Rapid Growth of Metal–Metal Oxide Core–Shell Structures through Joule Resistive Heating: Morphological, Structural, and Luminescence Characterization
Abstract
:1. Introduction
2. Experimental Method
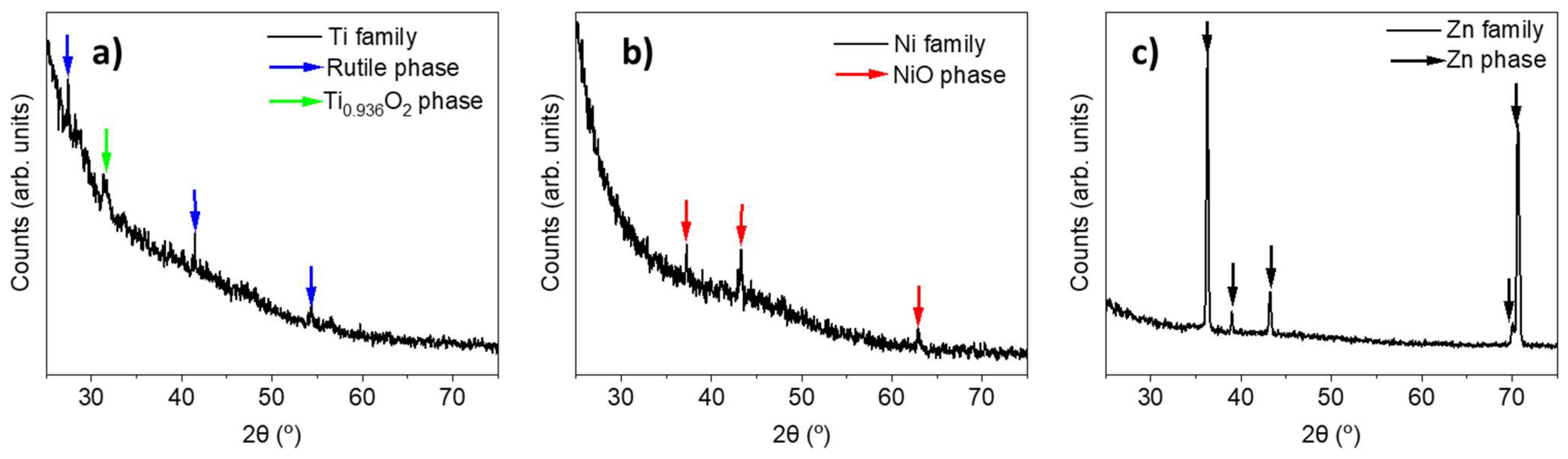
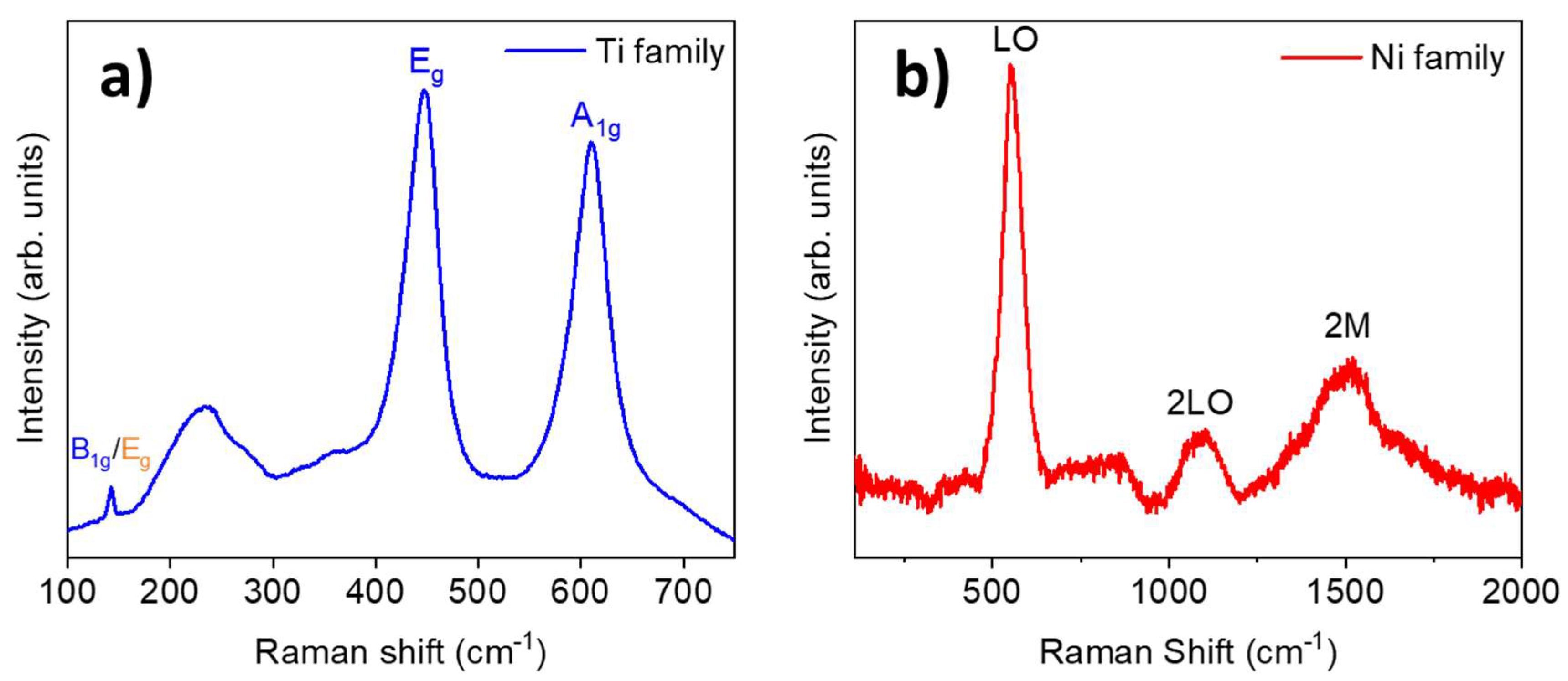
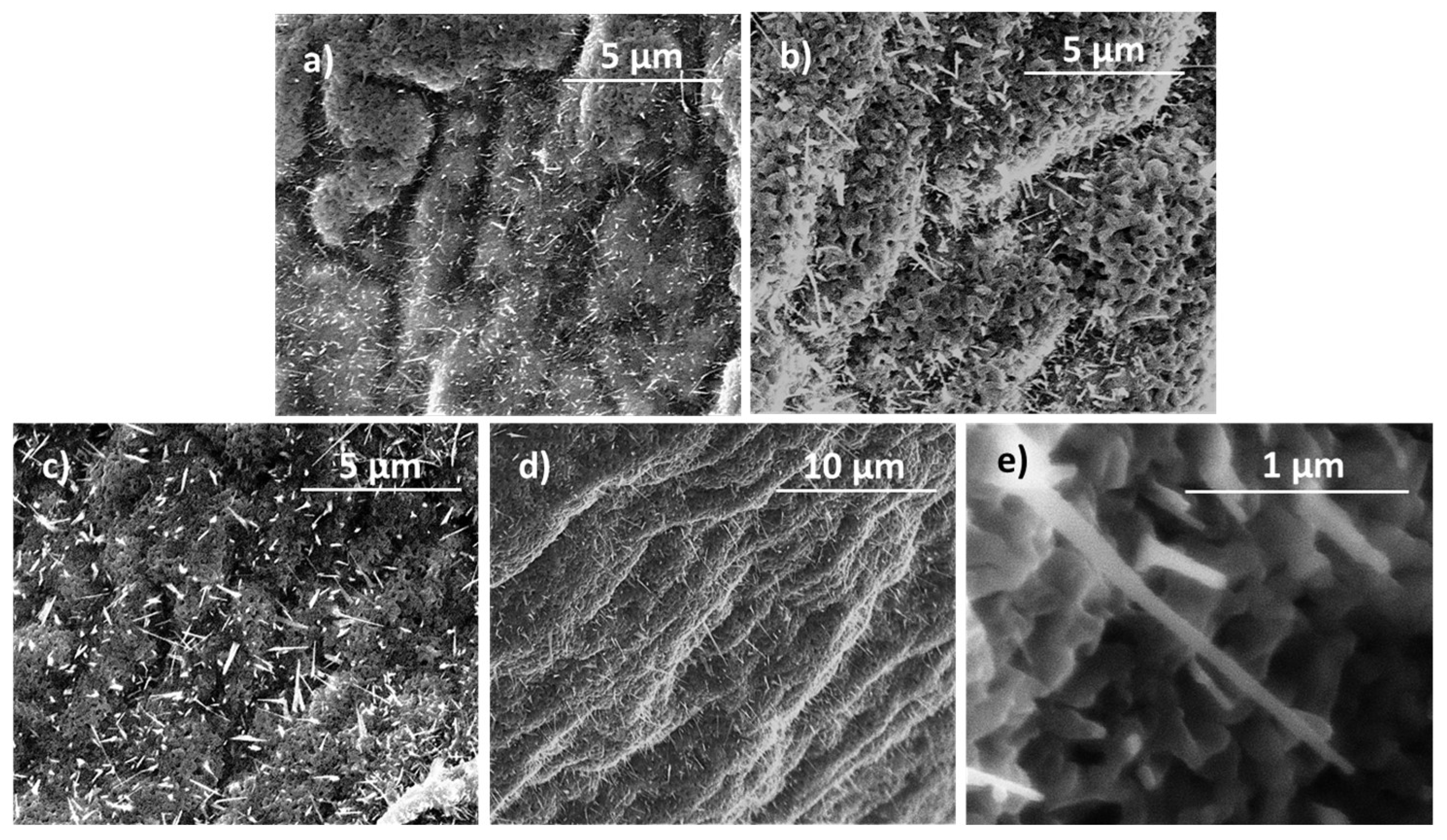
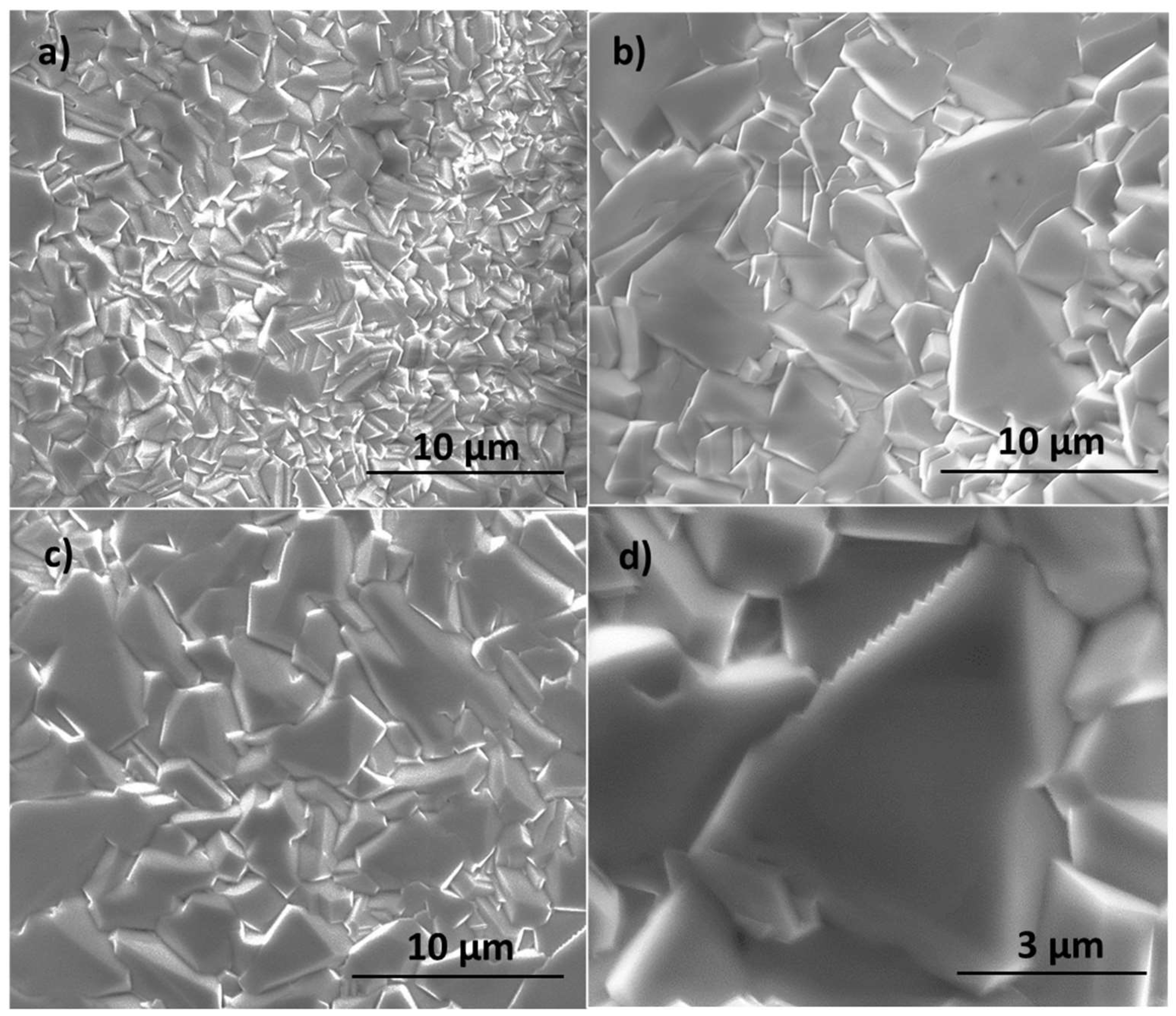
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meyer, J.; Hamwi, S.; Kröger, M.; Kowalsky, W.; Riedl, T.; Kahn, A. Transition metal oxides for organic electronics: Energetics, device physics and applications. Adv. Mater. 2012, 24, 5408–5427. [Google Scholar] [CrossRef] [PubMed]
- Danish, M.S.S.; Bhattacharya, A.; Stepanova, D.; Mikhaylov, A.; Grilli, M.L.; Khosravy, M.; Senjyu, T. A systematic review of metal oxide applications for energy and environmental sustainability. Metals 2020, 10, 1604. [Google Scholar] [CrossRef]
- De Prado, E.; Florian, C.; Sotillo, B.; Siegel, J.; Solis, J.; Fernández, P. Optical spectroscopy study of nano-and microstructures fabricated by femtosecond laser pulses on ZnO based systems. CrystEngComm 2018, 20, 2952–2960. [Google Scholar] [CrossRef]
- Urbieta, A.; Sánchez, V.; Fernández, P.; Piqueras, J. Fast Growth of Undoped and Sn-and Tb-doped ZnO Nanowires by Joule Heating of Zn. CrystEngComm 2018, 20, 4449–4454. [Google Scholar] [CrossRef]
