Characterization of Magnesium and Zinc Forms of Sodalite Coatings on Ti6Al4V ELI for Potential Application in the Release of Drugs for Osteoporosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
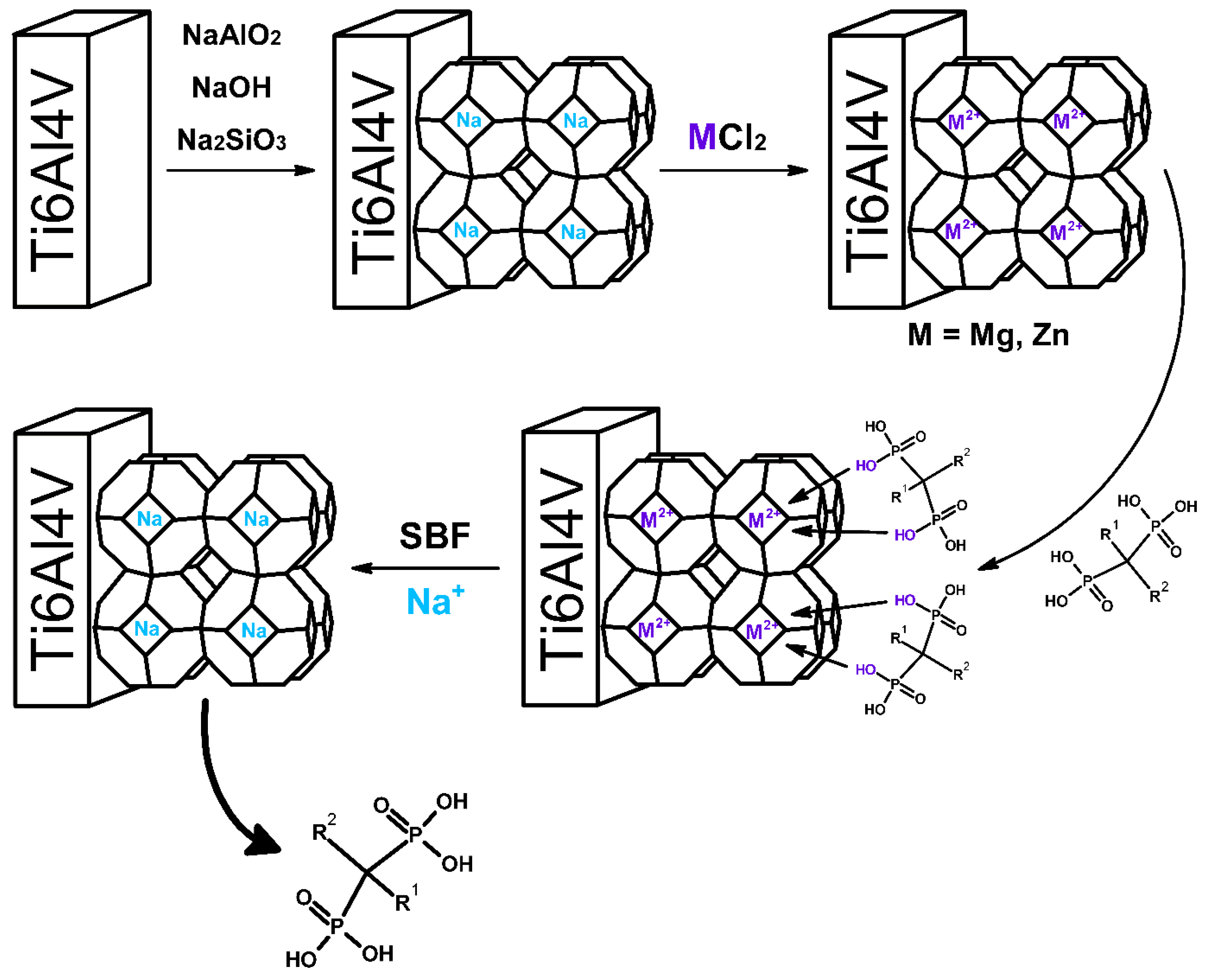
2.2. Sodium Zeolite (Sodalite) Coating on Titanium Alloy
2.3. Preparation of Magnesium or Zinc Ions Containing Sodalite Coating on Titanium Alloy
- Ti-Zeo-Mg;
- Ti-Zeo-Zn.
