Improved Lithium Storage Performance of a TiO2 Anode Material Doped by Co
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Synthesis
2.2. Materials Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
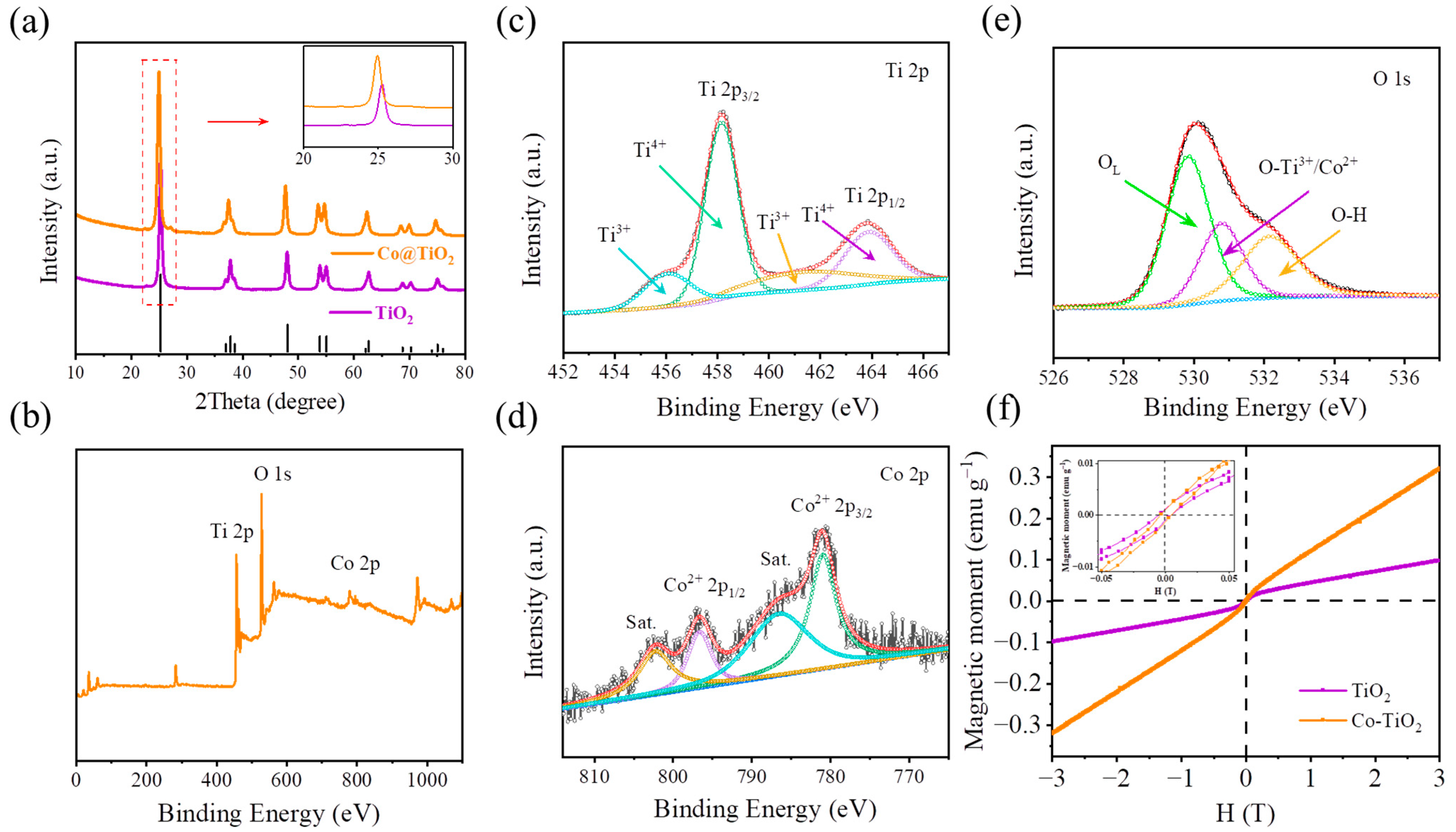
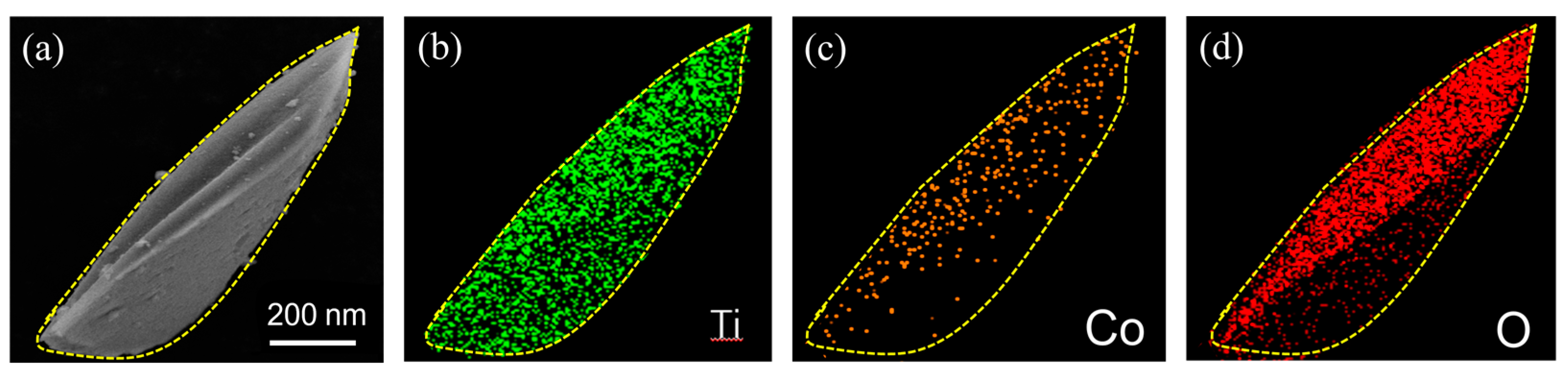
3.1. Materials Characterizations
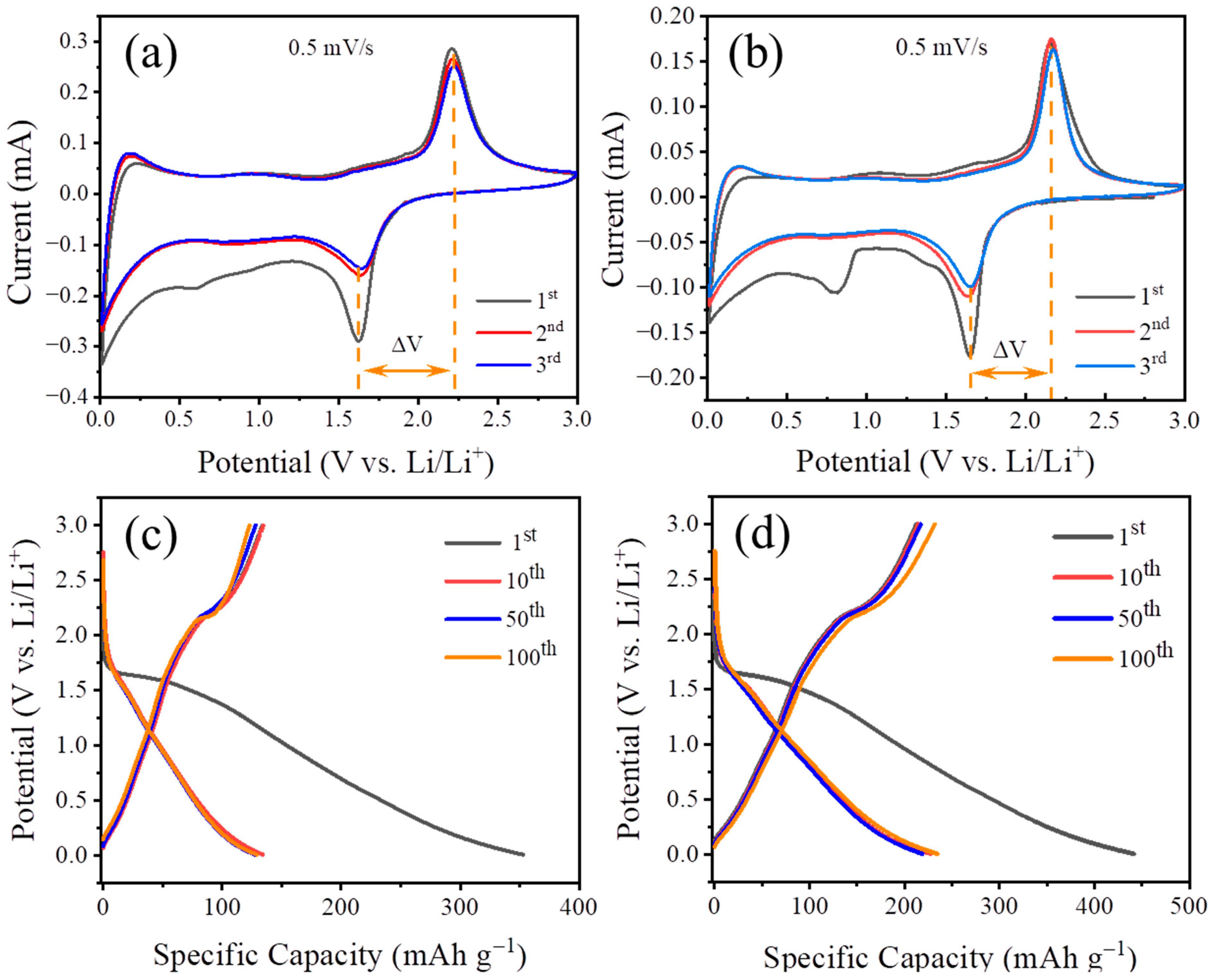
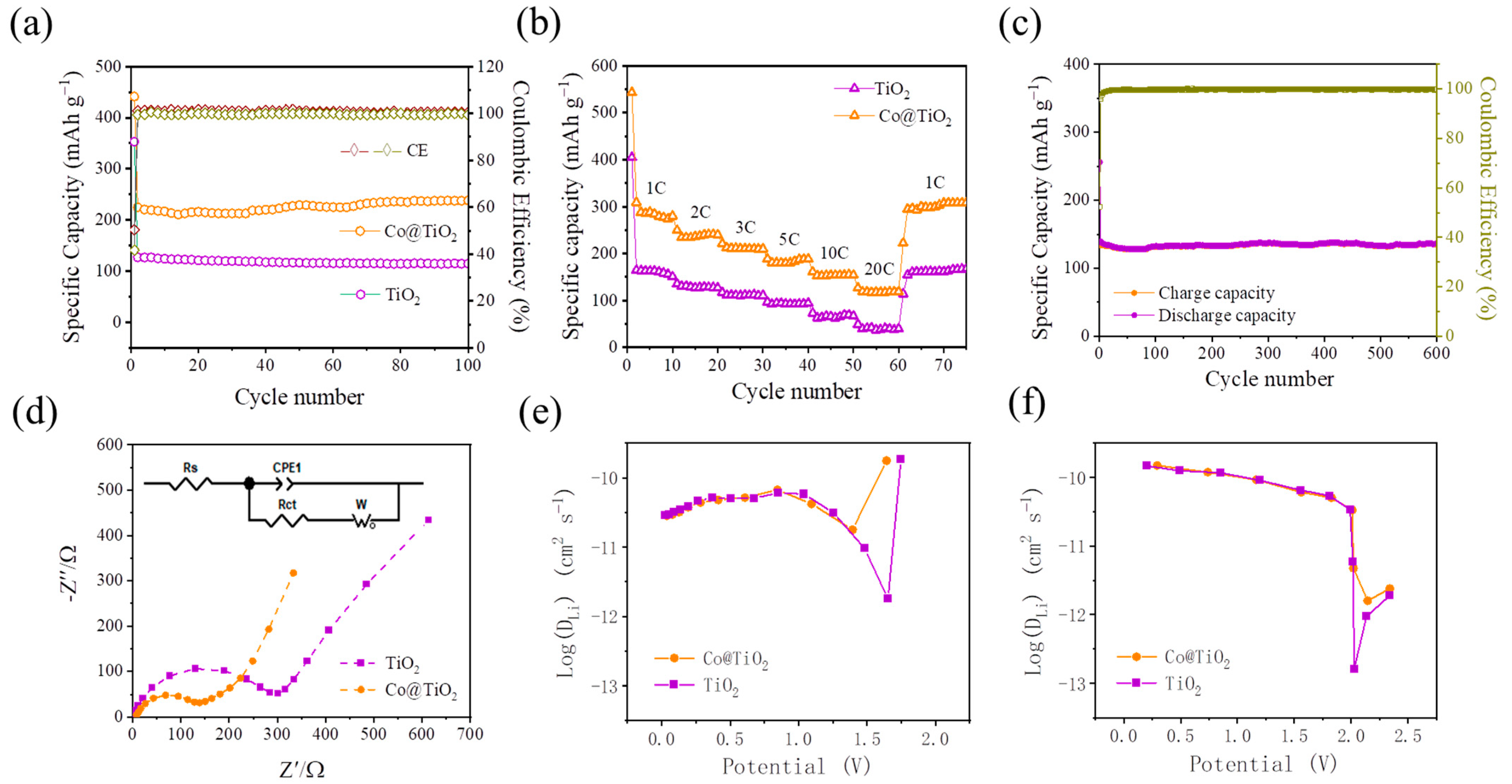
3.2. Electrochemical Performance and Kinetic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Croguennec, L.; Palacin, M.R. Recent Achievements on Inorganic Electrode Materials for Lithium-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 3140–3156. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-M.; Chen, J.-Y.; Li, H.; Wang, C.-R. Recent progress in Li-ion batteries with TiO2 nanotube anodes grown by electrochemical anodization. Rare Met. 2021, 40, 249–271. [Google Scholar] [CrossRef]
- Teng, X.-L.; Sun, X.-T.; Guan, L.; Hu, H.; Wu, M.-B. Self-supported transition metal oxide electrodes for electrochemical energy storage. Tungsten 2020, 2, 337–361. [Google Scholar] [CrossRef]
- Liang, S.; Wang, X.; Cheng, Y.-J.; Xia, Y.; Mueller-Buschbaum, P. Anatase titanium dioxide as rechargeable ion battery electrode- A review. Energy Storage Mater. 2022, 45, 201–264. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.; Hogg, B.-I.; Wohlfahrt-Mehrens, M. Li plating as unwanted side reaction in commercial Li-ion cells—A review. J. Power Sources 2018, 384, 107–124. [Google Scholar] [CrossRef]
- Nazari, A.; Farhad, S. Heat generation in lithium-ion batteries with different nominal capacities and chemistries. Appl. Therm. Eng. 2017, 125, 1501–1517. [Google Scholar] [CrossRef]
- Ren, H.; Yu, R.; Wang, J.; Jin, Q.; Yang, M.; Mao, D.; Kisailus, D.; Zhao, H.; Wang, D. Muftishelled TiO2 Hollow Microspheres as Anodes with Superior Reversible Capacity for Lithium Ion Batteries. Nano Lett. 2014, 14, 6679–6684. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yu, L.; Gao, X.; Lin, Z.; Lou, X.W. Hierarchical tubular structures constructed from ultrathin TiO2(B) nanosheets for highly reversible lithium storage. Energy Environ. Sci. 2015, 8, 1480–1483. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.