The Properties of Activated Carbons Functionalized with an Antibacterial Agent and a New SufA Protease Inhibitor
Abstract
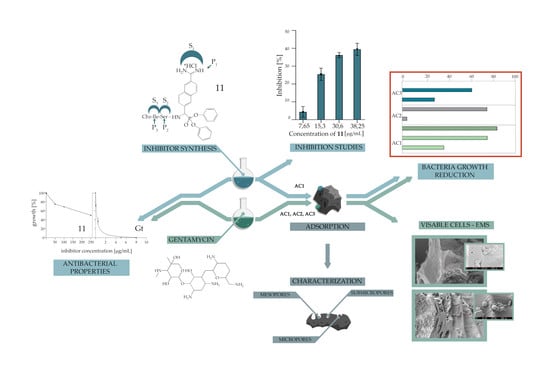
:1. Introduction
2. Materials and Methods
2.1. Carbon Activation Method
2.2. Characteristics of the Porous Structure of Activated Carbons
2.3. Elemental Content Studies of Active Carbons
2.4. Adsorption of Antibacterial Agents on Activated Carbons
2.5. Bacterial Growth Reduction
2.6. Quantitative Analysis of Live Bacterial Cells Adsorbed on Activated Carbons
2.7. Electron Microscopy Studies of Bacterial Sorption on Active Carbon
2.8. Synthesis Procedure of Cbz-Ile-Ser-6-AmNphthP(OPh)2
2.8.1. 6-(Methoxycarbonyl)-2-naphthoic Acid (2)
2.8.2. Methyl 6-carbamoyl-2-naphthoate (3)
2.8.3. Methyl 6-cyano-2-naphthoate (4)
2.8.4. 6-(Hydroxymethyl)-2-naphthonitrile (5)
2.8.5. 6-Formyl-2-naphthonitrile (6)
2.8.6. Cbz-6-CN-NphthP(OPh)2 (7)
2.8.7. Cbz-6-AmNphthP(OPh)2 (8)
2.8.8. Cbz-Ile-Ser(t-Bu)-OH
2.8.9. NH2-6-AmNphthP(OPh)2 (9)
2.8.10. Cbz-Ile-Ser-6-AmNphthP(OPh)2 (11)
2.9. SufA Inhibition Studies
3. Results
3.1. AC Characteristics
3.2. Inhibition Studies of Cbz-Ile-Ser-6-AmNphthP(OPh)2
3.3. Adsorption Studies
3.4. Bacterial Growth Reduction
3.5. Quantitative Analysis of Live Bacterial Cells
3.6. Electron Microscopy Studies of Bacterial Sorption on Active Carbon
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howell, C.; Sandeman, S.; Zheng, Y.; Sergey, V.; Mikhalovsky, V.G.N. New dextran coated activated carbons for medical use Mendeley. Carbon 2015, 97, 134–146. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, J.H.; Wang, S.H.; Ko, T.H.; Tseng, G.C. Evaluation of silver-containing activated carbon fiber for wound healing study: In vitro and in vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Shon, H.K.; Ngo, H.H. Adsorption characteristics of antibiotics trimethoprim on powdered and granular activated carbon. J. Ind. Eng. Chem. 2010, 16, 344–349. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Hsu, H.-C.; Lin, C.-J. Treatment of Chronic Wounds With the Silver-Containing Activated Carbon Fiber Dressing: Three Cases. J. Med. Cases 2014, 5, 587–591. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Burchacka, E.; Łukaszewicz, M.; Kułażyński, M. Determination of mechanisms of action of active carbons as a feed additive. Bioorg. Chem. 2019, 93, 102804. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-Garca, M.A.; Moreno-Castilla, C. Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. J. Chem. Technol. Biotechnol. 2001, 76, 1209–1215. [Google Scholar] [CrossRef]
- Baghdadi, M. UT (University of Tehran) isotherm as a novel and useful adsorption isotherm for investigation of adsorptive removal of pollutants. J. Environ. Chem. Eng. 2017, 5, 1906–1919. [Google Scholar] [CrossRef]
- Burchacka, E.; Pstrowska, K.; Kułażyński, M.; Beran, E.; Fałtynowicz, H.; Chojnacka, K. Antibacterial agents adsorbed on active carbon: A new approach for S. aureus and E. coli pathogen elimination. Pathogens 2021, 10, 1066. [Google Scholar] [CrossRef]
