The Emergence of Carbon Nanomaterials as Effective Nano-Avenues to Fight against COVID-19
Abstract
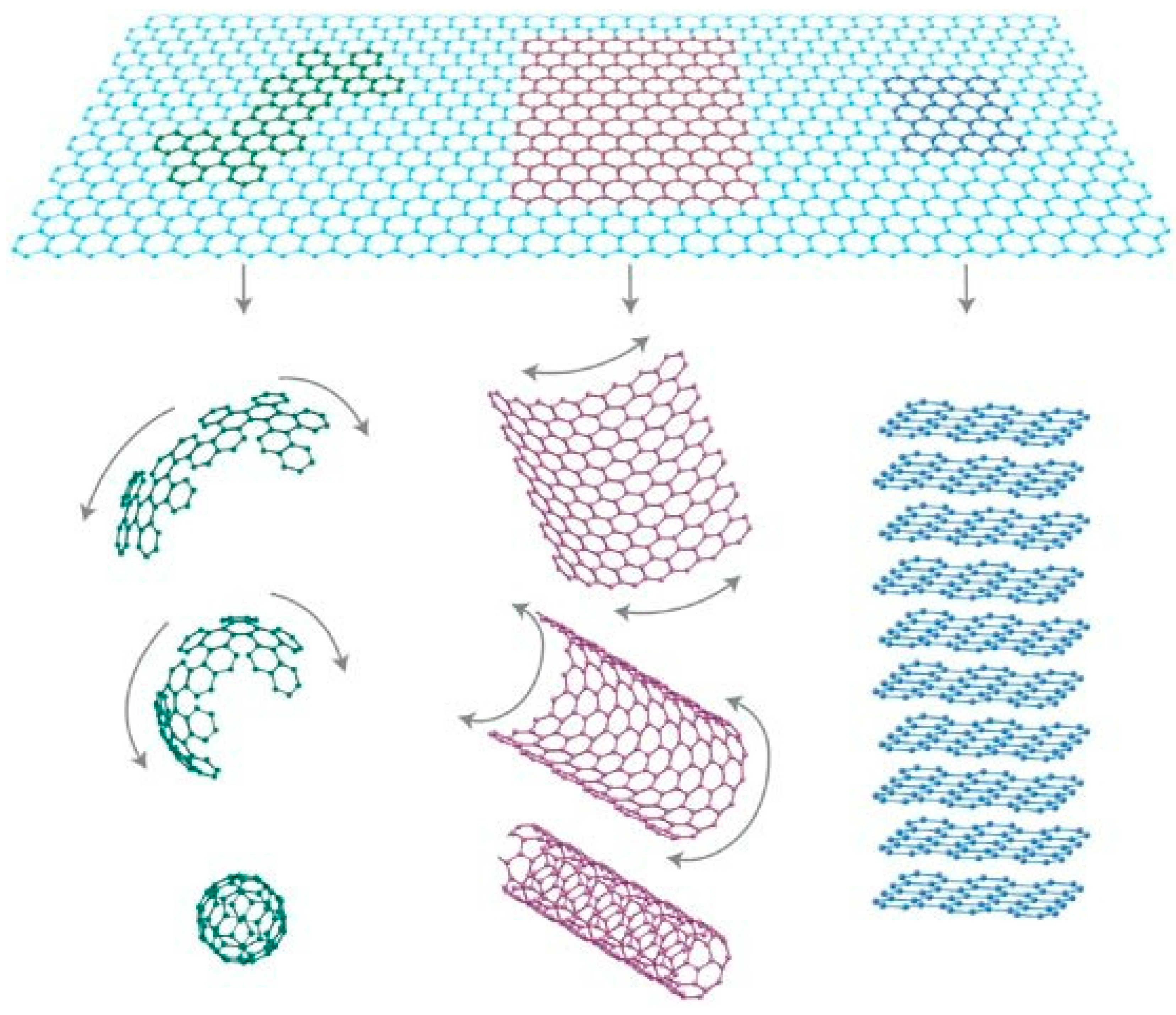
:1. Introduction
2. Applications of Carbon Nanomaterials against COVID-19
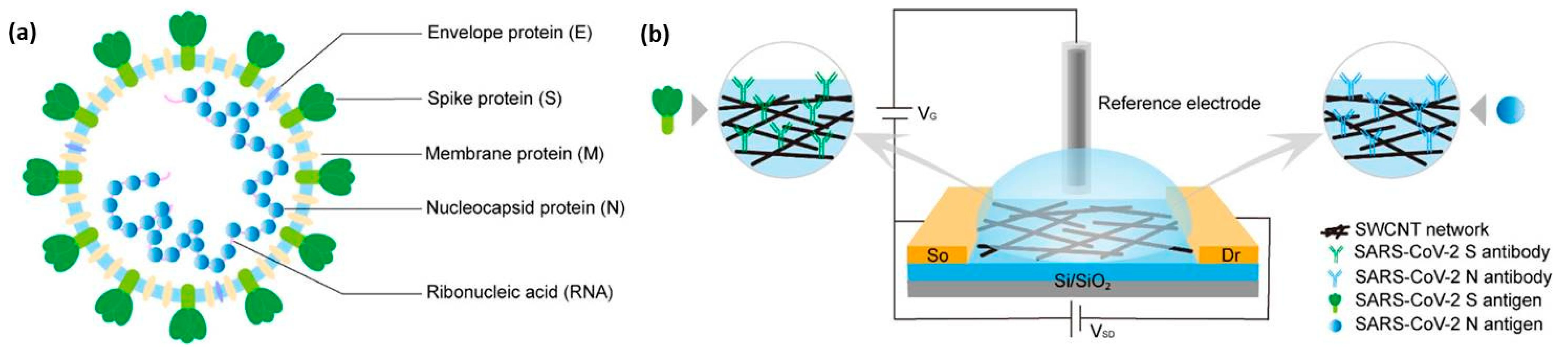
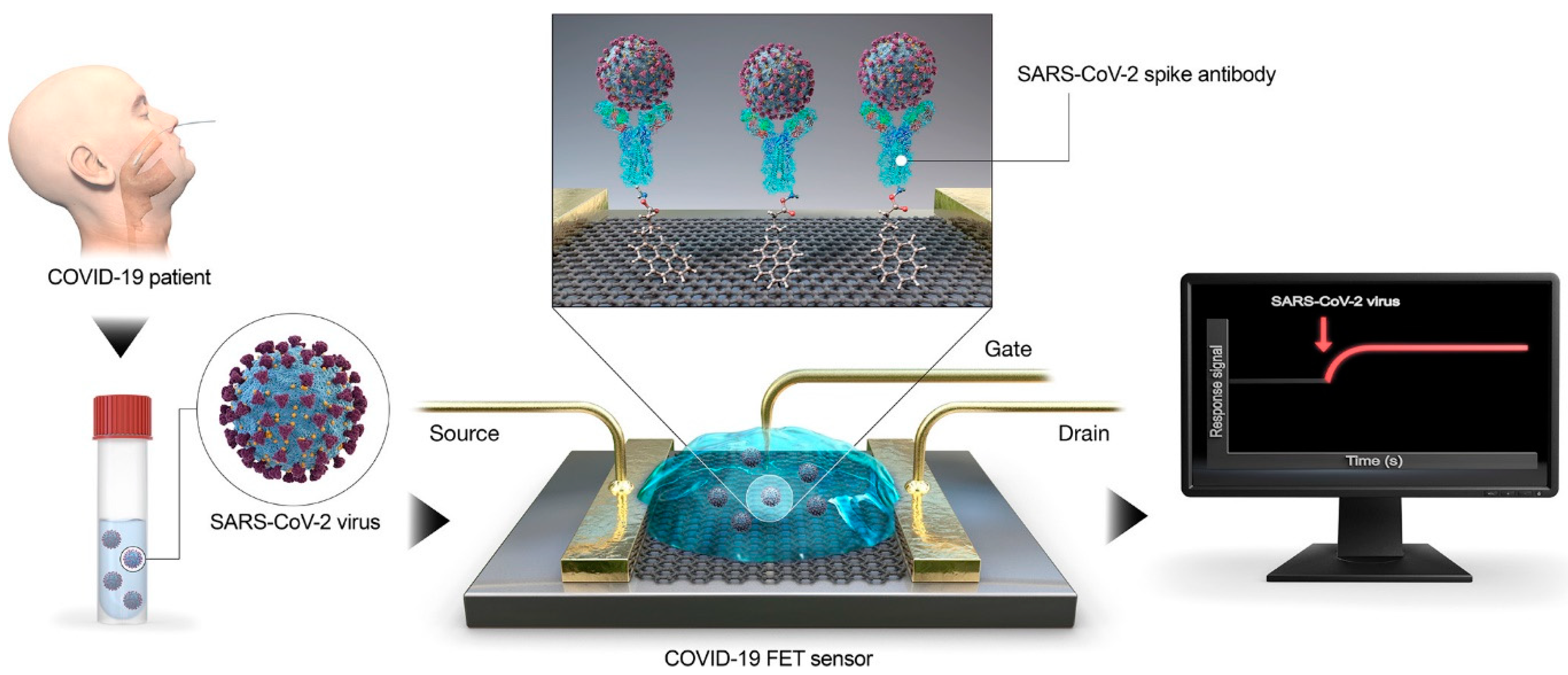
2.1. Carbon Nanomaterials as Sensor for Detection of SARS-CoV-2
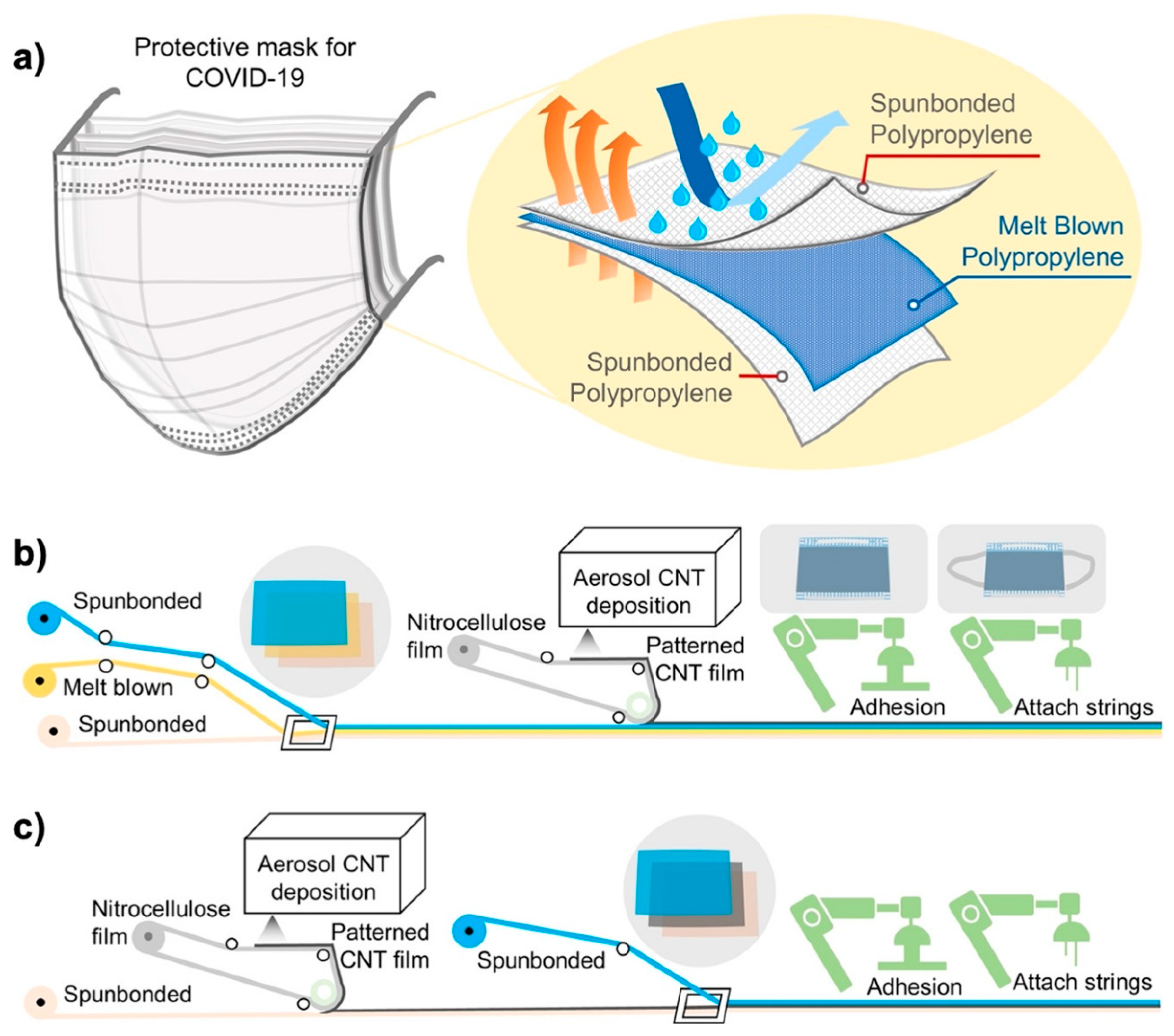
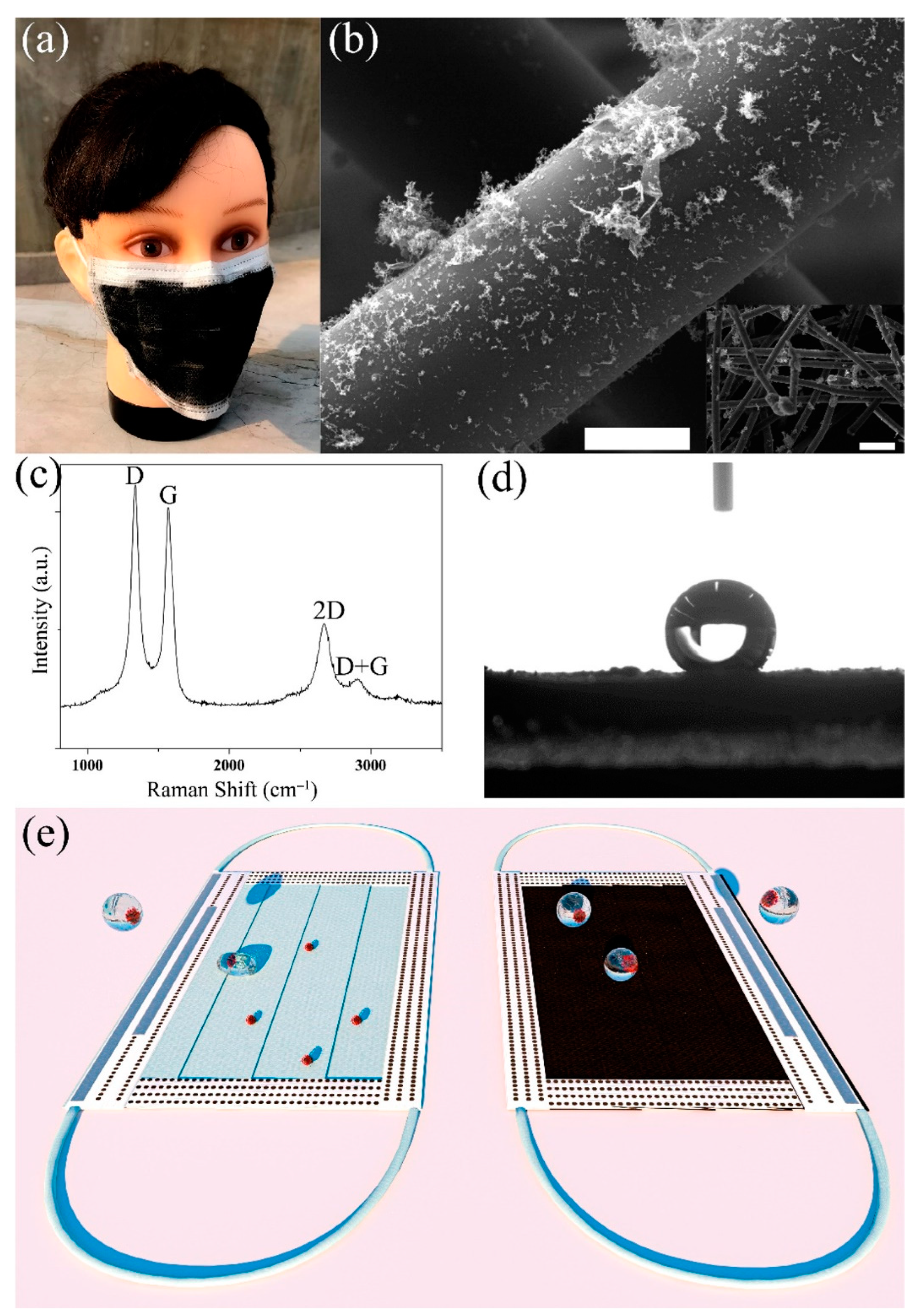
2.2. Carbon Nanomaterials in Personal Protection to Restrict SARS-CoV-2 Transmission
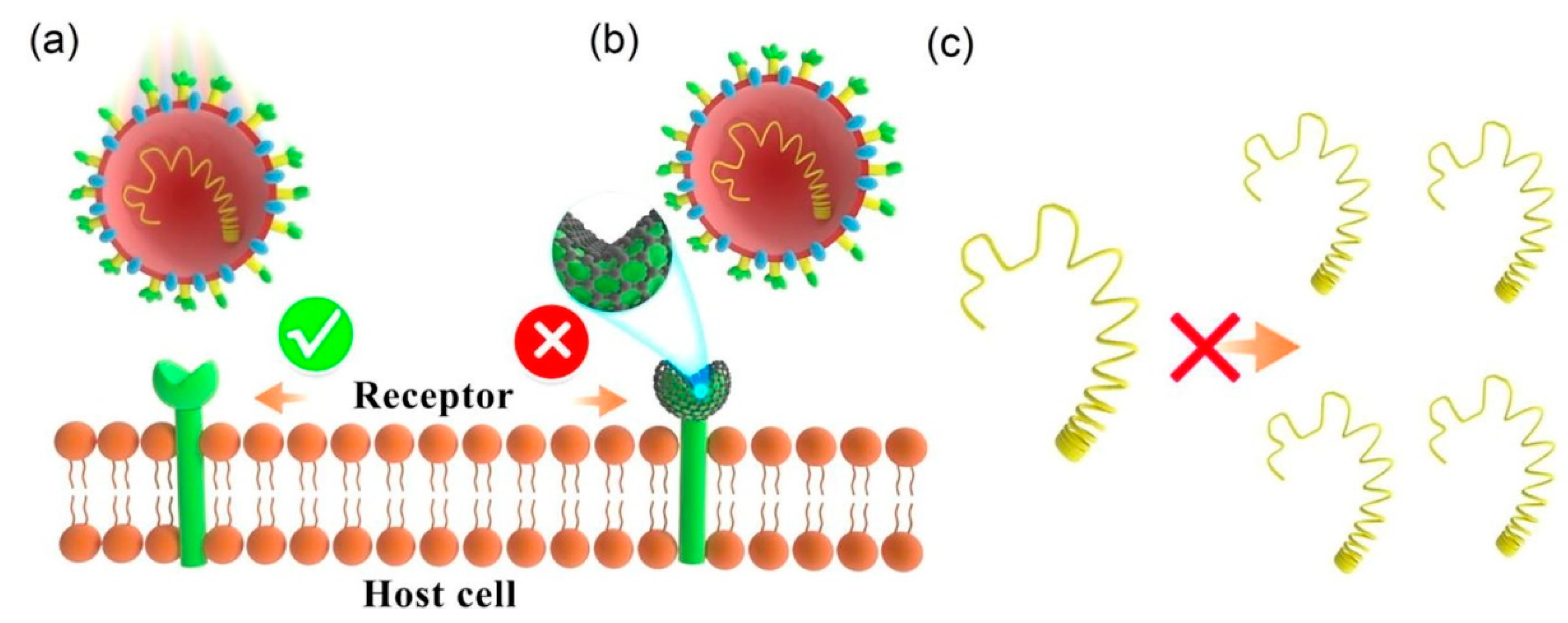
2.3. Carbon Nanomaterials as Antiviral Agents for Removal of the SARS-CoV-2
2.4. Carbon Nanomaterials in Making Vaccines for SARS-CoV-2
3. Challenges and Future Scope
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Onyancha, R.B.; Ukhurebor, K.E.; Aigbe, U.O.; Osibote, O.A.; Kusuma, H.S.; Darmokoesoemo, H. A Methodical Review on Carbon-Based Nanomaterials in Energy-Related Applications. Adsorpt. Sci. Technol. 2022, 2022, e4438286. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon Nanotubes: Properties, Synthesis, Purification, and Medical Applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.; Boivin, G. Pandemics Throughout History. Front. Microbiol. 2021, 11, 631736. [Google Scholar] [CrossRef]
- Adil, M.T.; Rahman, R.; Whitelaw, D.; Jain, V.; Al-Taan, O.; Rashid, F.; Munasinghe, A.; Jambulingam, P. SARS-CoV-2 and the Pandemic of COVID-19. Postgrad. Med. J. 2021, 97, 110–116. [Google Scholar] [CrossRef]
- Wang, H.; Paulson, K.R.; Pease, S.A.; Watson, S.; Comfort, H.; Zheng, P.; Aravkin, A.Y.; Bisignano, C.; Barber, R.M.; Alam, T.; et al. Estimating Excess Mortality Due to the COVID-19 Pandemic: A Systematic Analysis of COVID-19-Related Mortality, 2020–2021. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef] [PubMed]
- Pak, A.; Adegboye, O.A.; Adekunle, A.I.; Rahman, K.M.; McBryde, E.S.; Eisen, D.P. Economic Consequences of the COVID-19 Outbreak: The Need for Epidemic Preparedness. Front. Public Health 2020, 8, 241. [Google Scholar] [CrossRef]
- Excler, J.-L.; Saville, M.; Berkley, S.; Kim, J.H. Vaccine Development for Emerging Infectious Diseases. Nat. Med. 2021, 27, 591–600. [Google Scholar] [CrossRef]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global Impact of the First Year of COVID-19 Vaccination: A Mathematical Modelling Study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- COVID Research: A Year of Scientific Milestones. Nature 2021. [CrossRef]
- Huang, X.; Kon, E.; Han, X.; Zhang, X.; Kong, N.; Mitchell, M.J.; Peer, D.; Tao, W. Nanotechnology-Based Strategies against SARS-CoV-2 Variants. Nat. Nanotechnol. 2022, 17, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Varghese, R.; Salvi, S.; Sood, P.; Karsiya, J.; Kumar, D. Carbon Nanotubes in COVID-19: A Critical Review and Prospects. Colloid Interface Sci. Commun. 2022, 46, 100544. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Wang, H.-B.; Zhang, H.-D.; Xu, L.-L.; Gan, T.; Huang, K.-J.; Liu, Y.-M. Electrochemical Biosensor for Simultaneous Determination of Guanine and Adenine Based on Dopamine-Melanin Colloidal Nanospheres–Graphene Composites. J. Solid State Electrochem. 2014, 18, 2435–2442. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Y.; Shi, M.; Lu, X.; Qin, C.; Li, C.; Ye, M. Fast and Facile Preparation of Graphene Oxide and Reduced Graphene Oxide Nanoplatelets. Chem. Mater. 2009, 21, 3514–3520. [Google Scholar] [CrossRef]
