Modified Bamboo Charcoal as a Bifunctional Material for Methylene Blue Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
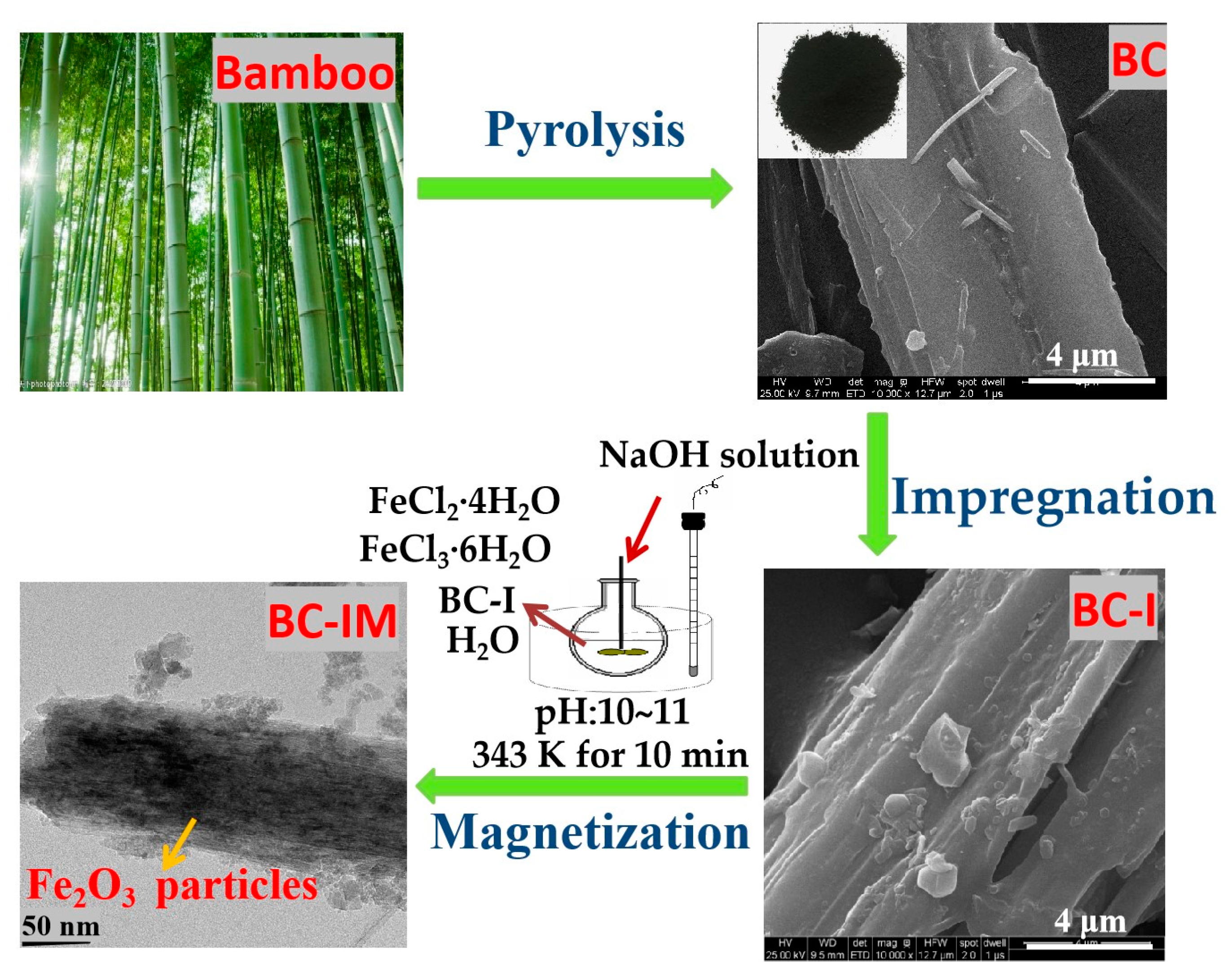
2.2. Sample Preparation
2.3. Sample Characterization
2.4. Batch Adsorption Experiments
2.5. Catalytic Degradation Experiments
3. Results
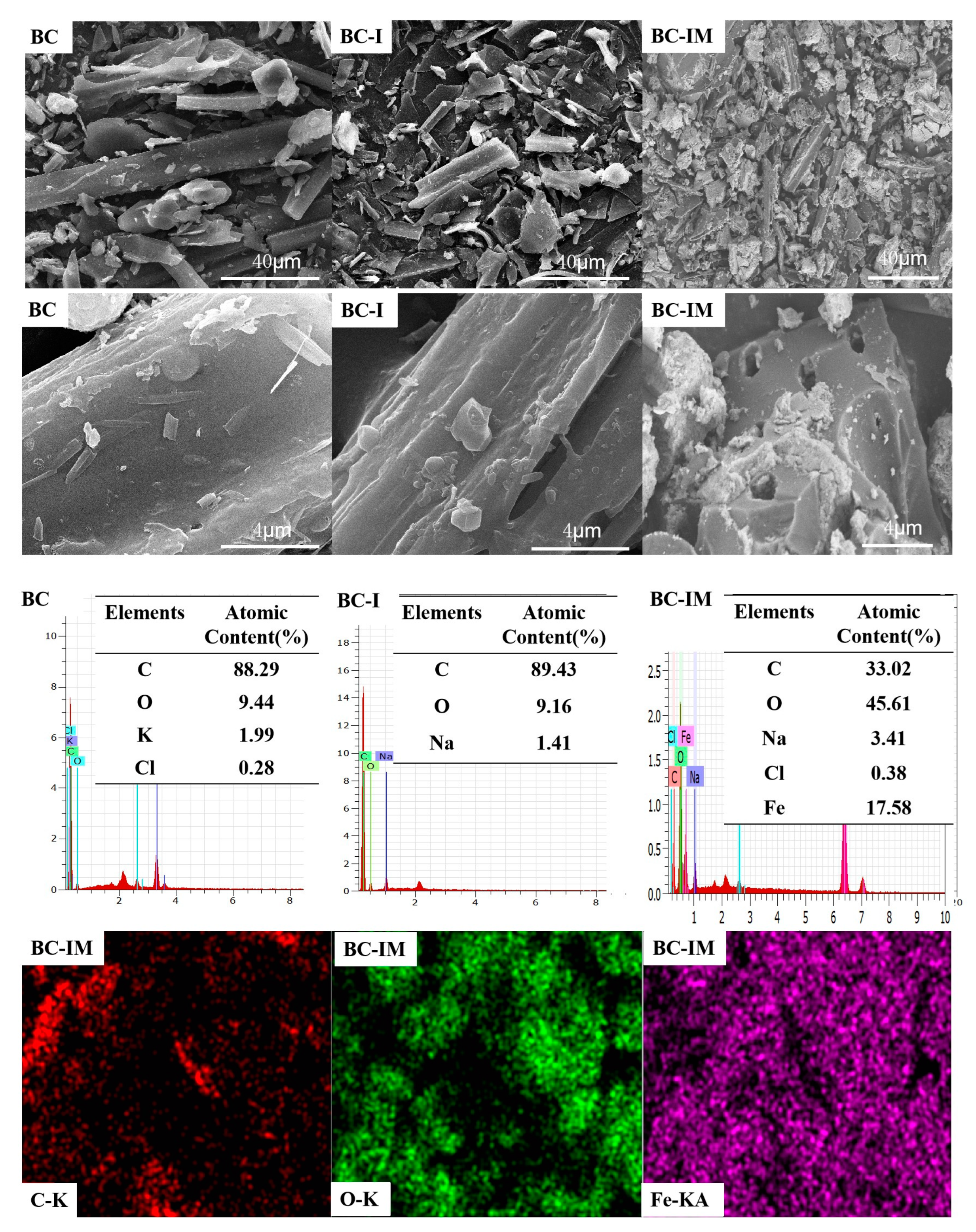
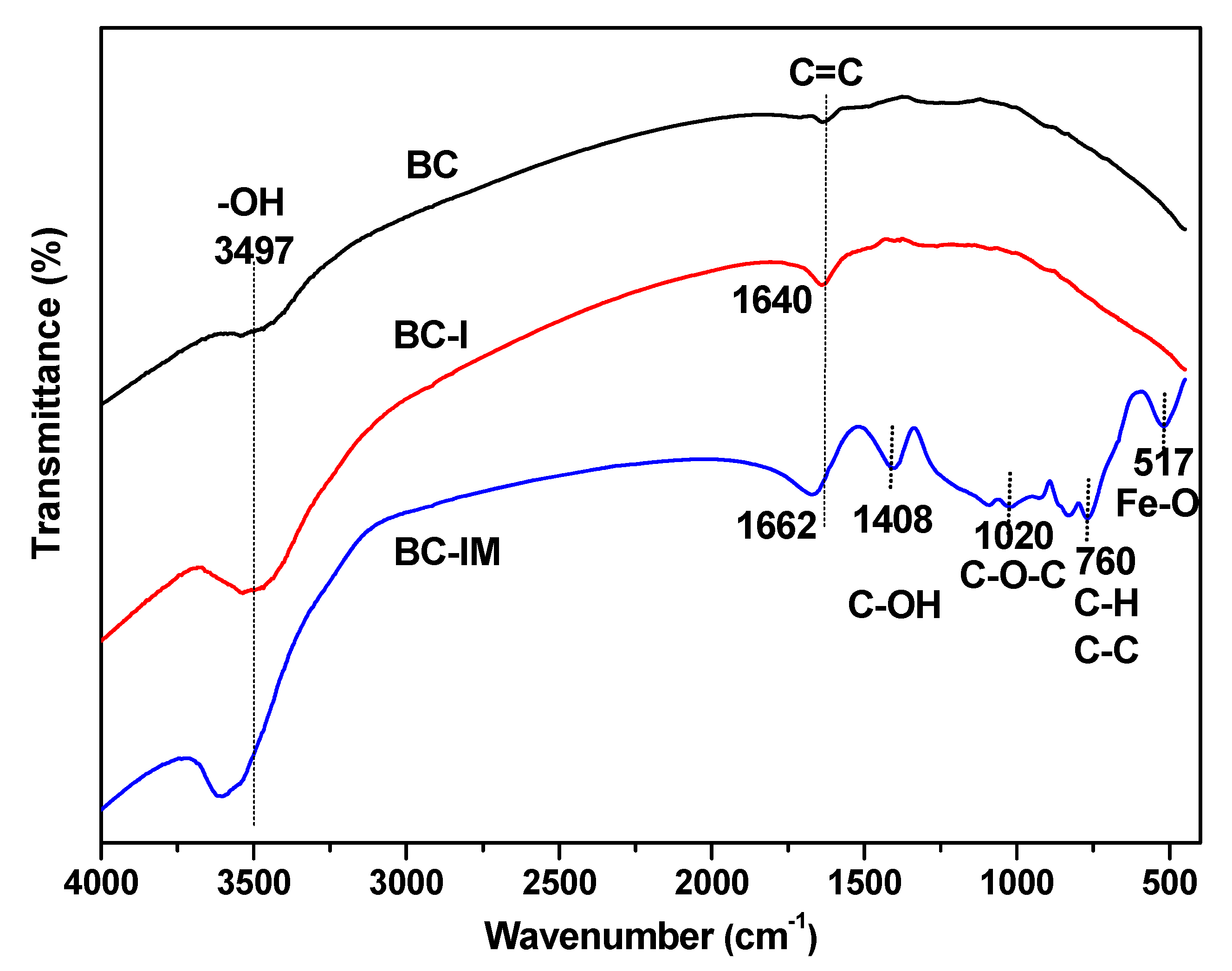
3.1. Characterization Results of the Samples
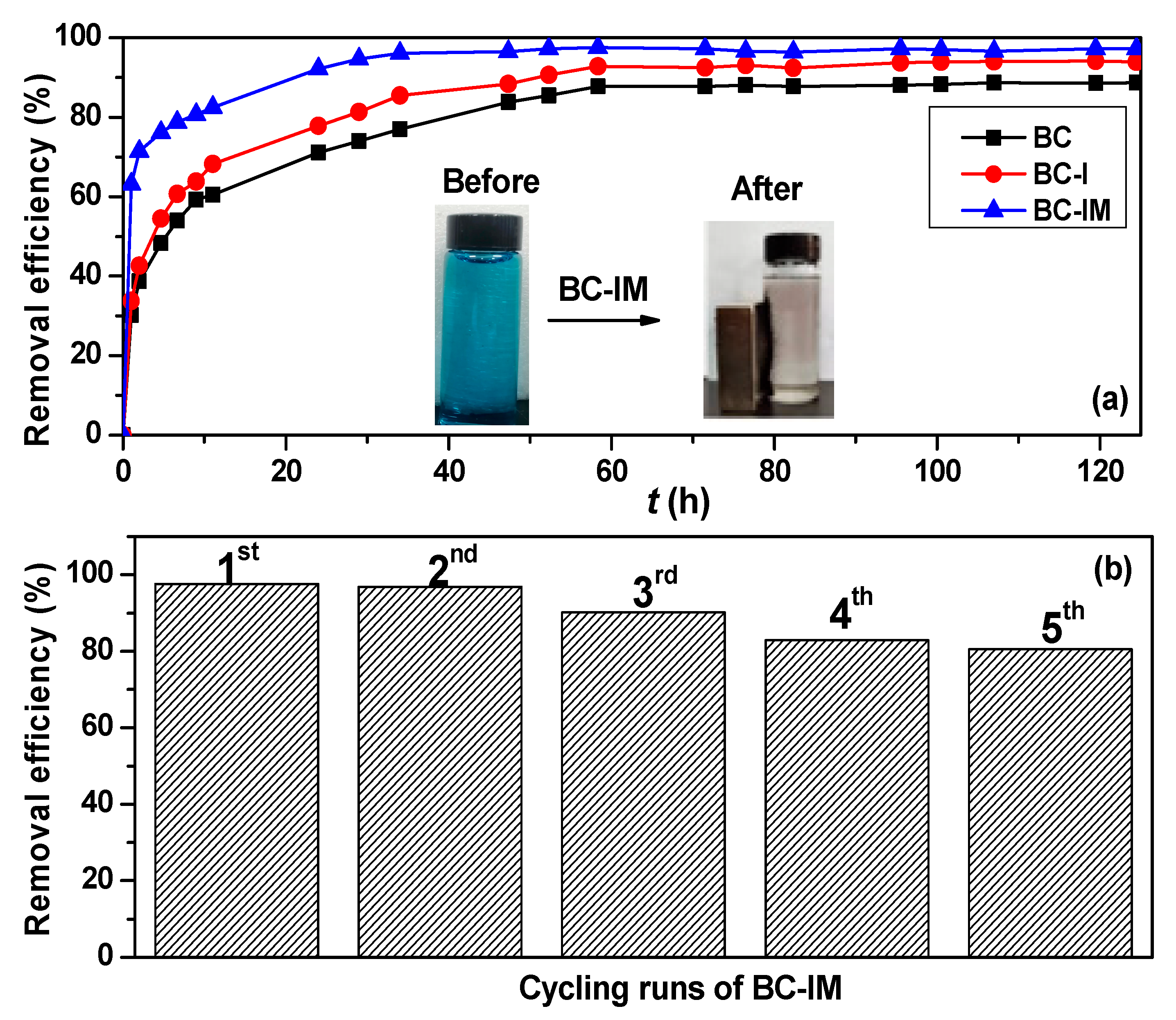
3.2. Adsorption Properties of Samples
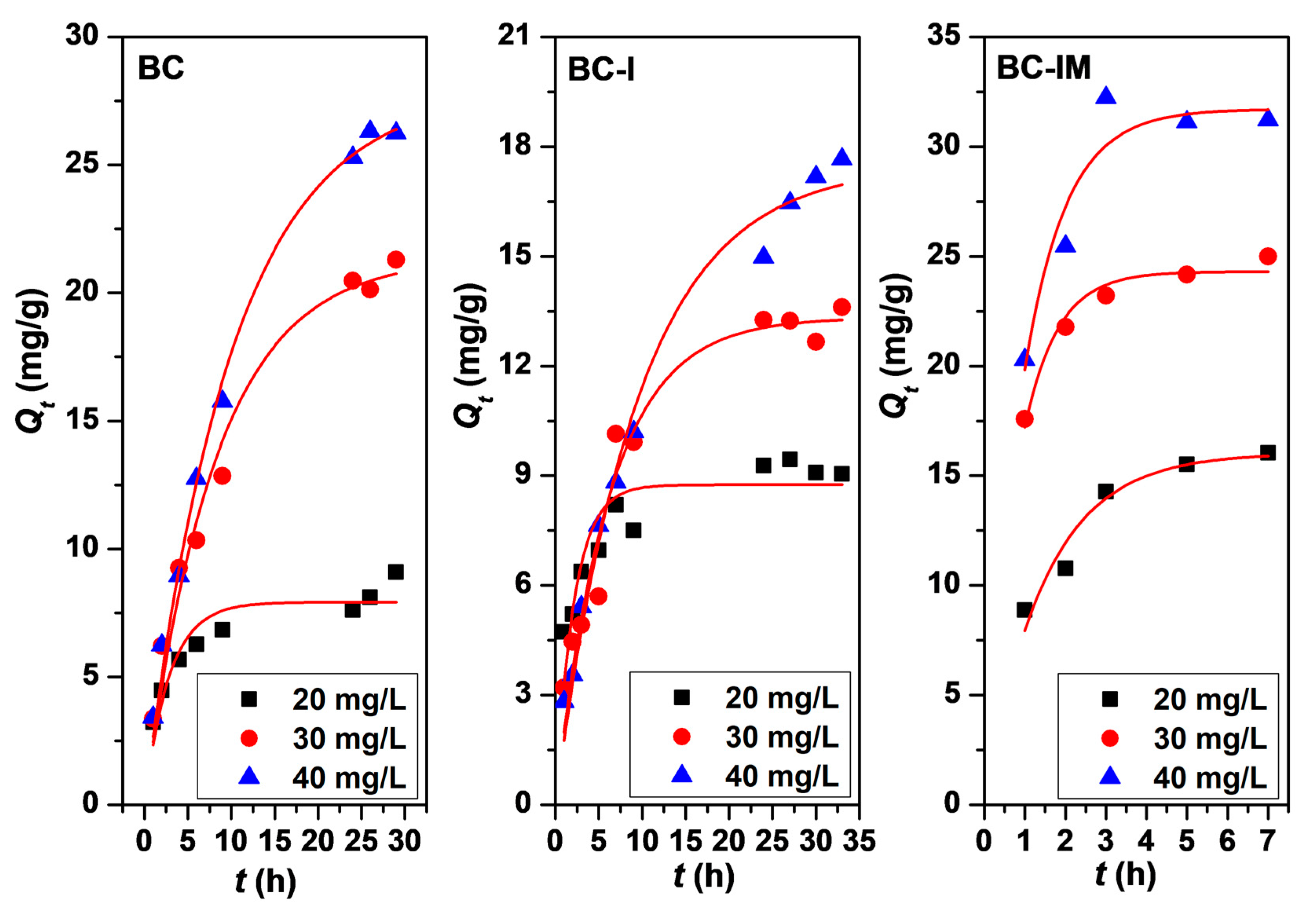
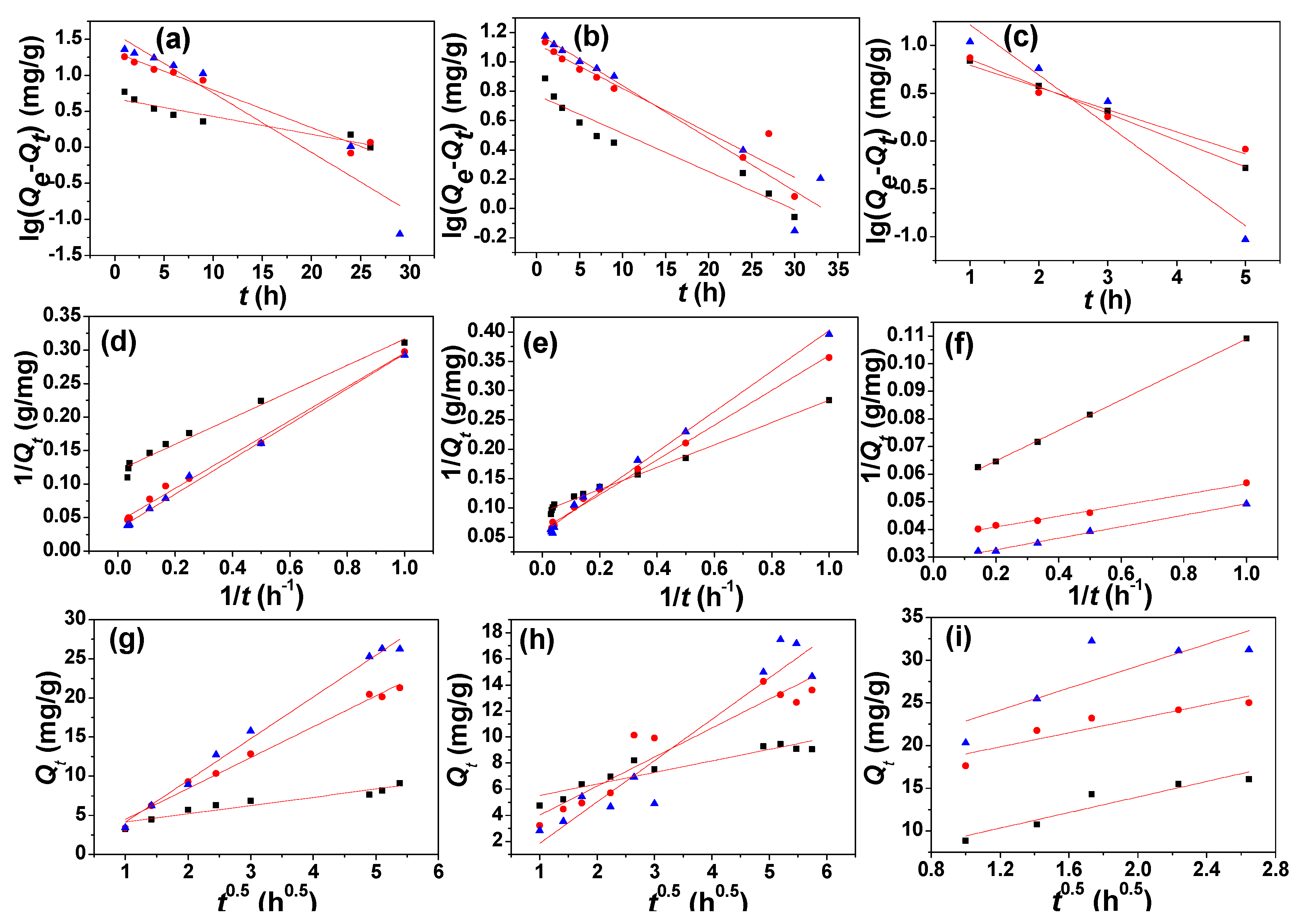
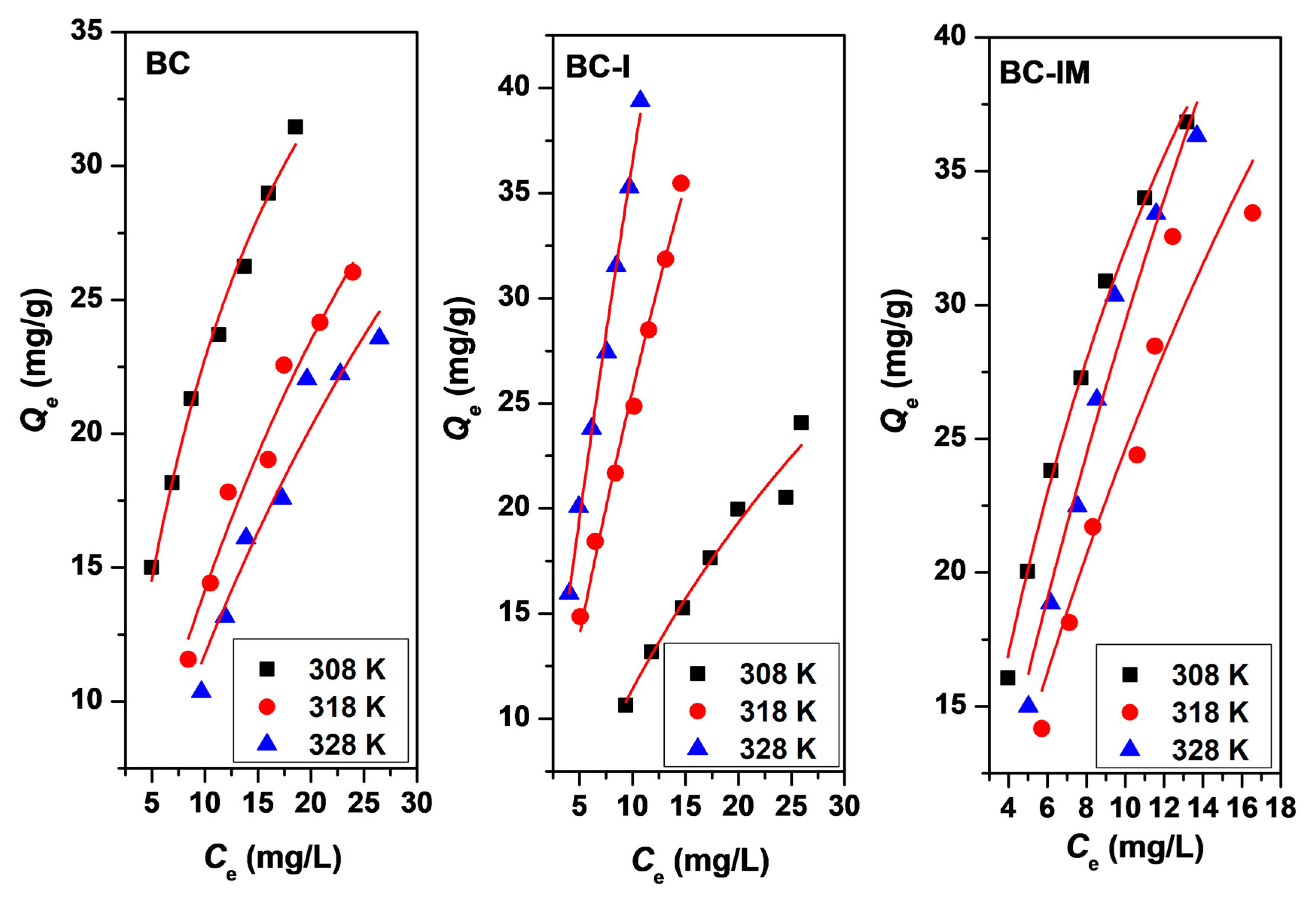
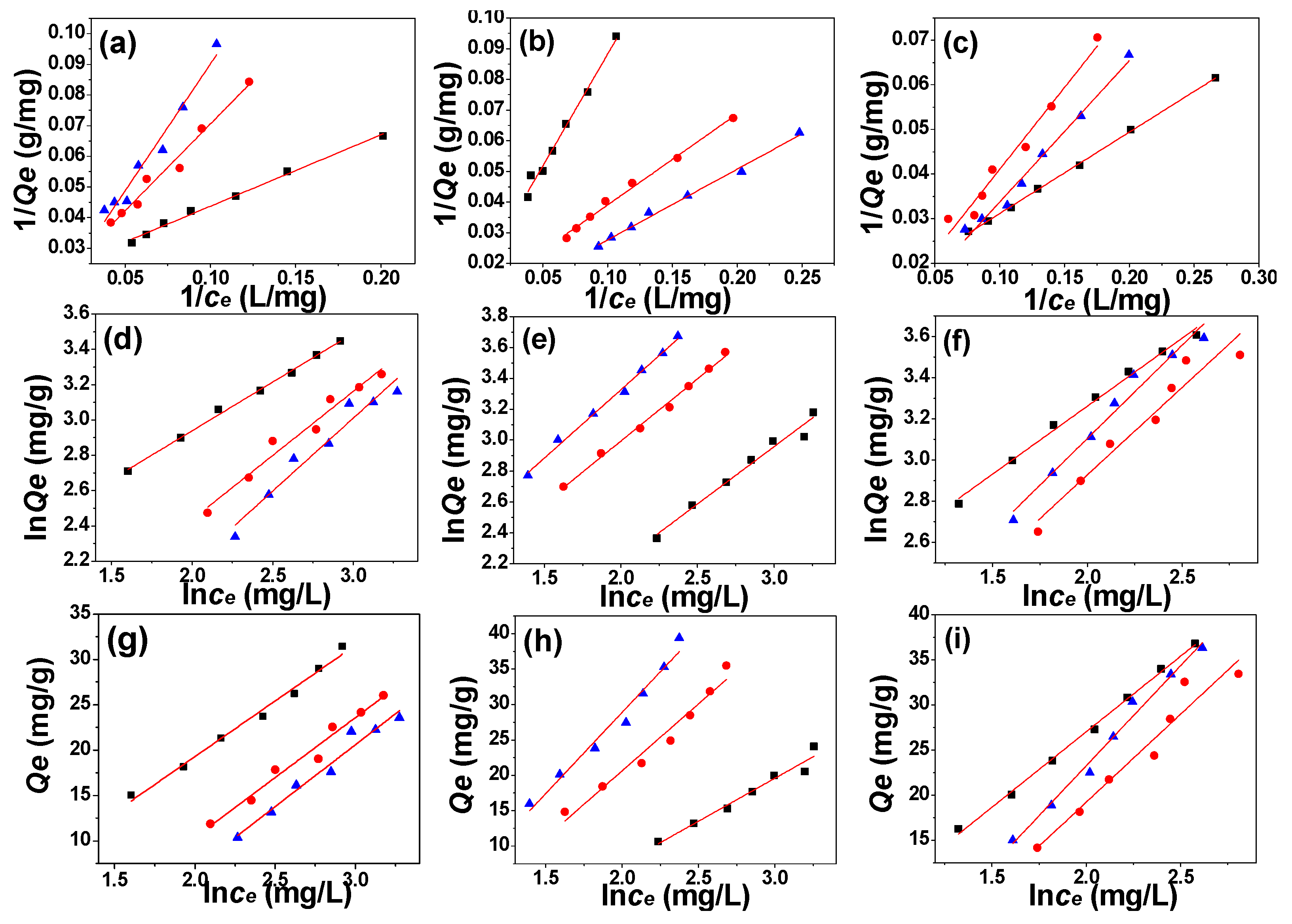
3.3. Adsorption Kinetics, Adsorption Isotherm, and Adsorption Thermodynamics
3.4. Catalytic Properties and Reusability of Samples
3.5. Mechanism Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.W.; Deng, W.Y.; Lin, X.C.; Huang, X.G.; Wei, L.; Gong, L.; Liu, C.X.; Liu, G.B.; Liu, Q. Solid-state preparation of mesoporous Ce-Mn-Co ternary mixed oxide nanoparticles for the catalytic degradation of methylene blue. J. Rare Earth. 2021, 31, 826–834. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, W. Microwave-enhanced membrane filtration for water treatment. J. Membr. Sci. 2018, 568, 97–104. [Google Scholar] [CrossRef]
- Zupko, R.; Kamath, D.; Coscarelli, E.; Rouleau, M.; Minakata, D. Agent-Based model to predict the fate of the degradation of organic compounds in the aqueous-phase UV/H2O2 advanced oxidation process. Process. Saf. Environ. 2020, 136, 49–55. [Google Scholar] [CrossRef]
- Malik, M.; Len, T.; Luque, R.; Osman, S.M.; Paone, E.; Khan, M.I.; Wattoo, M.A.; Jamshaid, M.; Anum, A.; Rehman, A.U. Investigation on synthesis of ternary g-C3N4/ZnO-W/M nanocomposites integrated heterojunction II as efficient photocatalyst for environmental applications. Environ. Res. 2023, 217, 114621. [Google Scholar] [CrossRef] [PubMed]
- Jamshaid, M.; Khan, M.I.; Fernandez, J.; Shanableh, A.; Hussaine, T.; Rehman, A.U. Synthesis of Ti4+ doped Ca-BiFO3 for the enhanced photodegradation of moxifloxacin. New J. Chem. 2022, 46, 19848. [Google Scholar] [CrossRef]
- Haounati, R.; Alakhras, F.; Ouachtak, H.; Saleh, T.A.; Al-Mazaideh, G.; Alhajri, E.; Jada, A.; Hafifid, N.; Addi, A.A. Synthesized of zeolite@Ag2O nanocomposite as superb stability photocatalysis toward hazardous rhodamine B dye from water. Arab. J. Sci. Eng. 2023, 48, 169–179. [Google Scholar] [CrossRef]