- Uikey, P.; Vishwakarma, K. Review of Zinc Oxide (ZnO) Nanoparticles Applications and Properties. Int. J. Emerg. Technol. Comput. Sci. Electron. 2016, 21, 976–1353. [Google Scholar]
- Malhotra, S.P.K.; Mandal, T.K. Zinc oxide nanostructure and its application as agricultural and industrial material. Contam. Agric. Environ. Health Risks Remediat. 2019, 1, 216–226. [Google Scholar] [CrossRef]
- Ji, S.; Ye, C. Synthesis, Growth Mechanism, and Applications of Zinc Oxide Nanomaterials. Artic. J. Mater. Sci. Technol. Shenyang 2008, 24, 457. [Google Scholar]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, A.P. Modified titanium oxide (TiO2) nanocomposites and its array of applications: A review. Toxicol. Environ. Chem. 2015, 97, 491–514. [Google Scholar] [CrossRef]
- Sibum, H. Titanium and Titanium Alloys—From Raw Material to Semi-finished Products. Adv. Eng. Mater. 2003, 5, 393–398. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Narender, S.S.; Varma, V.V.S.; Srikar, C.S.; Ruchitha, J.; Varma, P.A.; Praveen, B.V.S. Nickel Oxide Nanoparticles: A Brief Review of Their Synthesis, Characterization, and Applications. Chem. Eng. Technol. 2022, 45, 397–409. [Google Scholar] [CrossRef]
- Ramos-Justicia, J.F.; Ballester-Andújar, J.L.; Urbieta, A.; Fernández, P. Growth of Zr/ZrO2 Core–Shell Structures by Fast Thermal Oxidation. Appl. Sci. 2023, 13, 3714. [Google Scholar] [CrossRef]
