Self-Assembly TiO2-Ti3C2Tx Ball–Plate Structure for Highly Efficient Electromagnetic Interference Shielding
Abstract
:1. Introduction
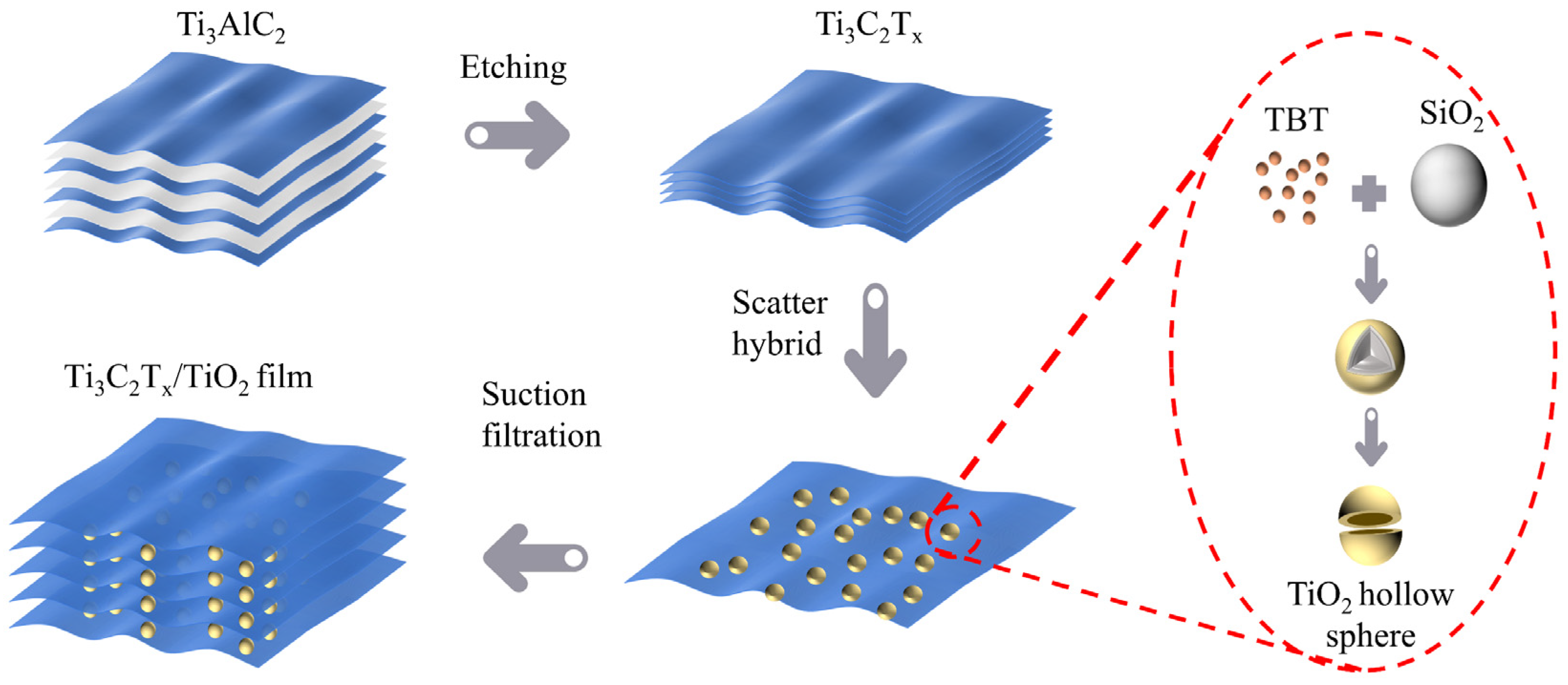
2. Design Principles and Synthesis of the Ball–Plate EMI Shielding Materials
3. Results and Discussion
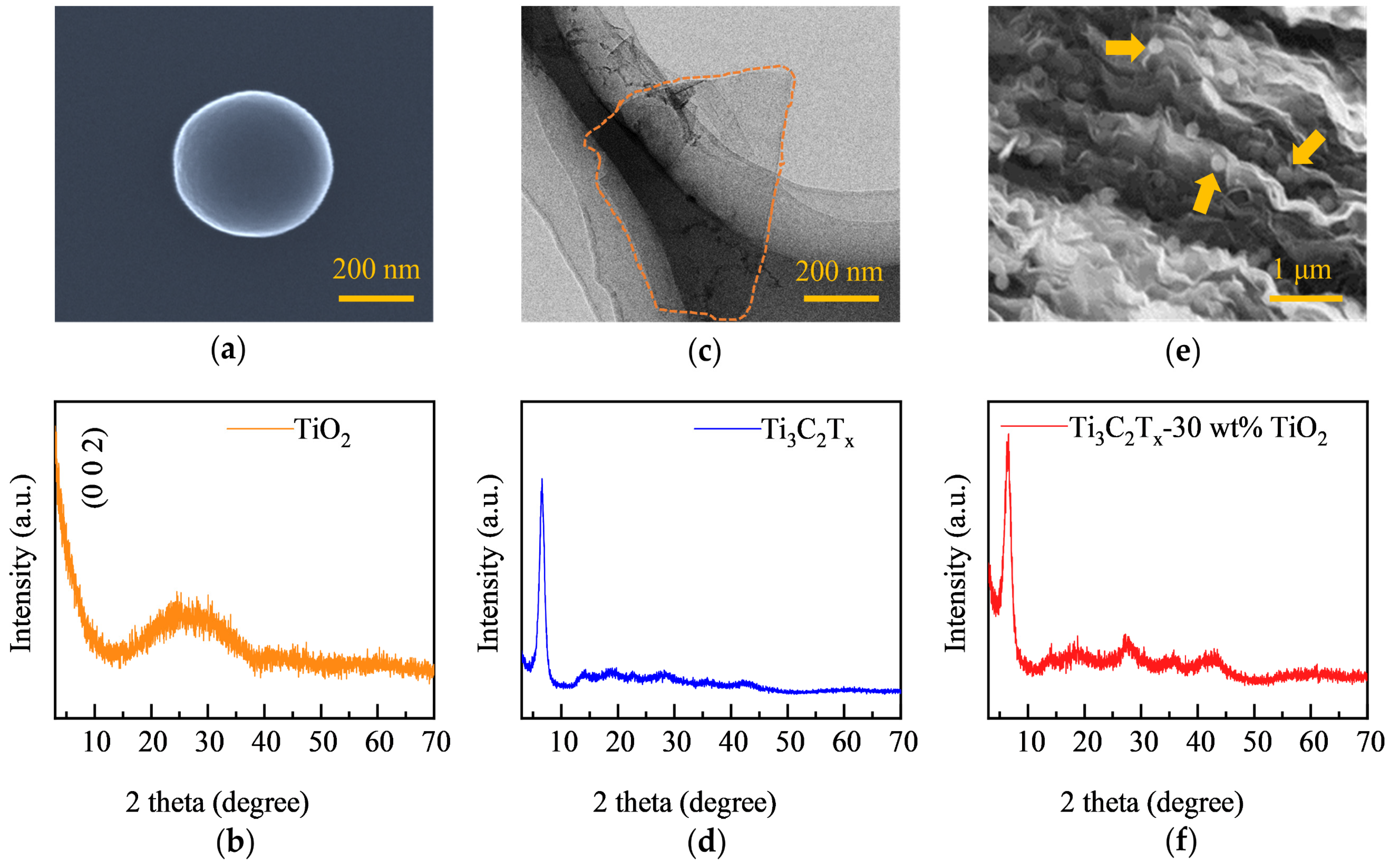
3.1. Structure Characterization
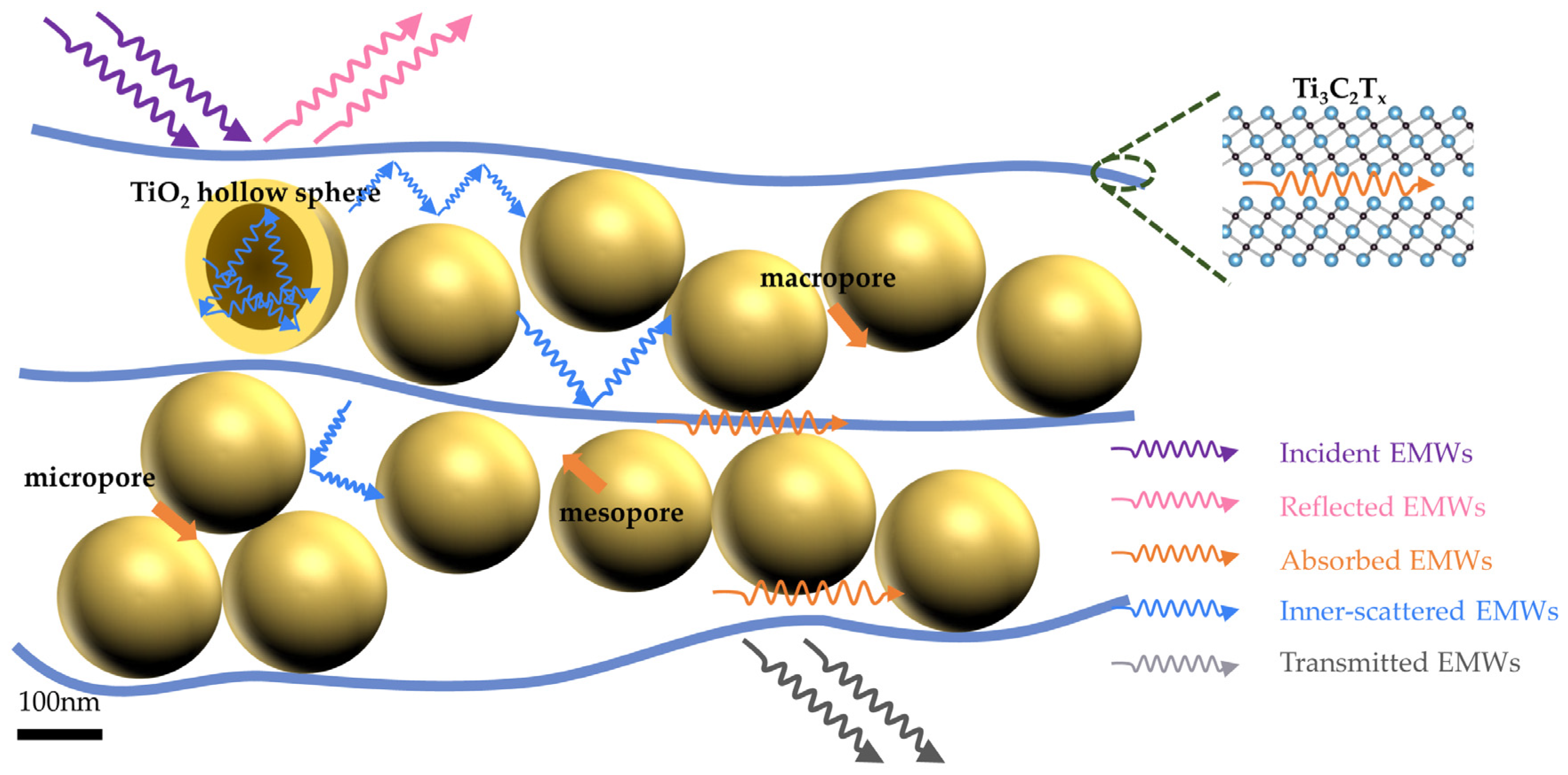
3.2. EMI Shielding Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Materials
Appendix A.2. Synthesis of Ti3C2Tx
Appendix A.3. Synthesis of TiO2 and Hollow Spheres
Appendix A.4. Fabrication of Ti3C2Tx/SiO2 Nanocomposite Films
Appendix A.5. Characterizations
References
- Raagulan, K.; Kim, B.M.; Chai, K.Y. Recent Advancement of Electromagnetic Interference (EMI) Shielding of Two Dimensional (2D) MXene and Graphene Aerogel Composites. Nanomaterials 2020, 10, 702. [Google Scholar] [CrossRef]
- Nazir, A.; Yu, H.; Wang, L.; Haroon, M.; Ullah, R.; Fahad, S.; Elshaarani, T.; Khan, A.; Usman, M. Recent progress in the modification of carbon materials and their application in composites for electromagnetic interference shielding. J. Mater. Sci. 2018, 53, 8699–8719. [Google Scholar] [CrossRef]
- Ramirez-Herrera, C.A.; Gonzalez, H.; Torre, F.; Benitez, L.; Cabanas-Moreno, J.G.; Lozano, K. Electrical Properties and Electromagnetic Interference Shielding Effectiveness of Interlayered Systems Composed by Carbon Nanotube Filled Carbon Nanofiber Mats and Polymer Composites. Nanomaterials 2019, 9, 238. [Google Scholar] [CrossRef]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Man Hong, S.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef]
- Fan, Z.M.; Wang, D.L.; Yuan, Y.; Wang, Y.S.; Cheng, Z.J.; Liu, Y.Y.; Xie, Z.M. A lightweight and conductive MXene/graphene hybrid foam for superior electromagnetic interference shielding. Chem. Eng. J. 2020, 381, 122696. [Google Scholar] [CrossRef]
