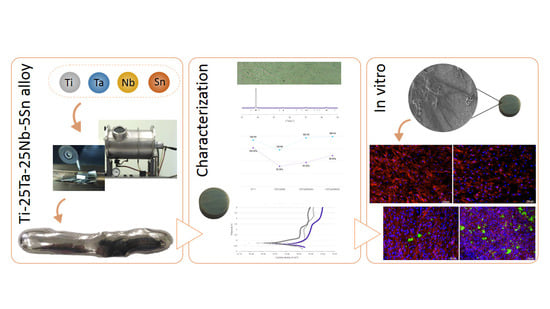
Mechanical Properties, Corrosion Behavior, and In Vitro Cell Studies of the New Ti-25Ta-25Nb-5Sn Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ti-25Ta-25Nb-5Sn Alloy Preparation
2.2. Microstructural and Mechanical Characterization
2.3. Electrochemical Measurements
2.4. In Vitro Studies
3. Results and Discussion
3.1. Alloy Characterization
3.2. Mechanical Properties
3.3. Corrosion Behavior
3.4. In Vitro Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Aherwar, A.; Kumar Singh, A.; Patnaik, A. Cobalt Based Alloy: A Better Choice Biomaterial for Hip Implants. Trends Biomater. Artif. Organs 2016, 30, 50–55. Available online: https://www.researchgate.net/profile/Amit-Aherwar/publication/320452685_Cobalt_based_alloy_A_better_choice_biomaterial_for_hip_implants/links/5b30ce270f7e9b0df5c754e5/Cobalt-based-alloy-A-better-choice-biomaterial-for-hip-implants.pdf (accessed on 19 January 2023).
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0079642508001126 (accessed on 19 January 2023). [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0928493118338232 (accessed on 19 January 2023). [CrossRef] [PubMed]
- Wang, K. The use of titanium for medical applications in the, U.S.A. Mater. Sci. Eng. A 1996, 213, 134–137. Available online: https://linkinghub.elsevier.com/retrieve/pii/0921509396102434 (accessed on 19 January 2023). [CrossRef]
- Walker, P.R.; LeBlanc, J.; Sikorska, M. Effects of aluminum and other cations on the structure of brain and liver chromatin. Biochemistry 1989, 28, 3911–3915. Available online: https://pubs.acs.org/doi/abs/10.1021/bi00435a043 (accessed on 19 January 2023). [CrossRef]
- Rack, H.J.; Qazi, J.I. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0928493105002237 (accessed on 19 January 2023).
- Carobolante, J.P.A.; da Silva, K.B.; Chaves, J.A.M.; Dias Netipanyj, M.F.; Popat, K.C.; Alves Claro, A.P.R. Nanoporous layer formation on the Ti10Mo8Nb alloy surface using anodic oxidation. Surf. Coat. Technol. 2020, 386, 125467. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0257897220301365 (accessed on 19 January 2023). [CrossRef]
- Ferrandini, P.L.; Cardoso, F.F.; Souza, S.A.; Afonso, C.R.; Caram, R. Aging response of the Ti–35Nb–7Zr–5Ta and Ti–35Nb–7Ta alloys. J. Alloy. Compd. 2007, 433, 207–210. Available online: https://linkinghub.elsevier.com/retrieve/pii/S092583880600819X (accessed on 19 January 2023). [CrossRef]
- Geetha, M.; Kamachi Mudali, U.; Gogia, A.K.; Asokamani, R.; Raj, B. Influence of microstructure and alloying elements on corrosion behavior of Ti–13Nb–13Zr alloy. Corros. Sci. 2004, 46, 877–892. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0010938X03001860 (accessed on 19 January 2023). [CrossRef]
- Seixas, M.R.; Bortolini, C., Jr.; Konatu, R.T.; Pereira, A., Jr.; Rosifini Alves Claro, A.P. Mechanical and Microstructural Characterization of the Ti-25Ta-25Nb Alloy for Dental Applications. Mater. Sci. Forum 2016, 869, 935–939. Available online: https://www.scientific.net/MSF.869.935 (accessed on 19 January 2023).
