Mechanical Properties of 3D-Printed Liquid Crystalline Polymers with Low and High Melting Temperatures
Abstract
:1. Introduction
1.1. Fused Filament Fabrication with Conventional High-Performance Polymers
1.2. Fused Filament Fabrication with Liquid Crystalline Polymers (LCPs)
2. Materials and Methods
2.1. Chemical Composition of the Utilized High- and Low-Melting-Point LCPs
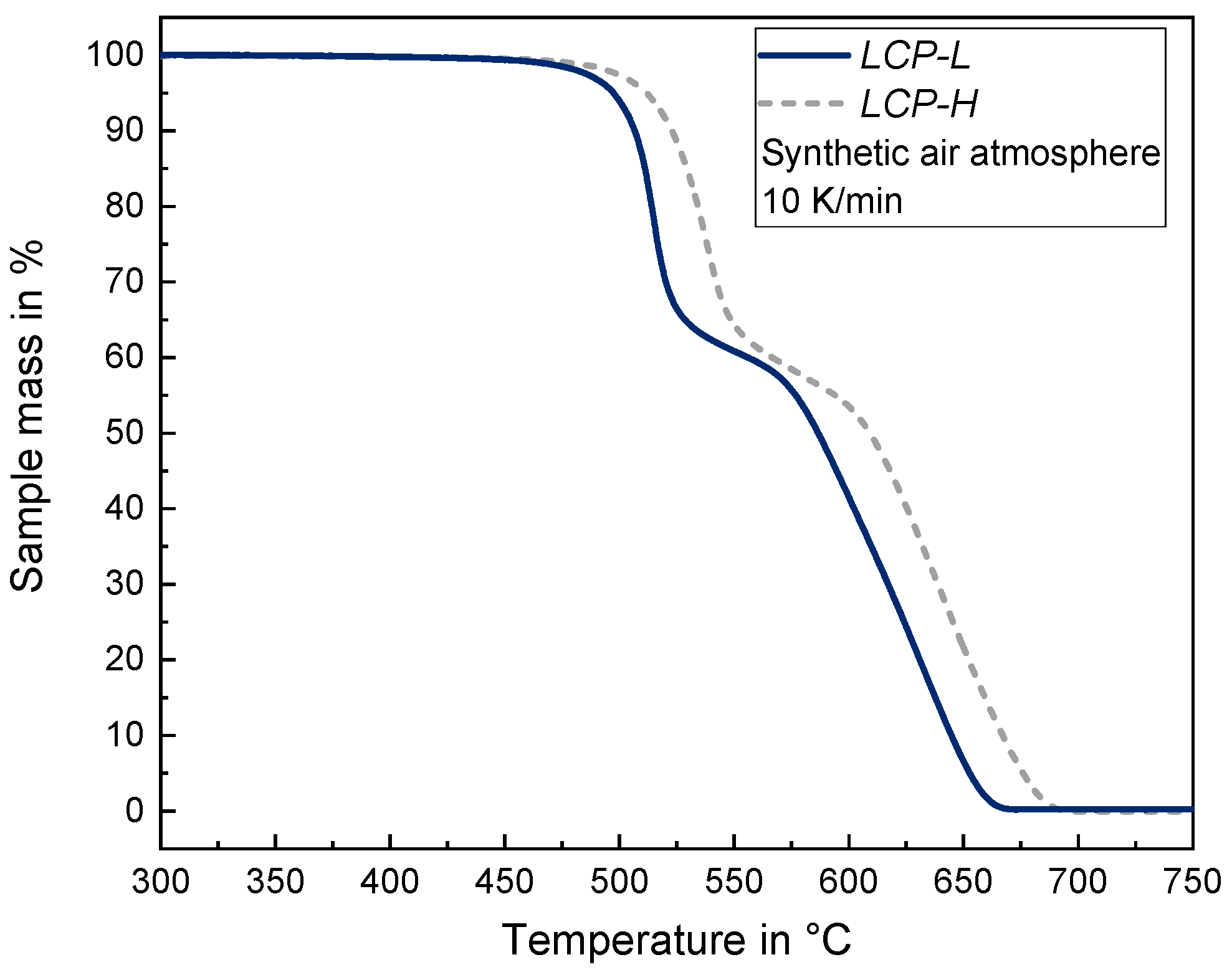
2.2. Thermal, Chemical and Mechanical Characterization Methods
2.3. Fused Filament Fabrication Procedure
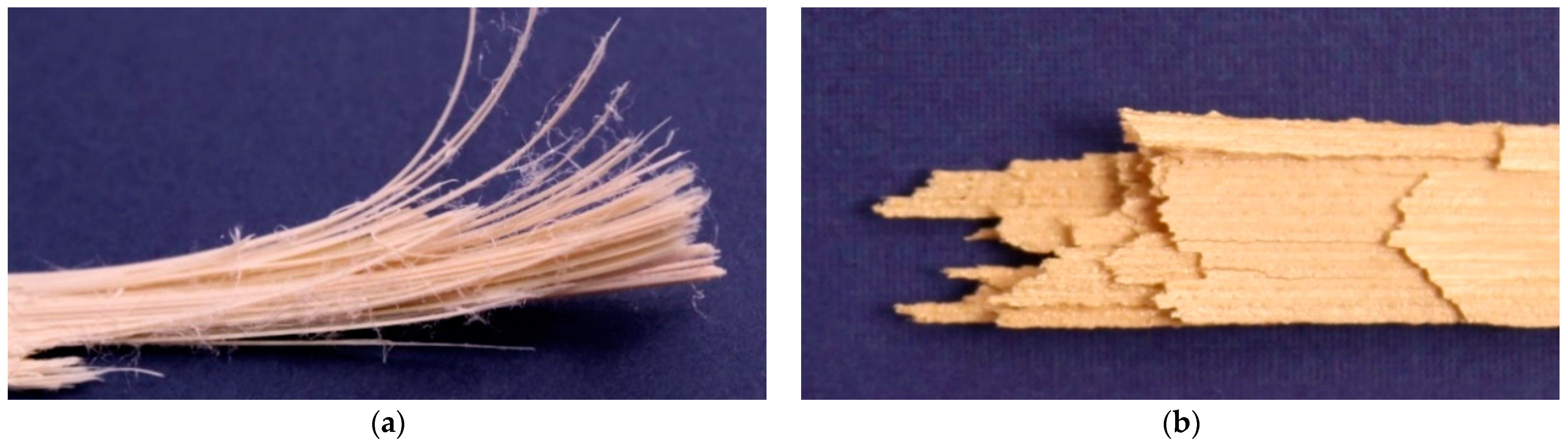
3. Results and Discussion
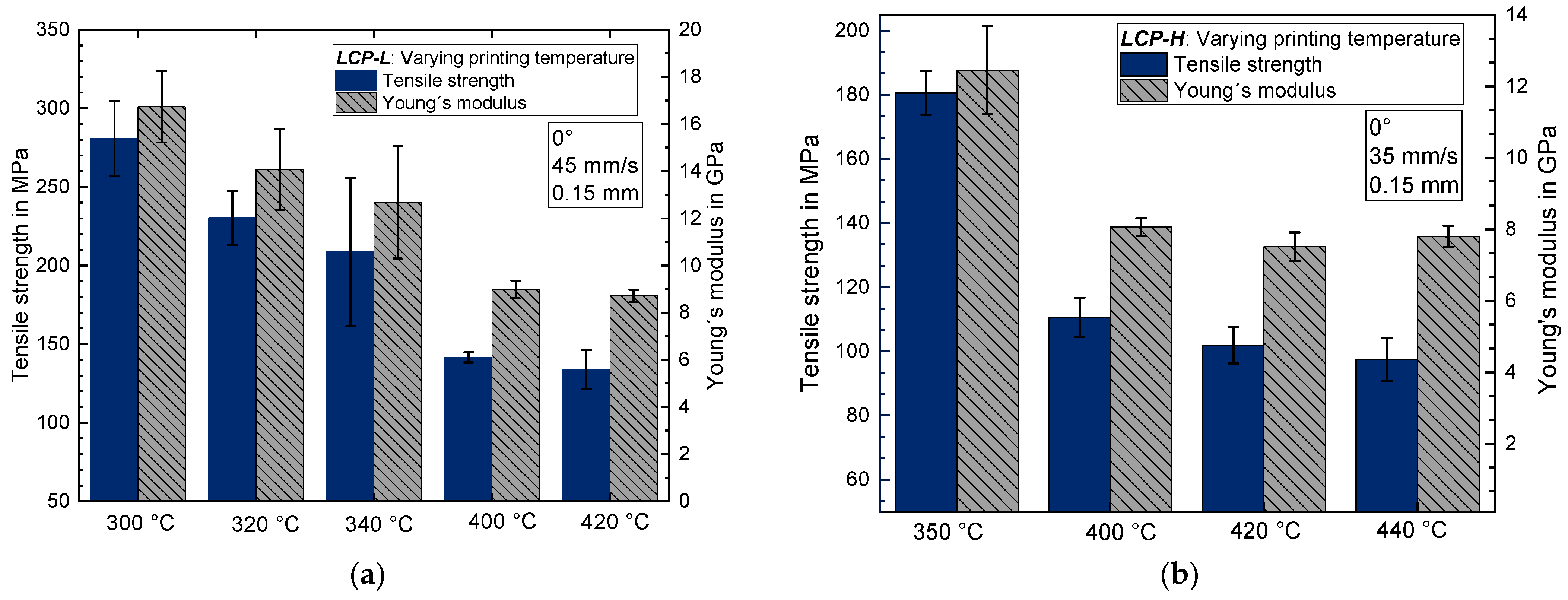
3.1. Influence of the Printing Temperature
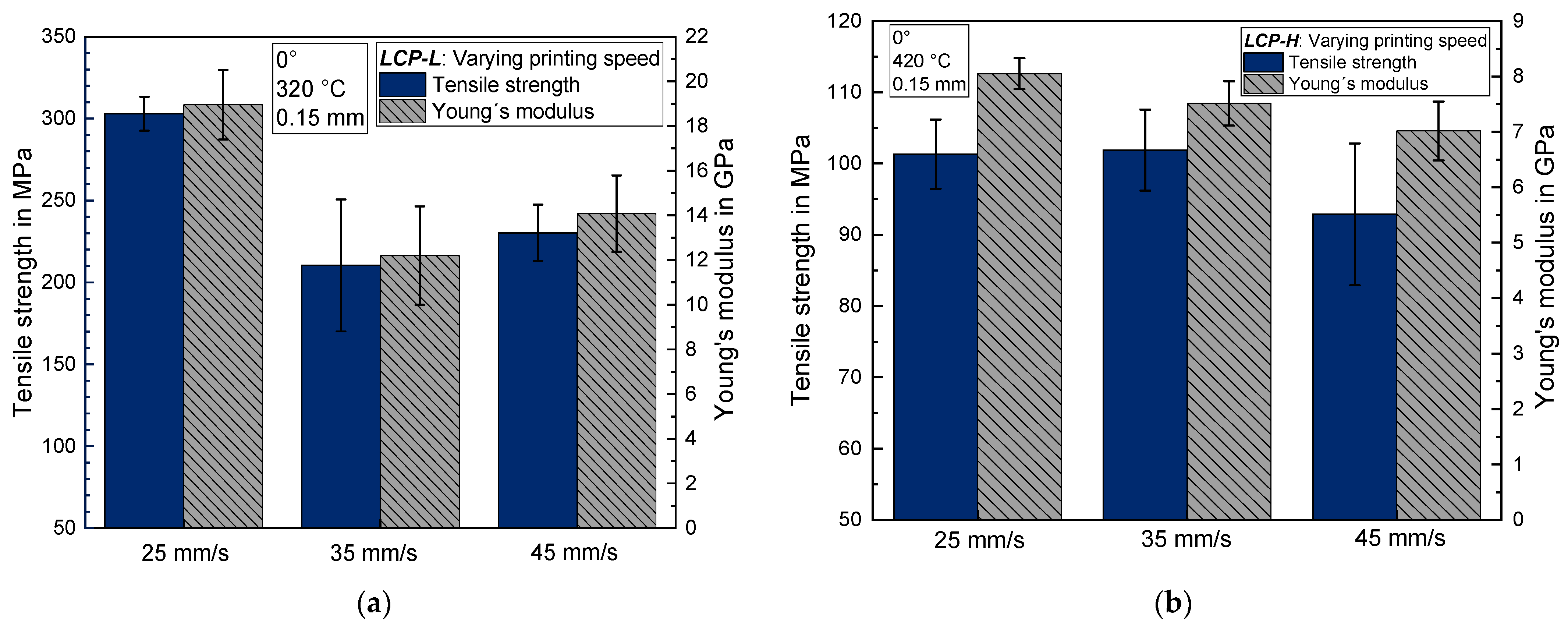
3.2. Influence of the Printing Speed
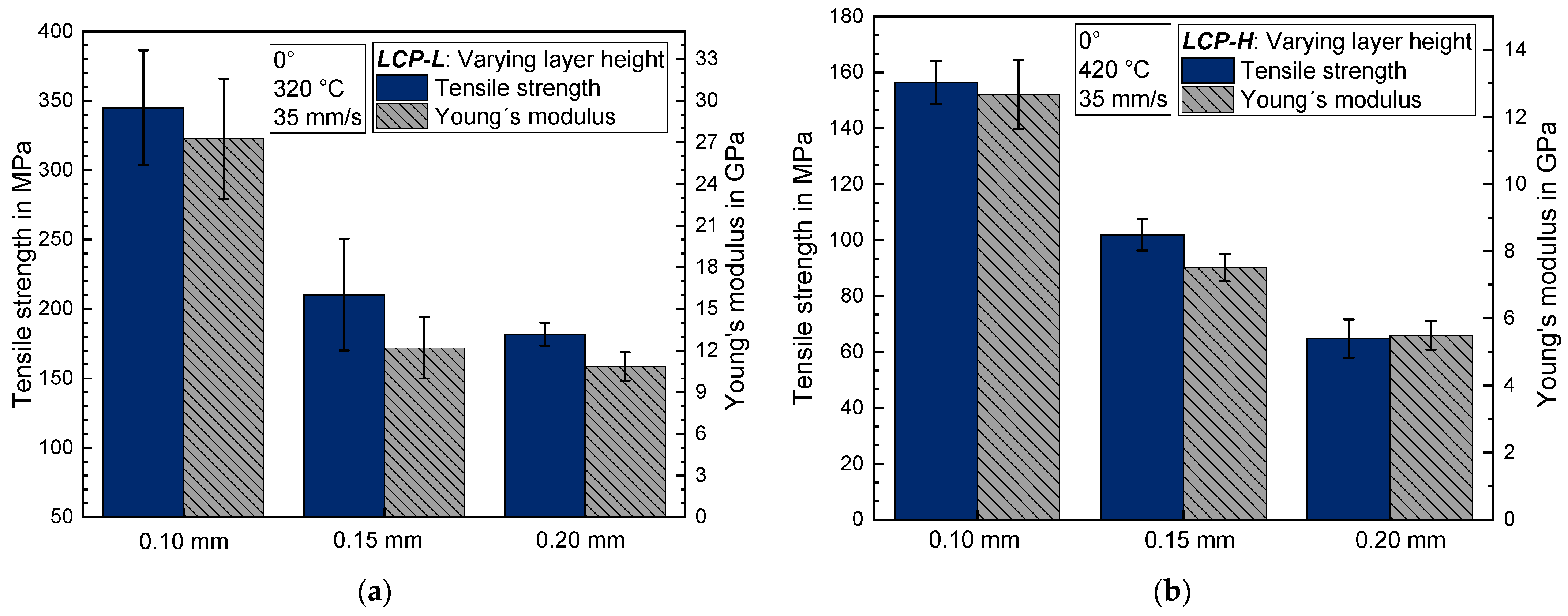
3.3. Influence of the Layer Height
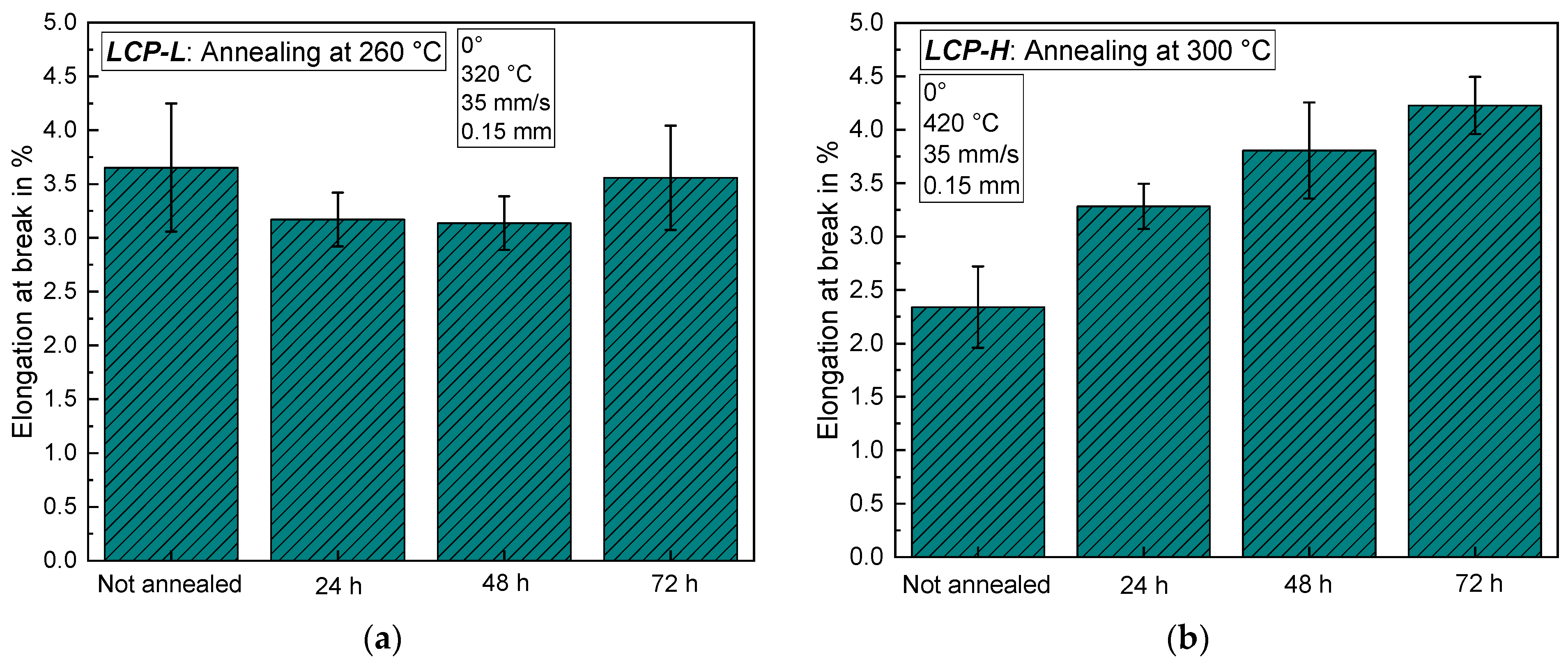
3.4. Influence of a Subsequent Annealing Step at Elevated Temperatures
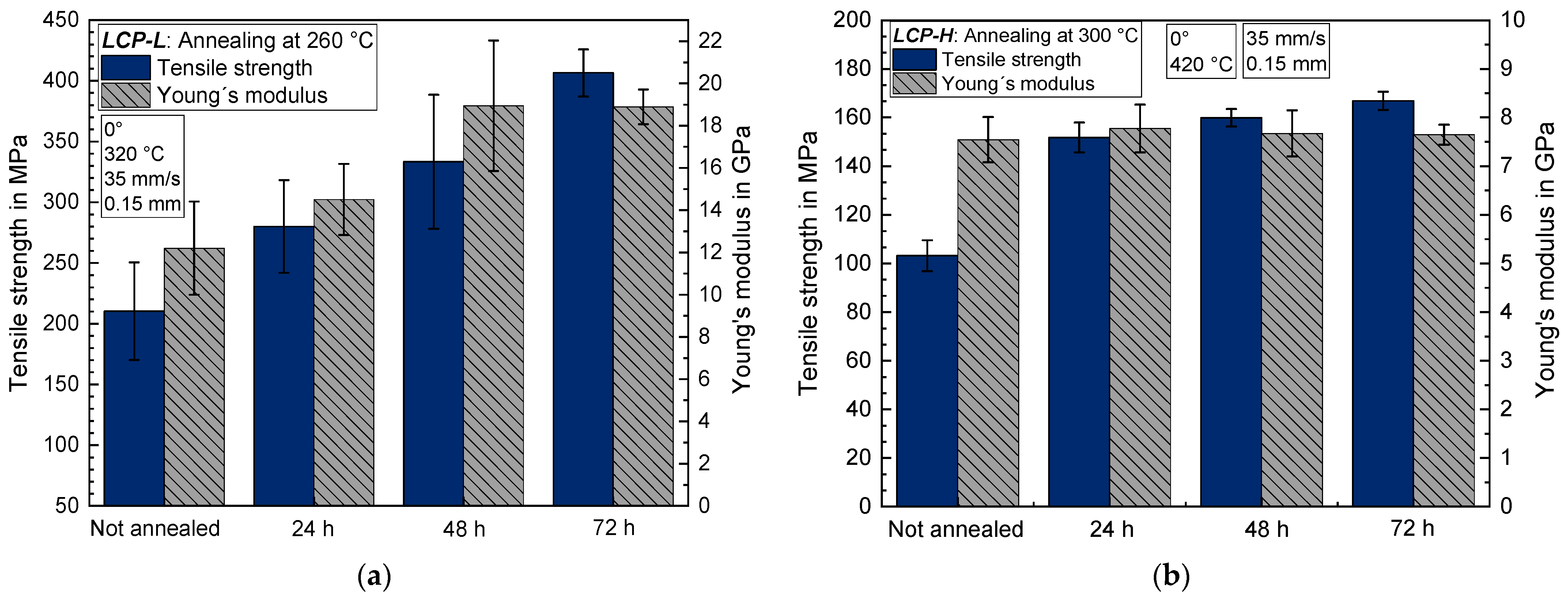
3.4.1. Mechanical Characterization of Annealed Samples
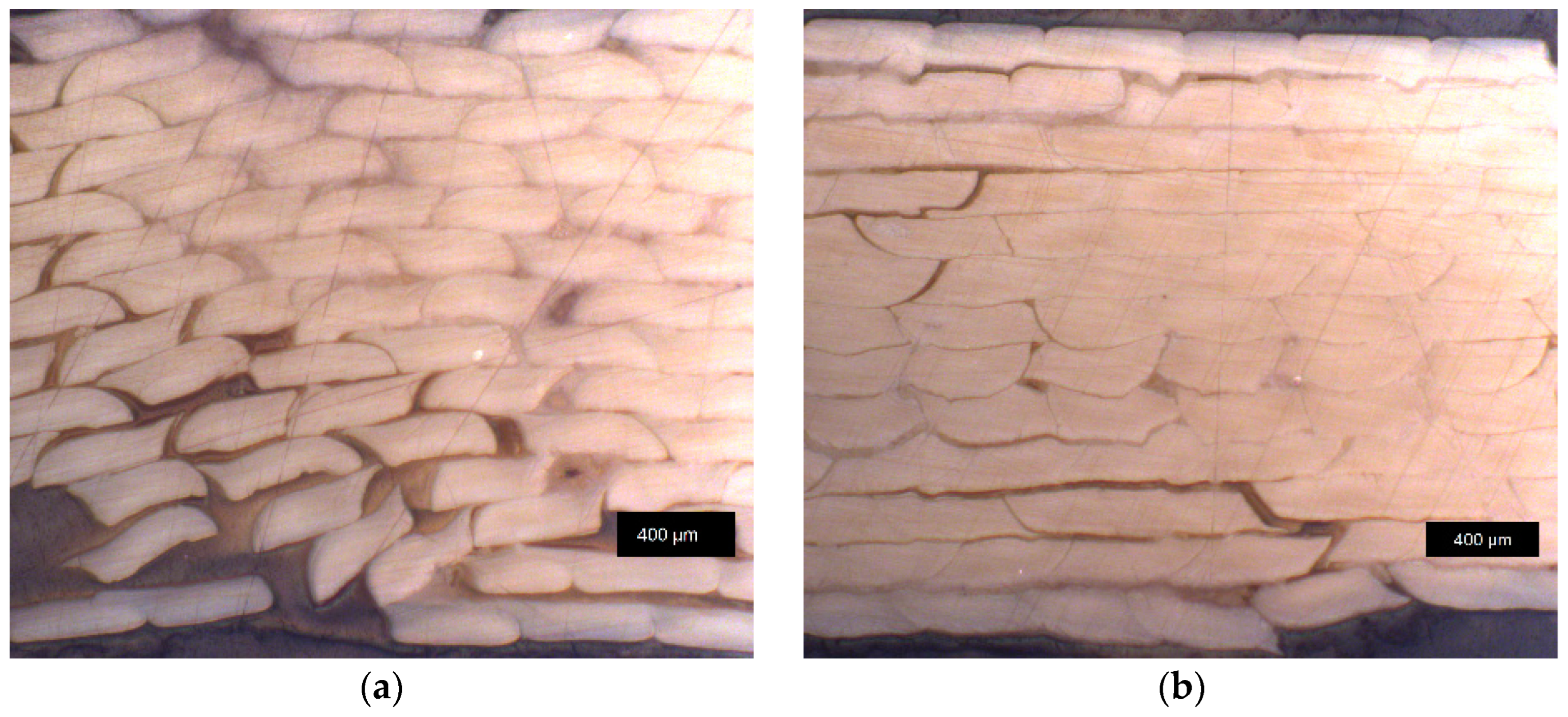
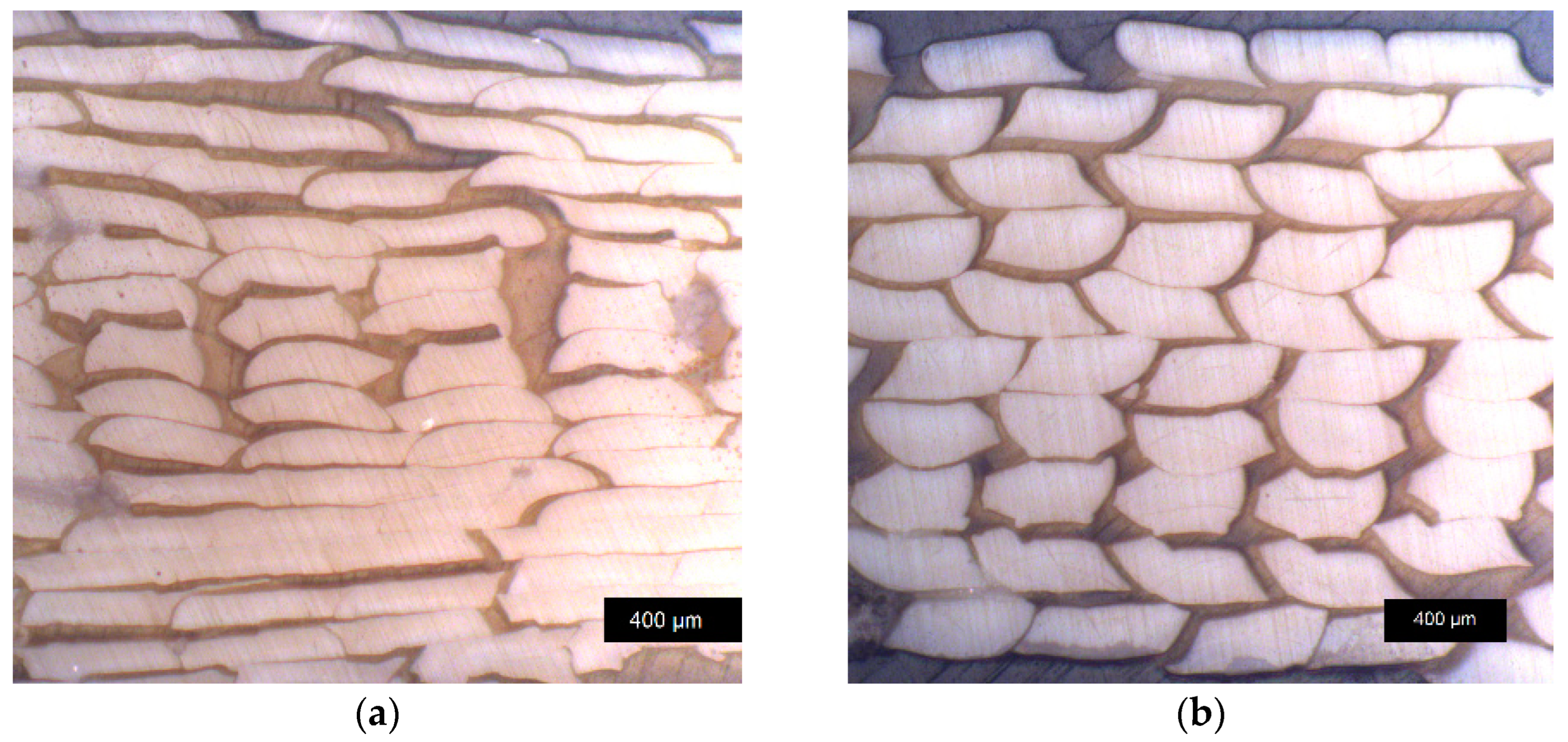
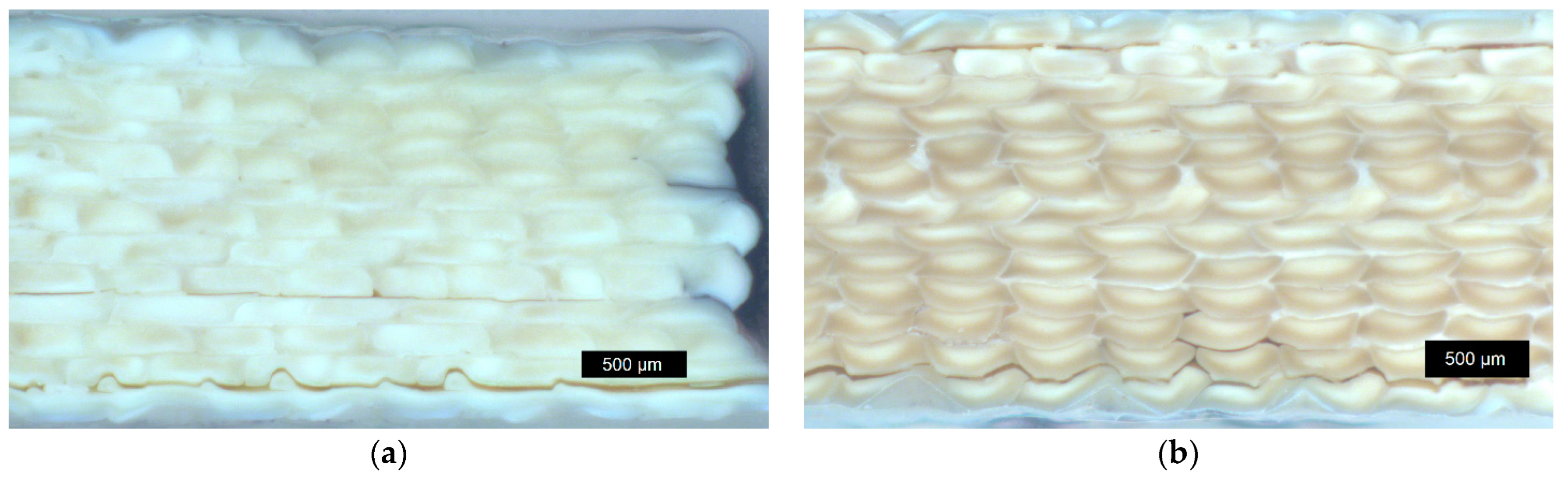
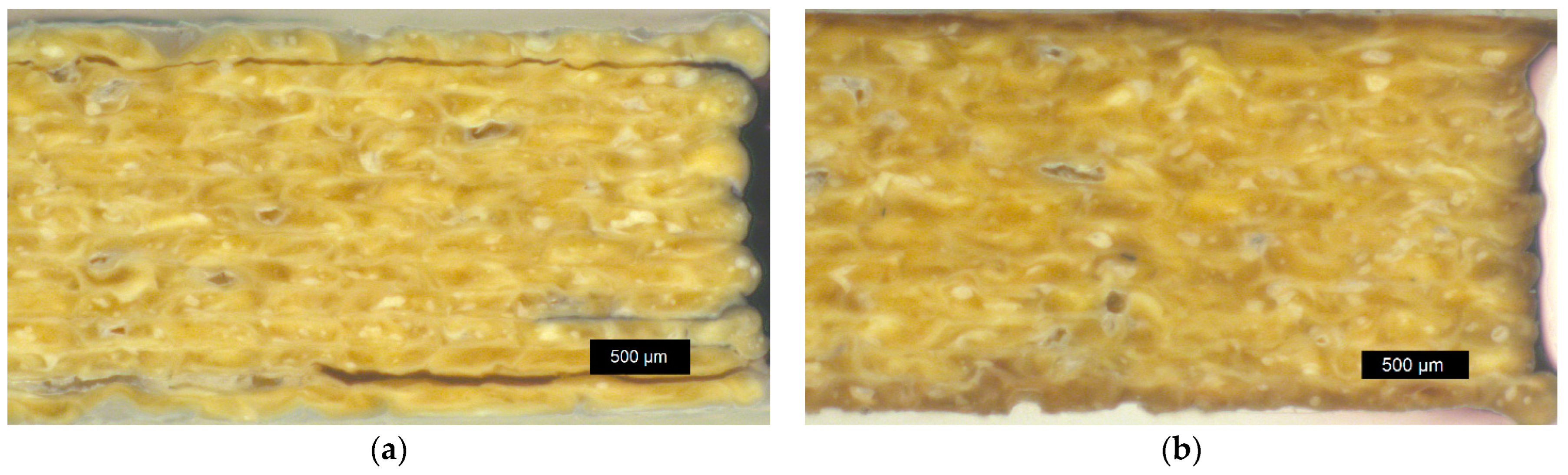
3.4.2. Sample Density of Annealed Samples and Optical Microscopy
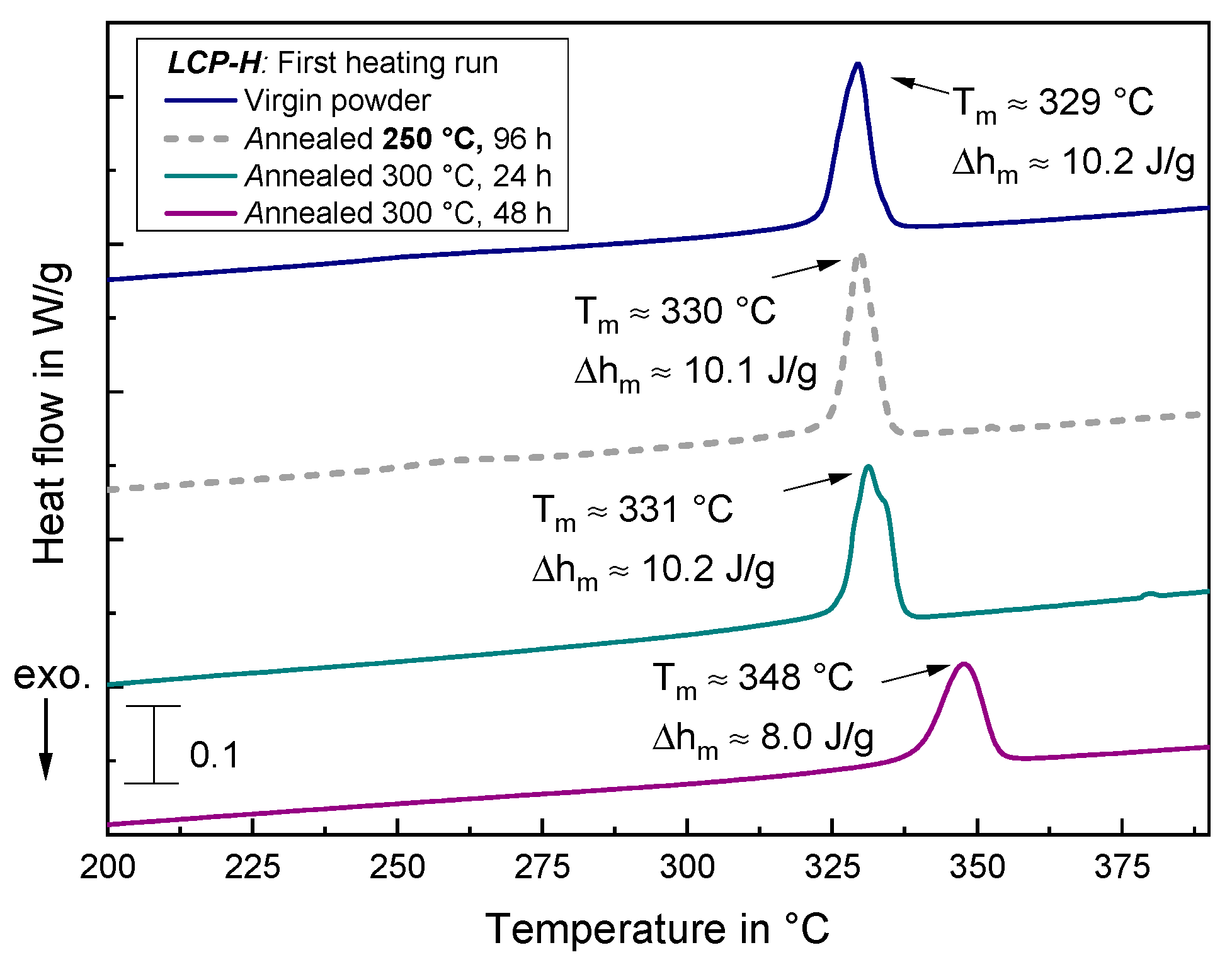
3.4.3. DSC Measurements of Annealed Samples
3.4.4. IR Measurements of Annealed Samples
4. Conclusions and Outlook
4.1. Conclusions
4.2. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonten, C. Plastics Technology: Introduction and Fundamentals; Carl Hanser Verlag: München, Germany, 2019; ISBN 978-1-56990-767-2. [Google Scholar]