- Harun-Ur-Rashid, M.; Foyez, T.; Jahan, I.; Pal, K.; Bin Imran, A. Rapid Diagnosis of COVID-19 via Nano-Biosensor-Implemented Biomedical Utilization: A Systematic Review. RSC Adv. 2022, 12, 9445–9465. [Google Scholar] [CrossRef]
- Functionalized Carbon Nanomaterials for Theranostic Applications—1st Edition. Available online: https://www.elsevier.com/books/functionalized-carbon-nanomaterials-for-theranostic-applications/mallakpour/978-0-12-824366-4 (accessed on 7 December 2022).
- Yang, H.; Liu, Y.; Guo, Z.; Lei, B.; Zhuang, J.; Zhang, X.; Liu, Z.; Hu, C. Hydrophobic Carbon Dots with Blue Dispersed Emission and Red Aggregation-Induced Emission. Nat. Commun. 2019, 10, 1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, J.; Zhang, X.; Li, L.; Regmi, S.; Yang, G.; Tang, S. Development of Fluorescent Lateral Flow Immunoassay for SARS-CoV-2-Specific IgM and IgG Based on Aggregation-Induced Emission Carbon Dots. Front. Bioeng. Biotechnol. 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, Z.; Alafeef, M.; Moitra, P.; Ray, P.; Pan, D. N-Gene-Complementary Antisense-Oligonucleotide Directed Molecular Aggregation of Dual-Colour Carbon Dots, Leading to Efficient Fluorometric Sensing of SARS-COV-2 RNA. Nanoscale 2022, 14, 5112–5120. [Google Scholar] [CrossRef]
- Xu, L.-D.; Zhu, J.; Ding, S.-N. Immunoassay of SARS-CoV-2 Nucleocapsid Proteins Using Novel Red Emission-Enhanced Carbon Dot-Based Silica Spheres. Analyst 2021, 146, 5055–5060. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, J.-C.; Seo, G.; Woo, J.H.; Lee, M.; Nam, J.; Sim, J.Y.; Kim, H.-R.; Park, E.C.; Park, S. Computational Method-Based Optimization of Carbon Nanotube Thin-Film Immunosensor for Rapid Detection of SARS-CoV-2 Virus. Small Sci. 2022, 2, 2100111. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, J.; Hussain, C.M. Graphene-Based Field-Effect Transistor Biosensors for the Rapid Detection and Analysis of Viruses: A Perspective in View of COVID-19. Carbon Trends 2021, 2, 100011. [Google Scholar] [CrossRef]
- Zamzami, M.A.; Rabbani, G.; Ahmad, A.; Basalah, A.A.; Al-Sabban, W.H.; Nate Ahn, S.; Choudhry, H. Carbon Nanotube Field-Effect Transistor (CNT-FET)-Based Biosensor for Rapid Detection of SARS-CoV-2 (COVID-19) Surface Spike Protein S1. Bioelectrochemistry 2022, 143, 107982. [Google Scholar] [CrossRef]
- Thanihaichelvan, M.; Surendran, S.N.; Kumanan, T.; Sutharsini, U.; Ravirajan, P.; Valluvan, R.; Tharsika, T. Selective and Electronic Detection of COVID-19 (Coronavirus) Using Carbon Nanotube Field Effect Transistor-Based Biosensor: A Proof-of-Concept Study. Mater. Today Proc. 2022, 49, 2546–2549. [Google Scholar] [CrossRef]
