Efficient Chlorostannate Modification of Magnetite Nanoparticles for Their Biofunctionalization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Degassing of Water
2.3. Magnetite Nanoparticles Synthesis
2.4. Functionalization of MNP with Amino Groups (MNP-NH2)
2.5. Functionalization of MNP-NH2 with Carboxyl Groups (MNP-COOH)
2.6. Covalent Grafting of mAb-FA to MNP-COOH
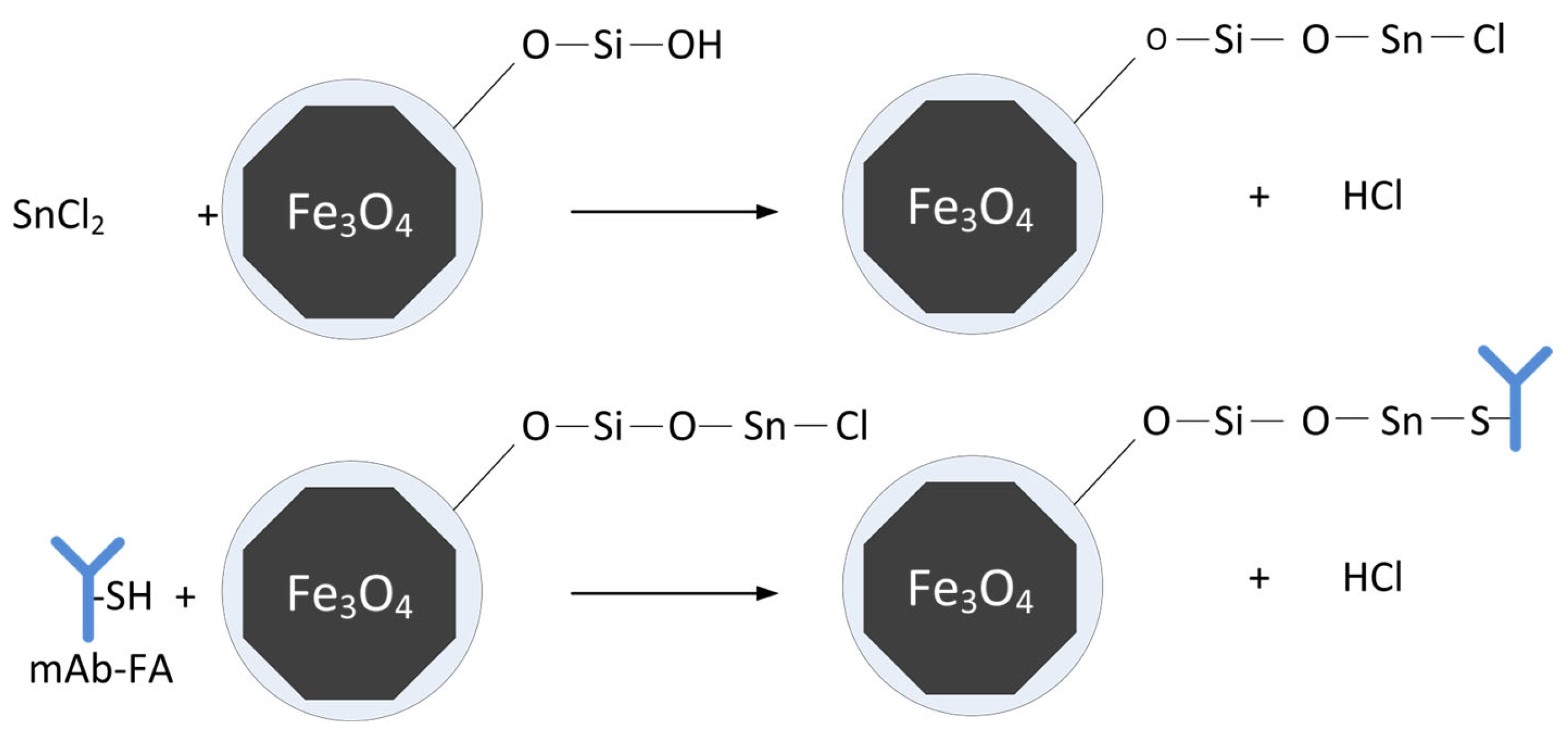
2.7. Functionalization of MNP with SnCl
2.8. Non-Covalent Grafting of mAb-FA to MNP-X
2.9. Study of mAb-FA Conjugation with MNP