- Karlsson, C.; Mörgelin, M.; Coilin, M.; Lood, R.; Andersson, M.L.; Schmidtchen, A.; Björck, L.; Frick, I.M. SufA—A bacterial enzyme that cleaves fibrinogen and blocks fibrin network formation. Microbiology 2009, 155, 238–248. [Google Scholar] [CrossRef]
- Karlsson, C.; Andersson, M.L.; Collin, M.; Schmidtchen, A.; Björck, L.; Frick, I.M. SufA—A novel subtilisin-like serine proteinase of Finegoldia magna. Microbiology 2007, 153, 4208–4218. [Google Scholar] [CrossRef]
- Stephens, P.; Wall, I.B.; Wilson, M.J.; Hill, K.E.; Davies, C.E.; Hill, C.M.; Harding, K.G.; Thomas, D.W. Anaerobic cocci populating the deep tissues of chronic wounds impair cellular wound healing responses in vitro. Br. J. Dermatol. 2003, 148, 456–466. [Google Scholar] [CrossRef]
- Hansson, C.; Hoborn, J.; Möller, A.; Swanbeck, G. The microbial flora in venous leg ulcers without clinical signs of infection. Repeated culture using a validated standardised microbiological technique. Acta Derm. Venereol. 1995, 75, 24–30. [Google Scholar]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef]
- Neut, C.; Lesieur, V.; Romond, C.; Beerens, H. Analysis of gram-positive anaerobic cocci in oral, fecal and vaginal flora. Eur. J. Clin. Microbiol. 1985, 4, 435–437. [Google Scholar] [CrossRef]
- Higaki, S.; Kitagawa, T.; Morohashi, M.; Yamagishi, T. Anaerobes isolated from infectious skin diseases. Anaerobe 1999, 5, 583–587. [Google Scholar] [CrossRef]
- Karlsson, C.; Eliasson, M.; Olin, A.I.; Mörgelin, M.; Karlsson, A.; Malmsten, M.; Egesten, A.; Frick, I.M. SufA of the opportunistic pathogen finegoldia magna modulates actions of the antibacterial chemokine MIG/CXCL9, promoting bacterial survival during epithelial inflammation. J. Biol. Chem. 2009, 284, 29499–29508. [Google Scholar] [CrossRef]
- Pietrusewicz, E.; Sieńczyk, M.; Oleksyszyn, J. Novel diphenyl esters of peptidyl α-aminoalkylphosphonates as inhibitors of chymotrypsin and subtilisin. J. Enzym. Inhib. Med. Chem. 2009, 24, 1229–1236. [Google Scholar] [CrossRef]
- Winiarski, Ł.; Oleksyszyn, J.; Sieńczyk, M. Human neutrophil elastase phosphonic inhibitors with improved potency of action. J. Med. Chem. 2012, 55, 6541–6553. [Google Scholar] [CrossRef]
- Burchacka, E.; Sieńczyk, M.; Frick, I.-M.; Wysocka, M.; Lesner, A.; Oleksyszyn, J. Substrate profiling of Finegoldia magna SufA protease, inhibitor screening and application to prevent human fibrinogen degradation and bacteria growth in vitro. Biochimie 2014, 103, 137–143. [Google Scholar] [CrossRef]
- Sieńczyk, M.; Oleksyszyn, J. Inhibition of trypsin and urokinase by Cbz-amino(4-guanidinophenyl)methanephosphonate aromatic ester derivatives: The influence of the ester group on their biological activity. Bioorganic Med. Chem. Lett. 2006, 16, 2886–2890. [Google Scholar] [CrossRef] [PubMed]