-G.; Hu, Y.-S.; Sigle, W.; Maier, J. Superior electrode performance of nanostructured mesoporous TiO2 (anatase) through efficient hierarchical mixed conducting networks. Adv. Mater. 2007, 19, 2087–2091. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, X.; Jing, M.; Hou, H.; Zhu, Y.; Fang, L.; Yang, X.; Chen, Q.; Banks, C.E. Carbon dots supported upon N-doped TiO2 nanorods applied into sodium and lithium ion batteries. J. Mater. Chem. A 2015, 3, 5648–5655. [Google Scholar] [CrossRef]
- Liu, B.; Deng, D.; Lee, J.Y.; Aydil, E.S. Oriented single-crystalline TiO2 nanowires on titanium foil for lithium ion batteries. J. Mater. Res. 2010, 25, 1588–1594. [Google Scholar] [CrossRef]
- Nam, S.H.; Shim, H.-S.; Kim, Y.-S.; Dar, M.A.; Kim, J.G.; Kim, W.B. Ag or Au Nanoparticle-Embedded One-Dimensional Composite TiO2 Nanofibers Prepared via Electrospinning for Use in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2010, 2, 2046–2052. [Google Scholar] [CrossRef]
- Bao, S.-J.; Bao, Q.-L.; Li, C.-M.; Dong, Z.-L. Novel porous anatase TiO2 nanorods and their high lithium electroactivity. Electrochem. Commun. 2007, 9, 1233–1238. [Google Scholar] [CrossRef]
- Qiu, Y.; Yan, K.; Yang, S.; Jin, L.; Deng, H.; Li, W. Synthesis of Size-Tunable Anatase TiO2 Nanospindles and Their Assembly into Anatase@Titanium Oxynitride/Titanium Nitride-Graphene Nanocomposites for Rechargeable Lithium Ion Batteries with High Cycling Performance. ACS Nano 2010, 4, 6515–6526. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-G.; Hu, Y.-S.; Maier, J. Synthesis of hierarchically mesoporous anatase spheres and their application in lithium batteries. Chem. Commun. 2006, 2783–2785. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Tang, Y. Electrochemical lithium storage of TiO2 hollow microspheres assembled by nanotubes. J. Power Sources 2010, 195, 6893–6896. [Google Scholar] [CrossRef]
- Jiao, H.; Sun, G.; Wang, Y.; Zhang, Z.; Wang, Z.; Wang, H.; Li, H.; Feng, M. Defective TiO2 hollow nanospheres as photo-electrocatalysts for photo-assisted Li-O2 batteries. Chin. Chem. Lett. 2022, 33, 4008–4012. [Google Scholar] [CrossRef]