- Yun, T.; Kim, H.; Iqbal, A.; Cho, Y.S.; Lee, G.S.; Kim, M.K.; Kim, S.J.; Kim, D.; Gogotsi, Y.; Kim, S.O.; et al. Electromagnetic Interference Shielding: Electromagnetic Shielding of Monolayer MXene Assemblies. Adv. Mater. 2020, 32, 2070064. [Google Scholar] [CrossRef]
- Singh, A.K.; Shishkin, A.; Koppel, T.; Gupta, N. A review of porous lightweight composite materials for electromagnetic interference shielding. Compos. Part B 2018, 149, 188–197. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Legum, B.; Anasori, B.; Wang, K.; Lelyukh, P.; Gogotsi, Y.; Randall, C.A. Cold Sintered Ceramic Nanocomposites of 2D MXene and Zinc Oxide. Adv. Mater. 2018, 30, e1801846. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.X.; Wang, J.F.; Dai, L.Z.; Liu, X.Y.; Li, L.; Yang, Y.Y.; Cao, Y.X.; Wang, W.J.; Wu, H.; Guo, S.Y. Flame-retardant poly(vinyl alcohol)/MXene multilayered films with outstanding electromagnetic interference shielding and thermal conductive performances. Chem. Eng. J. 2020, 380, 122475. [Google Scholar] [CrossRef]
- Iqbal, A.; Shahzad, F.; Hantanasirisakul, K.; Kim, M.K.; Kwon, J.; Hong, J.; Kim, H.; Kim, D.; Gogotsi, Y.; Koo, C.M. Anomalous absorption of electromagnetic waves by 2D transition metal carbonitride Ti3CNTx (MXene). Science 2020, 369, 446–450. [Google Scholar] [CrossRef]
- Koo, C.M.; Sambyal, P.; Iqbal, A.; Shahzad, F.; Hong, J. Two-Dimensional Materials for Electromagnetic Shielding; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Lee, S.H.; Yu, S.; Shahzad, F.; Kim, W.N.; Park, C.; Hong, S.M.; Koo, C.M. Density-tunable lightweight polymer composites with dual-functional ability of efficient EMI shielding and heat dissipation. Nanoscale 2017, 9, 13432–13440. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Shim, J.J. Cutting edge composite materials based on MXenes: Synthesis and electromagnetic interference shielding applications. Compos. Part B Eng. 2023, 2023, 110874. [Google Scholar] [CrossRef]