- Ouachtak, H.; Guerdaoui, A.E.; Haounati, R.; Akhouairi, S.; Haouti, R.E.; Hafifid, N.; Addi, A.A.; Šljukic, B.; Santos, D.M.F.; Taha, M.L. Highly effificient and fast batch adsorption of orange G dye from polluted water using superb organo-montmorillonite: Experimental study and molecular dynamics investigation. J. Mol. Liq. 2021, 335, 116560. [Google Scholar] [CrossRef]
- Osagie, C.; Othmani, A.; Ghosh, S.; Malloum, A.; Esfahani, Z.K.; Ahmadi, S. Dyes adsorption from aqueous media through the nanotechnology: A review. J. Mater. Res. Technol. 2021, 14, 2195–2218. [Google Scholar] [CrossRef]
- Li, T.; Ge, L.; Peng, X.; Wang, W.; Zhang, W. Enhanced degradation of sulfamethoxazole by a novel Fenton-like system with significantly reduced consumption of H2O2 activated by g-C3N4/MgO composite. Water Res. 2021, 190, 116777. [Google Scholar] [CrossRef]
- Li, X.P.; Wang, C.B.; Zhang, J.G.; Liu, J.P.; Liu, B.; Chen, G.Y. Preparation and application of magnetic biochar in water treatment: A critical review. Sci. Total Environ. 2020, 711, 134847. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Godlewska, P.; Reible, D.; Kraska, P. Bioaccessibility of polycyclic aromatic hydrocarbons in activated carbon or biochar amended vegetated (Salix viminalis) soil. Environ. Pollution. 2017, 227, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Wang, J.; Song, H.; Chen, W. Removal of methylene blue from aqueous solutions using biochar derived from a fallen leaf by slow pyrolysis: Behavior and mechanism. J. Environ. Chem. Eng. 2019, 7, 103036. [Google Scholar] [CrossRef]
- Sun, L.; Wan, S.; Luo, W. Biochars prepared from anaerobic digestion residue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: Characterization, equilibrium, and kinetic studies. Bioresour. Technol. 2013, 140, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Tseng, R.L. High adsorption capacity NaOH-activated carbon for dye removal from aqueous solution. J. Hazard. Mater. 2008, 152, 1256–1267. [Google Scholar] [CrossRef]