- Fang, J.; Liu, W.; Yu, F.; Qin, F.; Wang, M.; Zhang, K.; Lai, Y. Fe, S co-doped anatase TiO2 nanotubes as anodes with improved electrochemical performance for lithium ion batteries. Rsc Adv. 2016, 6, 70133–70140. [Google Scholar] [CrossRef]
- Ortiz, G.F.; Hanzu, I.; Djenizian, T.; Lavela, P.; Tirado, J.L.; Knauth, P. Alternative Li-Ion Battery Electrode Based on Self-Organized Titania Nanotubes. Chem. Mater. 2009, 21, 63–67. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Zhao, J.; Qiao, Z.-J.; Wang, J.-M.; Sun, S.-H.; Fu, W.-X.; Zhang, X.-Y.; Yu, Z.-Y.; Dou, Y.-H.; Kang, J.-L.; et al. Nonsolvent-induced phase separation-derived TiO2 nanotube arrays/porous Ti electrode as high-energy-density anode for lithium-ion batteries. Rare Met. 2021, 40, 393–399. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; He, P.; Hosono, E.; Zhou, H. Nano active materials for lithium-ion batteries. Nanoscale 2010, 2, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Um, J.H.; Ahn, J.; Yu, S.-H.; Sung, Y.-E.; Lee, J.-K. Soft Template Strategy to Synthesize Iron Oxide-Titania Yolk-Shell Nanoparticles as High-Performance Anode Materials for Lithium-Ion Battery Applications. Chem.-A Eur. J. 2015, 21, 7954–7961. [Google Scholar] [CrossRef] [PubMed]
- An, L.P.; Gao, X.P.; Li, G.R.; Yan, T.Y.; Zhu, H.Y.; Shen, P.W. Electrochemical lithium storage of titania nanotubes modified with NiO nanoparticles. Electrochim. Acta 2008, 53, 4573–4579. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, J.-H.; Kang, Y.C. One-pot rapid synthesis of core-shell structured NiO@TiO2 nanopowders and their excellent electrochemical properties as anode materials for lithium ion batteries. Nanoscale 2013, 5, 12645–12650. [Google Scholar] [CrossRef]
- Lai, Y.; Liu, W.; Fang, J.; Qin, F.; Wang, M.; Yu, F.; Zhang, K. Fe-doped anatase TiO2/carbon composite as an anode with superior reversible capacity for lithium storage. RSC Adv. 2015, 5, 93676–93683. [Google Scholar] [CrossRef]
- Li, J.; Cai, Y.; Yao, X.; Tian, H.; Su, Z. Europium modified TiO2 as a high-rate long-cycle life anode material for lithium-ion batteries. New J. Chem. 2022, 46, 2266–2271. [Google Scholar] [CrossRef]