- Piqueras, J.; Hidalgo, P. Growth of Metal Oxide Nanostructures by Thermal Oxidation of Metals Under Influence of External Electric Fields and Electric Current Flow. Phys. Status Solidi (A) Appl. Mater. Sci. 2021, 218, 2100323. [Google Scholar] [CrossRef]
- Yuk, J.M.; Kim, K.; Lee, Z.; Watanabe, M.; Zettl, A.; Kim, T.W.; No, Y.S.; Choi, W.K.; Lee, J.Y. Direct fabrication of zero-and one-dimensional metal nanocrystals by thermally assisted electromigration. ACS Nano 2010, 4, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Choopun, S.; Hongsith, N.; Wongrat, E. Metal-oxide Nanowires by Thermal Oxidation Reaction Technique. In Nanowires; InTech: Munich, Germany, 2010. [Google Scholar]
- Hauser, J.S.; Schwichtenberg, J.; Marz, M.; Sürgers, C.; Seiler, A.; Gerhards, U.; Messerschmidt, F.; Hensel, A.; Dittmeyer, R.; Löhneysen, H.V.; et al. Controlled electromigration and oxidation of free-standing copper wires. Appl. Phys. A 2016, 122, 1068. [Google Scholar] [CrossRef]
- Rodríguez, B.; Hidalgo, P.; Piqueras, J.; Méndez, B. Influence of an external electric field on the rapid synthesis of MoO3 micro-and nanostructures by Joule heating of Mo wires. RSC Adv. 2020, 10, 11892–11897. [Google Scholar] [CrossRef]
- Zhao, C.; Xue, Y.; Jin, Y. Effect of electric field on Fe2O3 nanowire growth during thermal oxidation. Mod. Phys. Lett. B 2016, 30, 1650054. [Google Scholar] [CrossRef]