- Jabbari-Farouji, S. Static and dynamic scaling behavior of a polymer melt model with triple-well bending potential. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1376–1392. [Google Scholar] [CrossRef]
- Elias, H.-G. Macromolecules 1: Structure and Properties, 7th ed.; Plenum Press: New York, NY, USA, 1984; ISBN 978-1-4615-7369-2. [Google Scholar]
- Strobl, G. The Physics of Polymers: Concepts for Understanding Their Stuctures and Behavior, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-25278-8. [Google Scholar]
- Eder, G.; Janeschitz-Kriegl, H. Theory of shear-induced crystallization of polymer melts. Colloid Polym. Sci. 1988, 266, 1087–1094. [Google Scholar] [CrossRef]
- Calignano, F.; Manfredi, D.; Ambrosio, E.P.; Biamino, S.; Lombardi, M.; Atzeni, E.; Salmi, A.; Minetola, P.; Iuliano, L.; Fino, P. Overview on Additive Manufacturing Technologies. Proc. IEEE 2017, 105, 593–612. [Google Scholar] [CrossRef]
- Wu, W.; Geng, P.; Li, G.; Zhao, D.; Zhang, H.; Zhao, J. Influence of Layer Thickness and Raster Angle on the Mechanical Properties of 3D-Printed PEEK and a Comparative Mechanical Study between PEEK and ABS. Materials 2015, 8, 5834–5846. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, M.; Yang, S. Extrusion-based additive manufacturing of PEEK for biomedical applications. Virtual Phys. Prototyp. 2015, 10, 123–135. [Google Scholar] [CrossRef]
- Rahman, K.M.; Letcher, T.; Reese, R. Mechanical Properties of Additively Manufactured PEEK Components Using Fused Filament Fabrication. In Volume 2A: Advanced Manufacturing, Proceedings of the ASME 2015 International Mechanical Engineering Congress and Exposition, Houston, TX, USA, 13–19 November 2015; American Society of Mechanical Engineers: New York, NY, USA, 2015; p. 11132015. ISBN 978-0-7918-5735-9. [Google Scholar]