2.4. Characterization of Materials
2.4.1. Scanning Electron Microscopy (SEM) and Energy Dispersion Spectroscopy (EDS)
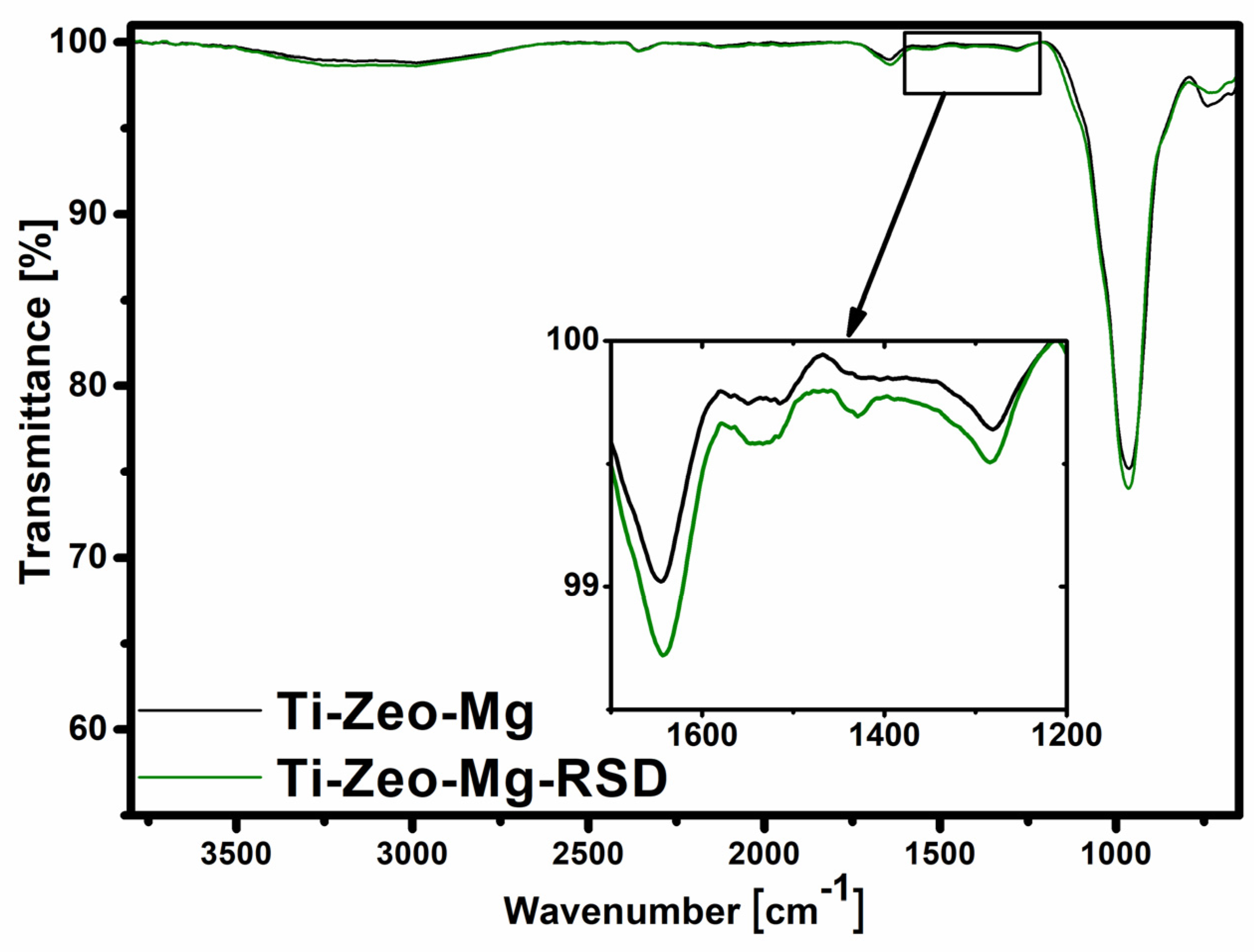