- Opra, D.P.; Gnedenkov, S.V.; Sinebryukhov, S.L.; Podgorbunsky, A.B.; Sokolov, A.A.; Ustinov, A.Y.; Kuryavyi, V.G.; Mayorov, V.Y.; Zheleznov, V.V. Doping of titania with manganese for improving cycling and rate performances in lithium-ion batteries. Chem. Phys. 2020, 538, 110864. [Google Scholar] [CrossRef]
- Kashale, A.A.; Dwivedi, P.K.; Sathe, B.R.; Shelke, M.V.; Chang, J.Y.; Ghule, A.V. Biomass-Mediated Synthesis of Cu-Doped TiO2 Nanoparticles for Improved-Performance Lithium-Ion Batteries. ACS Omega 2018, 3, 13676–13684. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-H.; Jung, D.-W.; Shin, E.W.; Oh, E.-S. Boron-doped TiO2 anode materials for high-rate lithium ion batteries. J. Alloys Compd. 2014, 604, 226–232. [Google Scholar] [CrossRef]
- Andriamiadamanana, C.; Laberty-Robert, C.; Sougrati, M.T.; Casale, S.; Davoisne, C.; Patra, S.; Sauvage, F. Room-temperature synthesis of iron-doped anatase TiO2 for lithium-ion batteries and photocatalysis. Inorg. Chem. 2014, 53, 10129–10139. [Google Scholar] [CrossRef]
- Geng, H.; Ming, H.; Ge, D.; Zheng, J.; Gu, H. Designed fabrication of fluorine-doped carbon coated mesoporous TiO2 hollow spheres for improved lithium storage. Electrochim. Acta 2015, 157, 1–7. [Google Scholar] [CrossRef]
- Tawa, S.; Matsumoto, K.; Hagiwara, R. Reaction Pathways of Iron Trifluoride Investigated by Operation at 363 K Using an Ionic Liquid Electrolyte. J. Electrochem. Soc. 2019, 166, A2105–A2110. [Google Scholar] [CrossRef]
- Seok, D.I.; Wu, M.; Shim, K.B.; Kang, Y.; Jung, H.K. High-rate performance of Ti3+ self-doped TiO2 prepared by imidazole reduction for Li-ion batteries. Nanotechnology 2016, 27, 435401. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, F.; Yan, X.; Jin, Y.; Zhu, K.; Wang, X.; Li, H.; Chen, G.; Wang, C.; Wei, Y. Improvements in the electrochemical kinetic properties and rate capability of anatase titanium dioxide nanoparticles by nitrogen doping. ACS Appl. Mater. Interfaces 2014, 6, 4458–4465. [Google Scholar] [CrossRef]
- Ishikawa, K.; Yoshikawa, K.; Okada, N. Size effect on the ferroelectric phase transition in PbTiO3 ultrafine particles. Phys. Rev. B Condens. Matter 1988, 37, 5852–5855. [Google Scholar] [CrossRef]