- Zanjanijam, A.R.; Major, I.; Lyons, J.G.; Lafont, U.; Devine, D.M. Fused Filament Fabrication of PEEK: A Review of Process-Structure-Property Relationships. Polymers 2020, 12, 1665. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Ball, J.; Bremner, T.; Sue, H.-J. Crystallization behavior and morphological characterization of poly(ether ether ketone). Polymer 2014, 55, 5255–5265. [Google Scholar] [CrossRef]
- Cicala, G.; Ognibene, G.; Portuesi, S.; Blanco, I.; Rapisarda, M.; Pergolizzi, E.; Recca, G. Comparison of Ultem 9085 Used in Fused Deposition Modelling (FDM) with Polytherimide Blends. Materials 2018, 11, 285. [Google Scholar] [CrossRef]
- Zaldivar, R.J.; Witkin, D.B.; McLouth, T.; Patel, D.N.; Schmitt, K.; Nokes, J.P. Influence of processing and orientation print effects on the mechanical and thermal behavior of 3D-Printed ULTEM® 9085 Material. Addit. Manuf. 2017, 13, 71–80. [Google Scholar] [CrossRef]
- Fischer, M.; Schöppner, V. Fatigue Behavior of FDM Parts Manufactured with Ultem 9085. JOM 2017, 69, 563–568. [Google Scholar] [CrossRef]
- Forés-Garriga, A.; Pérez, M.A.; Gómez-Gras, G.; Reyes-Pozo, G. Role of infill parameters on the mechanical performance and weight reduction of PEI Ultem processed by FFF. Mater. Des. 2020, 193, 108810. [Google Scholar] [CrossRef]
- Demus, D.; Goodby, J.; Gray, G.W.; Spiess, H.-W.; Vill, V. Handbook of Liquid Crystals—Vol. 1: Fundamentals; WILEY-VCH Verlag GmbH: Weinheim, Germany, 1998; ISBN 3-537-29270-5. [Google Scholar]
- Wang, X.; Zhou, Q. Liquid Crystalline Polymers; World Scientific Pub. Co.: River Edge, NJ, USA, 2004; ISBN 9812384103. [Google Scholar]