- Idumah, C.I. Recent advancements in electromagnetic interference shielding of polymer and MXene nanocomposites. Polym.-Plast. Technol. Mater. 2023, 62, 19–53. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, J.; Zou, Y.; Zheng, W.; Wang, Y.; Liu, Y.; Zhang, H.; Zhang, D.; Ji, X. Current advances and future perspectives of MXene-based electromagnetic interference shielding materials. Adv. Compos. Hybrid Mater. 2023, 6, 172. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Tan, Y.; Luo, H.; Zhou, X.; Peng, S.; Zhang, H. Dependences of microstructure on electromagnetic interference shielding properties of nano-layered Ti3AlC2 ceramics. Sci. Rep. 2018, 8, 7935. [Google Scholar] [CrossRef]
- Sambyal, P.; Iqbal, A.; Hong, J.; Kim, H.; Kim, M.K.; Hong, S.M.; Han, M.; Gogotsi, Y.; Koo, C.M. Ultralight and mechanically robust Ti3C2Tx hybrid aerogel reinforced by carbon nanotubes for electromagnetic interference shielding. ACS Appl. Mater. Interfaces 2019, 11, 38046–38054. [Google Scholar] [CrossRef]
- Aïssa, B.; Sinopoli, A.; Ali, A.; Zakaria, Y.; Zekri, A.; Helal, M.; Nedil, M.; Rosei, F.; Mansour, S.; Mahmoud, K.A. Nanoelectromagnetic of a highly conductive 2D transition metal carbide (MXene)/Graphene nanoplatelets composite in the EHF M-band frequency. Carbon 2021, 173, 528–539. [Google Scholar] [CrossRef]
- Iqbal, A.; Sambyal, P.; Kwon, J.; Han, M.; Hong, J.; Kim, S.J.; Kim, M.K.; Gogotsi, Y.; Koo, C.M. Enhanced absorption of electromagnetic waves in Ti3C2Tx MXene films with segregated polymer inclusions. Compos. Sci. Technol. 2021, 213, 108878. [Google Scholar] [CrossRef]
- Liang, L.; Han, G.; Li, Y.; Zhao, B.; Zhou, B.; Feng, Y.; Ma, J.; Wang, Y.; Zhang, R.; Liu, C. Promising Ti3C2Tx MXene/Ni Chain Hybrid with Excellent Electromagnetic Wave Absorption and Shielding Capacity. ACS Appl. Mater. Interfaces 2019, 11, 25399–25409. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Perelshtein, I.; Margel, S.; Gedanken, A. Acoustic green synthesis of graphene-gallium nanoparticles and PEDOT: PSS hybrid coating for textile to mitigate electromagnetic radiation pollution. ACS Appl. Nano Mater. 2022, 5, 1644–1655. [Google Scholar] [CrossRef]