- Deng, L.; Wang, S.; Liu, D.; Zhu, B.; Huang, W.; Wu, S.; Zhang, S. Synthesis, Characterization of Fe-doped TiO2 Nanotubes with High Photocatalytic Activity. Catal. Lett. 2009, 129, 513–518. [Google Scholar] [CrossRef]
- Hong, Z.; Kang, M.; Chen, X.; Zhou, K.; Huang, Z.; Wei, M. Synthesis of Mesoporous Co2+-Doped TiO2 Nanodisks Derived from Metal Organic Frameworks with Improved Sodium Storage Performance. ACS Appl. Mater. Interfaces 2017, 9, 32071–32079. [Google Scholar] [CrossRef]
- Hong, Z.; Zhou, K.; Zhang, J.; Huang, Z.; Wei, M. Facile synthesis of rutile TiO2 mesocrystals with enhanced sodium storage properties. J. Mater. Chem. A 2015, 3, 17412–17416. [Google Scholar] [CrossRef]
- Hong, Z.; Zhou, K.; Huang, Z.; Wei, M. Iso-Oriented Anatase TiO2 Mesocages as a High Performance Anode Material for Sodium-Ion Storage. Sci. Rep. 2015, 5, 11960. [Google Scholar] [CrossRef]
- Sanjines, R.; Tang, H.; Berger, H.; Gozzo, F.; Margaritondo, G.; Levy, F. Electronic-structure of anatase TiO2 oxide. J. Appl. Phys. 1994, 75, 2945–2951. [Google Scholar] [CrossRef]
- Akshay, V.R.; Arun, B.; Manda, G.; Mutta, G.R.; Chanda, A.; Vasundhara, M. Observation of Optical Band-Gap Narrowing and Enhanced Magnetic Moment in Co-Doped Sol-Gel-Derived Anatase TiO2 Nanocrystals. J. Phys. Chem. C 2018, 122, 26592–26604. [Google Scholar] [CrossRef]
- Huang, W.; Zuo, Z.; Han, P.; Li, Z.; Zhao, T. XPS and XRD investigation of Co/Pd/TiO2 catalysts by different preparation methods. J. Electron Spectrosc. Relat. Phenom. 2009, 173, 88–95. [Google Scholar] [CrossRef]
- Zhuang, J.; Weng, S.; Dai, W.; Liu, P.; Liu, Q. Effects of Interface Defects on Charge Transfer and Photoinduced Properties of TiO2 Bilayer Films. J. Phys. Chem. C 2012, 116, 25354–25361. [Google Scholar] [CrossRef]
- Li, X.; Su, J.; Li, Z.; Zhao, Z.; Zhang, F.; Zhang, L.; Ye, W.; Li, Q.; Wang, K.; Wang, X.; et al. Revealing interfacial space charge storage of Li+/Na+/K+ by operando magnetometry. Sci. Bull. 2022, 67, 1145–1153. [Google Scholar] [CrossRef]
