Rapid Solidification of Invar Alloy
Abstract
:1. Introduction
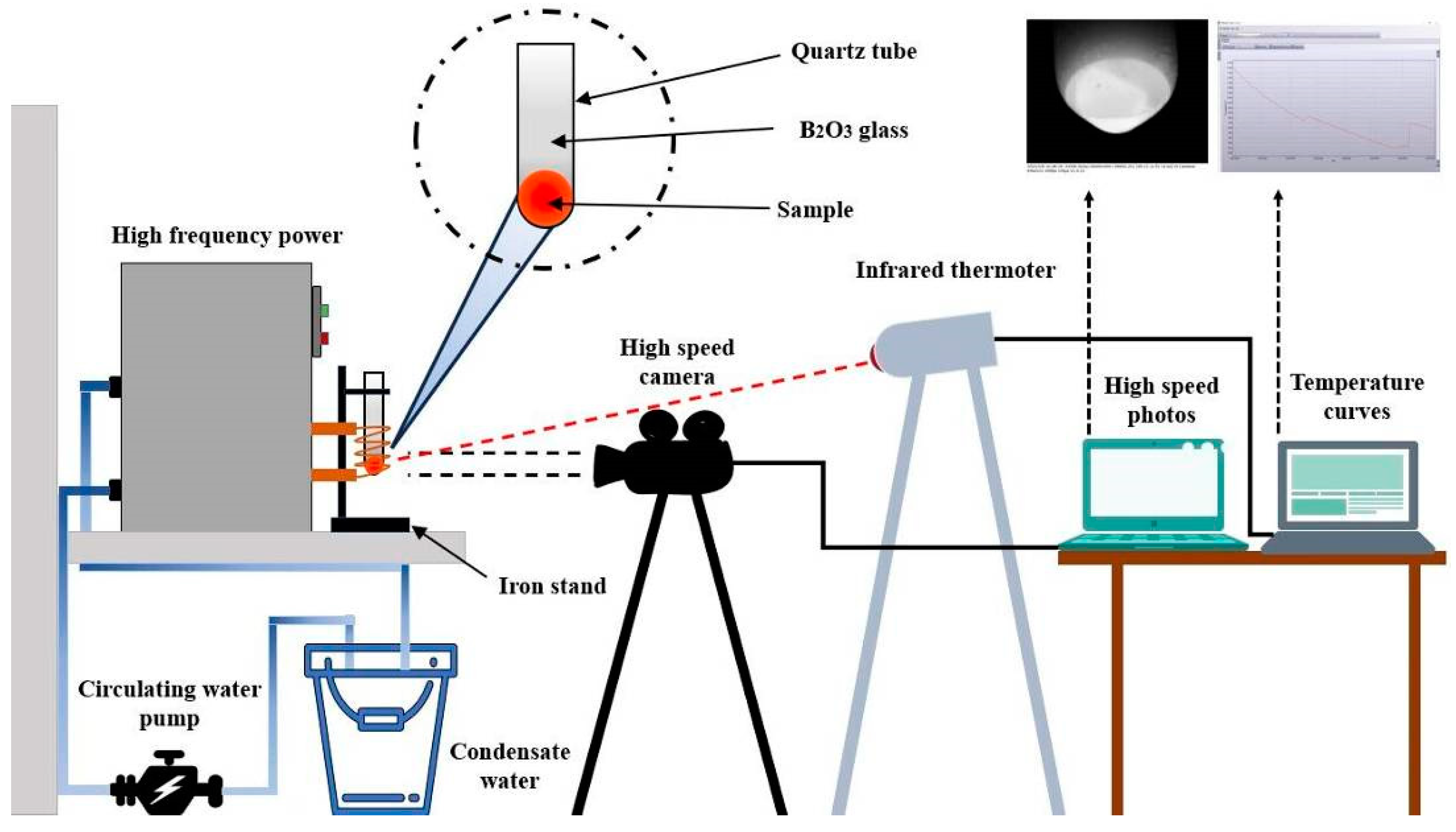
2. Experimental Section
3. Results
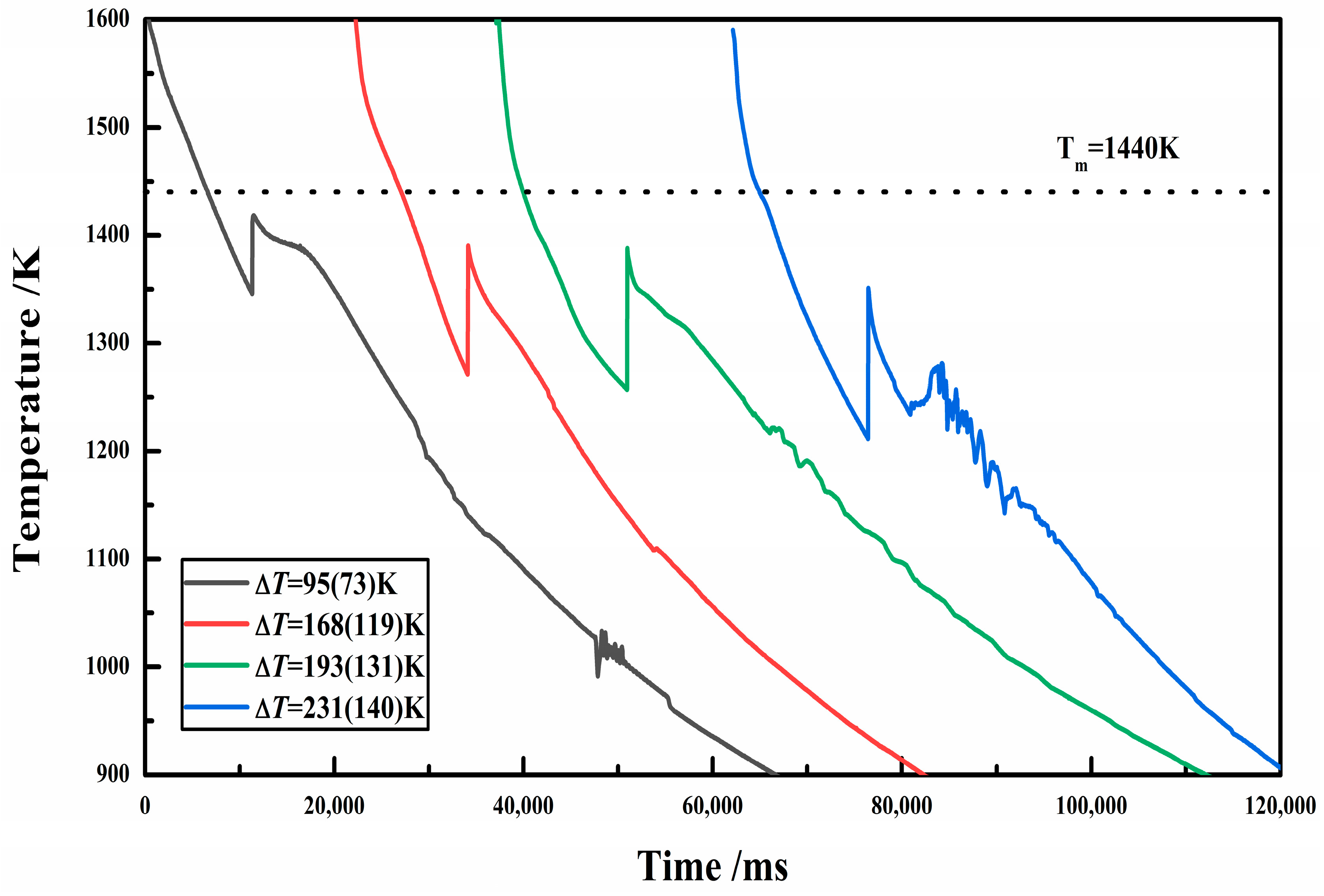
3.1. Cooling Curves
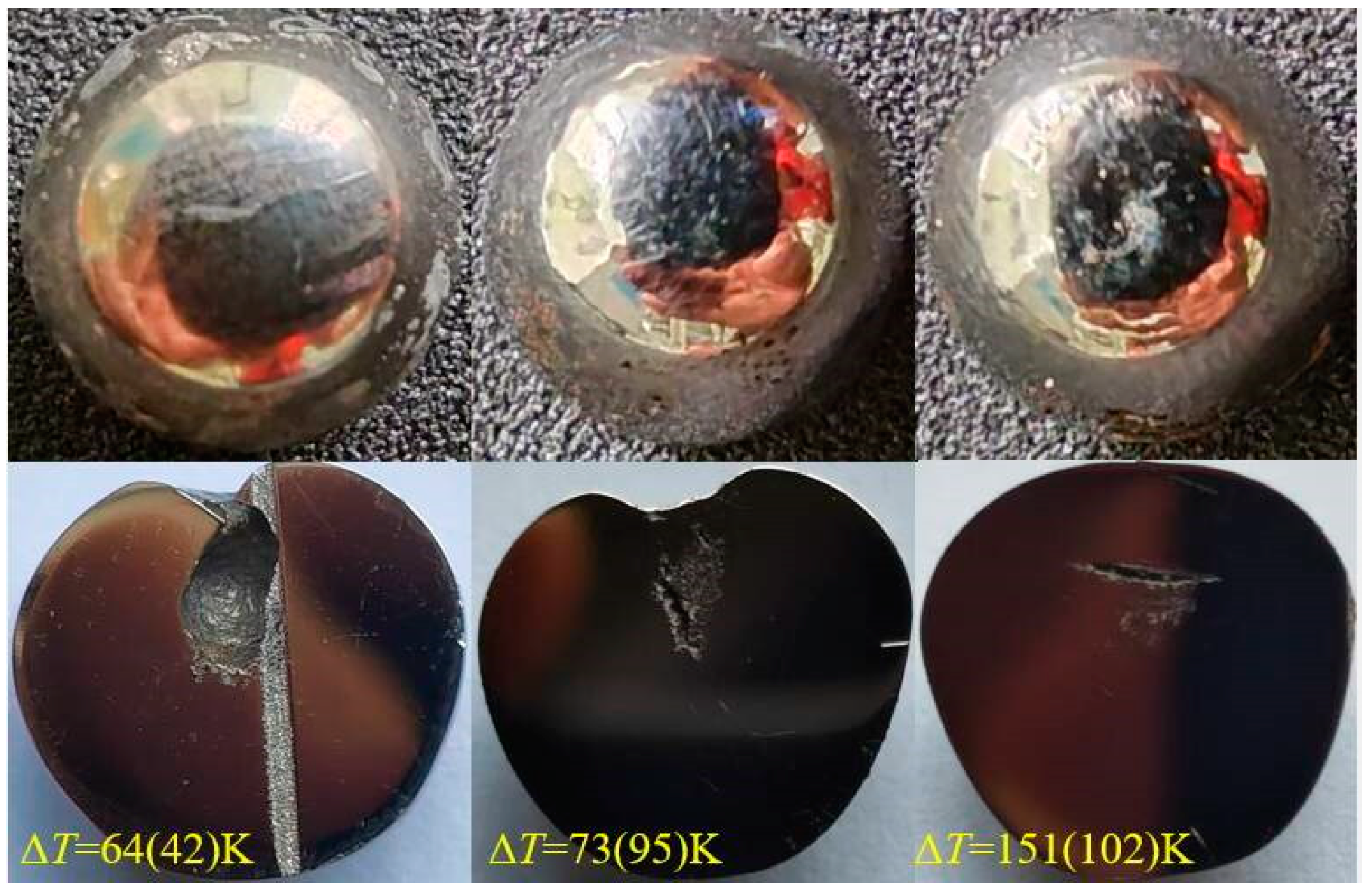
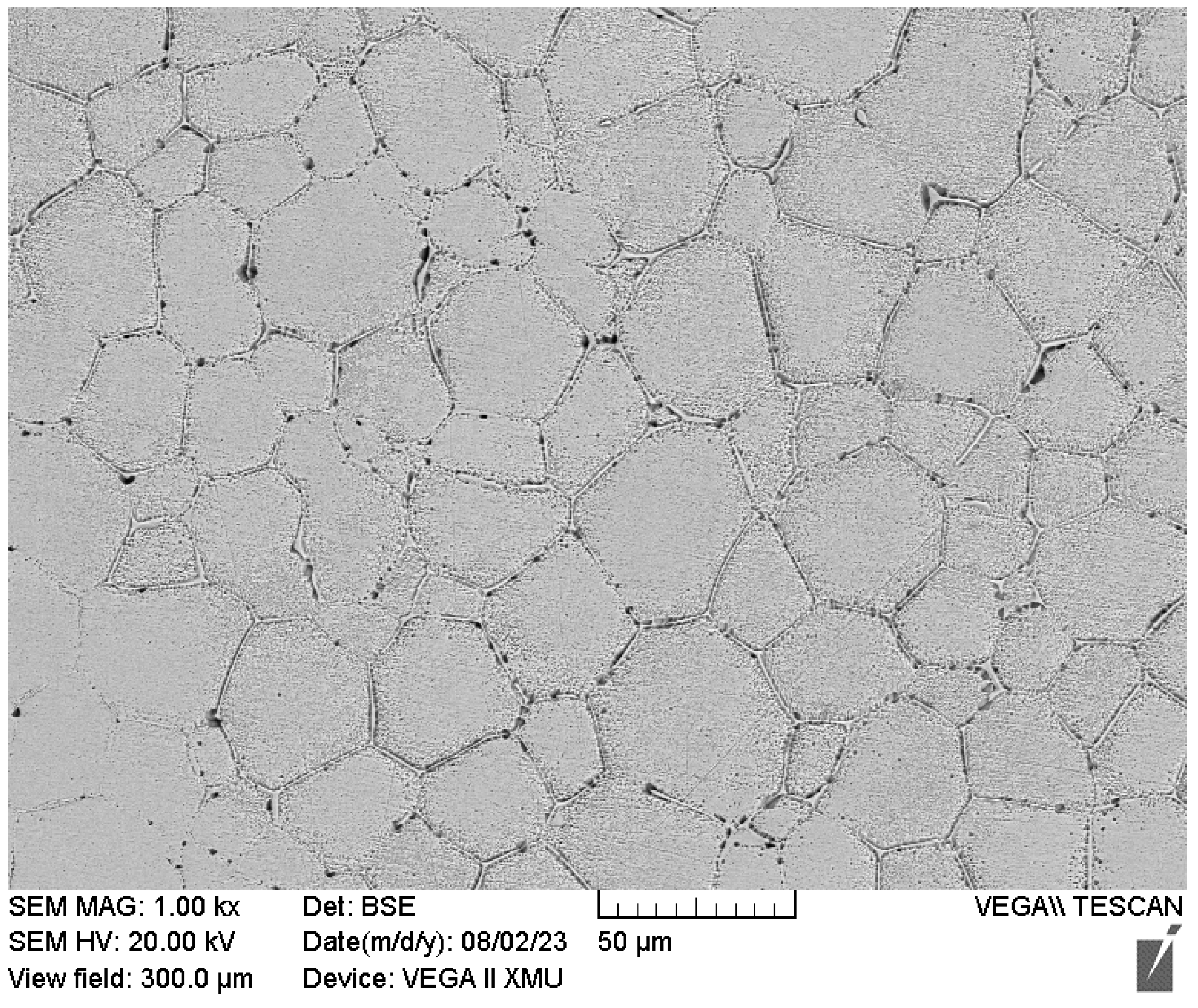
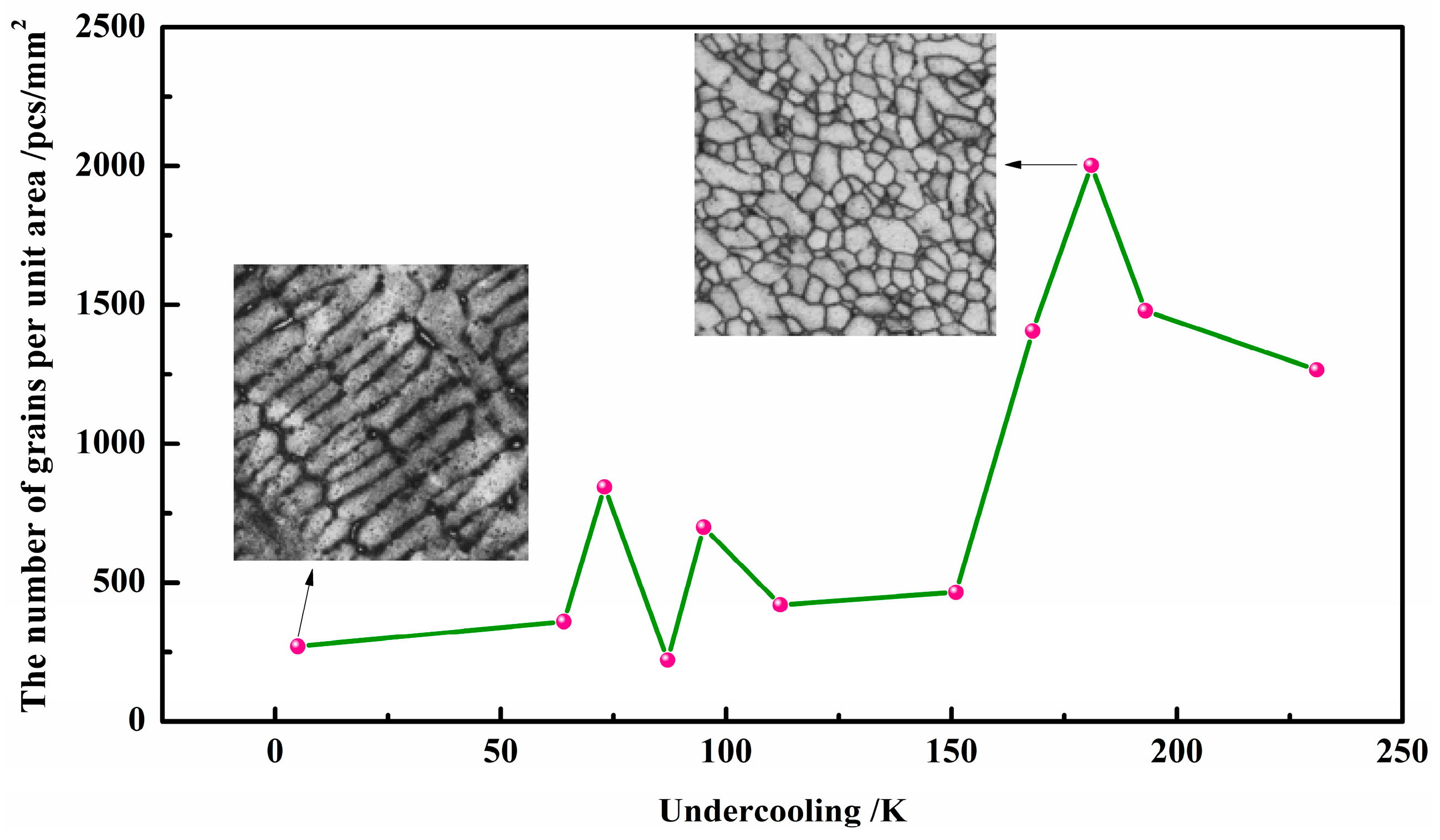
3.2. Microstructures
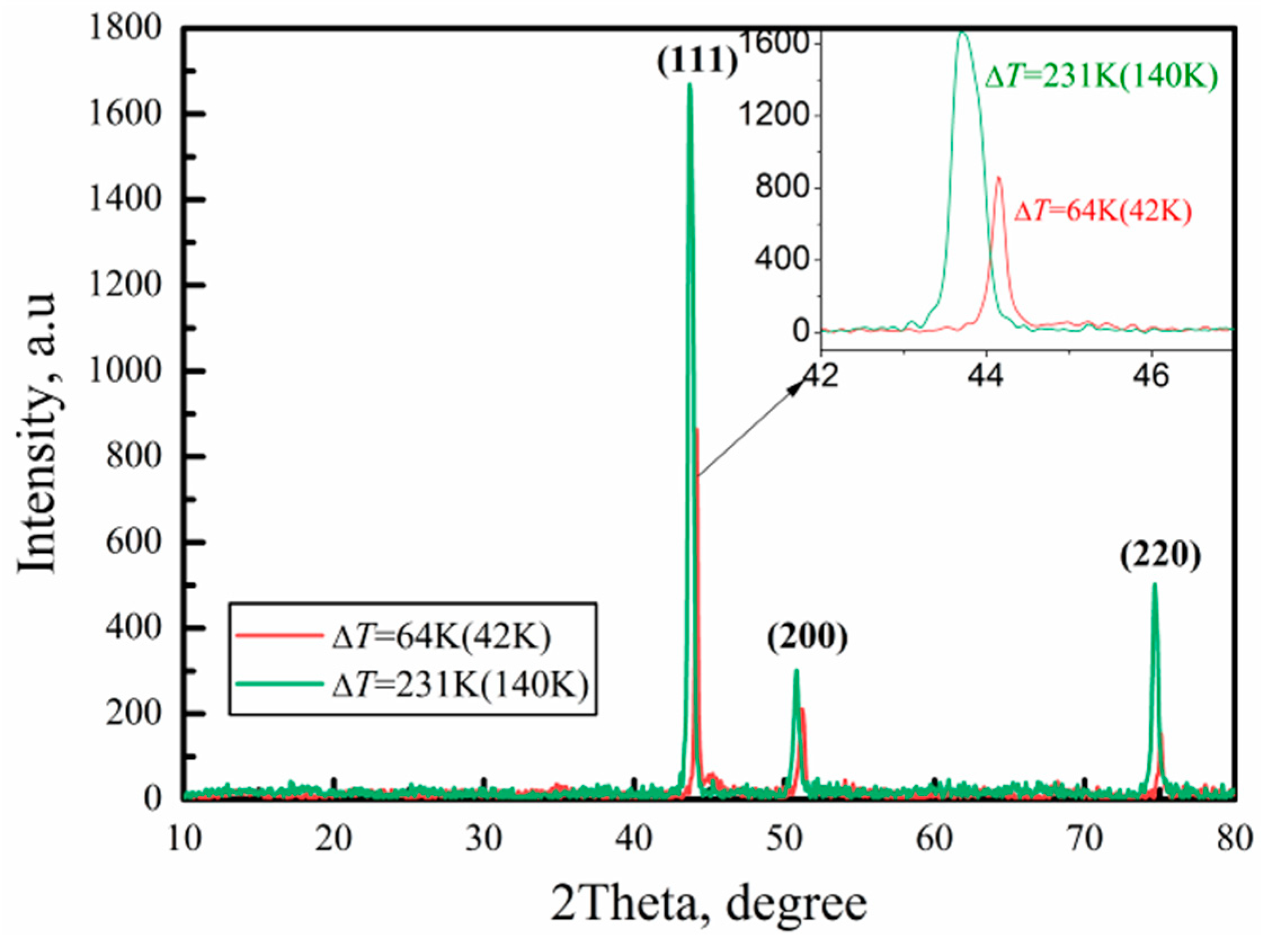
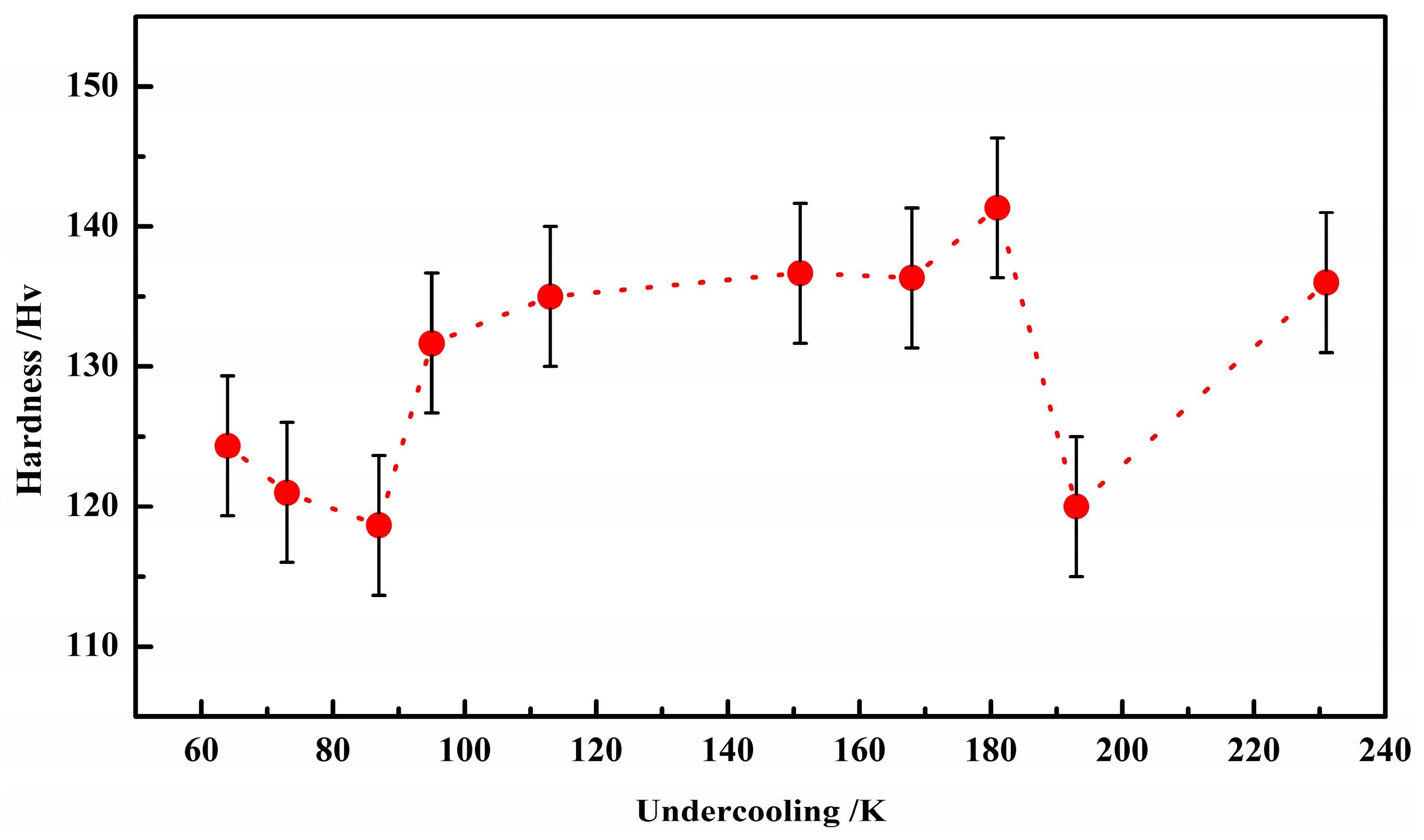
3.3. Microstructures
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sui, Q.; He, J.; Zhang, X.; Sun, Z.; Zhang, Y.; Wu, Y.; Zhu, Z.; Zhang, Q.; Peng, H. Strengthening of the Fe-Ni Invar Alloy through Chromium. Materials 2019, 12, 1297. [Google Scholar] [CrossRef] [PubMed]
