkali Activator. Sustain. Mater. Technol. 2020, 23, e00131. [Google Scholar] [CrossRef]
- Sharmin, A.; Alengaram, U.J.; Jumaat, M.Z.; Yusuf, M.O.; Kabir, S.M.A.; Bashar, I.I. Influence of Source Materials and the Role of Oxide Composition on the Performance of Ternary Blended Sustainable Geopolymer Mortar. Constr. Build. Mater. 2017, 144, 608–623. [Google Scholar] [CrossRef]
- Nimwinya, E.; Arjharn, W.; Horpibulsuk, S.; Phoo-ngernkham, T.; Poowancum, A. A Sustainable Calcined Water Treatment Sludge and Rice Husk Ash Geopolymer. J. Clean. Prod. 2016, 119, 128–134. [Google Scholar] [CrossRef]
- Yang, T.; Wang, Y.; Sheng, L.; He, C.; Sun, W.; He, Q. Enhancing Cd(II) Sorption by Red Mud with Heat Treatment: Performance and Mechanisms of Sorption. J. Environ. Manag. 2020, 255, 109866. [Google Scholar] [CrossRef]
- Shi, W.; Ren, H.; Huang, X.; Li, M.; Tang, Y.; Guo, F. Low Cost Red Mud Modified Graphitic Carbon Nitride for the Removal of Organic Pollutants in Wastewater by the Synergistic Effect of Adsorption and Photocatalysis. Sep. Purif. Technol. 2020, 237, 116477. [Google Scholar] [CrossRef]
- Buruberri, L.H.; Tobaldi, D.M.; Caetano, A.; Seabra, M.P.; Labrincha, J.A. Evaluation of Reactive Si and Al Amounts in Various Geopolymer Precursors by a Simple Method. J. Build. Eng. 2019, 22, 48–55. [Google Scholar] [CrossRef]
- Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S. The Mechanical Properties of Fly Ash-Based Geopolymer Concrete with Alkaline Activators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar] [CrossRef]
- Cong, P.; Cheng, Y. Advances in Geopolymer Materials: A Comprehensive Review. J. Traffic Transp. Eng. (Engl. Ed.) 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Helmy, A.I.I. Intermittent Curing of Fly Ash Geopolymer Mortar. Constr. Build. Mater. 2016, 110, 54–64. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer Concrete: A Review of Some Recent Developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Krishna, R.S.; Mishra, J.; Zribi, M.; Adeniyi, F.; Saha, S.; Baklouti, S.; Shaikh, F.U.A.; Gökçe, H.S. A Review on Developments of Environmentally Friendly Geopolymer Technology. Materialia 2021, 20, 101212. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 5th ed.; Geopolymer Institute: Saint-Quentin, France, 2020; ISBN 978-2-9544531-1-8. [Google Scholar]
- Bušatlić, N.; Petrovski, P.; Busatlić, I. The Investigation of Possibilities of Mono Al Phosphate and AlCr Phosphate Synthesis–the Binders for Refractories. In Proceedings of the 18th International Research/Expert Conference ”Trends in the Development of Machinery and Associated Technology” TMT 2014, Budapest, Hungary, 10–12 September 2014; pp. 145–148. [Google Scholar]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Khan, T.M.Y.; Dawood Abdul Khadar, S. Molarity Activity Effect on Mechanical and Microstructure Properties of Geopolymer Concrete: A Review. Case Stud. Constr. Mater. 2022, 16, e01014. [Google Scholar] [CrossRef]
- Liu, J.; Doh, J.-H.; Dinh, H.L.; Ong, D.E.L.; Zi, G.; You, I. Effect of Si/Al Molar Ratio on the Strength Behavior of Geopolymer Derived from Various Industrial Waste: A Current State of the Art Review. Constr. Build. Mater. 2022, 329, 127134. [Google Scholar] [CrossRef]
- Wang, H.; Wu, H.; Xing, Z.; Wang, R.; Dai, S. The Effect of Various Si/Al, Na/Al Molar Ratios and Free Water on Micromorphology and Macro-Strength of Metakaolin-Based Geopolymer. Materials 2021, 14, 3845. [Google Scholar] [CrossRef] [PubMed]