- Jiang, D.W.; Murugadoss, V.; Wang, Y.; Lin, J.; Ding, T.; Wang, Z.C.; Shao, Q.; Wang, C.; Liu, H.; Lu, N.; et al. Electromagnetic Interference Shielding Polymers and Nanocomposites—A Review. Polym. Rev. 2019, 59, 280–337. [Google Scholar] [CrossRef]
- Shahzad, F.; Shahzad, F.; Shahzad, F.; Koo, C.M.; Koo, C.M.; Koo, C.M. Controlling the Electromagnetic and Electrochemical Sensing Properties of Graphene via Heteroatom Doping. In Handbook of Graphene; Wiley: Hoboken, NJ, USA, 2019; pp. 663–682. [Google Scholar] [CrossRef]
- Geetha, S.; Kumar, K.K.S.; Rao, C.R.K.; Vijayan, M.; Trivedi, D.C. EMI Shielding: Methods and Materials—A Review. J. Appl. Polym. Sci. 2009, 112, 2073–2086. [Google Scholar] [CrossRef]
- De Temmerman, L. New Metallized Materials for EMI/RFI Shielding. J. Coat. Fabr. 2016, 21, 191–198. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2TX MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Sambyal, P.; Singh, A.P.; Verma, M.; Farukh, M.; Singh, B.P.; Dhawan, S.K. Tailored polyaniline/barium strontium titanate/expanded graphite multiphase composite for efficient radar absorption. Rsc Adv. 2014, 4, 12614–12624. [Google Scholar] [CrossRef]
- Pitkanen, O.; Tolvanen, J.; Szenti, I.; Kukovecz, A.; Hannu, J.; Jantunen, H.; Kordas, K. Lightweight Hierarchical Carbon Nanocomposites with Highly Efficient and Tunable Electromagnetic Interference Shielding Properties. ACS Appl. Mater. Interfaces 2019, 11, 19331–19338. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Cao, M.; Cai, Y.; Shu, J.; Cao, W.; Yuan, J. Self-assembling flexible 2D carbide MXene film with tunable integrated electron migration and group relaxation toward energy storage and green EMI shielding. Carbon 2020, 157, 80–89. [Google Scholar] [CrossRef]