- Bassett, D.C.; Mitchell, G.R.; Windle, A.H. Developments in Crystalline Polymers: Chapter 3, Orientation in Liquid Crystal Polymers; Elsevier Applied Science Publishers Ltd.: Barking, UK, 1988; ISBN 978-94-010-7096-6. [Google Scholar]
- Geiger, K. Verformungsinduzierte Selbstorganisation Steifer Makromoleküle in Thermotropen Flüssigkristallinen Polymerschmelzen. Ph.D. Thesis, Fakultät Energie-, Verfahrens- und Biotechnik, University of Stuttgart, Stuttgart, Germany, 2012. [Google Scholar]
- Zhou, W.-J.; Kornfield, J.A.; Ugaz, V.M.; Burghardt, W.R.; Link, D.R.; Clark, N.A. Dynamics and Shear Orientation Behavior of a Main-Chain Thermotropic Liquid Crystalline Polymer. Macromolecules 1999, 32, 5581–5593. [Google Scholar] [CrossRef]
- Rendon, S.; Burghardt, W.R.; Bubeck, R.A. Orientation dynamics in commercial thermotropic liquid crystalline polymers in transient shear flows. Rheol. Acta 2007, 46, 945–956. [Google Scholar] [CrossRef]
- Shibaev, V. Liquid Crystalline Polymers. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128035818. [Google Scholar]
- Ambulo, C.P.; Burroughs, J.J.; Boothby, J.M.; Kim, H.; Shankar, M.R.; Ware, T.H. Four-dimensional Printing of Liquid Crystal Elastomers. ACS Appl. Mater. Interfaces 2017, 9, 37332–37339. [Google Scholar] [CrossRef] [PubMed]
- Kotikian, A.; Truby, R.L.; Boley, J.W.; White, T.J.; Lewis, J.A. 3D Printing of Liquid Crystal Elastomeric Actuators with Spatially Programed Nematic Order. Adv. Mater. 2018, 30, 1706164. [Google Scholar] [CrossRef] [PubMed]