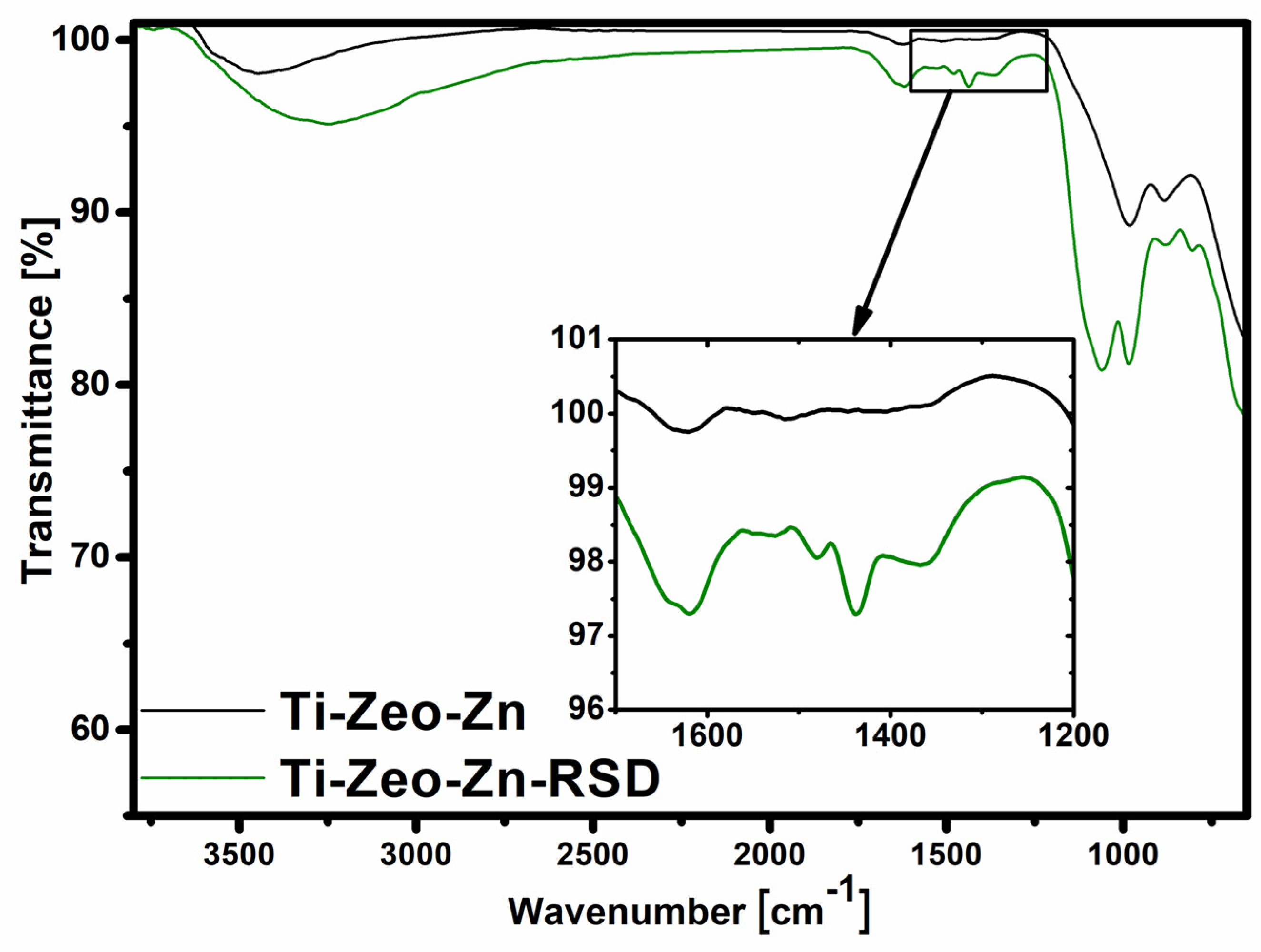
2.4.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4.3. UV-Vis Spectroscopy
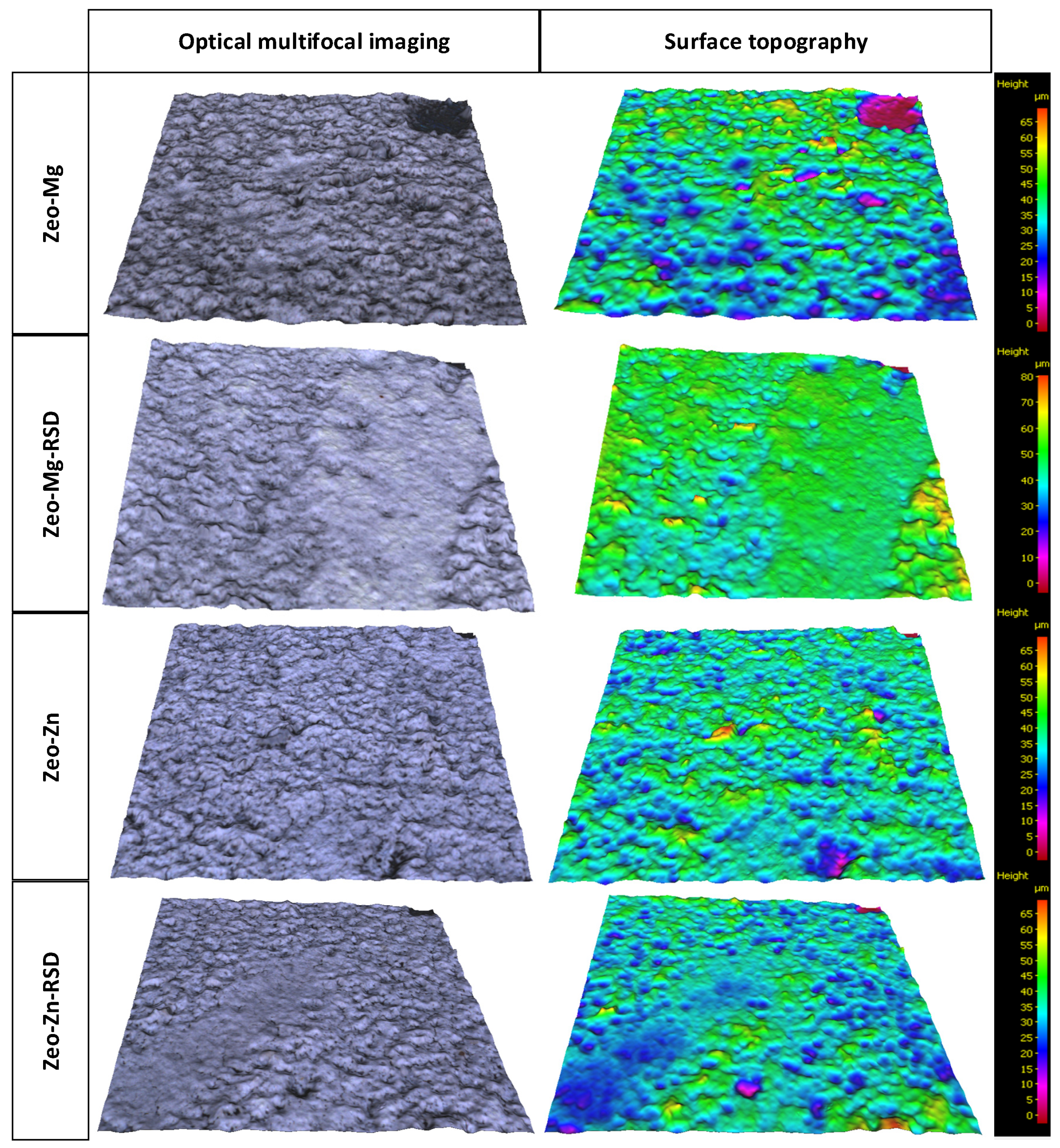
2.4.4. Optical Surface Profilometry