- Balakrishnan, V.; Dinh, T.; Phan, H.-P.; Dao, D.V.; Nguyen, N.-T. A generalized analytical model for Joule heating of segmented wires. J. Heat. Transf. 2018, 140, 072001. [Google Scholar] [CrossRef]
- Rekha, K.; Nirmala, M.; Nair, M.G.; Anukaliani, A. Structural, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide nanoparticles. Phys. B Phys. Condens. Matter 2010, 405, 3180–3185. [Google Scholar] [CrossRef]
- Du, G.H.; Chen, Q.; Che, R.C.; Yuan, Z.Y.; Peng, L.-M. Preparation and structure analysis of titanium oxide nanotubes. Appl. Phys. Lett. 2001, 79, 3702–3704. [Google Scholar] [CrossRef]
- Marciuš, M.; Ristić, M.; Ivanda, M.; Musić, S. Formation and microstructure of nickel oxide films. J. Alloys Compd. 2012, 541, 238–243. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Hamdan, S.A.; Ibrahim, I.M.; Ali, I.M. COMPARISON OF ANATASE AND RUTILE TiO2 NANOSTRUCTURE FOR GAS SENSING APPLICATION. Dig. J. Nanomater. Biostruct. 2020, 15, 1001–1008. [Google Scholar] [CrossRef]
- Fievet, F.; Germi, P.; De Bergevin, F.; Figlarz, M. Lattice Parameter, Microstrains and Non-Stoichiometry in NiO. Comparison between Mosaic Microcrystals and Quasi-Perfect Single Microcrystals. J. Appl. Cryst. 1979, 12, 387–394. [Google Scholar] [CrossRef]
- Lan, T.; Tang, X.; Fultz, B. Phonon anharmonicity of rutile TiO2 studied by Raman spectrometry and molecular dynamics simulations. Phys. Rev. B 2012, 85, 094305. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Melendres, C.A.; Narayanasamy, A.; Maroni, V.A.; Siegel, R.W. Raman spectroscopy of nanophase TiO2. J. Mater. Res. 1989, 4, 1246–1250. [Google Scholar] [CrossRef]