- López-Valdeolivas, M.; Liu, D.; Broer, D.J.; Sánchez-Somolinos, C. 4D Printed Actuators with Soft-Robotic Functions. Macromol. Rapid Commun. 2018, 39, 1700710. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, Y.; He, Q.; Wang, Y.; Cai, S. Three-dimensionals printing of functionally graded liquid crystal elastomer. Sci. Adv. 2020, 6, eabc0034. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.W., IV; Baird, D.G.; Bøhn, J.H. Effects of Processing Conditions on Prototypes Reinforced with TLCPs for Fused Deposition Modeling. Int. Solid Free. Fabr. Symp. 1997, 449–456. [Google Scholar]
- Ansari, M.Q.; Redmann, A.; Osswald, T.A.; Bortner, M.J.; Baird, D.G. Application of thermotropic liquid crystalline polymer reinforced acrylonitrile butadiene styrene in fused filament fabrication. Addit. Manuf. 2019, 29, 100813. [Google Scholar] [CrossRef]
- Ansari, M.Q. Generation of Thermotropic Liquid Crystalline Polymer (TLCP)—Thermoplastic Composite Filaments and Their Processing in Fused Filament Fabrication (FFF). Ph.D. Thesis, Faculty of Virginia Polytechnic Institute and State University, Blacksburg, WV, USA, 2019. [Google Scholar]
- Ansari, M.Q.; Bortner, M.J.; Baird, D.G. Generation of Polyphenylene Sulfide Reinforced with a Thermotropic Liquid Crystalline Polymer for Application in Fused Filament Fabrication. Addit. Manuf. 2019, 29, 100814. [Google Scholar] [CrossRef]
- Ansari, M.Q.; Baird, D.G. Generation of high performance polyphenylene sulfide-thermotropic liquid crystalline polymer composite filaments for use in fused filament fabrication. In AIP Conference Proceedings; Proceedings of PPS-33; AIP Publishing: Melville, NY, USA, 2019; p. 2139. [Google Scholar]
- Song, M.; Wang, X.; Du, R.; Zhou, Z.; Li, X.; Li, G.; Luo, Y. Effects of liquid crystal polymer (LCP) on the structure and performance of PEEK/CF composites. RSC Adv. 2022, 12, 12446–12452. [Google Scholar] [CrossRef] [PubMed]