- Bertrand, E.; Gloriant, T.; Gordin, D.M.; Vasilescu, E.; Drob, P.; Vasilescu, C.; Drob, S.I. Synthesis and characterisation of a new superelastic Ti–25Ta–25Nb biomedical alloy. J. Mech. Behav. Biomed. Mater. 2010, 3, 559–564. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1751616110000834 (accessed on 19 January 2023). [CrossRef]
- Watanabe, I.; Topham, D.S. Tensile strength and elongation of laser-welded Ti and Ti-6AL-7NB. J. Biomed. Mater. Res. 2004, 71B, 46–51. Available online: https://onlinelibrary.wiley.com/doi/10.1002/jbm.b.30058 (accessed on 19 January 2023). [CrossRef] [PubMed]
- Johansson, C.B.; Albrektsson, T. A removal torque and histomorphometric study of commercially pure niobium and titanium implants in rabbit bone. Clin. Oral Implant. Res. 1991, 2, 24–29. Available online: http://doi.wiley.com/10.1034/j.1600-0501.1991.020103.x (accessed on 19 January 2023). [CrossRef] [PubMed]
- Seixas, M.R.; Bortolini, C.; Pereira, A.; Nakazato, R.Z.; Popat, K.C.; Alves Claro, A.P.R. Development of a new quaternary alloy Ti–25Ta–25Nb–3Sn for biomedical applications. Mater. Res. Express 2018, 5, 025402. Available online: https://iopscience.iop.org/article/10.1088/2053-1591/aa87c8 (accessed on 19 January 2023). [CrossRef] [Green Version]
- Li, S.J.; Cui, T.C.; Hao, Y.L.; Yang, R. Fatigue properties of a metastable β-type titanium alloy with reversible phase transformation. Acta Biomater. 2008, 4, 305–317. Available online: https://linkinghub.elsevier.com/retrieve/pii/S174270610700164X (accessed on 19 January 2023). [CrossRef]
- Zhang, D.C.; Yang, S.; Wei, M.; Mao, Y.F.; Tan, C.G.; Lin, J.G. Effect of Sn addition on the microstructure and superelasticity in Ti–Nb–Mo–Sn Alloys. J. Mech. Behav. Biomed. Mater. 2012, 13, 156–165. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1751616112001385 (accessed on 19 January 2023). [CrossRef]
- Ma, B.; Ju, D.; Liu, Q. Design, Simulation and Performance Research of New Biomaterial Mg30Zn30Sn30Sr5Bi5. Coatings 2022, 12, 531. [Google Scholar] [CrossRef]
- da Silva Dias, C.; Rossi, M.C.; Apolonio, E.V.; dos Santos Rosa, G.; Pfeifer, J.P.; Hussni, C.A.; Watanabe, M.J.; Alves, A.L. Low Mg content on Ti-Nb-Sn alloy when in contact with eBMMSCs promotes improvement of its biological functions. J. Mater. Sci. Mater. Med. Dez 2021, 32, 144. [Google Scholar] [CrossRef]
- Çakmak, Ö.; Kaya, M. Effect of sintering procedure on microstructure and mechanical properties of biomedical TiNbSn alloy produced via powder metallurgy. Appl. Phys. A 2021, 127, 561. [Google Scholar] [CrossRef]
- Khrunyk, Y.Y.; Ehnert, S.; Grib, S.V.; Illarionov, A.G.; Stepanov, S.I.; Popov, A.A.; Ryzhkov, M.A.; Belikov, S.V.; Xu, Z.; Rupp, F.; et al. Synthesis and Characterization of a Novel Biocompatible Alloy, Ti-Nb-Zr-Ta-Sn. Int. J. Mol. Sci. 2021, 22, 10611. [Google Scholar] [CrossRef]
- Sabino, R.M.; Mondini, G.; Kipper, M.J.; Martins, A.F.; Popat, K.C. Tanfloc/heparin polyelectrolyte multilayers improve osteogenic differentiation of adipose-derived stem cells on titania nanotube surfaces. Carbohydr. Polym. 2021, 251, 117079. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0144861720312522 (accessed on 19 January 2023). [CrossRef]