- Jia, Z.; Zeng, W.; Xu, H.; Li, S.; Peng, Y. Adsorption removal and reuse of phosphate from wastewater using a novel adsorbent of lanthanum-modified platanus biochar. Process. Saf. Environ. 2020, 140, 221–232. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I.; Hosseini-Bandegharaei, A.; Giannakoudakis, D.A.; Robalds, A.; Usman, M.; Escudero, L.B.; Zhou, Y.; Colmenares, J.C.; Núňez-Delgado, A.; et al. Agricultural biomass/waste as adsorbents for toxic metal decontamination of aqueous solutions. J. Mol. Liq. 2019, 295, 111684. [Google Scholar] [CrossRef]
- Popa, N.; Visa, M. The synthesis, activation and characterization of charcoal powder for the removal of methylene blue and cadmium from wastewater. Adv. Powder Technol. 2017, 28, 1866–1876. [Google Scholar] [CrossRef]
- Lalhruaitluanga, H.; Prasad, M.N.V.; Radha, K. Potential of chemically activated and raw charcoals of Melocannabaccifera for removal of Ni(II) and Zn(II) from aqueous solutions. Desalination 2011, 271, 301–308. [Google Scholar] [CrossRef]
- Islama, M.A.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous activated carbon prepared from NaOH activation of rattan (Lacospermasecundiflorum) hydrochar for methylene blue removal. Ecotox. Environ. Safe. 2017, 138, 279–285. [Google Scholar] [CrossRef]
- Oginni, O.; Singh, K.; Oporto, G.; Dawson-Andoh, B.; McDonald, L.; Sabolsky, E. Influence of one-step and two-step KOH activation on activated carbon characteristics. Bioresour. Technol. 2019, 7, 100266. [Google Scholar] [CrossRef]
- Xu, H.; Shen, B.; Yuan, P.; Lu, F.; Tian, L.; Zhang, X. The adsorption mechanism of elemental mercury by HNO3-modified bamboo char. Fuel Process. Technol. 2016, 154, 139–146. [Google Scholar] [CrossRef]
- Wu, C.; Li, L.; Zhou, H.; Ai, J.; Zhang, H.; Tao, J.; Wang, D.; Zhang, W. Effects of chemical modification on physicochemical properties and adsorption behavior of sludge-based activated carbon. J. Environ. Sci. 2021, 100, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Peng, K.; Liang, S.; Jia, X.; Jiang, X.; Qian, L. One-step synthesis of biomass activated char supported copper nanoparticles for catalytic cracking of biomass primary tar. Energy 2019, 180, 584–593. [Google Scholar] [CrossRef]
- Thines, K.R.; Abdullah, E.C.; Mubarak, N.M.; Ruthiraan, M. Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for waste water and polymer application: A review. Renew. Sust. Energ. Rev. 2017, 67, 257–276. [Google Scholar] [CrossRef]