- Matsimbe, J.; Dinka, M.; Olukanni, D.; Musonda, I. Geopolymer: A Systematic Review of Methodologies. Materials 2022, 15, 6852. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Mohammed, A.A.; Rafiq, S.; Mohammed, A.S.; Mosavi, A.; Sor, N.H.; Qaidi, S.M.A. Compressive Strength of Sustainable Geopolymer Concrete Composites: A State-of-the-Art Review. Sustainability 2021, 13, 13502. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Ghoshal, S.K.; Ariffin, M.A.M. Effect of Metakaolin Replaced Granulated Blast Furnace Slag on Fresh and Early Strength Properties of Geopolymer Mortar. Ain Shams Eng. J. 2018, 9, 1557–1566. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.; Kamarudin, H.; Bnhussain, M.; Khairul Nizar, I.; Rafiza, A.R.; Zarina, Y. The Relationship of NaOH Molarity, Na2SiO3/NaOH Ratio, Fly Ash/Alkaline Activator Ratio, and Curing Temperature to the Strength of Fly Ash-Based Geopolymer. Adv. Mater. Res. 2011, 328–330, 1475–1482. [Google Scholar] [CrossRef]
- Pavithra, P.; Srinivasula Reddy, M.; Dinakar, P.; Hanumantha Rao, B.; Satpathy, B.K.; Mohanty, A.N. Effect of the Na2SiO3/NaOH Ratio and NaOH Molarity on the Synthesis of Fly Ash-Based Geopolymer Mortar. In Proceedings of the Geo-Chicago 2016, Chicago, IL, USA, 8 August 2016; American Society of Civil Engineers: Chicago, IL, USA, 2016; pp. 336–344. [Google Scholar]
- Xie, J.; Kayali, O. Effect of Initial Water Content and Curing Moisture Conditions on the Development of Fly Ash-Based Geopolymers in Heat and Ambient Temperature. Constr. Build. Mater. 2014, 67, 20–28. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, H.; Zhang, T.; Fernández, C.A. Alkali-Activated Copper Tailings-Based Pastes: Compressive Strength and Microstructural Characterization. J. Mater. Res. Technol. 2020, 9, 6557–6567. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. Sulfuric Acid Resistance of Fly Ash Based Geopolymer Concrete. Constr. Build. Mater. 2017, 146, 136–143. [Google Scholar] [CrossRef]
- Gunasekara, C.; Law, D.W.; Setunge, S. Long Term Permeation Properties of Different Fly Ash Geopolymer Concretes. Constr. Build. Mater. 2016, 124, 352–362. [Google Scholar] [CrossRef]
- Assaedi, H.; Shaikh, F.U.A.; Low, I.M. Influence of Mixing Methods of Nano Silica on the Microstructural and Mechanical Properties of Flax Fabric Reinforced Geopolymer Composites. Constr. Build. Mater. 2016, 123, 541–552. [Google Scholar] [CrossRef]
- Abdulkareem, O.A.; Mustafa Al Bakri, A.M.; Kamarudin, H.; Khairul Nizar, I.; Saif, A.A. Effects of Elevated Temperatures on the Thermal Behavior and Mechanical Performance of Fly Ash Geopolymer Paste, Mortar and Lightweight Concrete. Constr. Build. Mater. 2014, 50, 377–387. [Google Scholar] [CrossRef]
- Yunsheng, Z.; Wei, S.; Zongjin, L. Composition Design and Microstructural Characterization of Calcined Kaolin-Based Geopolymer Cement. Appl. Clay Sci. 2010, 47, 271–275. [Google Scholar] [CrossRef]
- Riahi, S.; Nemati, A.; Khodabandeh, A.R.; Baghshahi, S. The Effect of Mixing Molar Ratios and Sand Particles on Microstructure and Mechanical Properties of Metakaolin-Based Geopolymers. Mater. Chem. Phys. 2020, 240, 122223. [Google Scholar] [CrossRef]
- Steveson, M.; Sagoe-Crentsil, K. Relationships between Composition, Structure and Strength of Inorganic Polymers: Part I Metakaolin-Derived Inorganic Polymers. J. Mater. Sci. 2005, 40, 2023–2036. [Google Scholar] [CrossRef]
- Ramujee, K. Development of Low Calcium Flyash Based Geopolymer Concrete. Int. J. Eng. Technol. 2014, 6, 14. [Google Scholar] [CrossRef]
- Ghafoor, M.T.; Khan, Q.S.; Qazi, A.U.; Sheikh, M.N.; Hadi, M.N.S. Influence of Alkaline Activators on the Mechanical Properties of Fly Ash Based Geopolymer Concrete Cured at Ambient Temperature. Constr. Build. Mater. 2021, 273, 121752. [Google Scholar] [CrossRef]
- Fang, G.; Ho, W.K.; Tu, W.; Zhang, M. Workability and Mechanical Properties of Alkali-Activated Fly Ash-Slag Concrete Cured at Ambient Temperature. Constr. Build. Mater. 2018, 172, 476–487. [Google Scholar] [CrossRef]