- Xia, Q.; Li, X.; Wang, K.; Li, Z.; Liu, H.; Wang, X.; Ye, W.; Li, H.; Teng, X.; Pang, J.; et al. Unraveling the Evolution of Transition Metals during Li Alloying- Dealloying by In-Operando Magnetometry. Chem. Mater. 2022, 34, 5852–5859. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Li, X.; Gu, F.; Zhang, L.; Liu, H.; Xia, Q.; Li, Q.; Ye, W.; Ge, C.; et al. Reacquainting the Electrochemical Conversion Mechanism of FeS2 Sodium-Ion Batteries by Operando Magnetometry. J. Am. Chem. Soc. 2021, 143, 12800–12808. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Z.; Xia, Q.; Zhang, Q.; Ge, C.; Chen, Y.; Li, X.; Zhang, L.; Wang, K.; Li, H.; et al. Li-ionic control of magnetism through spin capacitance and conversion. Matter 2021, 4, 3605–3620. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Liu, Y.; Liu, H.; Zhao, Z.; Zheng, Y.; Chen, L.; Ye, W.; Li, H.; Li, Q. Transition metal catalysis in lithium-ion batteries studied by operando magnetometry. Chin. J. Catal. 2022, 43, 158–166. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Venkatesan, M.; Fitzgerald, C.B. Donor impurity band exchange in dilute ferromagnetic oxides. Nat. Mater. 2005, 4, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, X.; Wei, X.; Dai, J.; Li, W. Ferromagnetic Property of Co and Ni Doped TiO2 Nanoparticles. J. Nanomater. 2015, 2015, 371582. [Google Scholar] [CrossRef]
- Dakhel, A.A.; Hamad, H.; Jaafar, A. Investigation to the Structural, Optical, and Magnetic Properties of Synthesized Ni-Doped Anatase Nanoparticles: Essential Role of Treatment in Hydrogen on Long-Range Ferromagnetic Order. J. Supercond. Nov. Magn. 2019, 32, 253–260. [Google Scholar] [CrossRef]
- Santara, B.; Giri, P.K.; Dhara, S.; Imakita, K.; Fujii, M. Oxygen vacancy-mediated enhanced ferromagnetism in undoped and Fe-doped TiO2 nanoribbons. J. Phys. D-Appl. Phys. 2014, 47, 235304. [Google Scholar] [CrossRef]
- Bao, N.N.; Yi, J.B.; Fan, H.M.; Qin, X.B.; Zhang, P.; Wang, B.Y.; Ding, J.; Li, S. Vacancy-induced room-temperature ferromagnetism in Ga-TiO2. Scr. Mater. 2012, 66, 821–824. [Google Scholar] [CrossRef]
- Zhang, S.; Ogale, S.B.; Yu, W.; Gao, X.; Liu, T.; Ghosh, S.; Das, G.P.; Wee, A.T.S.; Greene, R.L.; Venkatesan, T. Electronic Manifestation of Cation-Vacancy-Induced Magnetic Moments in a Transparent Oxide Semiconductor: Anatase Nb:TiO2. Adv. Mater. 2009, 21, 2282–2287. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, Q. First principle study of the cation vacancy in anatase TiO2. Phys. Status Solidi-Rapid Res. Lett. 2008, 2, 25–27. [Google Scholar] [CrossRef]