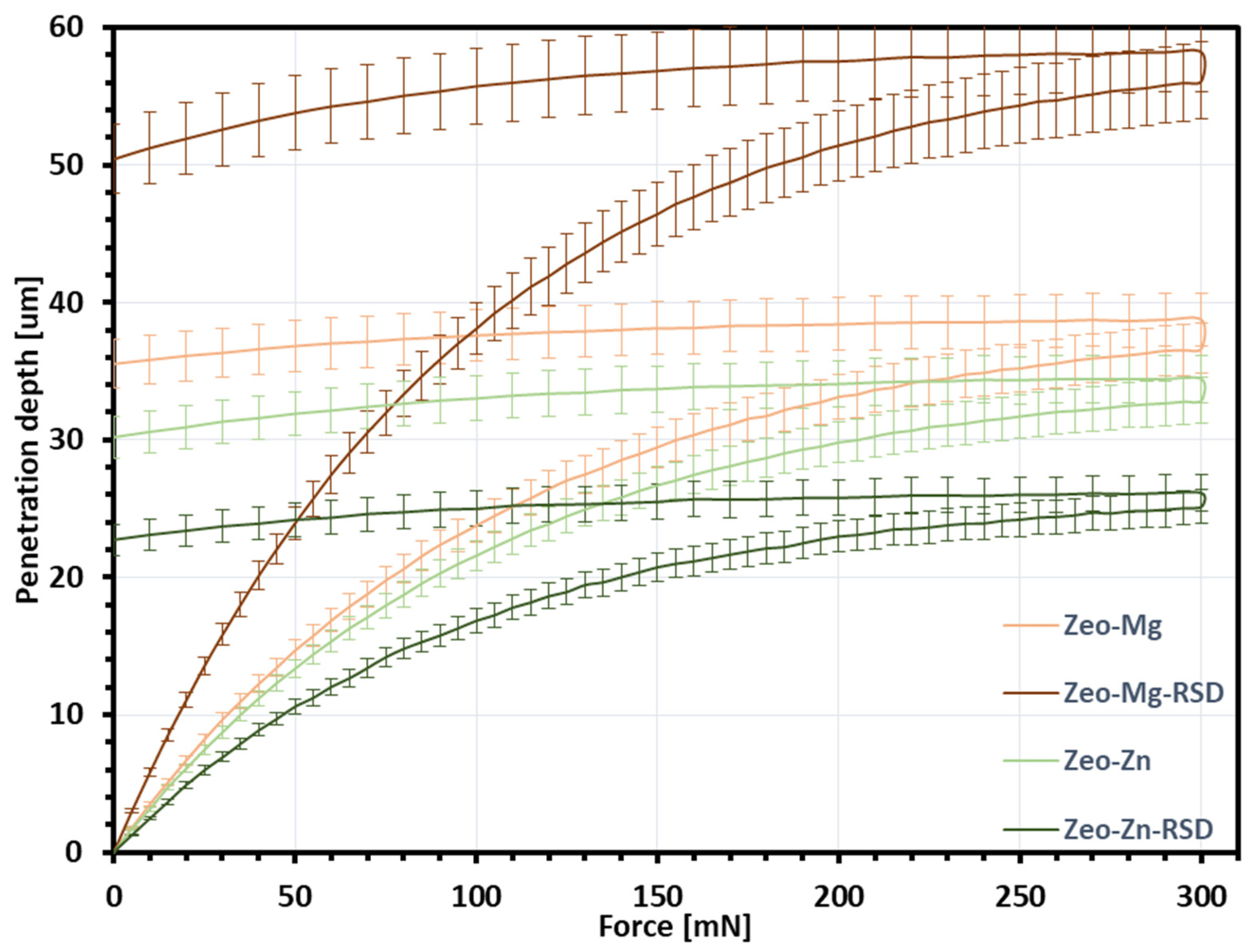
2.4.5. Nanoindentation
2.5. Risedronate Loading and Release Experiments
2.5.1. Drug Sorption
- Ti-Zeo-Mg-RSD;
- Ti-Zeo-Zn-RSD.