- Ibrahim, M.; Johari, M.A.M.; Maslehuddin, M.; Rahman, M.K. Influence of Nano-SiO2 on the Strength and Microstructure of Natural Pozzolan Based Alkali Activated Concrete. Constr. Build. Mater. 2018, 173, 573–585. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, S.; He, Z. Mechanical and Fracture Properties of Fly Ash Geopolymer Concrete Addictive with Calcium Aluminate Cement. Materials 2019, 12, 2982. [Google Scholar] [CrossRef]
- Vora, P.R.; Dave, U.V. Parametric Studies on Compressive Strength of Geopolymer Concrete. Procedia Eng. 2013, 51, 210–219. [Google Scholar] [CrossRef]
- Nuaklong, P.; Jongvivatsakul, P.; Pothisiri, T.; Sata, V.; Chindaprasirt, P. Influence of Rice Husk Ash on Mechanical Properties and Fire Resistance of Recycled Aggregate High-Calcium Fly Ash Geopolymer Concrete. J. Clean. Prod. 2020, 252, 119797. [Google Scholar] [CrossRef]
- Xie, T.; Ozbakkaloglu, T. Behavior of Low-Calcium Fly and Bottom Ash-Based Geopolymer Concrete Cured at Ambient Temperature. Ceram. Int. 2015, 41, 5945–5958. [Google Scholar] [CrossRef]
- Zhang, L.; Ahmari, S.; Zhang, J. Synthesis and Characterization of Fly Ash Modified Mine Tailings-Based Geopolymers. Constr. Build. Mater. 2011, 25, 3773–3781. [Google Scholar] [CrossRef]
- Perera, D.S.; Uchida, O.; Vance, E.R.; Finnie, K.S. Influence of Curing Schedule on the Integrity of Geopolymers. J. Mater. Sci. 2007, 42, 3099–3106. [Google Scholar] [CrossRef]
- Al-Azzawi, M.; Yu, T.; Hadi, M.N.S. Factors Affecting the Bond Strength Between the Fly Ash-Based Geopolymer Concrete and Steel Reinforcement. Structures 2018, 14, 262–272. [Google Scholar] [CrossRef]
- Singh, S.; Aswath, M.U.; Ranganath, R.V. Effect of Mechanical Activation of Red Mud on the Strength of Geopolymer Binder. Constr. Build. Mater. 2018, 177, 91–101. [Google Scholar] [CrossRef]
- Jindal, B.B.; Parveen; Singhal, D.; Goyal, A. Predicting Relationship between Mechanical Properties of Low Calcium Fly Ash-Based Geopolymer Concrete. Trans. Indian Ceram. Soc. 2017, 76, 258–265. [Google Scholar] [CrossRef]
- Khan, M.S.H.; Castel, A.; Akbarnezhad, A.; Foster, S.J.; Smith, M. Utilisation of Steel Furnace Slag Coarse Aggregate in a Low Calcium Fly Ash Geopolymer Concrete. Cem. Concr. Res. 2016, 89, 220–229. [Google Scholar] [CrossRef]
- Rowles, M.; O’Connor, B. Chemical Optimisation of the Compressive Strength of Aluminosilicate Geopolymers Synthesised by Sodium Silicate Activation of Metakaolinite. J. Mater. Chem. 2003, 13, 1161–1165. [Google Scholar] [CrossRef]
- Vignesh, P.; Vivek, K. An Experimental Investigation on Strength Parameters of Flyash Based Geopolymer Concrete with GGBS. Int. Res. J. Eng. Technol. 2015, 2, 135–142. [Google Scholar]
- Joseph, B.; Mathew, G. Influence of Aggregate Content on the Behavior of Fly Ash Based Geopolymer Concrete. Sci. Iran. 2012, 19, 1188–1194. [Google Scholar] [CrossRef]
- Falayi, T. Effect of Potassium Silicate and Aluminate on the Stabilisation of Gold Mine Tailings. Proc. Inst. Civ. Eng. Waste Resour. Manag. 2019, 172, 56–63. [Google Scholar] [CrossRef]
- Jaydeep, S.; Chakravarthy, B.J. Study on Fly Ash Based Geo-Polymer Concrete Using Admixtures. Int. J. Eng. Trends Technol. 2013, 4, 4614–4617. [Google Scholar]
- Nuruddin, M.N.; Kusbiantoro, A.K.; Qazi, S.Q.; Darmawan, M.D.; Husin, N.H. Development of Geopolymer Concrete with Different Curing Conditions. IPTEK J. Technol. Sci. 2011, 22. [Google Scholar] [CrossRef]
- Liu, M.Y.J.; Alengaram, U.J.; Santhanam, M.; Jumaat, M.Z.; Mo, K.H. Microstructural Investigations of Palm Oil Fuel Ash and Fly Ash Based Binders in Lightweight Aggregate Foamed Geopolymer Concrete. Constr. Build. Mater. 2016, 120, 112–122. [Google Scholar] [CrossRef]