- Shao, W.; Shurin, M.R.; Wheeler, S.E.; He, X.; Star, A. Rapid Detection of SARS-CoV-2 Antigens Using High-Purity Semiconducting Single-Walled Carbon Nanotube-Based Field-Effect Transistors. ACS Appl. Mater. Interfaces 2021, 13, 10321–10327. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; González-Grandío, E.; Navarro, N.; Pinals, R.L.; Ledesma, F.; Yang, D.; Landry, M.P. Extraction of Viral Nucleic Acids with Carbon Nanotubes Increases SARS-CoV-2 Quantitative Reverse Transcription Polymerase Chain Reaction Detection Sensitivity. ACS Nano 2021, 15, 10309–10317. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajan, K.; Rajini, G.K.; Maji, D. Flexible, Highly Sensitive Paper-Based Screen Printed MWCNT/PDMS Composite Breath Sensor for Human Respiration Monitoring. IEEE Sens. J. 2021, 21, 13985–13995. [Google Scholar] [CrossRef] [PubMed]
- Pinals, R.L.; Ledesma, F.; Yang, D.; Navarro, N.; Jeong, S.; Pak, J.E.; Kuo, L.; Chuang, Y.-C.; Cheng, Y.-W.; Sun, H.-Y.; et al. Rapid SARS-CoV-2 Spike Protein Detection by Carbon Nanotube-Based Near-Infrared Nanosensors. Nano Lett. 2021, 21, 2272–2280. [Google Scholar] [CrossRef]
- Hasan, R.R.; Saleque, A.M.; Anwar, A.B.; Rahman, M.A.; Tsang, Y.H. Multiwalled Carbon Nanotube-Based On-Body Patch Antenna for Detecting COVID-19-Affected Lungs. ACS Omega 2022, 7, 28265–28274. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Alves, J.F.; Frasco, M.F.; Piloto, A.M.; Serrano, V.; Mateus, D.; Sebastião, A.I.; Matos, A.M.; Carmo, A.; Cruz, T.; et al. An Ultra-Sensitive Electrochemical Biosensor Using the Spike Protein for Capturing Antibodies against SARS-CoV-2 in Point-of-Care. Mater. Today Bio 2022, 16, 100354. [Google Scholar] [CrossRef]
- Curti, F.; Fortunati, S.; Knoll, W.; Giannetto, M.; Corradini, R.; Bertucci, A.; Careri, M. A Folding-Based Electrochemical Aptasensor for the Single-Step Detection of the SARS-CoV-2 Spike Protein. ACS Appl. Mater. Interfaces 2022, 14, 19204–19211. [Google Scholar] [CrossRef]
- Li, T.; Soelberg, S.D.; Taylor, Z.; Sakthivelpathi, V.; Furlong, C.E.; Kim, J.-H.; Ahn, S.; Han, P.D.; Starita, L.M.; Zhu, J.; et al. Highly Sensitive Immunoresistive Sensor for Point-Of-Care Screening for COVID-19. Biosensors 2022, 12, 149. [Google Scholar] [CrossRef]
- Mujica, M.L.; Tamborelli, A.; Castellaro, A.; Barcudi, D.; Rubianes, M.D.; Rodríguez, M.C.; Saka, H.A.; Bocco, J.L.; Dalmasso, P.R.; Rivas, G.A. Impedimetric and Amperometric Genosensors for the Highly Sensitive Quantification of SARS-CoV-2 Nucleic Acid Using an Avidin-Functionalized Multi-Walled Carbon Nanotubes Biocapture Platform. Biosens. Bioelectron. X 2022, 12, 100222. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.; Kandeil, A.; Gomaa, M.; Mohamed El Nashar, R.; El-Sherbiny, I.M.; Hassan, R.Y.A. SARS-CoV-2-Impedimetric Biosensor: Virus-Imprinted Chips for Early and Rapid Diagnosis. ACS Sens. 2021, 6, 4098–4107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Luo, X. Detection of Covid-19 through a Heptanal Biomarker Using Transition Metal Doped Graphene. J. Phys. Chem. B 2022, 126, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.A.; Hu, C.; Jahan, S.; Yuan, B.; Saleh, M.S.; Ju, E.; Gao, S.-J.; Panat, R. Sensing of COVID-19 Antibodies in Seconds via Aerosol Jet Nanoprinted Reduced-Graphene-Oxide-Coated 3D Electrodes. Adv. Mater. 2021, 33, 2006647. [Google Scholar] [CrossRef]