- Tian, K.; Hu, D.R.; Wei, Q.; Fu, Q.; Deng, H. Recent progress on multifunctional electromagnetic interference shielding polymer composites. J. Mater. Sci. Technol. 2023, 134, 106–131. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Wang, J.; Yang, Y.; Wu, S.; You, C.; Tian, N.; Li, Y. Structural evolution of MXenes and their composites for electromagnetic interference shielding applications. Nanoscale 2022, 14, 9218–9247. [Google Scholar] [CrossRef]
- Kusior, A.; Warchal, A.; Komornicki, S.; Radecka, M. Hard-template synthesis of titanium dioxide hollow spheres. Micro Nano Lett. 2014, 9, 721–725. [Google Scholar] [CrossRef]
- Han, M.; Shuck, C.E.; Rakhmanov, R.; Parchment, D.; Anasori, B.; Koo, C.M.; Friedman, G.; Gogotsi, Y. Beyond Ti3C2Tx: MXenes for Electromagnetic Interference Shielding. ACS Nano 2020, 14, 5008–5016. [Google Scholar] [CrossRef]
- Schelkunoff, S.A. Ultrashort electromagnetic waves IV—Guided propagation. Electr. Eng. 1943, 62, 235–246. [Google Scholar] [CrossRef]
- Schulz, R.B.; Plantz, V.C.; Brush, D.R. Shielding theory and practice. IEEE Trans. Electromagn. Compat. 1988, 30, 187–201. [Google Scholar] [CrossRef]
- Takaura, N.; Ohyanagi, T.; Tai, M.; Kinoshita, M.; Akita, K.; Morikawa, T.; Shirakawa, H.; Araidai, M.; Shiraishi, K.; Saito, Y.; et al. 55-μA Ge xTe1-x/Sb2Te3 superlattice topological-switching random access memory (TRAM) and study of atomic arrangement in Ge-Te and Sb-Te structures. In Proceedings of the International Electron Devices Meeting, San Francisco, CA, USA, 15–17 December 2014; IEEE: Piscataway, NJ, USA, 2014; p. 686. [Google Scholar]
- Zhao, B.; Hamidinejad, M.; Wang, S.; Bai, P.W.; Che, R.C.; Zhang, R.; Park, C.B. Advances in electromagnetic shielding properties of composite foams. J. Mater. Chem. A 2021, 9, 8896–8949. [Google Scholar] [CrossRef]