- Ehn, A.; Alling, B.; Abrikosov, I.A. First-principles theory of the pressure-induced Invar effect in FeNi alloys. Phys. Rev. B 2023, 107, 104422. [Google Scholar] [CrossRef]
- Huang, J.J.; Lin, K.L. Electrorecrystallization of Invar 36 Alloy. Mater. Today Commun. 2023, 35, 106392. [Google Scholar] [CrossRef]
- Acharya, S.S.; Medicherla, V.R.R.; Bapna, K.; Ali, K.; Biswas, D.; Rawat, R.; Maiti, K. Mixed ground state in Fe-Ni Invar alloys. J. Alloys Comp. 2021, 863, 158605. [Google Scholar] [CrossRef]
- Li, X.; Chen, N.; Li, J.; He, X.; Liu, H.; Zheng, X.; Chen, J. Effect of temperature and strain rate on deformation behavior of Invar 36 alloy. Acta Met. Sin. 2017, 53, 968–974. [Google Scholar] [CrossRef]
- Liu, H.; Lv, S.; Xuan, Y.; Oliveira, J.P.; Schell, N.; Shen, J.N.; Deng, J.; Wang, Y.; Yang, J. Effects of Heat Input on Weld Microstructure and Properties in Keyhole TIG Welding of Invar 36 Alloy. Materials 2023, 16, 3692. [Google Scholar] [CrossRef]
- Park, S.J.; Jo, S.H.; Oh, S.; Oh, Y.S.; Kim, S.J.; Lee, H.W.; Kang, S.H.; Moon, Y.H.; Jung, J. Microstructure-dependent etching behavior of a partially recrystallized Invar alloy. Mater. Des. 2022, 217, 110631. [Google Scholar] [CrossRef]