- Porto, S.P.S.; Fleury, P.A.; Damen, T.C. Raman spectra of TiO2, MgF2, ZnF2, FeF2, and MnF2. Phys. Rev. 1967, 154, 522. [Google Scholar] [CrossRef]
- Mironova-Ulmane, N.; Kuzmin, A.; Steins, I.; Grabis, J.; Sildos, I.; Pärs, M. Raman scattering in nanosized nickel oxide NiO. J. Phys. Conf. Ser. 2007, 93, 012039. [Google Scholar] [CrossRef]
- Mironova-Ulmane, N.; Kuzmin, A.; Sildos, I.; Pärs, M. Polarisation dependent Raman study of single-crystal nickel oxide. Open Phys. 2011, 9, 1096–1099. [Google Scholar] [CrossRef]
- Sokol, A.A.; French, S.A.; Bromley, S.T.; Catlow, C.R.A.; van Dam, H.J.J.; Sherwood, P. Point defects in ZnO. Faraday Discuss. 2006, 134, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Galdámez-Martinez, A.; Santana, G.; Güell, F.; Martínez-Alanis, P.R.; Dutt, A. Photoluminescence of ZnO nanowires: A review. Nanomaterials 2020, 10, 857. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Li, C.; Chai, Z. Defect luminescence and its mediated physical properties in ZnO. J. Lumin. 2019, 208, 225–237. [Google Scholar] [CrossRef]
- Lima, S.A.M.; Sigoli, F.A.; Jafelicci, M., Jr.; Davolos, M.R. Luminescent properties and lattice defects correlation on zinc oxide. Int. J. Inorg. Mater. 2001, 3, 749–754. [Google Scholar] [CrossRef]