2.10. Sample Preparation for X-ray Diffraction Measurements and Vibrating Sample Magnetometry
2.11. X-ray Diffraction Measurements
2.12. Transmission Electron Microscopy (TEM)
2.13. Vibrating Sample Magnetometry
2.14. Magnetic Particle Quantification
2.15. Colloidal Properties
3. Results and Discussion
3.1. MNP Characterization
3.1.1. XRD Study of MNP
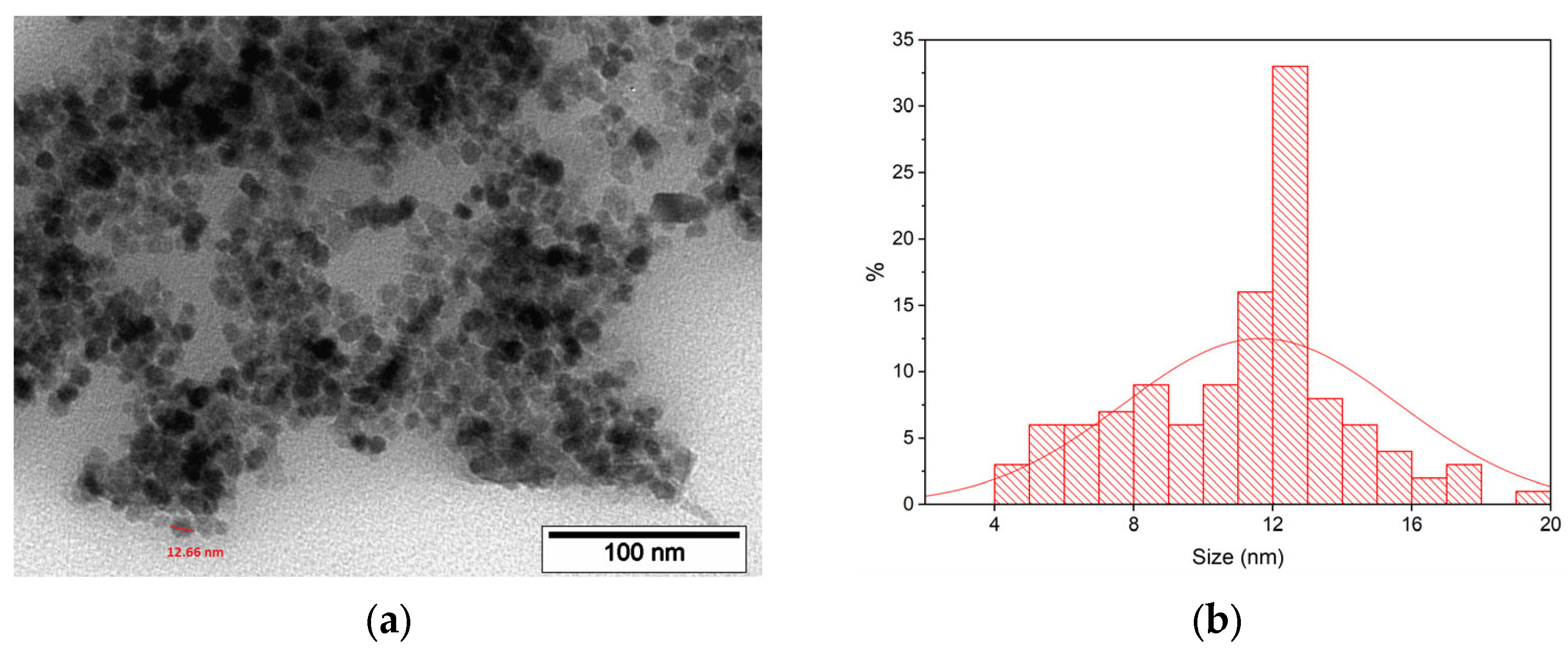
3.1.2. TEM Study of MNP
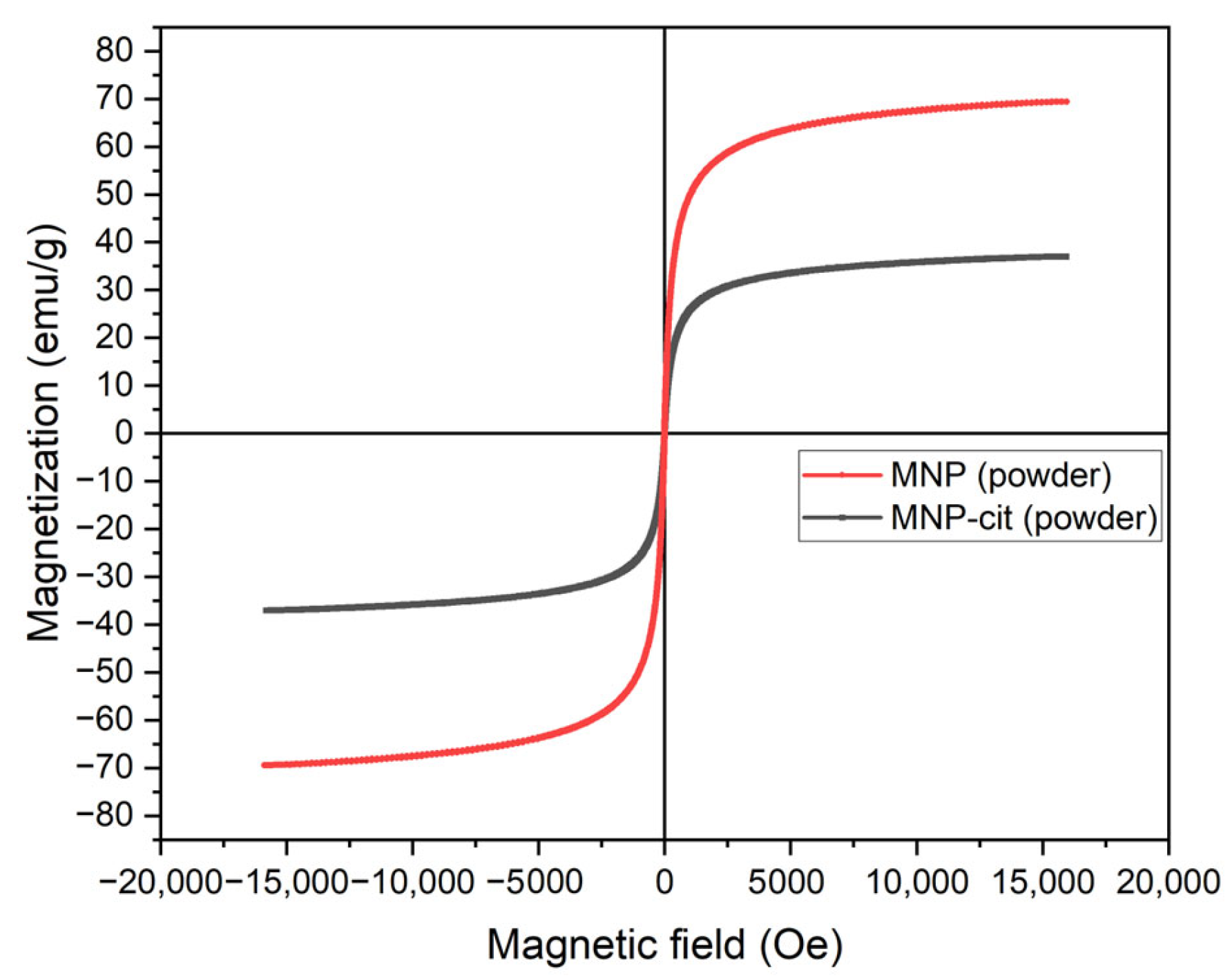
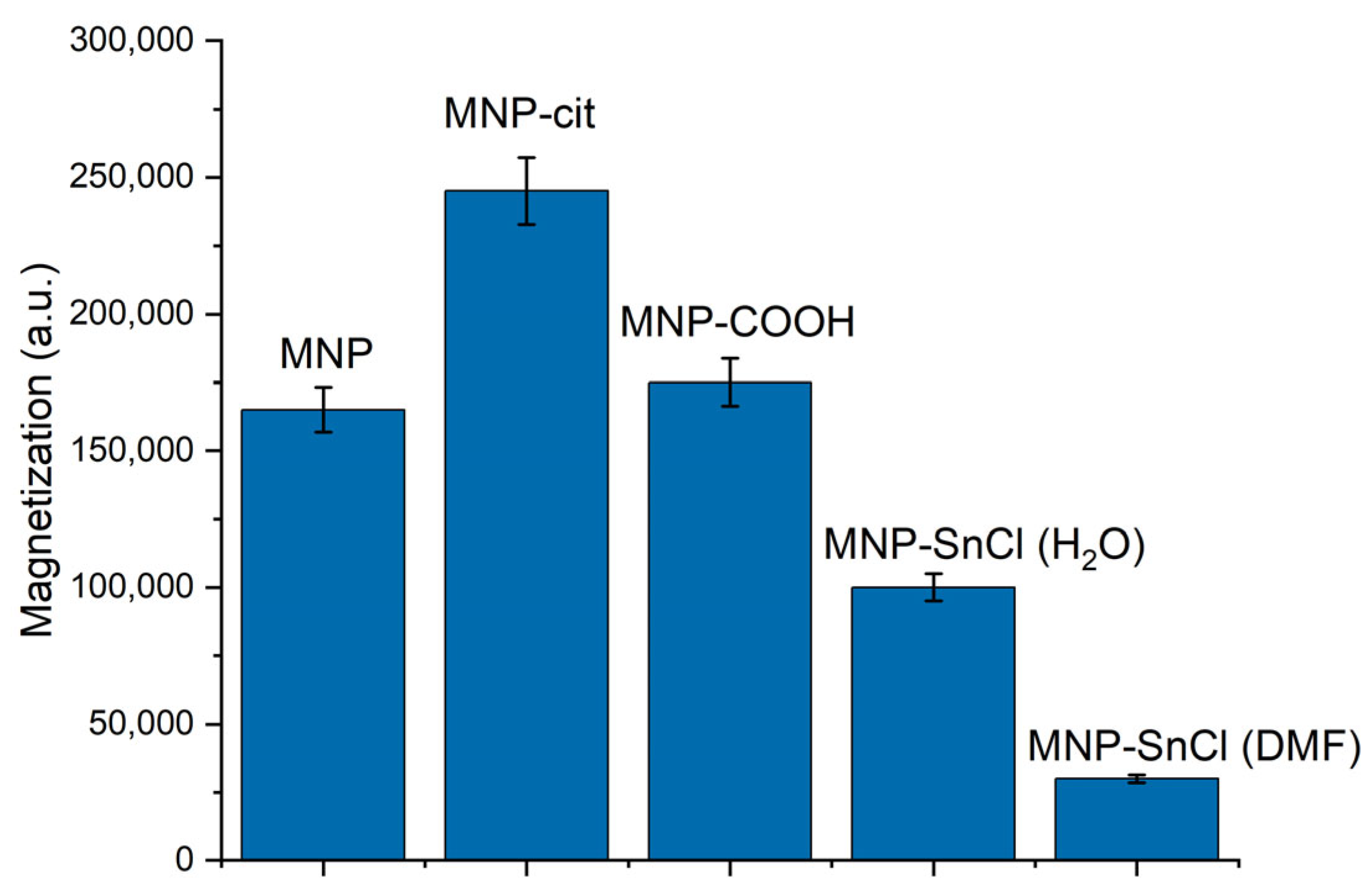