- El-Said, W.A.; Al-Bogami, A.S.; Alshitari, W. Synthesis of Gold Nanoparticles@reduced Porous Graphene-Modified ITO Electrode for Spectroelectrochemical Detection of SARS-CoV-2 Spike Protein. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120237. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Qiu, J.; Gao, J.; Liu, H.; Zhang, Y.; Han, L. Ultrasensitive, High-Throughput, and Rapid Simultaneous Detection of SARS-CoV-2 Antigens and IgG/IgM Antibodies within 10 Min through an Immunoassay Biochip. Microchim. Acta 2021, 188, 262. [Google Scholar] [CrossRef]
- Mattioli, I.A.; Castro, K.R.; Macedo, L.J.A.; Sedenho, G.C.; Oliveira, M.N.; Todeschini, I.; Vitale, P.M.; Ferreira, S.C.; Manuli, E.R.; Pereira, G.M.; et al. Graphene-Based Hybrid Electrical-Electrochemical Point-of-Care Device for Serologic COVID-19 Diagnosis. Biosens. Bioelectron. 2022, 199, 113866. [Google Scholar] [CrossRef]
- Krsihna, B.V.; Ahmadsaidulu, S.; Teja, S.S.T.; Jayanthi, D.; Navaneetha, A.; Reddy, P.R.; Prakash, M.D. Design and Development of Graphene FET Biosensor for the Detection of SARS-CoV-2. Silicon 2022, 14, 5913–5921. [Google Scholar] [CrossRef]
- Ban, D.K.; Bodily, T.; Karkisaval, A.G.; Dong, Y.; Natani, S.; Ramanathan, A.; Ramil, A.; Srivastava, S.; Bandaru, P.; Glinsky, G.; et al. Rapid Self-Test of Unprocessed Viruses of SARS-CoV-2 and Its Variants in Saliva by Portable Wireless Graphene Biosensor. Proc. Natl. Acad. Sci. USA 2022, 119, e2206521119. [Google Scholar] [CrossRef]
- Shahdeo, D.; Chauhan, N.; Majumdar, A.; Ghosh, A.; Gandhi, S. Graphene-Based Field-Effect Transistor for Ultrasensitive Immunosensing of SARS-CoV-2 Spike S1 Antigen. ACS Appl. Bio Mater. 2022, 5, 3563–3572. [Google Scholar] [CrossRef]
- Lucas Garrote, B.; Lopes, L.C.; Pinzón, E.F.; Mendonça-Natividade, F.C.; Martins, R.B.; Santos, A.; Arruda, E.; Bueno, P.R. Reagentless Quantum-Rate-Based Electrochemical Signal of Graphene for Detecting SARS-CoV-2 Infection Using Nasal Swab Specimens. ACS Sens. 2022, 7, 2645–2653. [Google Scholar] [CrossRef]
- Kadadou, D.; Tizani, L.; Wadi, V.S.; Banat, F.; Alsafar, H.; Yousef, A.F.; Hasan, S.W. Detection of SARS-CoV-2 in Clinical and Environmental Samples Using Highly Sensitive Reduced Graphene Oxide (RGO)-Based Biosensor. Chem. Eng. J. 2023, 453, 139750. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, S.; Ke, Y.; Chen, H.; Dang, J.; Huang, C.; Liu, W.; Cui, D.; Wang, J.; Zhi, X.; et al. A HiPAD Integrated with RGO/MWCNTs Nano-Circuit Heater for Visual Point-of-Care Testing of SARS-CoV-2. Adv. Funct. Mater. 2021, 31, 2100801. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, M.A.; Khan, S.A.; Rehman, A. Screen-Printed Graphene/Carbon Electrodes on Paper Substrates as Impedance Sensors for Detection of Coronavirus in Nasopharyngeal Fluid Samples. Diagnostics 2021, 11, 1030. [Google Scholar] [CrossRef] [PubMed]