- Kabir, S.M.A.; Alengaram, U.J.; Jumaat, M.Z.; Yusoff, S.; Sharmin, A.; Bashar, I.I. Performance Evaluation and Some Durability Characteristics of Environmental Friendly Palm Oil Clinker Based Geopolymer Concrete. J. Clean. Prod. 2017, 161, 477–492. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Use of OPC to Improve Setting and Early Strength Properties of Low Calcium Fly Ash Geopolymer Concrete Cured at Room Temperature. Cem. Concr. Compos. 2015, 55, 205–214. [Google Scholar] [CrossRef]
- Cui, Y.; Gao, K.; Zhang, P. Experimental and Statistical Study on Mechanical Characteristics of Geopolymer Concrete. Materials 2020, 13, 1651. [Google Scholar] [CrossRef]
- Patil, A.A.; Chore, H.S.; Dodeb, P.A. Effect of Curing Condition on Strength of Geopolymer Concrete. Adv. Concr. Constr. 2014, 2, 29–37. [Google Scholar] [CrossRef]
- Kumaravel, S. Development of Various Curing Effect of Nominal Strength Geopolymer Concrete. J. Eng. Sci. Technol. Rev. 2014, 7, 116–119. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Jiao, Z. Influence of Curing on the Strength Development of Calcium-Containing Geopolymer Mortar. Materials 2013, 6, 5069–5076. [Google Scholar] [CrossRef]
- Nurruddin, M.F.; Haruna, S.; Mohammed, B.S.; Sha’aban, I.G. Methods of Curing Geopolymer Concrete: A Review. Int. J. Adv. Appl. Sci. 2018, 5, 31–36. [Google Scholar] [CrossRef]
- Zeyad, A.M.; Tayeh, B.A.; Adesina, A.; De Azevedo, A.R.G.; Amin, M.; Hadzima-Nyarko, M.; Saad Agwa, I. Review on Effect of Steam Curing on Behavior of Concrete. Clean. Mater. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Triwulan; Ekaputri, J.J.; Priyanka, N.F. The Effect of Temperature Curing on Geopolymer Concrete. In Proceedings of the Engineering Technology International Conference 2016 (ETIC 2016), Ho Chi Minh City, Vietnam, 5–6 August 2016. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.; Blanco, M. Alkali-Activated Fly Ashes: A Cement for the Future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Heah, C.Y.; Kamarudin, H.; Bakri, A.M.M.A.; Binhussain, M.; Luqman, M.; Nizar, I.K.; Ruzaidi, C.M.; Liew, Y.M. Effect of Curing Profile on Kaolin-Based Geopolymers. Phys. Procedia 2011, 22, 305–311. [Google Scholar] [CrossRef]
- Yunsheng, Z.; Wei, S.; Qianli, C.; Lin, C. Synthesis and Heavy Metal Immobilization Behaviors of Slag Based Geopolymer. J. Hazard. Mater. 2007, 143, 206–213. [Google Scholar] [CrossRef]
- Hardjito, D.; Rangan, B.V. Development and Properties of Low-Calcium Fly Ash-Based Geopolymer Concrete; Curtin University of Technology: Perth, Australia, 2005; p. 94. [Google Scholar]
- Pasupathy, K.; Berndt, M.; Castel, A.; Sanjayan, J.; Pathmanathan, R. Carbonation of a Blended Slag-Fly Ash Geopolymer Concrete in Field Conditions after 8 Years. Constr. Build. Mater. 2016, 125, 661–669. [Google Scholar] [CrossRef]
- Elyamany, H.E.; Abd Elmoaty, A.E.M.; Elshaboury, A.M. Magnesium Sulfate Resistance of Geopolymer Mortar. Constr. Build. Mater. 2018, 184, 111–127. [Google Scholar] [CrossRef]
- Yang, T.; Yao, X.; Zhang, Z. Quantification of Chloride Diffusion in Fly Ash–Slag-Based Geopolymers by X-ray Fluorescence (XRF). Constr. Build. Mater. 2014, 69, 109–115. [Google Scholar] [CrossRef]
- Sun, P.; Wu, H.-C. Chemical and Freeze–Thaw Resistance of Fly Ash-Based Inorganic Mortars. Fuel 2013, 111, 740–745. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of Geopolymer Materials to Acid Attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Marvila, M.T.; Azevedo, A.R.G.; Delaqua, G.C.G.; Mendes, B.C.; Pedroti, L.G.; Vieira, C.M.F. Performance of Geopolymer Tiles in High Temperature and Saturation Conditions. Constr. Build. Mater. 2021, 286, 122994. [Google Scholar] [CrossRef]
- Ariffin, M.A.M.; Bhutta, M.A.R.; Hussin, M.W.; Mohd Tahir, M.; Aziah, N. Sulfuric Acid Resistance of Blended Ash Geopolymer Concrete. Constr. Build. Mater. 2013, 43, 80–86. [Google Scholar] [CrossRef]