2.5.2. Drug Release
3. Results and Discussion
- 3 h;
- 1 day;
- 4 days;
- 7 days.
- 47.04% of the drug was released from the surface modified with magnesium zeolite;
- 24.58% of the drug was released from the surface was modified with zinc zeolite.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sozen, T.; Ozisik, L.; Basaran, N.C. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.; Varacallo, M. Osteoporosis. StatPearls Publishing, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441901/ (accessed on 11 December 2022).
- Russell, R.G.G. Bisphosphonates: The first 40years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Giger, E.V.; Castagner, B.; Leroux, J.-C. Biomedical applications of bisphosphonates. J. Control. Release 2013, 167, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Gałęzowska, J. Interactions between Clinically Used Bisphosphonates and Bone Mineral: From Coordination Chemistry to Biomedical Applications and Beyond. Chemmedchem 2018, 13, 289–302. [Google Scholar] [CrossRef]
- Sandomierski, M.; Zielińska, M.; Voelkel, A. A long-term controlled release of the drug for osteoporosis from the surface of titanium implants coated with calcium zeolite. Mater. Chem. Front. 2021, 5, 5718–5725. [Google Scholar] [CrossRef]
- Sandomierski, M.; Jakubowski, M.; Ratajczak, M.; Voelkel, A. Zeolitic Imidazolate Framework-8 (ZIF-8) modified titanium alloy for controlled release of drugs for osteoporosis. Sci. Rep. 2022, 12, 9103. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Chen, L.-Y. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef] [Green Version]
- Sandomierski, M.; Jakubowski, M.; Ratajczak, M.; Voelkel, A. Drug distribution evaluation using FT-IR imaging on the surface of a titanium alloy coated with zinc titanate with potential application in the release of drugs for osteoporosis. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2022, 281, 121575. [Google Scholar] [CrossRef]
- Rack, H.; Qazi, J. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Niinomi, M. Recent research and development in titanium alloys for biomedical applications and healthcare goods. Sci. Technol. Adv. Mater. 2003, 4, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Jakubowski, M.; Voelkel, A.; Sandomierski, M. Crystalline Zeolite Layers on the Surface of Titanium Alloys in Biomedical Applications: Current Knowledge and Possible Directions of Development. Crystals 2022, 12, 1520. [Google Scholar] [CrossRef]
- Sandomierski, M.; Zielińska, M.; Voelkel, A. Calcium zeolites as intelligent carriers in controlled release of bisphosphonates. Int. J. Pharm. 2020, 578, 119117. [Google Scholar] [CrossRef] [PubMed]
- Lutzweiler, G.; Zhang, Y.; Gens, F.; Echalard, A.; Ladam, G.; Hochart, J.; Janicot, T.; Mofaddel, N.; Louis, B. Deciphering the role of faujasite-type zeolites as a cation delivery platform to sustain the functions of MC3T3-E1 pre-osteoblastic cells. Mater. Adv. 2022, 3, 8616–8628. [Google Scholar] [CrossRef]