3.1.3. Magnetization Measurements of MNPs
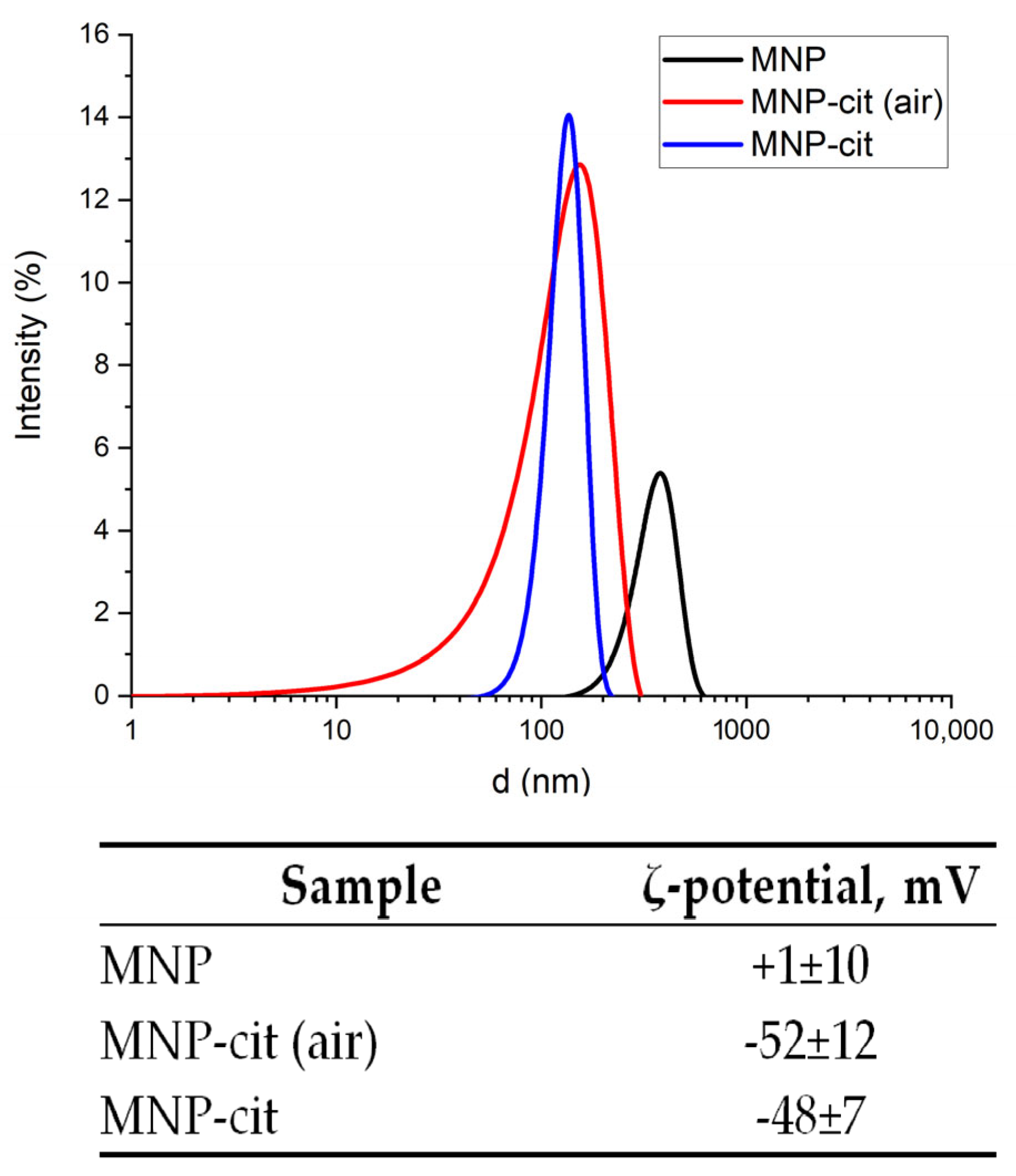
3.1.4. Colloidal Properties of As-Synthesized and Citrate-Stabilized MNP
3.1.5. Colloidal Properties of MNP-SiO2
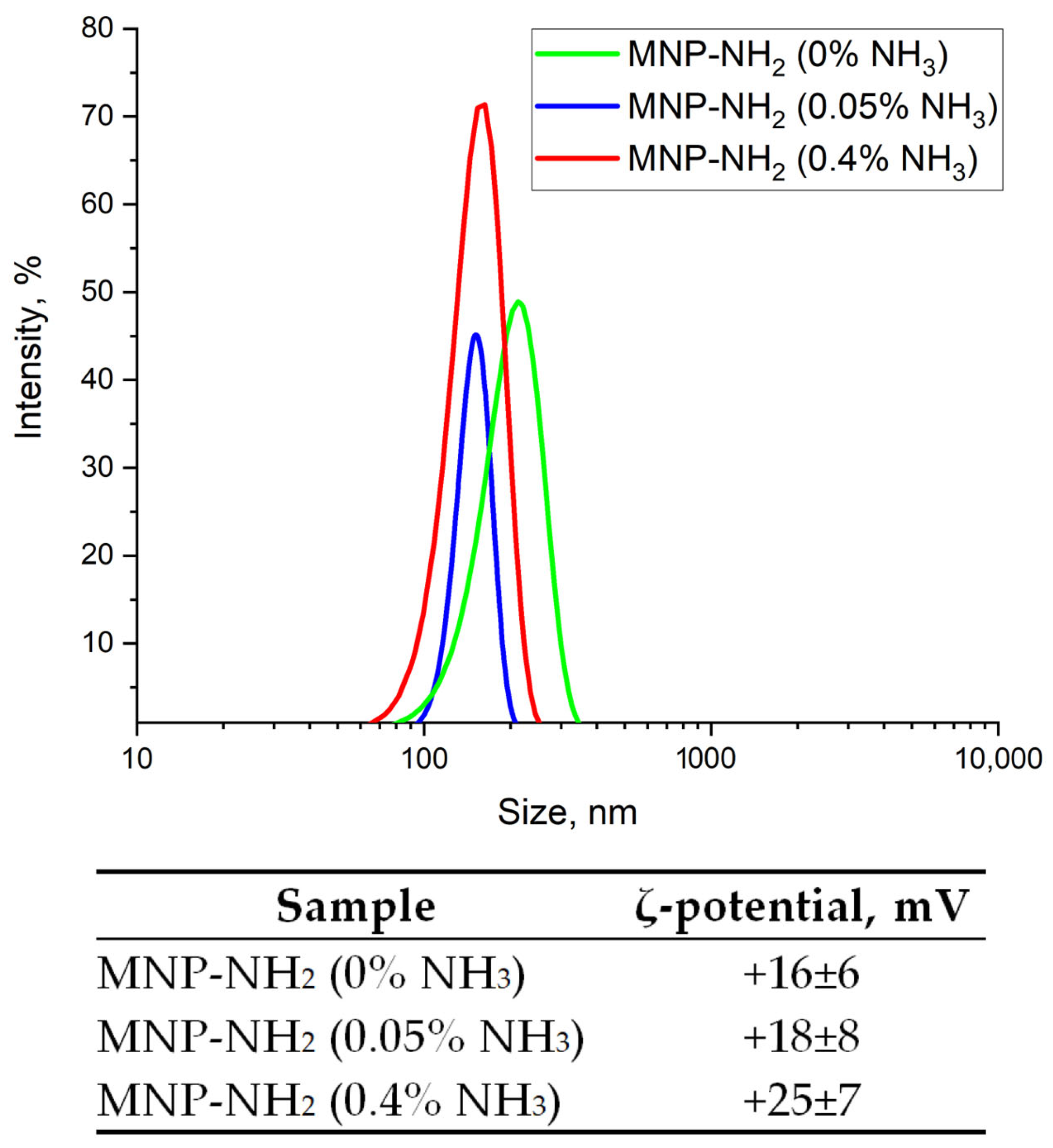
3.1.6. Colloidal Properties of MNP-NH2