- Chung, D.D.L. Materials for electromagnetic interference shielding. Mater. Chem. Phys. 2020, 255, 123587. [Google Scholar] [CrossRef]
- Zhao, H.; Yun, J.; Zhang, Y.; Ruan, K.; Huang, Y.; Zheng, Y.; Chen, L.; Gu, J. Pressure-Induced Self-Interlocked Structures for Expanded Graphite Composite Papers Achieving Prominent EMI Shielding Effectiveness and Outstanding Thermal Conductivities. ACS Appl. Mater. Interfaces 2022, 14, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V. Review of electromagnetic interference shielding materials fabricated by iron ingredients. Nanoscale Adv. 2019, 1, 1640–1671. [Google Scholar] [CrossRef] [PubMed]
- Sohi, N.J.S.; Rahaman, M.; Khastgir, D. Dielectric Property and Electromagnetic Interference Shielding Effectiveness of Ethylene Vinyl Acetate-Based Conductive Composites: Effect of Different Type of Carbon Fillers. Polym. Compos. 2011, 32, 1148–1154. [Google Scholar] [CrossRef]
- Al-Saleh, M.H.; Sundararaj, U. Electromagnetic interference shielding mechanisms of CNT/polymer composites. Carbon 2009, 47, 1738–1746. [Google Scholar] [CrossRef]
- Iqbal, A.; Sambyal, P.; Koo, C.M. 2D MXenes for Electromagnetic Shielding: A Review. Adv. Funct. Mater. 2020, 30, 2000883. [Google Scholar] [CrossRef]
- Wen, C.; Li, X.; Zhang, R.; Xu, C.; You, W.; Liu, Z.; Zhao, B.; Che, R. High-Density Anisotropy Magnetism Enhanced Microwave Absorption Performance in Ti3C2Tx MXene@Ni Microspheres. ACS Nano 2022, 16, 1150–1159. [Google Scholar] [CrossRef]
- Yang, H.J.; Cao, W.Q.; Zhang, D.Q.; Su, T.J.; Shi, H.L.; Wang, W.Z.; Yuan, J.; Cao, M.S. NiO hierarchical nanorings on SiC: Enhancing relaxation to tune microwave absorption at elevated temperature. ACS Appl. Mater. Interfaces 2015, 7, 7073–7077. [Google Scholar] [CrossRef]