- Esparham, A.; Ghalatian, F. The Features of Geopolymer Concrete as a Novel Approach for Utilization in Green Urban Structures. J. Compos. Compd. 2022, 4, 89–96. [Google Scholar] [CrossRef]
- Palomo, A.; Krivenko, P.; Garcia-Lodeiro, I.; Kavalerova, E.; Maltseva, O.; Fernández-Jiménez, A. A Review on Alkaline Activation: New Analytical Perspectives. Mater. De Construcción 2014, 64, e022. [Google Scholar] [CrossRef]
- Aldred, J.; Day, J. Is Geopolymer Concrete a Suitable Alternative To Traditional Concrete? In Proceedings of the 37th Conference on Our World in Concrete & Structures, Singapore, 29–31 August 2012; pp. 1–14. [Google Scholar]
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential Applications of Geopolymer Concrete in Construction: A Review. Case Stud. Constr. Mater. 2021, 15, e00733. [Google Scholar] [CrossRef]
- Albitar, M.; Mohamed Ali, M.S.; Visintin, P.; Drechsler, M. Durability Evaluation of Geopolymer and Conventional Concretes. Constr. Build. Mater. 2017, 136, 374–385. [Google Scholar] [CrossRef]
- Komnitsas, K.A. Potential of Geopolymer Technology towards Green Buildings and Sustainable Cities. Procedia Eng. 2011, 21, 1023–1032. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, B.; Yi, Z.; Du, F.; Zhang, Y. The Properties and Latest Application of Geopolymers. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Kazimierz Dolny, Poland, 21–23 November 2019; Volume 472. [Google Scholar]
- Mo, K.H.; Alengaram, U.J.; Jumaat, M.Z. Structural Performance of Reinforced Geopolymer Concrete Members: A Review. Constr. Build. Mater. 2016, 120, 251–264. [Google Scholar] [CrossRef]
- Tayeh, B.A.; Saffar, D.M.A.; Alyousef, R. The Utilization of Recycled Aggregate in High Performance Concrete: A Review. J. Mater. Res. Technol. 2020, 9, 8469–8481. [Google Scholar] [CrossRef]
- Etim, M.-A.; Babaremu, K.; Lazarus, J.; Omole, D. Health Risk and Environmental Assessment of Cement Production in Nigeria. Atmosphere 2021, 12, 1111. [Google Scholar] [CrossRef]
- Shehata, N.; Sayed, E.T.; Abdelkareem, M.A. Recent Progress in Environmentally Friendly Geopolymers: A Review. Sci. Total Environ. 2021, 762, 143166. [Google Scholar] [CrossRef]
- Barcelo, L.; Kline, J.; Walenta, G.; Gartner, E. Cement and Carbon Emissions. Mater. Struct. 2014, 47, 1055–1065. [Google Scholar] [CrossRef]
- Amran, M.; Debbarma, S.; Ozbakkaloglu, T. Fly Ash-Based Eco-Friendly Geopolymer Concrete: A Critical Review of the Long-Term Durability Properties. Constr. Build. Mater. 2021, 270, 121857. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P. Technical and Commercial Progress in the Adoption of Geopolymer Cement. Miner. Eng. 2012, 29, 89–104. [Google Scholar] [CrossRef]
- Alsalman, A.; Assi, L.N.; Kareem, R.S.; Carter, K.; Ziehl, P. Energy and CO2 Emission Assessments of Alkali-Activated Concrete and Ordinary Portland Cement Concrete: A Comparative Analysis of Different Grades of Concrete. Clean. Environ. Syst. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Lloyd, N.; Rangan, B.V. Geopolymer concrete: A review of development and opportunities. In Proceedings of the 35th Conference on Our World in Concrete & Structures: “The Challenge of Low Carbon Age”, Singapore Concrete Institute, Singapore, 25–27 August 2010; pp. 25–27. [Google Scholar]
- Zain, H.; Abdullah, M.M.A.B.; Hussin, K.; Ariffin, N.; Bayuaji, R. Review on Various Types of Geopolymer Materials with the Environmental Impact Assessment. In Proceedings of the Engineering Technology International Conference 2016 (ETIC 2016), Ho Chi Minh City, Vietnam, 5–6 August 2016. [Google Scholar] [CrossRef]
- Dal Pozzo, A.; Carabba, L.; Bignozzi, M.C.; Tugnoli, A. Life Cycle Assessment of a Geopolymer Mixture for Fireproofing Applications. Int. J. Life Cycle Assess. 2019, 24, 1743–1757. [Google Scholar] [CrossRef]