- Wang, Y.G.; Lau, S.P.; Zhang, X.H.; Lee, H.W.; Yu, S.F.; Tay, B.K.; Hng, H.H. Evolution of visible luminescence in ZnO by thermal oxidation of zinc films. Chem. Phys. Lett. 2003, 375, 113–118. [Google Scholar] [CrossRef]
- Kofstad, P. Defects and transport properties of metal oxides. Oxid. Met. 1995, 44, 3–27. [Google Scholar] [CrossRef]
- Rempel, A.A.; Valeeva, A.A.; Vokhmintsev, A.S.; Weinstein, I.A. Titanium dioxide nanotubes: Synthesis, structure, properties and applications. Russ. Chem. Rev. 2021, 90, 1397–1414. [Google Scholar] [CrossRef]
- Pallotti, D.K.; Passoni, L.; Maddalena, P.; Di Fonzo, F.; Lettieri, S. Photoluminescence Mechanisms in Anatase and Rutile TiO2. J. Phys. Chem. C 2017, 121, 9011–9021. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, D.; Zhu, Q.; Wu, J.; Huang, Q.; Xie, C. Effect of Nickel Vacancies on the Room-Temperature NO2 Sensing Properties of Mesoporous NiO Nanosheets. J. Phys. Chem. C 2016, 120, 3936–3945. [Google Scholar] [CrossRef]
- Jang, W.-L.; Lu, Y.-M.; Hwang, W.-S.; Hsiung, T.-L.; Wang, H.P. Point defects in sputtered NiO films. Appl. Phys. Lett. 2009, 94, 062103. [Google Scholar] [CrossRef]
- Gandhi, A.C.; Wu, S.Y. Strong Deep-Level-Emission Photoluminescence in NiO Nanoparticles. Nanomaterials 2017, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Qi, H.; Lu, C.; Yang, Y.; Zhao, Y. Photoluminescence and magnetic properties of b-Ni(OH)2 nanoplates and NiO nanostructures. J. Mater. Sci. Mater. Electron. 2008, 20, 479–483. [Google Scholar] [CrossRef]