- Correa, D.R.N.; Vicente, F.B.; Donato, T.A.G.; Arana-Chavez, V.E.; Buzalaf, M.A.R.; Grandini, C.R. The effect of the solute on the structure, selected mechanical properties, and biocompatibility of Ti–Zr system alloys for dental applications. Mater. Sci. Eng. C 2014, 34, 354–359. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0928493113005389 (accessed on 19 January 2023). [CrossRef]
- Banumathy, S.; Mandal, R.K.; Singh, A.K. Structure of orthorhombic martensitic phase in binary Ti–Nb alloys. J. Appl. Phys. 2009, 106, 093518. Available online: http://aip.scitation.org/doi/10.1063/1.3255966 (accessed on 19 January 2023). [CrossRef]
- Bania, P.J. Beta titanium alloys and their role in the titanium industry. JOM 1994, 46, 16–19. Available online: http://link.springer.com/10.1007/BF03220742 (accessed on 19 January 2023). [CrossRef]
- Weiss, I.; Semiatin, S.L. Thermomechanical processing of alpha titanium alloys—An overview. Mater. Sci. Eng. A 1999, 263, 243–256. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0921509398011551 (accessed on 19 January 2023). [CrossRef]
- Severino Martins, J.R.; Grandini, C.R. Structural characterization of Ti-15Mo alloy used as biomaterial by Rietveld method. J. Appl. Phys. 2012, 111, 083535. Available online: http://aip.scitation.org/doi/10.1063/1.4707920 (accessed on 19 January 2023). [CrossRef] [Green Version]
- Zhang, L.; Chen, L. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1801215. Available online: https://onlinelibrary.wiley.com/doi/10.1002/adem.201801215 (accessed on 19 January 2023). [CrossRef] [Green Version]
- Wei, T.Y.; Huang, J.C.; Chao, C.-Y.; Wei, L.L.; Tsai, M.T.; Chen, Y.H. Microstructure and elastic modulus evolution of TiTaNb alloys. J. Mech. Behav. Biomed. Mater. 2018, 86, 224–231. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1751616118309627 (accessed on 19 January 2023). [CrossRef]
- da Silva, K.B.; Konatu, R.T.; de Oliveira, L.L.; Nakazato, R.Z.; Claro, A.P.R.A. Corrosion Resistance After Mechanical Deformation of the Ti30Ta Experimental Alloy for Using in Biomedical Applications. Mater. Res. 2017, 20, 1402–1405. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1516-14392017000501402&lng=en&tlng=en (accessed on 19 January 2023). [CrossRef] [Green Version]
- Al-Timimi, Z.; Tammemi, Z.J. Polymer Blends and Nanocomposite Materials Based on Polymethyl Methacrylate (PMMA) for Bone Regeneration and Repair. J. Sustain. Mater. Process. Manag. 2022, 2. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chuang, W.; Huang, J.; Wang, X.; Chou, H.; Lai, Y.; Lin, P. On the bio-corrosion and biocompatibility of TiTaNb medium entropy alloy films. Appl. Surf. Sci. 2020, 508, 145307. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0169433220300635 (accessed on 19 January 2023). [CrossRef]
- Tito Patricio, M.A.; Lustosa, C.J.R.; Chaves, J.A.M.; Marques, P.W.B.; Silva, P.S.; Almeida, A.; Vilar, R.; Florêncio, O. Relationship between microstructure, phase transformation, and mechanical behavior in Ti–40Ta alloys for biomedical applications. J. Mater. Res. Technol. 2021, 14, 210–219. Available online: https://linkinghub.elsevier.com/retrieve/pii/S2238785421005937 (accessed on 19 January 2023). [CrossRef]