- Yang, K.-H.; Song, J.-K.; Song, K.-I. Assessment of CO2 Reduction of Alkali-Activated Concrete. J. Clean. Prod. 2013, 39, 265–272. [Google Scholar] [CrossRef]
- Tahir, F.; Sbahieh, S.; Al-Ghamdi, S.G. Environmental Impacts of Using Recycled Plastics in Concrete. Mater. Today Proc. 2022, 62, 4013–4017. [Google Scholar] [CrossRef]
- Alsabri, A.; Tahir, F.; Al-Ghamdi, S.G. Environmental Impacts of Polypropylene (PP) Production and Prospects of Its Recycling in the GCC Region. Mater. Today Proc. 2022, 56, 2245–2251. [Google Scholar] [CrossRef]
- Saleem, J.; Tahir, F.; Baig, M.Z.K.; Al-Ansari, T.; McKay, G. Assessing the Environmental Footprint of Recycled Plastic Pellets: A Life-Cycle Assessment Perspective. Environ. Technol. Innov. 2023, 32, 103289. [Google Scholar] [CrossRef]
- Garces, J.I.T.; Dollente, I.J.; Beltran, A.B.; Tan, R.R.; Promentilla, M.A.B. Life Cycle Assessment of Self-Healing Geopolymer Concrete. Clean. Eng. Technol. 2021, 4, 100147. [Google Scholar] [CrossRef]
- Imtiaz, L.; Kashif-ur-Rehman, S.; Alaloul, W.S.; Nazir, K.; Javed, M.F.; Aslam, F.; Musarat, M.A. Life Cycle Impact Assessment of Recycled Aggregate Concrete, Geopolymer Concrete, and Recycled Aggregate-Based Geopolymer Concrete. Sustainability 2021, 13, 13515. [Google Scholar] [CrossRef]
- Garces, J.I.T.; Tan, R.R.; Beltran, A.B.; Ongpeng, J.M.C.; Promentilla, M.A.B. Environmental Life Cycle Assessment of Alkali-Activated Material with Different Mix Designs and Self-Healing Agents. Chem. Eng. Trans. 2021, 88, 835–840. [Google Scholar] [CrossRef]
- Fernando, S.; Gunasekara, C.; Law, D.W.; Nasvi, M.C.M.; Setunge, S.; Dissanayake, R. Life Cycle Assessment and Cost Analysis of Fly Ash–Rice Husk Ash Blended Alkali-Activated Concrete. J. Environ. Manag. 2021, 295, 113140. [Google Scholar] [CrossRef]
- Asadollahfardi, G.; Katebi, A.; Taherian, P.; Panahandeh, A. Environmental Life Cycle Assessment of Concrete with Different Mixed Designs. Int. J. Constr. Manag. 2021, 21, 665–676. [Google Scholar] [CrossRef]
- Meshram, R.B.; Kumar, S. Comparative Life Cycle Assessment (LCA) of Geopolymer Cement Manufacturing with Portland Cement in Indian Context. Int. J. Environ. Sci. Technol. 2022, 19, 4791–4802. [Google Scholar] [CrossRef]
- Heath, A.; Paine, K.; McManus, M. Minimising the Global Warming Potential of Clay Based Geopolymers. J. Clean. Prod. 2014, 78, 75–83. [Google Scholar] [CrossRef]
- Tang, W.; Pignatta, G.; Sepasgozar, S.M.E. Life-Cycle Assessment of Fly Ash and Cenosphere-Based Geopolymer Material. Sustainability 2021, 13, 11167. [Google Scholar] [CrossRef]




| Precursors | Reference | Composition (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | MnO | K2O | Na2O | P2O5 | TiO2 | SO3 | ||
| FA | [85] | 65.9 | 24 | 2.87 | 1.59 | 0.42 | 0.06 | 1.44 | 0.49 | 0.19 | 0.92 | - |
| FA | [86] | 52.83 | 21.50 | 10.49 | 6.44 | 0.89 | - | 1.76 | 0.82 | 1.75 | 1.6 | - |
| FA | [87] | 62.04 | 25.50 | 4.28 | 3.96 | 1.27 | - | - | 0.46 | 0.31 | 1.33 | 0.73 |
| FA | [88] | 61.86 | - | - | - | 0.86 | - | - | - | - | - | 0.28 |
| FA | [72] | 50.70 | 28.80 | 8.80 | 2.38 | 1.39 | - | 2.40 | 0.84 | - | - | 0.30 |
| BFS | [85] | 36 | 13.8 | 0.3 | 42.6 | 5.8 | 0.4 | 0.27 | 0.21 | 0.10 | 0.8 | 0.56 |
| BFS | [69] | 35.80 | 13.21 | 1.97 | 35.68 | 9.76 | - | 0.57 | 0.48 | - | - | 0.21 |
| BFS | [89] | 32.5 | 13.7 | 0.8 | 45.8 | 3.3 | 0.4 | 0.5 | 0.3 | 0 | 0.7 | 1.8 |
| BFS | [87] | 34.11 | 15.36 | 0.83 | 35.99 | 6.58 | 1.07 | 0.62 | 0.4 | - | 2.41 | 2.50 |
| BFS | [88] | 32.9 | - | 0.7 | 41.3 | 5.9 | - | - | 0.45 | - | - | 0.21 |
| RHA | [69] | 89.47 | 0.83 | 0.53 | 0.68 | 0.37 | - | 0.17 | 0.22 | - | - | 0.12 |
| RHA | [89] | 93.46 | 0.58 | 0.52 | 1.03 | 0.51 | - | 1.82 | 0.08 | 1.6 | 0 | 0.6 |
| RHA | [90] | 89.17 | 0 | 0.41 | 0.61 | 1.22 | - | 1.12 | 7.29 | - | 0.03 | - |