- Novodchuk, I.; Kayaharman, M.; Prassas, I.; Soosaipillai, A.; Karimi, R.; Goldthorpe, I.A.; Abdel-Rahman, E.; Sanderson, J.; Diamandis, E.P.; Bajcsy, M.; et al. Electronic Field Effect Detection of SARS-CoV-2 N-Protein before the Onset of Symptoms. Biosens. Bioelectron. 2022, 210, 114331. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Nam, J.-S.; Han, J.; Zhang, Q.; Kauppinen, E.I.; Jeon, I. Carbon Nanotube Mask Filters and Their Hydrophobic Barrier and Hyperthermic Antiviral Effects on SARS-CoV-2. ACS Appl. Nano Mater. 2021, 4, 8135–8144. [Google Scholar] [CrossRef]
- Soni, R.; Joshi, S.R.; Karmacharya, M.; Min, H.; Kim, S.-K.; Kumar, S.; Kim, G.-H.; Cho, Y.-K.; Lee, C.Y. Superhydrophobic and Self-Sterilizing Surgical Masks Spray-Coated with Carbon Nanotubes. ACS Appl. Nano Mater. 2021, 4, 8491–8499. [Google Scholar] [CrossRef]
- Patel, S.; Srivastav, A.K.; Gupta, S.K.; Kumar, U.; Mahapatra, S.K.; Gajjar, P.N.; Banerjee, I. Carbon Nanotubes for Rapid Capturing of SARS-COV-2 Virus: Revealing a Mechanistic Aspect of Binding Based on Computational Studies. RSC Adv. 2021, 11, 5785–5800. [Google Scholar] [CrossRef]
- Singh, S.; Shauloff, N.; Sharma, C.P.; Shimoni, R.; Arnusch, C.J.; Jelinek, R. Carbon Dot-Polymer Nanoporous Membrane for Recyclable Sunlight-Sterilized Facemasks. J. Colloid Interface Sci. 2021, 592, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Li, A.; Liu, Y.; Wang, L.; Qin, X.; Yu, J. Multi-Scale Nanoarchitectured Fibrous Networks for High-Performance, Self-Sterilization, and Recyclable Face Masks. Small 2022, 18, 2105570. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-C.; Yu, H.-Y.; Jiang, L.; Qi, D.; Zhang, X.; Chen, L.; Lv, W.; Xu, W.; Tam, K.C. Flexible, Anti-Damage, and Non-Contact Sensing Electronic Skin Implanted with MWCNT to Block Public Pathogens Contact Infection. Nano Res. 2022, 15, 2616–2625. [Google Scholar] [CrossRef] [PubMed]
- Goswami, M.; Yadav, A.K.; Chauhan, V.; Singh, N.; Kumar, S.; Das, A.; Yadav, V.; Mandal, A.; Tiwari, J.K.; Siddiqui, H.; et al. Facile Development of Graphene-Based Air Filters Mounted on a 3D Printed Mask for COVID-19. J. Sci. Adv. Mater. Devices 2021, 6, 407–414. [Google Scholar] [CrossRef]
- Zhong, H.; Zhu, Z.; Lin, J.; Cheung, C.F.; Lu, V.L.; Yan, F.; Chan, C.-Y.; Li, G. Reusable and Recyclable Graphene Masks with Outstanding Superhydrophobic and Photothermal Performances. ACS Nano 2020, 14, 6213–6221. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, Z.; Zhang, X.; Diao, D. Superhydrophobic, Photo-Sterilize, and Reusable Mask Based on Graphene Nanosheet-Embedded Carbon (GNEC) Film. Nano Res. 2021, 14, 1110–1115. [Google Scholar] [CrossRef]
- De Maio, F.; Rosa, E.; Perini, G.; Augello, A.; Niccolini, B.; Ciaiola, F.; Santarelli, G.; Sciandra, F.; Bozzi, M.; Sanguinetti, M.; et al. 3D-Printed Graphene Polylactic Acid Devices Resistant to SARS-CoV-2: Sunlight-Mediated Sterilization of Additive Manufactured Objects. Carbon 2022, 194, 34–41. [Google Scholar] [CrossRef]