- Meybodi, S.M.; Hosseini, S.; Rezaee, M.; Sadrnezhaad, S.; Mohammadyani, D. Synthesis of wide band gap nanocrystalline NiO powder via a sonochemical method. Ultrason. Sonochem. 2012, 19, 841–845. [Google Scholar] [CrossRef]
- Rackauskas, S.; Nasibulin, A.G.; Jiang, H.; Tian, Y.; I Kleshch, V.; Sainio, J.; Obraztsova, E.D.; Bokova, S.N.; Obraztsov, A.N.; Kauppinen, E.I. A novel method for metal oxide nanowire synthesis. Nanotechnology 2009, 20, 165603. [Google Scholar] [CrossRef]
- Tampo, H.; Fons, P.; Yamada, A.; Kim, K.-K.; Shibata, H.; Matsubara, K.; Niki, S.; Yoshikawa, H.; Kanie, H. Determination of crystallographic polarity of ZnO layers. Appl. Phys. Lett. 2005, 87, 141904. [Google Scholar] [CrossRef]
- Varea, D.M.; Varea, D.M. Crecimiento y Caracterización Mediante Técnicas de Microscopía, de Nano-y Microestructuras de SnO2 y TiO2; Universidad Complutense de Madrid: Madrid, Spain, 2007. [Google Scholar]
- Khoi, N.N.; Smeltzer, W.W.; Embury, J.D. Growth and Structure of Nickel Oxide on Nickel Crystal Faces. J. Electrochem. Soc. 1975, 122, 1495–1503. [Google Scholar] [CrossRef]

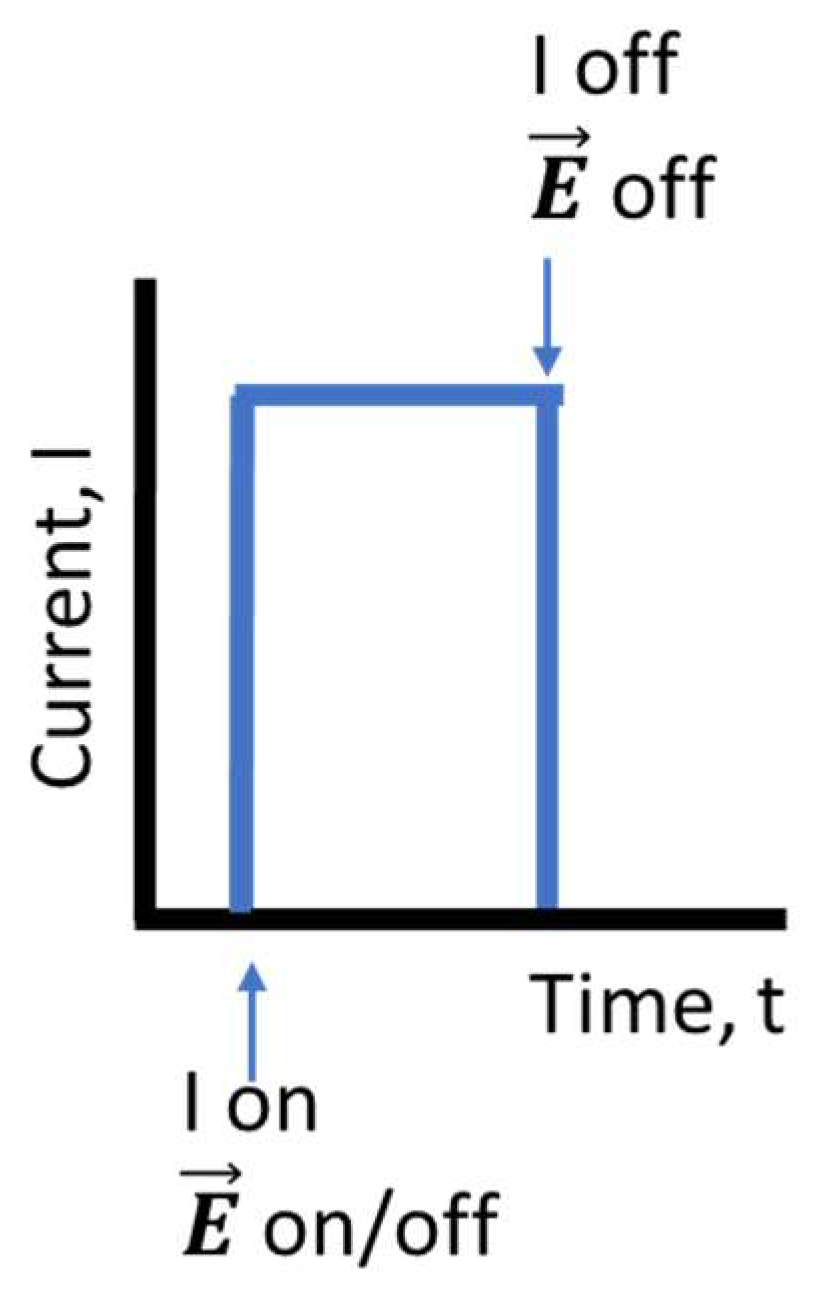
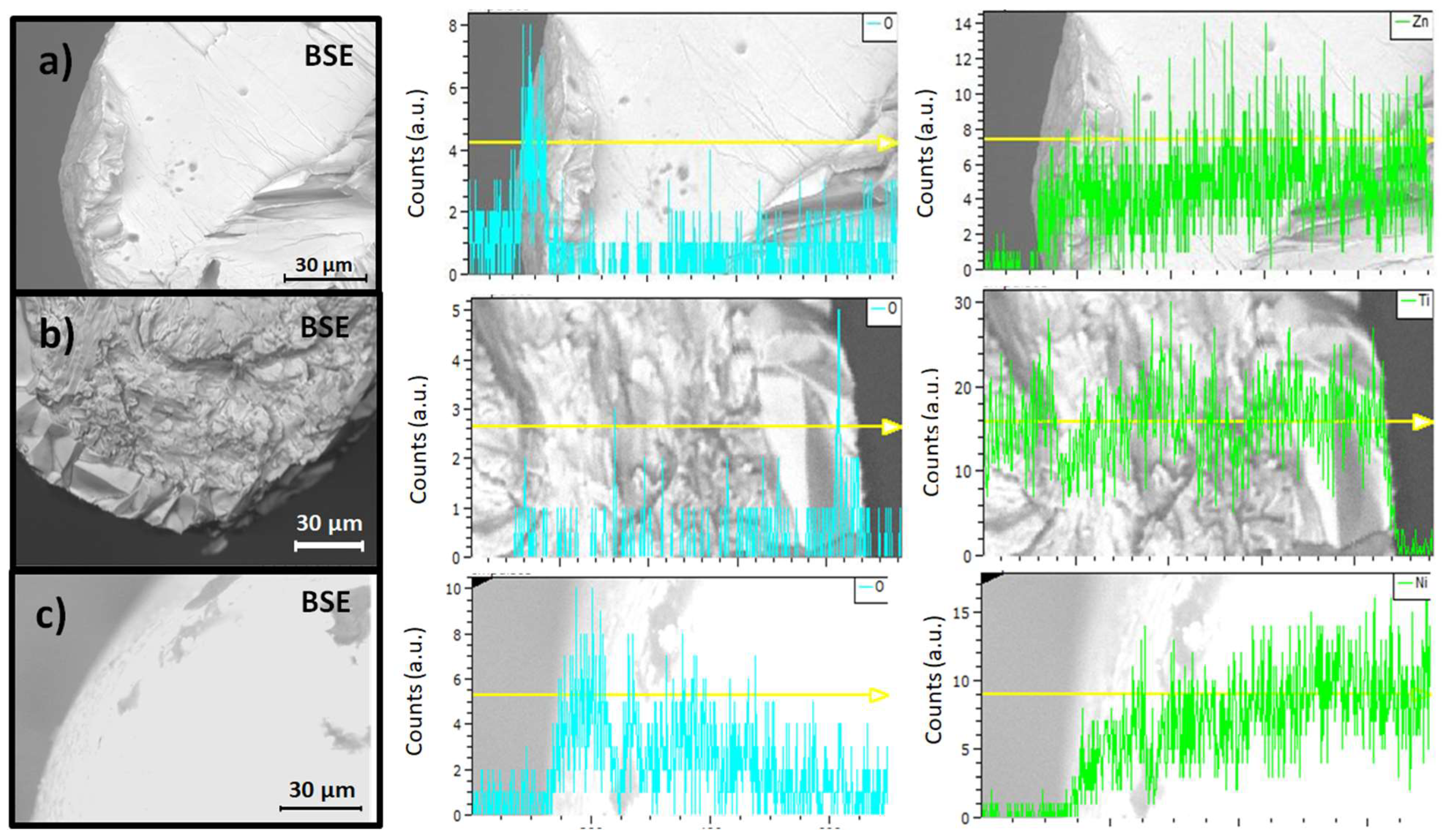
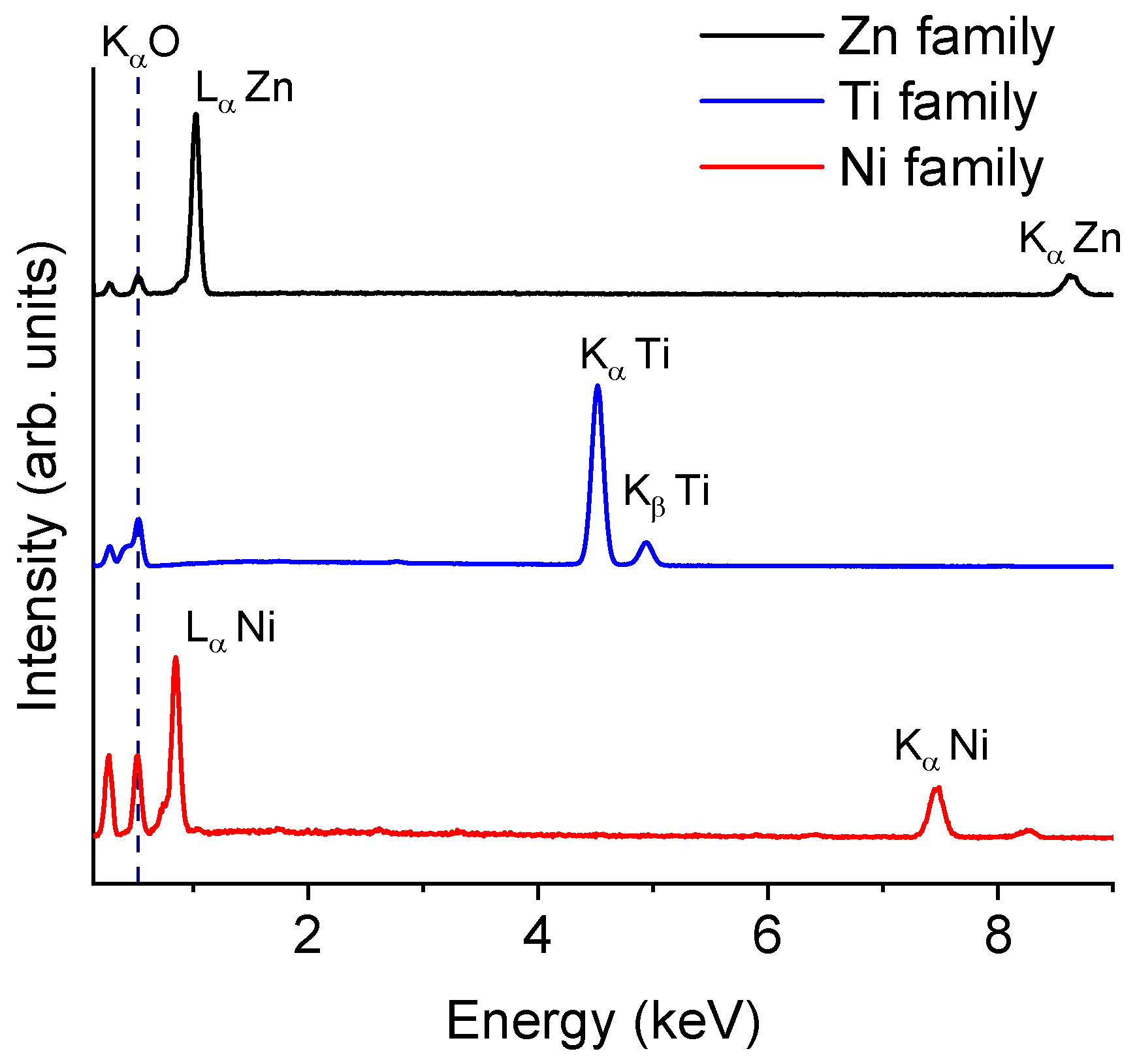
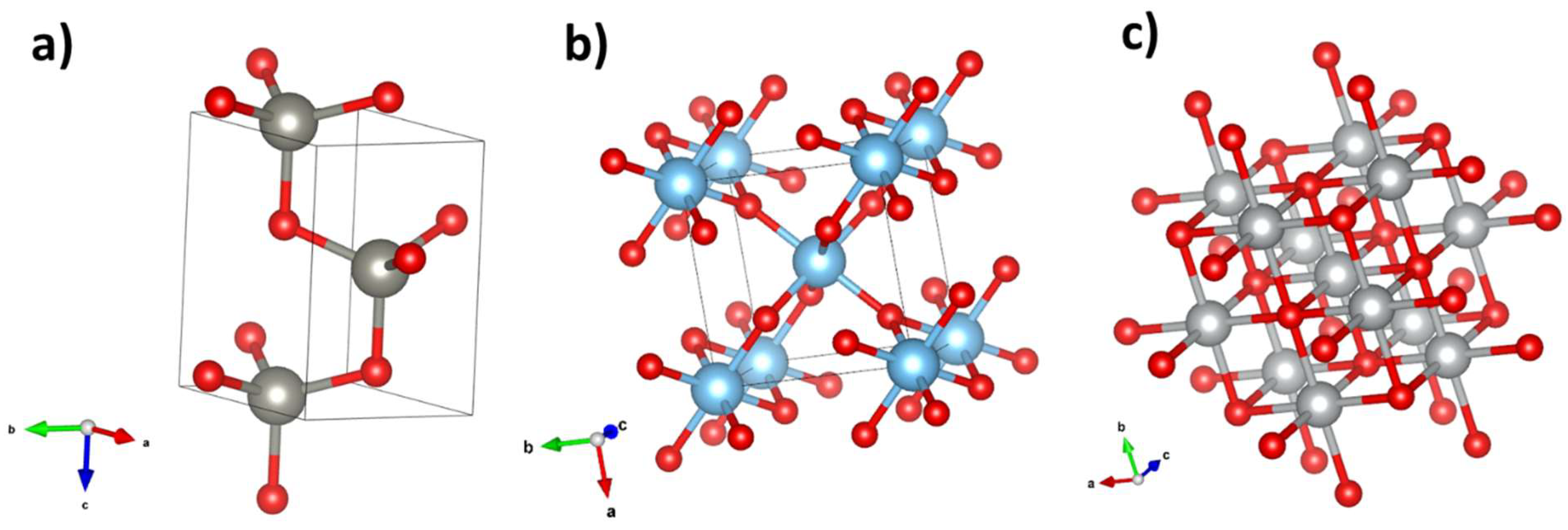