- Gantenbein, S.; Masania, K.; Woigk, W.; Sesseg, J.P.W.; Tervoort, T.A.; Studart, A.R. Three-dimensional printing of hierarchical liquid-crystal-polymer structures. Nature 2018, 561, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Gantenbein, S.; Mascolo, C.; Houriet, C.; Zboray, R.; Neels, A.; Masania, K.; Studart, A.R. Spin-Printing of Liquid Crystal Polymer into Recyclable and Strong All-Fiber Materials. Adv. Funct. Mater. 2021, 31, 2104574. [Google Scholar] [CrossRef]
- Ji, Y.; Bai, Y.; Liu, X.; Jia, K. Progress of liquid crystal polyester (LCP) for 5G application. Adv. Ind. Eng. Polym. Res. 2020, 3, 160–174. [Google Scholar] [CrossRef]
- Chen, B.-K.; Tsay, S.-Y.; Chen, J.-Y. Synthesis and properties of liquid crystalline polymers with low Tm and broad mesophase temperature ranges. Polymer 2005, 46, 8624–8633. [Google Scholar] [CrossRef]
- Jin, X.; Chung, T.-S. Thermal Decomposition Behavior of Main-Chain Thermotropic Liquid Crystalline Polymers, Vectra A-950, B-950, and Xydar SRT-900. J. Appl. Polym. Sci. 1999, 73, 2195–2207. [Google Scholar] [CrossRef]
- Thumsorn, S.; Prasong, W.; Ishigami, A.; Kurose, T.; Kobayashi, Y.; Ito, H. Influence of Ambient Temperature and Crystalline Structure on Fracture Toughness and Production of Thermoplastic by Enclosure FDM 3D Printer. J. Manuf. Mater. Process. 2023, 7, 44. [Google Scholar] [CrossRef]
- Rodzeń, K.; Harkin-Jones, E.; Wegrzyn, M.; Sharma, P.K.; Zhigunov, A. Improvement of the layer-layer adhesion in FFF 3D printed PEEK/carbon fibre composites. Compos. Part A Appl. Sci. Manuf. 2021, 149, 106532. [Google Scholar] [CrossRef]