- Capellato, P.; Vilela, F.B.; Fontenele, A.H.P.; Silva, G.; da Silva, K.B.; Carobolante, J.P.A.; Bejarano, E.G.M.; de Lourdes Noronha Motta Melo, M.; Claro, A.P.R.A.; Sachs, D. Evaluation of Microstructure and Mechanical Properties of a Ti10Mo8Nb Alloy for Biomedical Applications. Metals 2022, 12, 1065. [Google Scholar] [CrossRef]
- Weng, W.; Biesiekierski, A.; Li, Y.; Wen, C. Effects of selected metallic and interstitial elements on the microstructure and mechanical properties of beta titanium alloys for orthopedic applications. Materialia 2019, 6, 100323. Available online: https://linkinghub.elsevier.com/retrieve/pii/S258915291930119X (accessed on 19 January 2023). [CrossRef]
- Bahl, S.; Suwas, S.; Chatterjee, K. Comprehensive review on alloy design, processing, and performance of β Titanium alloys as biomedical materials. Int. Mater. Rev. 2021, 66, 114–139. Available online: https://www.tandfonline.com/doi/full/10.1080/09506608.2020.1735829 (accessed on 19 January 2023). [CrossRef]
- Bahl, S.; Krishnamurthy, A.S.; Suwas, S.; Chatterjee, K. Controlled nanoscale precipitation to enhance the mechanical and biological performances of a metastable β Ti-Nb-Sn alloy for orthopedic applications. Mater. Des. 2017, 126, 226–237. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0264127517303702 (accessed on 19 January 2022). [CrossRef]
- Bortolini Junior, C.; Pereira Junior, A.; Seixas, M.R.; Alves Claro, A.R. Mechanical Characterization of the Ti25Ta25Nb3Sn Experimental Quaternary Alloy for Biomedical Applications. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:52059646 (accessed on 22 March 2022).
- Chen, L.Y.; Cui, Y.W.; Zhang, L.C. Recent Development in Beta Titanium Alloys for Biomedical Applications. Metals 2020, 10, 1139. [Google Scholar] [CrossRef]
- Erdogan, A.; Döleker, K.M.; Zeytin, S. Effect of laser re-melting on electric current assistive sintered CoCrFeNiAlxTiy high entropy alloys: Formation, micro-hardness and wear behaviors. Surface and Coatings Technology 2020, 399, 126179. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0257897220308483 (accessed on 19 January 2023). [CrossRef]
- Dias-Netipanyj, M.F.; Cowden, K.; Sopchenski, L.; Cogo, S.C.; Elifio-Esposito, S.; Popat, K.C.; Soares, P. Effect of crystalline phases of titania nanotube arrays on adipose derived stem cell adhesion and proliferation. Mater. Sci. Eng. C 2019, 103, 109850. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0928493117347446 (accessed on 19 January 2023). [CrossRef]
- Carobolante, J.P.A.; Pereira Júnior, A.; Bortolini Junior, C.; Barboza da Silva, K.; Sabino, R.M.; Popat, K.C.; Claro, A.P.R.A. Processing and Characterization of a New Quaternary Alloy Ti10Mo8Nb6Zr for Potential Biomedical Applications. Materials 2022, 15, 8636. [Google Scholar] [CrossRef]
- Miura, K.; Yamada, N.; Hanada, S.; Jung, T.-K.; Itoi, E. The bone tissue compatibility of a new Ti–Nb–Sn alloy with a low Young’s modulus. Acta Biomater. 2011, 7, 2320–2326. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1742706111000675 (accessed on 19 January 2023).