| RM | [91] | 16.51 | 28.05 | 30.32 | 2.22 | 0.7 | 0.11 | 0.26 | 8.70 | - | 4.29 | - |
| RM | [92] | 27.544 | 30.591 | 4.603 | 25.478 | 0.818 | 0.012 | 3.82 | - | - | 5.151 | 1.422 |
| MK | [93] | 54.4 | 39.4 | 1.8 | 0.1 | - | 0.01 | 1.0 | - | 0.1 | 1.6 | - |
| MK | [75] | 50.995 | 42.631 | 2.114 | 1.287 | 0.127 | 0.006 | 0.337 | 0.284 | 0.051 | 1.713 | 0.439 |
| MK | [89] | 51.7 | 40.6 | 0.64 | 0.71 | 0.96 | 0.08 | 2 | 0.31 | 0.2 | 3 | 0.1 |
| SF | [86] | 92.39 | 1.41 | 0.154 | 0.547 | - | - | <1 | - | 2.32 | <1 | - |
| SF | [88] | 92.98 | - | 1.49 | 0.32 | 0.57 | - | 0.51 | 0.47 | - | - | 0.57 |
| SF | [72] | 93.67 | 0.83 | 1.30 | 0.31 | 0.84 | 0.84 | 1.10 | 0.40 | - | - | 0.16 |
| Reference | Source | Si/Al | M | CT (°C) | CD (h) | D (Day) | CS (MPa) |
|---|---|---|---|---|---|---|---|
| [111] | FA + OPC | 2.5 | 10 | 80 | 24 | 28–365 | 44–55 |
| [112] | FA | 1.5–3.9 | 15 | 80 | 24 | 3–365 | 22.5–60.7 |
| [113] | Nano silica + FA | 2.29–4.10 | 8 | 80 | 24 | 28 | 37.2–47.3 |
| [114] | FA | 2.89 | 12 | 70–800 | 24 | 28 | 11.93–17 |
| [115] | Metakaolin | 2–6 | 6–7 | 20 | - | 28 | 5.4–34.9 |
| [116] | Metakaolin | 2.25–4 | 10 | Ambient, 50 and 75 | 24 | 3–90 | 2–66 |
| [117] | Metakaolin | 3.5–3.8 | 12 | 85 | 2 | - | 2–48 |
| [118] | FA | 2.3 | 16 | Ambient and 60 | 24 | 3–28 | 8–50 |
| [119] | FA | 7.7 | 8–16 | Ambient (23) | - | 28 | 7.6–21.5 |
| [120] | FA + GBFS | - | 10–12 | Ambient (20) | - | 1–56 | 2–60.1 |
| [121] | Natural pozzolan+ nano-silica | 2.47–4.17 | 14 | 60 | 168 | 1–28 | 7.32–44.97 |
| [122] | FA | 1–1.88 | - | 75 | 16 | - | 33.45–41.02 |
| [123] | FA | 2.1 | 8–14 | 60–90 | 24–48 | 3–7 | 20–49 |
| [11] | Metakaolin | 1–5 | - | 60 | 6 | 7 | 2.1–36.8 |
| [124] | FA + RHA | 2 | - | Ambient | - | 7–90 | 17.2–48.7 |
| [125] | FA | 1.6 | 14 | 25 | 24 | 7–70 | 7.1–48.2 |
| [125] | Bottom Ash | 2.16 | 14 | 25 | 24 | 7–70 | 0.2–1.1 |
| [125] | FA + BA | 1.6–2.16 | 14 | 25 | 24 | 7–70 | 0.8–12.7 |
| [126] | Copper tailings + FA | 1.89–7.78 | 5–15 | 60 | - | 2–28 | 1.37–21.2 |
| [127] | Metakaolin | 1.86–2.11 | 7.2 | Ambient then 40–60 | 24 + 24 | 7 | 57–61 |
| [128] | FA | 1.5–5.1 | 12–16 | Ambient then 70 | 24 + 24 | 7 | 16–64 |
| [129] | RM + FA | 1.5–2.75 | 6–12 | 60 | 24 | 7 | 5.3–38 |
| [130] | FA + Alccofine | - | 16 | Ambient to 90 | 24 | 3–28 | 2.5–73 |
| [131] | FA + GBFS | - | 12 | 75 | 18 | 28 | 51.1–53.2 |
| [132] | Metakaolin | 1–3 | 11–18 | 75 | 24 | 7 | 0.4–64 |
| [133] | FA + GBFS | 1.8 | 8 | Ambient | - | 7–28 | 12.88–45.55 |
| [134] | FA | 2.1 | 8–16 | 24–120 | 6–72 | 3–28 | 13–56 |
| [135] | Gold mine tailing | 1–11 | 10 | 60–110 | - | 5 | 1.23–18.10 |
| [136] | FA | - | 3–9 | 50 | 72 | 3–7 | 45–81 |
| [137] | FA + RHA | 2.1 | 8 | Hot gunny then Ambient | 24 | 3–56 | 3.19–50.96 |
| [138] | Palm oil-fuel ash (POFA) + FA + oil-palm shell (OPS) | 3.43–6.17 | 14 | 65 | 48 | 3- 28 | 7.3–30.1 |
| [139] | GGBFS + MK + POFA | - | 14 | 65 | 24 | 3–28 | 24.7–41.5 |
| [140] | FA + OPC | 1.765–2.018 | 14 | 20–23 | - | 3–90 | 4–46 |
| [141] | FA | 2.6–2.9 | 12 | 80 | 24 | 7 | 28.99–46.18 |
| Reference | Type of Concrete | ADPF | GWP | ODP | HT | FWAE | MAE | TE | POCP | AP | EP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [34] | GPC | 1.19 | 168.5 | 1.39 × 10−5 | 105.4 | 27.01 | 0.000459 | 1.77 | 3.65 × 10−2 | 0.82 | 0.0796 |
| OPCC | 0.61 | 305.9 | 8.74 × 10−6 | 18.9 | 2.52 | 0.00968 | 0.45 | 1.67 × 10−2 | 0.45 | 0.0683 | |
| [181] | OPCC | 757.42 | 454.5937 | 4.66 × 10−9 | - | - | - | - | 4.49 × 10−2 | 0.8217 | 0.1647 |
| GPC | 2933.205 | 285.0813 | 2.24 × 10−8 | - | - | - | - | 9.30 × 10−2 | 1.6067 | 0.1337 | |
| SHGPC | 6428.252 | 417.1633 | 2.66 × 10−6 | - | - | - | - | 1.65 × 10−1 | 2.2264 | 0.2226 | |
| [182] | OPCC | - | 264.181 | 0 | 0.8952 | 1.78 × 10−7 | 4.58 × 10−5 | 6.32 × 10−31 | 9.63 × 10−2 | 1.01904 | 0.07922 |