- Agarwal, U.S.; Mashelkar, R.A. Diffusion of Rigid Rodlike Molecules across Interfaces: Implications in Welding of Liquid Crystalline Polymers. Macromolecules 1992, 25, 6703–6704. [Google Scholar] [CrossRef]
- Yang, T.-C.; Yeh, C.-H. Morphology and Mechanical Properties of 3D Printed Wood Fiber/Polylactic Acid Composite Parts Using Fused Deposition Modeling (FDM): The Effects of Printing Speed. Polymers 2020, 12, 1334. [Google Scholar] [CrossRef]
- Sawyer, L.C.; Jaffe, M. The structure of thermotropic copolyesters. J. Mater. Sci. 1986, 21, 1897–1913. [Google Scholar] [CrossRef]
- Taylor, J.E.; Romo-Uribe, A.; Libera, M.R. Molecular orientation gradients in thermotropic liquid crystalline fiber. Polym. Adv. Technol. 2003, 14, 595–600. [Google Scholar] [CrossRef]
- Kalfon-Cohen, E.; Pegoretti, A.; Marom, G. Annealing of drawn monofilaments of liquid crystalline polymer vectra/vapor grown carbon fiber nanocomposites. Polymer 2010, 51, 1033–1041. [Google Scholar] [CrossRef]
- Kocer, H.B.; Cerkez, I.; Broughton, R. Annealing studies on a thermotropic liquid crystalline polyester meltblown fabric. J. Ind. Text. 2017, 46, 1656–1667. [Google Scholar] [CrossRef]
- Schneggenburger, L.A.; Osenar, P.; Economy, J. Direct Evidence for Sequence Ordering of Random Semicrystalline Copolyesters during High-Temperature Annealing. Macromolecules 1997, 30, 3754–3758. [Google Scholar] [CrossRef]
- Lenz, W.R.; Jin, J.-I.; Feichtinger, K.A. Crystallization-induced reactions of copolymers: 6. Reorganization of polyesters in the liquid crystal state. Polymer 1983, 24, 327–334. [Google Scholar] [CrossRef]
- Frich, D.; Hall, A.; Economy, J. Nature of adhesive bonding via interchain transesterification reactions (ITR). Macromol. Chem. Phys. 1998, 199, 913–921. [Google Scholar] [CrossRef]