- Sonomura, H.; Ozaki, T.; Katagiri, K.; Hasegawa, Y.; Tanaka, T.; Kakitsuji, A. Invar alloy metallization of Al2O3 substrate by friction stirring. Ceram. Int. 2023, 49, 18624–18628. [Google Scholar] [CrossRef]
- Prică, C.V.; Neamţu, B.V.; Marinca, T.F.; Popa, F.; Sechel, A.N.; Isnard, O.; Chicinaş, I. Synthesis of Invar 36 type alloys from elemental and prealloyed powders by mechanical alloying. Powder Metall. 2019, 62, 155–161. [Google Scholar] [CrossRef]
- Wladysiak, R.; Kozuń, A.; Dębowska, K.; Pacyniak, T. Analysis of Crystallization Process of Intensive Cooled AlSi20CuNiCoMg Alloy. Arch. Foundry Eng. 2017, 17, 137–144. [Google Scholar] [CrossRef]
- Władysiak, R.; Kozuń APacyniak, T. Effect of Casting Die Cooling on Solidification Process and Microstructure of Hypereutectic Al-Si Alloy. Arch. Foundry Eng. 2016, 16, 175–180. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, W.; Zhan, X.; Ding, L.; Zhou, J. Parameter optimisation of laser cladding repair for an Invar alloy mould. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2019, 233, 1859–1871. [Google Scholar] [CrossRef]
- Zhan, X.; Ling, W.; Gao, Q.; Yan, T.; Liu, Z. Microstructure and mechanical properties of the MLMPW on Invar alloy. Mater. Res. Express 2019, 6, 046529. [Google Scholar] [CrossRef]
- Si, P.Z.; Choi, C.J. High hardness nanocrystalline Invar alloys prepared from Fe-Ni nanoparticles. Metals 2018, 8, 28. [Google Scholar] [CrossRef]