- Sayani, B.; Prashant, A. MXene: Evolutions in chemical synthesis and recent advances in applications. Surfaces 2022, 5, 1–34. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Cui, X.; Zeng, M.; Yu, R.; Wang, G. Flexible nanocomposites with enhanced microwave absorption properties based on Fe3O4/SiO2 nanorods and polyvinylidene fluoride. J. Mater. Chem. A 2015, 3, 12197–12204. [Google Scholar] [CrossRef]
- Han, M.K.; Yin, X.W.; Hantanasirisakul, K.; Li, X.L.; Iqbal, A.; Hatter, C.B.; Anasori, B.; Koo, C.M.; Torita, T.; Soda, Y.; et al. Anisotropic MXene Aerogels with a Mechanically Tunable Ratio of Electromagnetic Wave Reflection to Absorption. Adv. Opt. Mater. 2019, 7, 1900267. [Google Scholar] [CrossRef]



| Element | Sample | Binding Energy (eV) | Binding | Relative Content |
|---|---|---|---|---|
| Ti 2p | Ti3C2Tx | 455.5 (461.2) | C-Ti-Tx | 37.7% |
| 456.9 (462.4) | Ti2+ | 25.7% | ||
| 458.3 (463.9) | Ti3+ | 13.6% | ||
| 459.7 (465.5) | Ti-O | 23.0% | ||
| TiO2/Ti3C2Tx−30 wt.% | 455.2 (460.8) | C-Ti-Tx | 35.3% | |
| 456.4 (462.3) | Ti2+ | 19.8% | ||
| 457.8 (464.3) | Ti3+ | 10.4% | ||
| 459.5 (465.8) | Ti-O | 34.5% | ||
| O 1s | Ti3C2Tx | 530.7 | O-Ti | 21.6% |
| 533.3 | -OH | 72.4% | ||
| 534.8 | O-C/O=C | 6.0% | ||
| TiO2/Ti3C2Tx−30 wt.% | 530.7 | O-Ti | 51.7% | |
| 532.5 | -OH | 37.8% | ||
| 534.3 | O-C/O=C | 10.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Ning, X.; Liu, B.; Zhou, J.; Sun, Z. Self-Assembly TiO2-Ti3C2Tx Ball–Plate Structure for Highly Efficient Electromagnetic Interference Shielding. Materials 2024, 17, 72. https://doi.org/10.3390/ma17010072
Zhang Z, Ning X, Liu B, Zhou J, Sun Z. Self-Assembly TiO2-Ti3C2Tx Ball–Plate Structure for Highly Efficient Electromagnetic Interference Shielding. Materials. 2024; 17(1):72. https://doi.org/10.3390/ma17010072
Chicago/Turabian StyleZhang, Zhen, Xingyang Ning, Bin Liu, Jian Zhou, and Zhimei Sun. 2024. "Self-Assembly TiO2-Ti3C2Tx Ball–Plate Structure for Highly Efficient Electromagnetic Interference Shielding" Materials 17, no. 1: 72. https://doi.org/10.3390/ma17010072