- Chung, T.S.; Jin, X. Studies on the Phase Transition and Thermal Stability of Xydar and Zenite Series Liquid Crystalline Polymers. Polym. Eng. Sci. 2000, 40, 841–856. [Google Scholar] [CrossRef]
- Selby, J.C.; Shannon, M.A.; Xu, K.; Economy, J. Sub-micrometer solid-state adhesive bonding with aromatic thermosetting copolyesters for the assembly of polyimide membranes in silicon-based devices. J. Micromech. Microeng. 2001, 11, 672–685. [Google Scholar] [CrossRef]
- Hudson, S.D.; Lovinger, A.J. Transmission electron microscopic investigation of the morphology of a poly (hydroxybenzoate-co-hydroxynaphthoate) liquid crystal polymer. Polymer 1993, 34, 1123–1129. [Google Scholar] [CrossRef]
- Hsieh, T.-T.; Tiu, C.; Simon, G.P. Correlation Between Molecular Structure, Free Volume, and Physical Properties of a Wide Range of Main Chain Thermotropic Liquid Crystalline Polymers. J. Appl. Polym. Sci. 2001, 82, 2252–2267. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johann, K.S.; Wolf, A.; Bonten, C. Mechanical Properties of 3D-Printed Liquid Crystalline Polymers with Low and High Melting Temperatures. Materials 2024, 17, 152. https://doi.org/10.3390/ma17010152
Johann KS, Wolf A, Bonten C. Mechanical Properties of 3D-Printed Liquid Crystalline Polymers with Low and High Melting Temperatures. Materials. 2024; 17(1):152. https://doi.org/10.3390/ma17010152
Chicago/Turabian StyleJohann, Kai S., Andreas Wolf, and Christian Bonten. 2024. "Mechanical Properties of 3D-Printed Liquid Crystalline Polymers with Low and High Melting Temperatures" Materials 17, no. 1: 152. https://doi.org/10.3390/ma17010152