- Hao, Z.; Wang, C.; Yan, Z.; Jiang, H.; Xu, H. Magnetic particles modification of coconut shell-derived activated carbon and biochar for effective removal of phenol from water. Chemosphere 2018, 211, 962–969. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Wang, Z.; Zhang, Z.; Wang, X.; Yang, Z. Active biochar support nano zero-valent iron for efficient removal of U(VI) from sewage water. J. Alloy Compd. 2021, 852, 156993. [Google Scholar] [CrossRef]
- Liu, J.W.; Jiang, J.G.; Meng, Y.; Aihemaiti, A.; Xu, Y.W.; Xiang, H.L.; Gao, Y.; Chen, X.C. Preparation, environmental application and prospect of biochar-supported metal nanoparticles: A review. J. Hazard. Mater. 2020, 388, 122026. [Google Scholar] [CrossRef]
- Khataee, A.; Kalderis, D.; Gholami, P.; Fazli, A.; Moschogiannaki, M.; Binas, V.; Lykaki, M.; Konsolakis, M. Cu2O-CuO@biochar composite: Synthesis, characterization and its efficient photocatalytic performance. Appl. Surf. Sci. 2019, 498, 143846. [Google Scholar] [CrossRef]
- Zhai, S.; Li, M.; Wang, D.; Zhang, L.; Yang, Y.; Fu, S. In situ loading metal oxide particles on bio-chars: Reusable materials for efficient removal of methylene blue from wastewater. J. Clean. Prod. 2019, 220, 460–474. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.; Fu, B.; Song, Y. Facile synthesis of sludge-derived MnOx-N-biochar as an efficient catalyst for peroxymonosulfate activation. Appl. Catal. B Environ. 2019, 255, 117765. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Tsang, E.P.; Cheng, W.; Fang, J.; Fang, Z. Magnetic biochar for environmental remediation: A review. Bioresour. Technol. 2020, 298, 122468. [Google Scholar] [CrossRef]
- Oyedum, A.O.; Gebreegziabher, T.; Hui, C.W. Mechanism and modeling of bamboo pyrolysis. Fuel Process. Technol. 2013, 106, 595–604. [Google Scholar] [CrossRef]
- Regmi, P.; Moscoso, J.L.G.; Kumar, S.; Cao, X.; Mao, J.; Schafran, G. Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. J. Environ. Manag. 2012, 109, 61–69. [Google Scholar] [CrossRef]
- Karunanayake, A.G.; Navarathna, C.M.; Gunatilake, S.R.; Crowley, M.; Anderson, R.; Mohan, D.; Perez, F.; Pittman Jr, C.U.; Mlsna, T. Fe3O4 Nanoparticles Dispersed on Douglas Fir Biochar for Phosphate Sorption. ACS Appl. Nano. Mater. 2019, 2, 3467–3479. [Google Scholar] [CrossRef]