- Jakubowski, M.; Kucinska, M.; Ratajczak, M.; Pokora, M.; Murias, M.; Voelkel, A.; Sandomierski, M. Zinc forms of faujasite zeolites as a drug delivery system for 6-mercaptopurine. Microporous Mesoporous Mater. 2022, 343, 112194. [Google Scholar] [CrossRef]
- Servatan, M.; Zarrintaj, P.; Mahmodi, G.; Kim, S.-J.; Ganjali, M.R.; Saeb, M.R.; Mozafari, M. Zeolites in drug delivery: Progress, challenges and opportunities. Drug Discov. Today 2020, 25, 642–656. [Google Scholar] [CrossRef]
- Bacakova, L.; Vandrovcova, M.; Kopova, I.; Jirka, I. Applications of zeolites in biotechnology and medicine—A review. Biomater. Sci. 2018, 6, 974–989. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, Y.; Li, X.; Guo, Z. Improving the osteointegration of Ti6Al4V by zeolite MFI coating. Biochem. Biophys. Res. Commun. 2015, 460, 151–156. [Google Scholar] [CrossRef]
- Bedi, R.S.; Beving, D.E.; Zanello, L.P.; Yan, Y. Biocompatibility of corrosion-resistant zeolite coatings for titanium alloy biomedical implants. Acta Biomater. 2009, 5, 3265–3271. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Guo, S.; Zhang, J.; Song, Y.; Dong, X.; Wang, X.; Yu, J. Antibacterial and anti-adhesive zeolite coatings on titanium alloy surface. Microporous Mesoporous Mater. 2011, 146, 216–222. [Google Scholar] [CrossRef]
- Li, D.; Li, K.; Shan, H. Improving biocompatibility of titanium alloy scaffolds by calcium incorporated silicalite-1 coatings. Inorg. Chem. Commun. 2019, 102, 61–65. [Google Scholar] [CrossRef]
- Wang, S.; Li, R.; Li, D.; Zhang, Z.-Y.; Liu, G.; Liang, H.; Qin, Y.; Yu, J.; Li, Y. Fabrication of bioactive 3D printed porous titanium implants with Sr ion-incorporated zeolite coatings for bone ingrowth. J. Mater. Chem. B 2018, 6, 3254–3261. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg alloys for orthopedic implants—A review. J. Magnes. Alloy 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Zhuo, X.; Wu, Y.; Ju, J.; Liu, H.; Jiang, J.; Hu, Z.; Bai, J.; Xue, F. Recent progress of novel biodegradable zinc alloys: From the perspective of strengthening and toughening. J. Mater. Res. Technol. 2022, 17, 244–269. [Google Scholar] [CrossRef]
- Su, Y.; Cockerill, I.; Wang, Y.; Qin, Y.-X.; Chang, L.; Zheng, Y.; Zhu, D. Zinc-Based Biomaterials for Regeneration and Therapy. Trends Biotechnol. 2019, 37, 428–441. [Google Scholar] [CrossRef] [PubMed]
- ISO 25178-6:2010; Geometrical Product Specifications (GPS)—Surface Texture: Areal—Part 6: Classification of Methods for Measuring Surface Texture. ISO: Geneva, Switzerland, 2010. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/04/28/42896.html (accessed on 15 September 2022).
- Newton, L.; Senin, N.; Gomez, C.; Danzl, R.; Helmli, F.; Blunt, L.; Leach, R. Areal topography measurement of metal additive surfaces using focus variation microscopy. Addit. Manuf. 2018, 25, 365–389. [Google Scholar] [CrossRef]