- Shan, X.; Zhang, H.; Liu, C.; Yu, L.; Di, Y.; Zhang, X.; Dong, L.; Gan, Z. Reusable Self-Sterilization Masks Based on Electrothermal Graphene Filters. ACS Appl. Mater. Interfaces 2020, 12, 56579–56586. [Google Scholar] [CrossRef]
- Figerez, S.P.; Patra, S.; Rajalakshmi, G.; Narayanan, T.N. Graphene Oxide-Based Rechargeable Respiratory Masks. Oxf. Open Mater. Sci. 2021, 1, itab003. [Google Scholar] [CrossRef]
- Muñoz, A.; Illescas, B.M.; Luczkowiak, J.; Lasala, F.; Ribeiro-Viana, R.; Rojo, J.; Delgado, R.; Martín, N. Antiviral Activity of Self-Assembled Glycodendro [60]Fullerene Monoadducts. J. Mater. Chem. B 2017, 5, 6566–6571. [Google Scholar] [CrossRef] [Green Version]
- Gupta, I.; Azizighannad, S.; Farinas, E.T.; Mitra, S. Antiviral Properties of Select Carbon Nanostructures and Their Functionalized Analogs. Mater. Today Commun. 2021, 29, 102743. [Google Scholar] [CrossRef]
- Seifi, T.; Reza Kamali, A. Antiviral Performance of Graphene-Based Materials with Emphasis on COVID-19: A Review. Med. Drug Discov. 2021, 11, 100099. [Google Scholar] [CrossRef] [PubMed]
- Bagheri Novir, S.; Aram, M.R. Quantum Mechanical Simulation of Chloroquine Drug Interaction with C60 Fullerene for Treatment of COVID-19. Chem. Phys. Lett. 2020, 757, 137869. [Google Scholar] [CrossRef] [PubMed]
- Hurmach, V.V.; Platonov, M.O.; Prylutska, S.V.; Scharff, P.; Prylutskyy, Y.I.; Ritter, U. C60 Fullerene against SARS-CoV-2 Coronavirus: An in Silico Insight. Sci. Rep. 2021, 11, 17748. [Google Scholar] [CrossRef]
- Marforio, T.D.; Mattioli, E.J.; Zerbetto, F.; Calvaresi, M. Fullerenes against COVID-19: Repurposing C60 and C70 to Clog the Active Site of SARS-CoV-2 Protease. Molecules 2022, 27, 1916. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Urrehman, S.; Khalid, L.; Yaseen, M.; Khan, A.Q.; Jia, R. Metal Doped Fullerene Complexes as Promising Drug Delivery Materials against COVID-19. Chem. Pap. 2021, 75, 6487–6497. [Google Scholar] [CrossRef] [PubMed]
- Hurmach, V.; Platonov, M.; Prylutska, S.; Klestova, Z.; Cherepanov, V.; Prylutskyy, Y.; Ritter, U. Anticoronavirus Activity of Water-Soluble Pristine C60 Fullerenes: In Vitro and In Silico Screenings. Adv. Exp. Med. Biol. 2021, 1352, 159–172. [Google Scholar] [CrossRef]
- Bagheri Novir, S.; Aram, M.R. Quantum Mechanical Studies of the Adsorption of Remdesivir, as an Effective Drug for Treatment of COVID-19, on the Surface of Pristine, COOH-Functionalized and S-, Si- and Al- Doped Carbon Nanotubes. Phys. E Low-Dimens. Syst. Nanostructures 2021, 129, 114668. [Google Scholar] [CrossRef]
- Jomhori, M.; Mosaddeghi, H.; Farzin, H. Tracking the Interaction between Single-Wall Carbon Nanotube and SARS-Cov-2 Spike Glycoprotein: A Molecular Dynamics Simulations Study. Comput. Biol. Med. 2021, 136, 104692. [Google Scholar] [CrossRef]