- Oleksyszyn, J.; Powers, J.C. Amino acid and peptide phosphonate derivatives as specific inhibitors of serine peptidases. Methods Enzymol. 1994, 244, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Schechter, I.; Berger, A. On the size of the active site in proteases. I. Papain. Biochem. Biophys. Res. Commun. 1967, 27, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Cardona, A.F.; Wilson, S.E. Skin and Soft-Tissue Infections: A Critical Review and the Role of Telavancin in Their Treatment. Clin. Infect. Dis. 2015, 61, S69–S78. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. The Equation of the Characteristic Curve of Activated Charcoal. Proc. Acad. Sci. Phys. Chem. Sect. 1947, 55, 327–329. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Toda, Y.; Hatami, M.; Toyoda, S.; Yoshida, Y.; Honda, H. Micropore structure of coal. Fuel 1971, 50, 187–200. [Google Scholar] [CrossRef]
- Pierce, C. Computation of pore sizes from physical adsorption data. J. Phys. Chem. 1953, 57, 149–152. [Google Scholar] [CrossRef]
- Abramowitz, N.; Schechter, I.; Berger, A. On the size of the active site in proteases II. Carboxypeptidase-A. Biochem. Biophys. Res. Commun. 1967, 29, 862–867. [Google Scholar] [CrossRef]







| Sample | Moisture Content (%) | Ash Content (%) | SBET (m2/g) | Surface of Pores (m2/g) | ||
|---|---|---|---|---|---|---|
| SSUBMIC < 0.4 nm | SMIC 0.4–2 nm | SMES 2–50 nm | ||||
| AC1 | 4.2 | 4.4 | 416 | 24.0 | 472.0 | 43.0 |
| AC2 | 3.1 | 2.2 | 979 | 0 | 1107.0 | 44.0 |
| AC3 | 0.7 | 5.3 | 863 | 0 | 964.0 | 61.6 |
| Element | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Fe | Rn | Ca | K | S | P | Si | Al | Cu | Mg | Na | |
| Weight (%) | |||||||||||||
| AC1 | 84.7 ± 2.5 | 14.0 ±2.5 | - | - | 0.7 ± 0.7 | 0.3 ± 0.3 | 0.03 ± 0.06 | 0.05 ± 0.08 | 0.05 ± 0.05 | 0.02 ± 0.03 | - | 0.07 ± 0.08 | - |
| AC2 | 91.3 ± 0.8 | 6.1 ±1.5 | - | 0.05 ± 0.11 | - | 1.4 ± 0.5 | - | 0.03 ± 0.06 | 0.08 ± 0.08 | - | 1.0 ± 0.7 | - | 0.08 ± 0.13 |
| AC3 | 68.8 ± 24.1 | 11.3 ±1.4 | 2.0 ± 3.7 | - | 1.4 ± 1.3 | 0.5 ± 0.5 | 0.25 ± 0.35 | - | 8.9 ± 12.5 | 0.09 ± 0.01 | 5.9 ± 1.6 | 0.3 ± 0.2 | 0.8 ± 1.3 |
| Activated Carbon | Degree of Adsorption on 20 mg of AC (%) | Concentration Adsorbed on 20 mg of AC (µg/mL) | ||
|---|---|---|---|---|
| Gt | 11 | Gt | 11 | |
| AC1 | 89.52 | 100 | 0.448 | 100 |
| AC2 | 98.33 | 0.492 | ||
| AC3 | 89.76 | 0.449 | ||
| Gentamycin | The Number of Units That Form a Bacterial Colony (CFU/mL ×107) | ||
|---|---|---|---|
| AC1 | AC2 | AC3 | |
| + | 23 ± 0.53 | 2.2 ± 0.18 | 1.7 ± 0.08 |
| - | 41 ± 0.20 | 3.6 ± 0.38 | 2.9 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burchacka, E.; Pstrowska, K.; Bryk, M.; Maciejowski, F.; Kułażyński, M.; Chojnacka, K. The Properties of Activated Carbons Functionalized with an Antibacterial Agent and a New SufA Protease Inhibitor. Materials 2023, 16, 1263. https://doi.org/10.3390/ma16031263
Burchacka E, Pstrowska K, Bryk M, Maciejowski F, Kułażyński M, Chojnacka K. The Properties of Activated Carbons Functionalized with an Antibacterial Agent and a New SufA Protease Inhibitor. Materials. 2023; 16(3):1263. https://doi.org/10.3390/ma16031263
Chicago/Turabian StyleBurchacka, Ewa, Katarzyna Pstrowska, Michał Bryk, Filip Maciejowski, Marek Kułażyński, and Katarzyna Chojnacka. 2023. "The Properties of Activated Carbons Functionalized with an Antibacterial Agent and a New SufA Protease Inhibitor" Materials 16, no. 3: 1263. https://doi.org/10.3390/ma16031263