- Hou, Y.; Yan, S.; Huang, G.; Yang, Q.; Huang, S.; Cai, J. Fabrication of N-doped carbons from waste bamboo shoot shell with high removal efficiency of organic dyes from water. Bioresour. Technol. 2020, 303, 122939. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.R.; Huang, G.G.; Li, J.H.; Yang, Q.P.; Huang, S.R.; Cai, J.J. Hydrothermal conversion of bamboo shoot shell to biochar: Preliminary studies of adsorption equilibrium and kinetics for rhodamine B removal. J. Anal. Appl. Pyrol. 2019, 143, 104694. [Google Scholar] [CrossRef]
- Jena, L.; Soren, D.; Deheri, P.K.; Pattojoshi, P. Preparation, characterization and optical properties evaluations of bamboo charcoal. Curr. Res. Green Sustain. Chem. 2021, 4, 100077. [Google Scholar] [CrossRef]
- Li, Y.; Meas, A.; Shan, S.; Yang, R.; Gai, X. Production and optimization of bamboo hydrochars for adsorption of Congo red and 2-naphthol. Bioresour. Technol. 2016, 207, 379–386. [Google Scholar] [CrossRef]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Review on recent advances of carbon based adsorbent for methylene blue removal from waste water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Liu, S.S.; Ge, H.Y.; Wang, C.C.; Zou, Y.; Liu, J.Y. Agricultural waste/grapheme oxide 3D bio-adsorbent for highly efficient removal of methylene blue from water pollution. Sci. Total Environ. 2018, 628–629, 959–968. [Google Scholar] [CrossRef]
- Saleh, S.; Kamarudin, K.B.; Ghani, W.A.; Kheang, L.S. Removal of organic contaminant from aqueous solution using magnetic biochar. Procedia Eng. 2016, 148, 228–235. [Google Scholar] [CrossRef]
- Oh, W.D.; Lua, S.K.; Dong, Z.I.; Lim, T.T. Performance of magnetic activated carbon composites as peroxymonosulfate activator and regenerable adsorbent via sulfate radical-mediated oxidation processes. J. Hazard. Mater. 2015, 284, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Idohou, E.A.; Fatombi, J.K.; Osseni, S.A.; Agani, I.; Neumeyer, D.; Verelst, M.; Mauricot, R.; Aminou, T. Preparation of activated carbon/chitosan/carica papaya seeds composite for efficient adsorption of cationic dye from aqueous solution. Surf. Interfaces 2020, 21, 100741. [Google Scholar] [CrossRef]
- Panneerselvam, P.; Morad, N.; Tan, K.A. Magnetic nanoparticle (Fe3O4) impregnated onto tea waste for the removal of nickel(II) from aqueous solution. J. Hazard. Mater. 2011, 186, 160–168. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, Y.; Qin, Y.; Yi, Y.; Liu, Z.; Lv, L.; Xu, M. Evalution of perchlorate removal from aqueous solution by cross-linked magnetic chitosan/poly (vinyl alcohol) particles. J. Taiwan Inst. Chem. Eng. 2016, 65, 295–303. [Google Scholar] [CrossRef]