- Kalkal, A.; Allawadhi, P.; Pradhan, R.; Khurana, A.; Bharani, K.K.; Packirisamy, G. Allium Sativum Derived Carbon Dots as a Potential Theranostic Agent to Combat the COVID-19 Crisis. Sens. Int. 2021, 2, 100102. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Sharma, P.C.; Patibandla, S.; Gao, Y.; Ruppa-Kasani, V.; Goli, J.; Kumar, A.; Chatterjee, A.; Sinha, S.S.; Bates, J.T.; et al. Blocking SARS-CoV-2 Delta Variant (B.1.617.2) Spike Protein Receptor-Binding Domain Binding with the ACE2 Receptor of the Host Cell and Inhibiting Virus Infections Using Human Host Defense Peptide-Conjugated Graphene Quantum Dots. ACS Omega 2022, 7, 8150–8157. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Gu, M.; Wang, Z.; Tang, T.W.; Zhu, Z.; Yuan, Y.; Wang, D.; Shen, C.; Tang, B.Z.; Ye, R. Highly Efficient and Rapid Inactivation of Coronavirus on Non-Metal Hydrophobic Laser-Induced Graphene in Mild Conditions. Adv. Funct. Mater. 2021, 31, 2101195. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xu, S.; Wang, Z.; Xue, K.; Su, J.; Song, Y.; Chen, S.; Zhu, C.; Tang, B.Z.; Ye, R. Self-Reporting and Photothermally Enhanced Rapid Bacterial Killing on a Laser-Induced Graphene Mask. ACS Nano 2020, 14, 12045–12053. [Google Scholar] [CrossRef]
- Donskyi, I.S.; Nie, C.; Ludwig, K.; Trimpert, J.; Ahmed, R.; Quaas, E.; Achazi, K.; Radnik, J.; Adeli, M.; Haag, R.; et al. Graphene Sheets with Defined Dual Functionalities for the Strong SARS-CoV-2 Interactions. Small 2021, 17, 2007091. [Google Scholar] [CrossRef]
- Unal, M.A.; Bayrakdar, F.; Nazir, H.; Besbinar, O.; Gurcan, C.; Lozano, N.; Arellano, L.M.; Yalcin, S.; Panatli, O.; Celik, D.; et al. Graphene Oxide Nanosheets Interact and Interfere with SARS-CoV-2 Surface Proteins and Cell Receptors to Inhibit Infectivity. Small 2021, 17, 2101483. [Google Scholar] [CrossRef]
- Hassan, H.A.F.M.; Diebold, S.S.; Smyth, L.A.; Walters, A.A.; Lombardi, G.; Al-Jamal, K.T. Application of Carbon Nanotubes in Cancer Vaccines: Achievements, Challenges and Chances. J. Control. Release 2019, 297, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; He, L.; Cao, W.; Huang, X.; Jia, K.; Dai, J. Recent Progress of Graphene Oxide as a Potential Vaccine Carrier and Adjuvant. Acta Biomater. 2020, 112, 14–28. [Google Scholar] [CrossRef]
- Gao, A.; Liang, H.; Shen, Q.; Zhou, C.; Chen, X.M.; Tian, J.; Li, X.; Liu, Z.; Ni, J.; Cui, D. Designing a Novel Nano-Vaccine against SARS-CoV-2. Nano Biomed. Eng. 2020, 12, 321–324. [Google Scholar] [CrossRef]
- Cui, D.; Gao, A.; Liang, H.; Tian, J.; Li, X.; Shen, Q. Nano Coronavirus Recombinant Vaccine Taking Graphene Oxide as Carrier. 2021. [Google Scholar]
- Zhou, Q.; Gu, H.; Sun, S.; Zhang, Y.; Hou, Y.; Li, C.; Zhao, Y.; Ma, P.; Lv, L.; Aji, S.; et al. Large-Sized Graphene Oxide Nanosheets Increase DC–T-Cell Synaptic Contact and the Efficacy of DC Vaccines against SARS-CoV-2. Adv. Mater. 2021, 33, 2102528. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Carbon Nanomaterials to Combat Virus: A Perspective in View of COVID-19. Carbon Trends 2021, 2, 100019. [Google Scholar] [CrossRef]
- Sensing Tools and Techniques for COVID-19—1st Edition. Available online: https://www.elsevier.com/books/sensing-tools-and-techniques-for-covid-19/hussain/978-0-323-90280-9 (accessed on 7 December 2022).