- Azzeddine, H.; Tirsatine, K.; Baudin, T.; Mathon, M.H.; Helbert, A.L.; Brisset, F.; Bradai, D. On the stored energy evolution after accumulative roll-bonding of Invar alloy. Mater. Chem. Phys. 2017, 201, 408–415. [Google Scholar] [CrossRef]
- Dong, L.; Yu, Z.; Hu, X.; Feng, F. Effect of Mo do on the microstructure and properties of an Fe36Ni Invar alloy. Int. J. Mod. Phys. B 2020, 34, 2050297. [Google Scholar] [CrossRef]
- Coppock, J.; Waxter, Q.; Wolle, R.; Kane, B.E. Observation of Undercooling in a Levitated Nanoscale Liquid Au Droplet. J. Phys. Chem. C 2022, 126, 17990–17996. [Google Scholar] [CrossRef]
- Xu, J.; Yang, T.; Li, Z.; Wang, X.; Xiao, Y.; Jian, Z. The recalescence rate of cooling curve for undercooled solidification. Sci. Rep. 2020, 10, 1380. [Google Scholar] [CrossRef]
- Yao, K.; Min, X. Static and dynamic Hall‒Petch relations in {332}<113> TWIP Ti–15Mo alloy. Mater. Sci. Eng. A 2021, 827, 142044. [Google Scholar] [CrossRef]
- Herlach, D.M.; Eckler, K.; Karma, A.; Schwarz, M. Grain refinement through fragmentation of dendrites in undercooled melts. Mater. Sci. Eng. A 2001, 304, 20–25. [Google Scholar] [CrossRef]
- Ma, K.; Zhao, Y.; Xu, X.; Hou, H. Grain refinement mechanism at high undercooling of Ni80Cu20 alloy. Mater. Res. Express 2019, 6, 046522. [Google Scholar] [CrossRef]
- Thompson, C.V.; Spaepen, F. Homogeneous crystal nucleation in binary metallic melts. Acta Metall. 1983, 31, 2021–2027. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Yao, Z.; Li, X.; Xu, J. Rapid Solidification of Invar Alloy. Materials 2024, 17, 231. https://doi.org/10.3390/ma17010231
He H, Yao Z, Li X, Xu J. Rapid Solidification of Invar Alloy. Materials. 2024; 17(1):231. https://doi.org/10.3390/ma17010231
Chicago/Turabian StyleHe, Hanxin, Zhirui Yao, Xuyang Li, and Junfeng Xu. 2024. "Rapid Solidification of Invar Alloy" Materials 17, no. 1: 231. https://doi.org/10.3390/ma17010231