- Daou, T.; Begin-Colin, S.; Greneche, J.; Thomas, F.; Derory, A.; Ernhardt, P.; Legaré, P.; Pourroy, P.G. Phosphate adsorption properties of magnetite-based nanoparticles. Chem. Mater. 2007, 19, 4494–4505. [Google Scholar] [CrossRef]
- Navarathna, C.M.; Karunanayake, A.G.; Gunatilake, S.R.; Pittman Jr, C.U.; Perez, F.; Mohan, D.; Mlsna, T. Removal of Arsenic (III) from water using magnetite precipitated onto Douglas fir biochar. J. Environ. Manag. 2019, 250, 109429. [Google Scholar] [CrossRef]
- Luo, H.Y.; Lin, Q.T.; Zhang, X.F.; Huang, Z.F.; Fu, H.G.; Xiao, R.B.; Liu, S.S. Determining the key factors of nonradical pathway in activation of persulfate by metal-biochar nanocomposites for bisphenol A degradation. Chem. Eng. J. 2020, 391, 123555. [Google Scholar]
- Fan, S.S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Altintas, B.; Arica, M.Y. Adsorption kinetics and thermodynamic parameters of cationic dyes from aqueous solutions by using a new strong cation-exchange resin. Chem. Eng. J. 2009, 152, 339–346. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res. 2001, 35, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Pan, J.; Li, Y.H.; Zhang, P.F.; Li, M.X.; Zheng, H.; Zhang, X.P.; Li, H.; Du, Q.J. Methylene blue adsorption by activated carbon, nickel alginate/activated carbon aerogel, and nickel alginate/grapheme oxide aerogel: A comparison study. J. Mater. Res. Technol. 2020, 9, 12443–12460. [Google Scholar] [CrossRef]
- Moradi, H.; Azizpour, H.; Bahmanyar, H.; Mohammadi, M. Molecular dynamics simulation of H2S adsorption behavior on the surface of activated carbon. Inorg. Chem. Commun. 2020, 118, 108048. [Google Scholar] [CrossRef]
- Rehman, M.U.; Manan, A.; Uzair, M.; Khan, A.S.; Ullah, A.; Ahmad, A.S.; Wazir, A.H.; Qazi, I.; Khan, M.A. Physicochemical characterization of Pakistani clay for adsorption of methylene blue: Kinetic, isotherm and thermodynamic study. Mater. Chem. Phys. 2021, 269, 124722. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmiumion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Jamshaid, M.; Nazir, M.A.; Najam, T.; Shah, S.S.A.; Khan, H.M.; Rehman, A.U. Facile synthesis of Yb3+-Zn2+ substituted M type hexaferrites: Structural, electric and photocatalytic properties under visible light for methylene blue removal. Chem. Phy. Lett. 2022, 805, 139939. [Google Scholar] [CrossRef]