- Li, Y.; Xiong, J.; Wong, C.S.; Hodgson, P.D.; Wen, C. Ti6Ta4Sn Alloy and Subsequent Scaffolding for Bone Tissue Engineering. Tissue Eng. Part A 2009, 15, 3151–3159. Available online: https://www.liebertpub.com/doi/10.1089/ten.tea.2009.0150 (accessed on 19 January 2023). [CrossRef] [PubMed] [Green Version]
- Rangel, A.L.R.; Falentin-Daudré, C.; da Silva Pimentel, B.N.A.; Vergani, C.E.; Migonney, V.; Alves Claro, A.P.R. Nanostructured titanium alloy surfaces for enhanced osteoblast response: A combination of morphology and chemistry. Surf. Coat. Technol. 2020, 383, 125226. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0257897219312162 (accessed on 19 January 2023). [CrossRef]
- Park, J.-W.; Kim, H.-K.; Kim, Y.-J.; Jang, J.-H.; Song, H.; Hanawa, T. Osteoblast response and osseointegration of a Ti–6Al–4V alloy implant incorporating strontium. Acta Biomater. 2010, 6, 2843–2851. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1742706110000280 (accessed on 19 January 2023). [CrossRef] [PubMed]






| Ti25Ta25Nb5Sn | Rwp | GOF | RBragg | Phase | Content (wt%) | a (Å) | c (Å) | Volume (Å3) |
|---|---|---|---|---|---|---|---|---|
| As-cast | 9.92 | 2.46 | 4.959 | Alpha | 0.060 | 2.921 | 4.433 | 32.761 |
| 0.579 | Beta | 99.94 | 3.315 | - | 36.415 | |||
| Homogenized | 6.80 | 1.31 | 1.201 | Beta | 100.00 | 3.304 | - | 36.304 |
| Quenched | 6.04 | 1.34 | 4.635 | Alpha | 1.72 | 2.897 | 4.675 | 33.988 |
| 0.370 | Beta | 98.28 | 3.074 | - | 29.053 |
| Alloys | EOC (mV) | Ecorr (mV) | Jcorr (nA∙cm−2) | Jpass (μA∙cm−2) |
|---|---|---|---|---|
| CP Ti * | −383 | −376 | 37.40 | 28.00 |
| Ti25Ta25Nb3Sn * | −314 | −249 | 1.72 | 12.71 |
| Ti25Ta25Nb5Sn | −338 | −341 | 16.00 | 82.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, K.B.d.; Carobolante, J.P.A.; Rajan, S.S.; Júnior, C.B.; Sabino, R.M.; Seixas, M.R.; Nakazato, R.Z.; Popat, K.C.; Claro, A.P.R.A. Mechanical Properties, Corrosion Behavior, and In Vitro Cell Studies of the New Ti-25Ta-25Nb-5Sn Alloy. Materials 2023, 16, 1970. https://doi.org/10.3390/ma16051970
Silva KBd, Carobolante JPA, Rajan SS, Júnior CB, Sabino RM, Seixas MR, Nakazato RZ, Popat KC, Claro APRA. Mechanical Properties, Corrosion Behavior, and In Vitro Cell Studies of the New Ti-25Ta-25Nb-5Sn Alloy. Materials. 2023; 16(5):1970. https://doi.org/10.3390/ma16051970
Chicago/Turabian StyleSilva, Kerolene Barboza da, João Pedro Aquiles Carobolante, S. Sudhagara Rajan, Celso Bortolini Júnior, Roberta Maia Sabino, Maurício Rangel Seixas, Roberto Zenhei Nakazato, Ketul C. Popat, and Ana Paula Rosifini Alves Claro. 2023. "Mechanical Properties, Corrosion Behavior, and In Vitro Cell Studies of the New Ti-25Ta-25Nb-5Sn Alloy" Materials 16, no. 5: 1970. https://doi.org/10.3390/ma16051970