- Bao, N.N.; Fan, H.M.; Ding, J.; Yi, J.B. Room temperature ferromagnetism in N-doped rutile TiO2 films. J. Appl. Phys. 2011, 109, 07C302. [Google Scholar] [CrossRef]
- Zhou, S.; Cizmar, E.; Potzger, K.; Krause, M.; Talut, G.; Helm, M.; Fassbender, J.; Zvyagin, S.A.; Wosnitza, J.; Schmidt, H. Origin of magnetic moments in defective TiO2 single crystals. Phys. Rev. B 2009, 79, 113201. [Google Scholar] [CrossRef]
- Olson, C.L.; Nelson, J.; Islam, M.S. Defect chemistry, surface structures, and lithium insertion in anatase TiO2. J. Phys. Chem. B 2006, 110, 9995–10001. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.D.; Ma, C.L.; Wang, Y.D.; Li, H.D. Al-13-pillared anatase TiO2 as a cathode for a lithium battery. Nanotechnology 2004, 15, 1535–1538. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Z.; Shang, X.; Zhang, J.; Cai, L.; Pan, Y.; Li, Q.; Li, H.; Cao, Q.; Li, Q. Electrical control of ON–OFF magnetism and exchange bias via reversible ionic motion. Appl. Phys. Lett. 2022, 120, 082405. [Google Scholar] [CrossRef]
- Li, H.; Hu, Z.; Xia, Q.; Zhang, H.; Li, Z.; Wang, H.; Li, X.; Zuo, F.; Zhang, F.; Wang, X.; et al. Operando Magnetometry Probing the Charge Storage Mechanism of CoO Lithium-Ion Batteries. Adv. Mater. 2021, 33, e2006629. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Zhao, Z.; Zhang, Q.; Fu, X.; Li, X.; Gu, F.; Zhong, H.; Pan, Y.; Chen, G.; et al. Space-Charge Control of Magnetism in Ferromagnetic Metals: Coupling Giant Magnitude and Robust Endurance. Adv. Mater. 2022, e2207353. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Zhang, F.; Long, G.; Fan, S.; Zheng, Y.; Ye, W.; Li, Q.; Wang, X.; Li, H.; et al. Fe, N co-doped amorphous carbon as efficient electrode materials for fast and stable Na/K-storage. Electrochim. Acta 2021, 396, 139265. [Google Scholar] [CrossRef]
- Kim, K.-T.; Ali, G.; Chung, K.Y.; Yoon, C.S.; Yashiro, H.; Sun, Y.-K.; Lu, J.; Amine, K.; Myung, S.-T. Anatase Titania Nanorods as an Intercalation Anode Material for Rechargeable Sodium Batteries. Nano Lett. 2014, 14, 416–422. [Google Scholar] [CrossRef]