| RAC | - | 261.315 | 0 | 0.886 | 1.68 × 10−7 | 4.48 × 10−5 | 6.3 × 10−31 | 4.11 × 10−2 | 1.01165 | 0.0788 | |
| GPC | - | 112.743 | 5.59 × 10−5 | 33.7 | 40.94 | 136.45 | 0.0107 | 7.77 × 10−2 | 0.60119 | 0.11483 | |
| RAGC | - | 111.377 | 5.59 × 10−5 | 33.68249 | 40.94 | 136.45 | 0.0107 | 5.13 × 10−2 | 0.59769 | 0.11463 | |
| [19] | OPC | 1213 | 302 | 0.13 | - | - | - | - | 2.60 × 10−2 | 0.674 | 0.174 |
| GPC-S1 | 900 | 110 | 1.61 | - | - | - | - | 2.90 × 10−2 | 0.727 | 0.155 | |
| GPC-S2 | 1480 | 163 | 1.67 | - | - | - | - | 4.90 × 10−2 | 1.237 | 0.201 | |
| GPC-S3 | 2796 | 254 | 1.78 | - | - | - | - | 5.60 × 10−2 | 1.263 | 1.245 | |
| [183] | OPCC | 1892 | 333.65 | 4.19 × 10−6 | - | - | - | - | 1.30 × 10−2 | 0.78 | 0.265 |
| GPC-1 | 2443 | 207.51 | 1.10 × 10−5 | - | - | - | - | 4.30 × 10−2 | 1.21 | 0.265 | |
| GPC-2 | 1618 | 138.89 | 7.75 × 10−6 | - | - | - | - | 3.20 × 10−2 | 0.94 | 0.195 | |
| GPC-3 | 2127 | 178.1 | 9.50 × 10−6 | - | - | - | - | 3.90 × 10−2 | 1.11 | 0.243 | |
| SH1GPC-2 | 1817 | 171.076 | 8.73 × 10−6 | - | - | - | - | 3.60 × 10−2 | 1.09 | 0.2226 | |
| SH1GPC-3 | 2339 | 212.26 | 1.05 × 10−5 | - | - | - | - | 4.20 × 10−2 | 1.27 | 0.273 | |
| SH2GPC-2 | 4558 | 252.06 | 1.74 × 10−5 | - | - | - | - | 8.50 × 10−2 | 1.43 | 0.34 | |
| SH2GPC-3 | 5247 | 298.19 | 1.98 × 10−5 | - | - | - | - | 9.50 × 10−2 | 1.63 | 0.397 | |
| [184] | 100 PC | 1.45 × 10−2 | 319 | 9.96 × 10−6 | 20.7 | 6.95 × 10−1 | 2.65 × 100 | - | 1.89 × 10−2 | 0.65 | 0.251 |
| 100% FA | 2.32 × 100 | 327 | 3.63 × 10−5 | 298.4 | 7.77 × 101 | 1.13 × 10−1 | - | 2.66 × 10−2 | 0.758 | 0.209 | |
| 90%FA-10%RHA | 2.31 × 100 | 326 | 3.62 × 10−5 | 2.98 × 102 | 7.77 × 101 | 1.05 × 10−1 | - | 2.65 × 10−2 | 0.756 | 0.208 | |
| [185] | OPCC | - | 386.44 | - | 35.68 | - | - | - | - | 0.84 | 0.159 |
| GPC | - | 286.85 | - | 72.35 | - | - | - | - | 1.11 | 0.183 |
| Reference | Typer of Concrete/Cement | GWP | Reduction in GWP | Reference | Type of Concrete/Cement | GWP | Reduction in GWP |
|---|---|---|---|---|---|---|---|
| [186] | OPC | 895 | [34] | OPCC | 305.9 | ||
| GC/FA + slag | 267 | 70% | GPC | 168.5 | 44.92% | ||
| GC/FA + cement | 351 | 61% | [181] | OPCC | 454.5937 | ||
| [37] | OPCC | 354 | GPC | 285.0813 | 37.30% | ||
| GCC | 320 | 10% | SHGPC | 417.1633 | 8.23% | ||
| [188] | NAC | 704 | [182] | OPCC | 264.181 | ||
| G-C | 360 | 49% | GPC | 112.743 | 57% | ||
| G-FA | 477 | 32% | RAGC | 111.377 | 58% | ||
| [36] | OPCC | 381.17 | [19] | OPCC | 302 | ||
| GPC | 210.9 | 45% | GPC- S1 | 110 | 64% | ||
| [177] | OPCC | 323 | GPC- S2 | 163 | 46% | ||
| AA GGBS | 110 | 66% | GPC- S3 | 254 | 16% | ||
| AA FA | 160 | 50% | [183] | OPCC | 333.65 | ||
| AA MK | 187 | 42% | GPC-1 | 207.51 | 38% | ||
| [35] | OPC | 760 | GPC-2 | 138.89 | 58% | ||
| GP1 | 404 | 47% | GPC-3 | 178.1 | 47% | ||
| GP2 | 271 | 64% | SH1GPC-2 | 171.076 | 49% | ||
| GP3 | 310 | 59% | SH1GPC-3 | 212.26 | 36% | ||
| GP4 | 425 | 44% | SH2GPC-2 | 252.06 | 24% | ||
| [184] | OPCC | 319 | SH2GPC-3 | 298.19 | 11% | ||
| 100% FA | 327 | −3% | [185] | OPCC | 386.44 | ||
| 90%FA-10%RHA | 326 | −2% | GPC | 286.85 | 26% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sbahieh, S.; McKay, G.; Al-Ghamdi, S.G. Comprehensive Analysis of Geopolymer Materials: Properties, Environmental Impacts, and Applications. Materials 2023, 16, 7363. https://doi.org/10.3390/ma16237363
Sbahieh S, McKay G, Al-Ghamdi SG. Comprehensive Analysis of Geopolymer Materials: Properties, Environmental Impacts, and Applications. Materials. 2023; 16(23):7363. https://doi.org/10.3390/ma16237363
Chicago/Turabian StyleSbahieh, Sami, Gordon McKay, and Sami G. Al-Ghamdi. 2023. "Comprehensive Analysis of Geopolymer Materials: Properties, Environmental Impacts, and Applications" Materials 16, no. 23: 7363. https://doi.org/10.3390/ma16237363