- Jamshaid, M.; Rehman, A.U.; Kumar, O.P.; Iqbal, S.; Nazir, M.A.; Anum, A.; Khan, H.M. Design of dielectric and photocatalytic properties of Dy–Ni substituted Ca0.5 Pb0.5-xFe12-yO19 M-type hexaferrites. J Mater Sci: Mater Electron 2021, 32, 16255–16268. [Google Scholar] [CrossRef]
- Liu, Q.; Deng, W.; Wang, Q.; Lin, X.; Gong, L.; Liu, C.; Xiong, W.; Nie, X. An efficient chemical precipitation route to fabricate 3D flower-like CuO and 2D leaf-like CuO for degradation of methylene blue. Adv. Powder Technol. 2020, 31, 1391–1401. [Google Scholar] [CrossRef]
- Li, L.; Lai, C.; Huang, F.; Cheng, M.; Zeng, G.; Huang, D.; Li, B.; Liu, S.; Zhang, M.; Qin, L.; et al. Degradation of naphthalene with magnetic bio-char activate hydrogen peroxide: Synergism of bio-char and FeMn binary oxides. Water Res. 2019, 160, 238–248. [Google Scholar] [CrossRef]






| Catalyst | SBET (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| BC | 1.4 | \ | 0.001 |
| BC-I | 63.0 | 0.8/1.0/6.2 | 0.082 |
| BC-IM | 84.7 | 0.9/3.8 | 0.112 |
| Adsorbents | co (mg/L) | Pseudo-First-Order | Pseudo-Second-Order | Intra-Particle Diffusion | |||
|---|---|---|---|---|---|---|---|
| k1 (h−1) | R2 | k2 (g/(mg·h)) | R2 | kip (mg/(g·h0.5) | R2 | ||
| BC | 20 | 0.0246 | 0.8843 | 0.0735 | 0.9812 | 1.0560 | 0.8909 |
| 30 | 0.0522 | 0.9723 | 0.0074 | 0.9928 | 3.9489 | 0.9893 | |
| 40 | 0.0823 | 0.9145 | 0.0041 | 0.9944 | 5.3319 | 0.9916 | |
| BC-I | 20 | 0.0260 | 0.9209 | 0.0458 | 0.9931 | 0.8832 | 0.8515 |
| 30 | 0.0302 | 0.9281 | 0.0134 | 0.9931 | 2.2269 | 0.8910 | |
| 40 | 0.0359 | 0.9261 | 0.0087 | 0.9921 | 3.1691 | 0.9070 | |
| BC-IM | 20 | 0.0280 | 0.9979 | 0.0523 | 0.9990 | 4.5554 | 0.8668 |
| 30 | 0.0232 | 0.9477 | 0.0699 | 0.9910 | 4.1223 | 0.7974 | |
| 40 | 0.0525 | 0.9301 | 0.0389 | 0.9936 | 6.4335 | 0.5708 | |
| Adsorbent | T (K) | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qm (mg/g) | kL (L/mg) | R2 | kF | 1/n | R2 | AT (L/mg) | BT (mg/g) | R2 | ||
| BC | 308 | 48.92 | 0.08777 | 0.9937 | 6.2660 | 0.5518 | 0.9964 | 0.65082 | 12.2765 | 0.9866 |
| 318 | 71.48 | 0.02473 | 0.9741 | 2.6739 | 0.7261 | 0.9641 | 0.29622 | 13.2230 | 0.9695 | |