- Estevan, C.; Vilanova, E.; Sogorb, M.A. Case Study: Risk Associated to Wearing Silver or Graphene Nanoparticle-Coated Facemasks for Protection against COVID-19. Arch. Toxicol. 2022, 96, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Torres, I.; González-Tobío, B.; Ares, P.; Gómez-Herrero, J.; Zamora, F. Evaluation of the Degradation of the Graphene-Polypropylene Composites of Masks in Harsh Working Conditions. Mater. Today Chem. 2022, 26, 101146. [Google Scholar] [CrossRef] [PubMed]
- Handbook of Nanomaterials in Analytical Chemistry—1st Edition. Available online: https://www.elsevier.com/books/handbook-of-nanomaterials-in-analytical-chemistry/mustansar-hussain/978-0-12-816699-4 (accessed on 7 December 2022).
- Xiang, M.; Yang, Z.; Yang, J.; Lu, T.; Wu, D.; Liu, Z.; Xue, R.; Dong, S. Conductive Polymer Composites Fabricated by Disposable Face Masks and Multi-Walled Carbon Nanotubes: Crystalline Structure and Enhancement Effect. J. Renew. Mater. 2021, 10, 821–831. [Google Scholar] [CrossRef]

| Carbon Nanomaterial | Target | Sensor Type | Limit of Detection (LOD) | Detection Range | Detection Platform | Ref. |
|---|---|---|---|---|---|---|
| Carbon Dot | IgM and IgG | Optical | 100 pg/ml | 100 pg/mL to 100 μg/mL | Surface of carbon dot | [19] |
| RNA | Optical | 81 copies/μL. | NA | Surface of carbon dot | [20] | |
| Nucleocapsid proteins | Optical | 10 pg/mL | 10 pg/mL to 1 μg/mL | Surface of carbon dot | [21] | |
| Carbon Nanotube | Nucleocapsid proteins | Immunosensor | 5.62 fg/mL | NA | CNT thin-film | [22] |
| Spike protein (S1) | FET-based | 4.12 fg/mL | 4.12 fg/mL to 5.0 pg/mL | CNT surface | [24] | |
| RNA | FET-based | 10 fM | NA | CNT surface | [25] | |
| Spike antigen (SAg) and Nucleocapsid antigen (NAg) | FET-based | 0.55 fg/mL for SAg and 0.016 fg/mL for NAg | 0.55 fg/mL to 5.5 pg/mL for SAg and 16 fg/mL to 16 pg/mL for NAg | CNT surface | [26] | |
| Spike protein | Optical | 12.6 nM (receptor-binding-domain RBD) | NA | SWCNT substrate | [29] | |
| Antibodies to Spike protein | Electrochemical | 0.7 pg/mL | 0.7 pg/mL to 10.0 ng/mL | Electrode | [31] | |
| Spike protein S1 | Electrochemical | 7 nM | NA | Electrode | [32] | |
| Spike protein | Immunosensor | 350 genome equivalents/mL | NA | Electrode | [33] | |
| DNA | Electrochemical | aM level (for impedimetric) and zM level (for amperometric) | 1.0 × 10−18 M to 1.0 × 10−11 M | Electrode | [34] | |
| Whole Virus | Electrochemical | 57 pg/mL | NA | Electrode | [35] | |
| Graphene | Spike protein | FET-based | 1 fg/mL (in phosphate-buffered saline) and 100 fg/mL (in a clinical transport medium) and 1.6 × 10 pfu/mL (in culture medium) and 2.42 × 102 copies/mL (in clinical samples) | NA | Graphene surface | [37] |
| Antibodies to Spike protein S1 | Electrochemical | 2.8 × 10−15 M | NA | Electrode | [38] | |
| Spike protein S1 | Electrochemical | 39.5 fM | 39.5 nM/L to 500 fM | Electrode | [39] | |
| Spike antigen (SAg) and Nucleocapsid antigen (NAg) | Immunosensor | 0.3 pg/mL. | 0.3 to 103 pg/mL | Graphene oxide quantum dots surface | [40] | |
| IgG | Immunosensor | 1.0 pg/mL | 10−12 to 10−7 g/mL | Graphene surface | [41] | |
| Spike protein | FET-based | 0.002 fM | NA | rGO surface | [42] | |
| Spike (S) protein and Nucleocapsid (N) protein | FET-based | 1.28 (PFU)/mL (for S protein) and 1.45 PFU/mL (for N protein) | 100 fM to 100 nM | Graphene surface | [43] | |
| Spike S1 antigen | FET-based | 10 fM | 10 fM to 1 μM | Graphene surface | [44] | |
| Spike (S) protein and Nucleocapsid (N) protein | FET-based | 0.167 ag/mL | NA | Graphene surface | [45] | |
| Spike antibody | Immunosensor | 2.91 copies/mL (Synthetic) 0.5 fg/mL (raw wastewater) | Graphene surface | [46] | ||
| N-Gene | Optical | 25 copies/mL | 25 to 2.5 × 1010 copies/mL | NA | [47] | |
| Spike protein | Electrochemical | 0.25 fg/mL | 0.25 fg/mL to 1 µg/mL | Electrode | [48] | |
| Nucleocapsid protein | FET-based | 10 ag/mL | 10 ag/mL to 1 g/mL | Graphene surface | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sengupta, J.; Hussain, C.M. The Emergence of Carbon Nanomaterials as Effective Nano-Avenues to Fight against COVID-19. Materials 2023, 16, 1068. https://doi.org/10.3390/ma16031068
Sengupta J, Hussain CM. The Emergence of Carbon Nanomaterials as Effective Nano-Avenues to Fight against COVID-19. Materials. 2023; 16(3):1068. https://doi.org/10.3390/ma16031068
Chicago/Turabian StyleSengupta, Joydip, and Chaudhery Mustansar Hussain. 2023. "The Emergence of Carbon Nanomaterials as Effective Nano-Avenues to Fight against COVID-19" Materials 16, no. 3: 1068. https://doi.org/10.3390/ma16031068