| Zn family | Intensity | Time | Electric field on/off |
| Zn1 | 3.8 A | 35 s | Off |
| Zn2 | 3.8 A | 60 s | Off |
| Zn3 | 3.8 A | 80 s | Off |
| Zn4-E | 3.8 A | 80 s | On |
| Ti family | Intensity | Time | Electric field on/off |
| Ti1 | 2.7 A | 30 s | Off |
| Ti2 | 2.7 A | 60 s | Off |
| Ti3 | 2.7 A | 100 s | Off |
| Ti4 | 2.5 A | 180 s | Off |
| Ti5 | 2.5 A | 300 s | Off |
| Ti6-E | 2.5 A | 300 s | On |
| Ni family | Intensity | Time | Electric field on/off |
| Ni1 | 5.5 A | 30 s | Off |
| Ni2 | 5.5 A | 60 s | Off |
| Ni3-E | 5.5 A | 60 s | On |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Justicia, J.F.; Urbieta, A.; Fernández, P. Rapid Growth of Metal–Metal Oxide Core–Shell Structures through Joule Resistive Heating: Morphological, Structural, and Luminescence Characterization. Materials 2024, 17, 208. https://doi.org/10.3390/ma17010208
Ramos-Justicia JF, Urbieta A, Fernández P. Rapid Growth of Metal–Metal Oxide Core–Shell Structures through Joule Resistive Heating: Morphological, Structural, and Luminescence Characterization. Materials. 2024; 17(1):208. https://doi.org/10.3390/ma17010208
Chicago/Turabian StyleRamos-Justicia, Juan Francisco, Ana Urbieta, and Paloma Fernández. 2024. "Rapid Growth of Metal–Metal Oxide Core–Shell Structures through Joule Resistive Heating: Morphological, Structural, and Luminescence Characterization" Materials 17, no. 1: 208. https://doi.org/10.3390/ma17010208