| 328 | 135.13 | 0.00894 | 0.9633 | 1.7027 | 0.8259 | 0.9424 | 0.22519 | 13.6403 | 0.9593 | |
| BC-I | 308 | 65.75 | 0.02086 | 0.9866 | 2.0719 | 0.7433 | 0.9726 | 0.25067 | 12.1212 | 0.9571 |
| 318 | 107.76 | 0.03123 | 0.9917 | 3.9911 | 0.8051 | 0.9936 | 0.40348 | 18.9115 | 0.9594 | |
| 328 | 220.26 | 0.01968 | 0.9929 | 4.7295 | 0.8861 | 0.9936 | 0.47785 | 22.9148 | 0.9703 | |
| BC-IM | 308 | 77.46 | 0.07074 | 0.9992 | 6.9693 | 0.6600 | 0.9920 | 0.67924 | 16.7576 | 0.9967 |
| 318 | 227.79 | 0.01196 | 0.9671 | 3.4210 | 0.8490 | 0.9354 | 0.36229 | 19.5187 | 0.9381 | |
| 328 | 497.51 | 0.00633 | 0.9831 | 3.6430 | 0.9058 | 0.9689 | 0.38632 | 22.1840 | 0.9841 | |
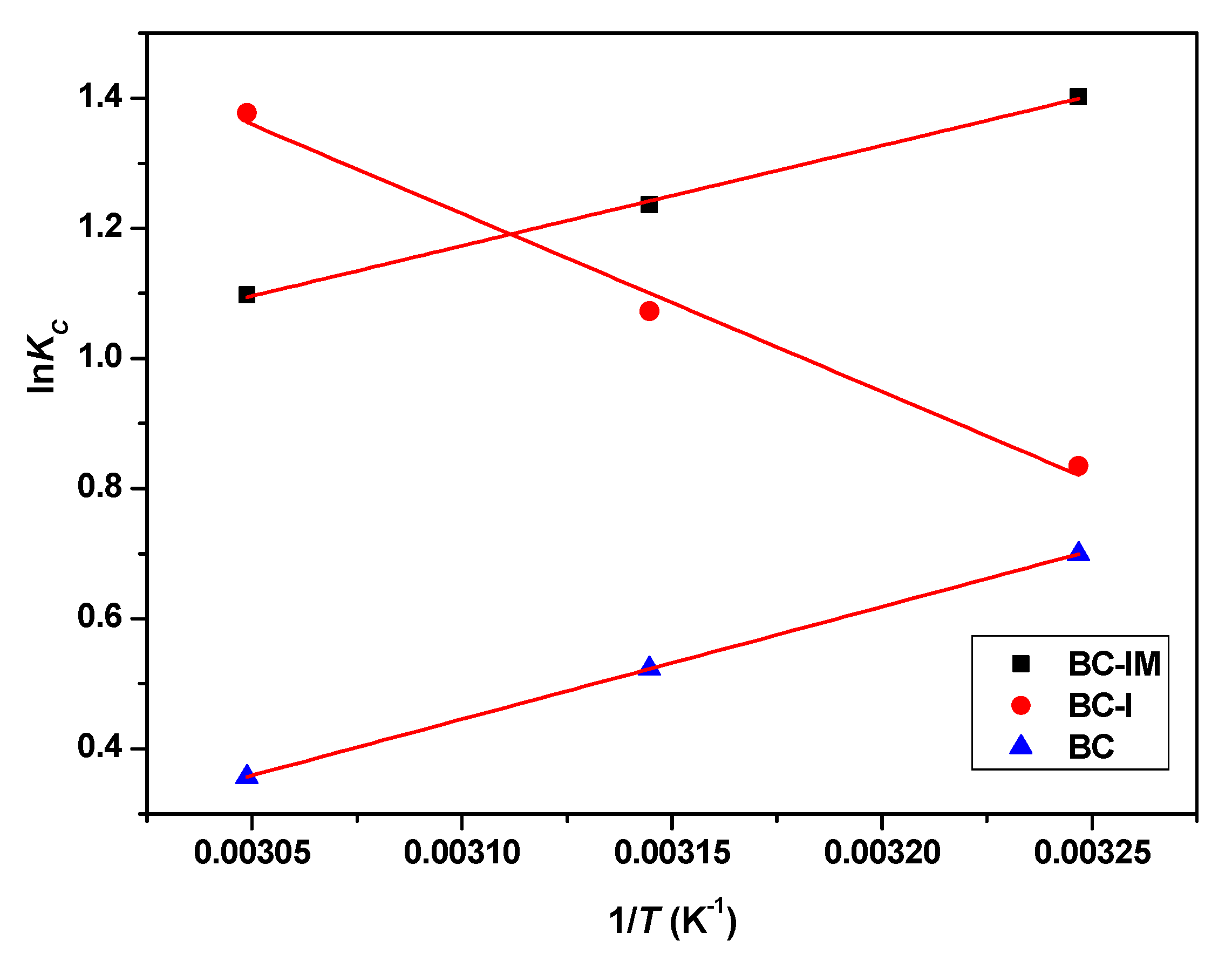
| Adsorbents | T (K) | Kc | ΔG0 (kJ/mol) | ΔH0 (kJ/mol) | ΔS0 (J/(mol K)) |
|---|---|---|---|---|---|
| BC | 308 | 2.01 | −1.79 | −0.21 | −0.59 |
| 318 | 1.69 | −1.38 | |||
| 328 | 1.43 | −0.97 | |||
| BC-I | 308 | 2.30 | −2.13 | 0.33 | 1.17 |
| 318 | 2.92 | −2.83 | |||
| 328 | 3,96 | −3.75 | |||
| BC-IM | 308 | 4.06 | −3.59 | −0.18 | −0.43 |
| 318 | 3.44 | −3.27 | |||
| 328 | 3.00 | −2.99 |
| Catalyst | Degradation Time and Temperature | Degradation Efficiency | k (h−1) | Reference |
|---|---|---|---|---|
| Ca0.5Pb0.4Yb0.1Zn1.0Fe11O19 | 120 min | 96.1% | 1.0788 | [56] |
| Ca0.5Pb0.4Dy0.1Ni1.0Fe11.9O19 | 105 min | 85% | 0.801 | [57] |
| CoMn30Ce10 | 360 min, 308 K | 90% | 0.251 | [1] |
| CuO | 210 min, 308 K | 96% | 0.8112 | [58] |
| BC-IM | 120 min, 308 K | 98.43% | 1.542 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Deng, W.-Y.; Zhang, L.-Y.; Liu, C.-X.; Jie, W.-W.; Su, R.-X.; Zhou, B.; Lu, L.-M.; Liu, S.-W.; Huang, X.-G. Modified Bamboo Charcoal as a Bifunctional Material for Methylene Blue Removal. Materials 2023, 16, 1528. https://doi.org/10.3390/ma16041528
Liu Q, Deng W-Y, Zhang L-Y, Liu C-X, Jie W-W, Su R-X, Zhou B, Lu L-M, Liu S-W, Huang X-G. Modified Bamboo Charcoal as a Bifunctional Material for Methylene Blue Removal. Materials. 2023; 16(4):1528. https://doi.org/10.3390/ma16041528
Chicago/Turabian StyleLiu, Qian, Wen-Yong Deng, Lie-Yuan Zhang, Chang-Xiang Liu, Wei-Wei Jie, Rui-Xuan Su, Bin Zhou, Li-Min Lu, Shu-Wu Liu, and Xi-Gen Huang. 2023. "Modified Bamboo Charcoal as a Bifunctional Material for Methylene Blue Removal" Materials 16, no. 4: 1528. https://doi.org/10.3390/ma16041528