- Fleck, N.; Muller, G.; Ashby, M.; Hutchinson, J. Strain gradient plasticity: Theory and experiment. Acta Met. Mater. 1994, 42, 475–487. [Google Scholar] [CrossRef]
- Nix, W.D.; Gao, H. Indentation size effects in crystalline materials: A law for strain gradient plasticity. J. Mech. Phys. Solids 1998, 46, 411–425. [Google Scholar] [CrossRef]
- Quarrell, A.G. The Hardness of Metals. Nature 1952, 170, 818. [Google Scholar] [CrossRef]
- Chu, L.; Jiang, G.; Hu, X.-L.; James, T.D.; He, X.-P.; Li, Y.; Tang, T. Osteogenesis, vascularization and osseointegration of a bioactive multiphase macroporous scaffold in the treatment of large bone defects. J. Mater. Chem. B 2018, 6, 4197–4204. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.-R.; Kim, M.-S.; Goh, T.S.; Lee, J.S.; Kim, Y.H.; Yoon, S.-Y.; Lee, C.-S. Evaluation of Structural and Mechanical Properties of Porous Artificial Bone Scaffolds Fabricated via Advanced TBA-Based Freeze-Gel Casting Technique. Appl. Sci. 2019, 9, 1965. [Google Scholar] [CrossRef] [Green Version]
- Errassifi, F.; Sarda, S.; Barroug, A.; Legrouri, A.; Sfihi, H.; Rey, C. Infrared, Raman and NMR investigations of risedronate adsorption on nanocrystalline apatites. J. Colloid Interface Sci. 2014, 420, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Sandomierski, M.; Jakubowski, M.; Ratajczak, M.; Pokora, M.; Zielińska, M.; Voelkel, A. Release of drugs used in the treatment of osteoporosis from zeolites with divalent ions—Influence of the type of ion and drug on the release profile. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022. [Google Scholar] [CrossRef] [PubMed]
- Forte, L.; Sarda, S.; Combes, C.; Brouillet, F.; Gazzano, M.; Marsan, O.; Boanini, E.; Bigi, A. Hydroxyapatite functionalization to trigger adsorption and release of risedronate. Colloids Surf. B Biointerfaces 2017, 160, 493–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Houdt, C.I.A.; Gabbai-Armelin, P.R.; Lopez-Perez, P.M.; Ulrich, D.J.O.; Jansen, J.A.; Renno, A.C.M.; Beucken, J.J.J.P.V.D. Alendronate release from calcium phosphate cement for bone regeneration in osteoporotic conditions. Sci. Rep. 2018, 8, 15398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damiati, L.; Eales, M.G.; Nobbs, A.H.; Su, B.; Tsimbouri, P.M.; Salmeron-Sanchez, M.; Dalby, M.J. Impact of surface topography and coating on osteogenesis and bacterial attachment on titanium implants. J. Tissue Eng. 2018, 9, 492–495. [Google Scholar] [CrossRef]
- Bollenl, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Larsson, C.; Thomsen, P.; Lausmaa, J.; Rodahl, M.; Kasemo, B.; Ericson, L. Bone response to surface modified titanium implants: Studies on electropolished implants with different oxide thicknesses and morphology. Biomaterials 1994, 15, 1062–1074. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-L.; He, F.-M.; Yang, X.-F.; Wang, X.-X.; Zhao, S.-F. Bone responses to titanium implants surface-roughened by sandblasted and double etched treatments in a rabbit model. Oral Sur. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, 516–524. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Suggested guidelines for the topographic evaluation of implant surfaces. Int. J. Oral Maxillofac. Implant. 2000, 15, 331–344. [Google Scholar]