- Yan, D.; Yu, C.; Li, D.; Zhang, X.; Li, J.; Lu, T.; Pan, L. Improved sodium-ion storage performance of TiO2 nanotubes by Ni2+ doping. J. Mater. Chem. A 2016, 4, 11077–11085. [Google Scholar] [CrossRef]
- Yadav, H.M.; Otari, S.V.; Koli, V.B.; Mali, S.S.; Hong, C.K.; Pawar, S.H.; Delekar, S.D. Preparation and characterization of copper-doped anatase TiO2 nanoparticles with visible light photocatalytic antibacterial activity. J. Photochem. Photobiol. A-Chem. 2014, 280, 32–38. [Google Scholar] [CrossRef]
- Zhu, J.; Zeng, K.; Lu, L. In-situ nanoscale mapping of surface potential in all-solid-state thin film Li-ion battery using Kelvin probe force microscopy. J. Appl. Phys. 2012, 111, 063723. [Google Scholar] [CrossRef]


| Anode | Cycle Performance | Rfs (Year) |
|---|---|---|
| Fe–S-doped anatase TiO2 nanotubes | 61.4 mAh g−1 at 1680 mA g−1 after 500 cycles | [20] (2016) |
| Fe-doped anatase TiO2/carbon composite Europium-modified TiO2 Mn-doped TiO2 Cu-doped TiO2 B-doped TiO2 F-doped carbon coated mesoporous TiO2 Co-doped TiO2 | 158.6 mAh g−1 at 1700 mA g−1 after 300 cycles 219.1 mAh g−1 at 850 mA g−1 after 600 cycles 113 mAh g−1 at 170 mA g−1 after 118 cycles 250 mAh g−1 at 500 mA g−1 after 100 cycles 119.4 mAh g−1 at 1680 mA g−1 after 100 cycles 210 mAh g−1 at 84 mA g−1 after 100 cycles 125 mAh g−1 at 1700 mA g−1 after 600 cycles | [27] (2015) [28] (2022) [29] (2020) [30] (2018) [31] (2014) [33] (2014) In this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, L.; Gu, F.-C.; Meng, S.-M.; Zhuang, A.-Q.; Dong, H.; Li, Z.-Z.; Guan, Z.-F.; Li, D.-S.; Li, Y.; Xu, X.-X.; et al. Improved Lithium Storage Performance of a TiO2 Anode Material Doped by Co. Materials 2023, 16, 1325. https://doi.org/10.3390/ma16041325
Cai L, Gu F-C, Meng S-M, Zhuang A-Q, Dong H, Li Z-Z, Guan Z-F, Li D-S, Li Y, Xu X-X, et al. Improved Lithium Storage Performance of a TiO2 Anode Material Doped by Co. Materials. 2023; 16(4):1325. https://doi.org/10.3390/ma16041325
Chicago/Turabian StyleCai, Li, Fang-Chao Gu, Shu-Min Meng, An-Qi Zhuang, Hang Dong, Zi-Zhe Li, Zhen-Feng Guan, De-Shuai Li, Yong Li, Xi-Xiang Xu, and et al. 2023. "Improved Lithium Storage Performance of a TiO2 Anode Material Doped by Co" Materials 16, no. 4: 1325. https://doi.org/10.3390/ma16041325