- Demirci, S.; Ustaoğlu, Z.; Yılmazer, G.A.; Şahin, F.; Bac, N. Antimicrobial Properties of Zeolite-X and Zeolite-A Ion-Exchanged with Silver, Copper, and Zinc Against a Broad Range of Microorganisms. Appl. Biochem. Biotechnol. 2013, 172, 1652–1662. [Google Scholar] [CrossRef]
- Ceylan, M.N.; Akdas, S.; Yazihan, N. Is Zinc an Important Trace Element on Bone-Related Diseases and Complications? A Meta-analysis and Systematic Review from Serum Level, Dietary Intake, and Supplementation Aspects. Biol. Trace Element Res. 2020, 199, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Avci, M.; Yilmaz, B.; Tezcaner, A.; Evis, Z. Strontium doped hydroxyapatite biomimetic coatings on Ti6Al4V plates. Ceram. Int. 2017, 43, 9431–9436. [Google Scholar] [CrossRef]
- Christoffersen, J.; Christoffersen, M.; Kolthoff, N.; Bärenholdt, O. Effects of strontium ions on growth and dissolution of hydroxyapatite and on bone mineral detection. Bone 1997, 20, 47–54. [Google Scholar] [CrossRef] [PubMed]

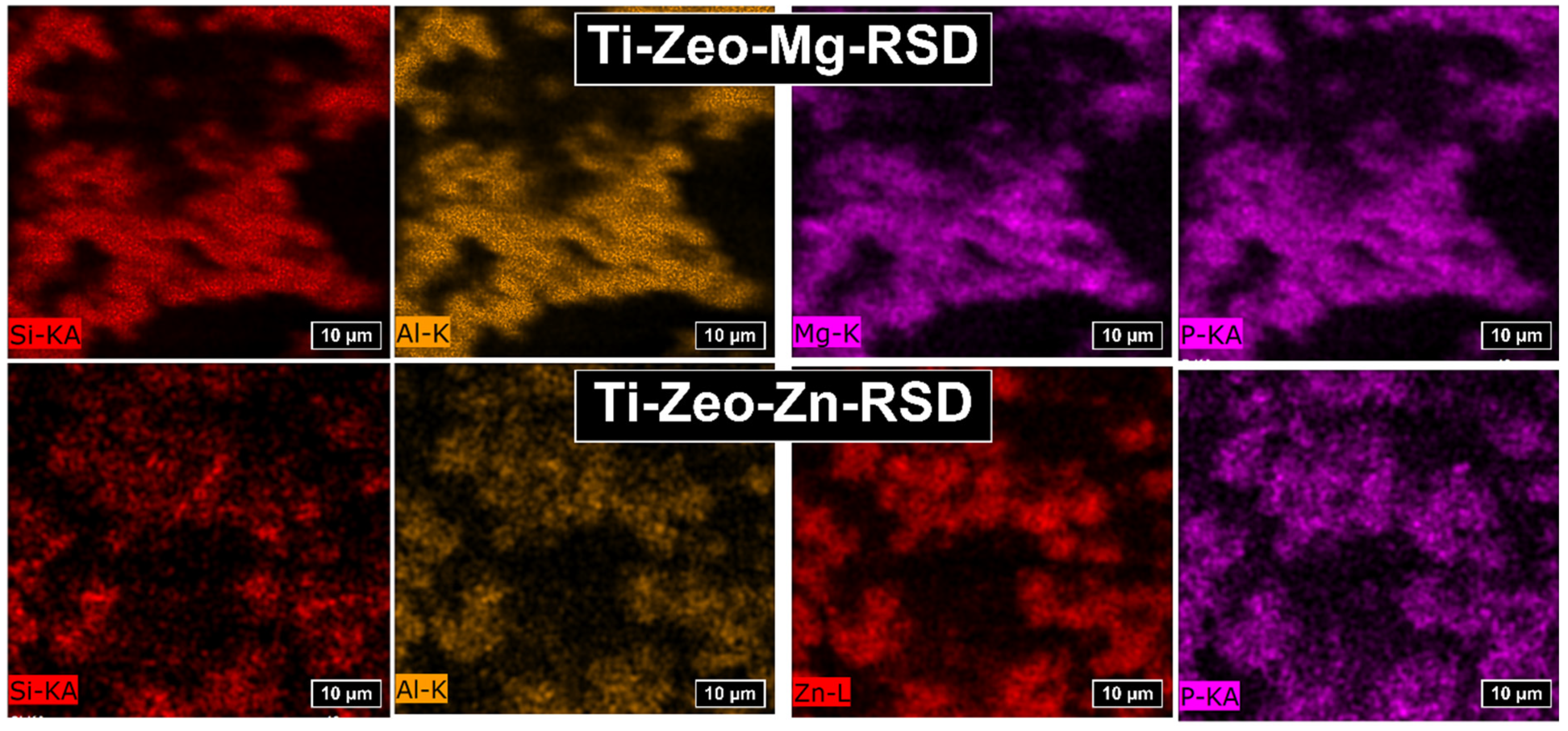


| Ti-Zeo-Mg-RSD | Ti-Zeo-Zn-RSD | |
|---|---|---|
| Mg | 0.66 ± 0.24 | - |
| Zn | - | 31.67 ± 5.99 |
| P | 1.81 ± 1.10 | 6.14 ± 2.04 |
| N | - | 1.75 ± 1.60 |
| Al | 11.00 ± 5.88 | 6.16 ± 1.69 |
| Si | 12.44 ± 6.62 | 3.04 ± 1.90 |
| Coating Thickness (μm) | Profile and Surface Topography Parameters | Indentation Depth (μm) | Estimated Coating Strength (MPa) | ||||
|---|---|---|---|---|---|---|---|
| Pa (μm) | Pq (μm) | Sa (μm) | Sq (μm) | ||||
| Ti-Zeo-Mg | 37.7 | 6.46 | 8.26 | 4.42 | 5.80 | 37.0 | 9.04 |
| Ti-Zeo-Mg-RSD | 58.5 | 4.95 | 5.05 | 2.85 | 3.99 | 55.9 | 3.95 |
| Ti-Zeo-Zn | 40.4 | 5.25 | 6.80 | 3.90 | 5.08 | 33.3 | 12.09 |
| Ti-Zeo-Zn-RSD | 34.9 | 4.51 | 5.76 | 2.98 | 3.96 | 25.0 | 21.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandomierski, M.; Stachowicz, W.; Patalas, A.; Grochalski, K.; Graboń, W.; Voelkel, A. Characterization of Magnesium and Zinc Forms of Sodalite Coatings on Ti6Al4V ELI for Potential Application in the Release of Drugs for Osteoporosis. Materials 2023, 16, 1710. https://doi.org/10.3390/ma16041710
Sandomierski M, Stachowicz W, Patalas A, Grochalski K, Graboń W, Voelkel A. Characterization of Magnesium and Zinc Forms of Sodalite Coatings on Ti6Al4V ELI for Potential Application in the Release of Drugs for Osteoporosis. Materials. 2023; 16(4):1710. https://doi.org/10.3390/ma16041710
Chicago/Turabian StyleSandomierski, Mariusz, Wiktoria Stachowicz, Adam Patalas, Karol Grochalski, Wiesław Graboń, and Adam Voelkel. 2023. "Characterization of Magnesium and Zinc Forms of Sodalite Coatings on Ti6Al4V ELI for Potential Application in the Release of Drugs for Osteoporosis" Materials 16, no. 4: 1710. https://doi.org/10.3390/ma16041710