3.1.7. Colloidal Properties of MNP-NH2 and MNP-COOH
3.2. Magnetic Immunochromatography Studies of MNP Conjugation with Antibodies
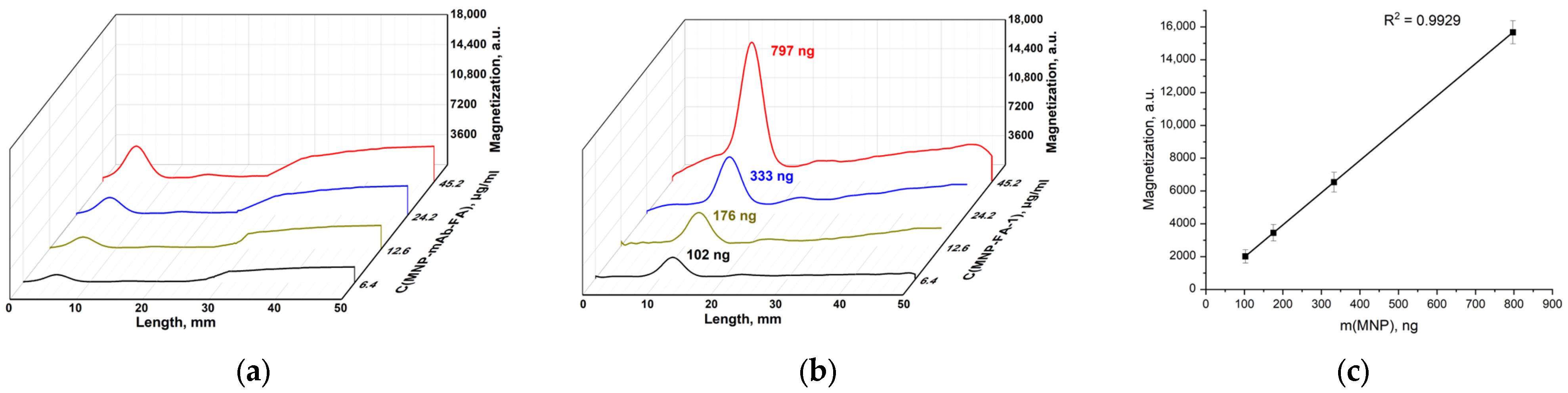
3.2.1. Conjugation of Pristine MNPs with mAb-FA
3.2.2. Conjugation of MNP-COOH with mAb-FA
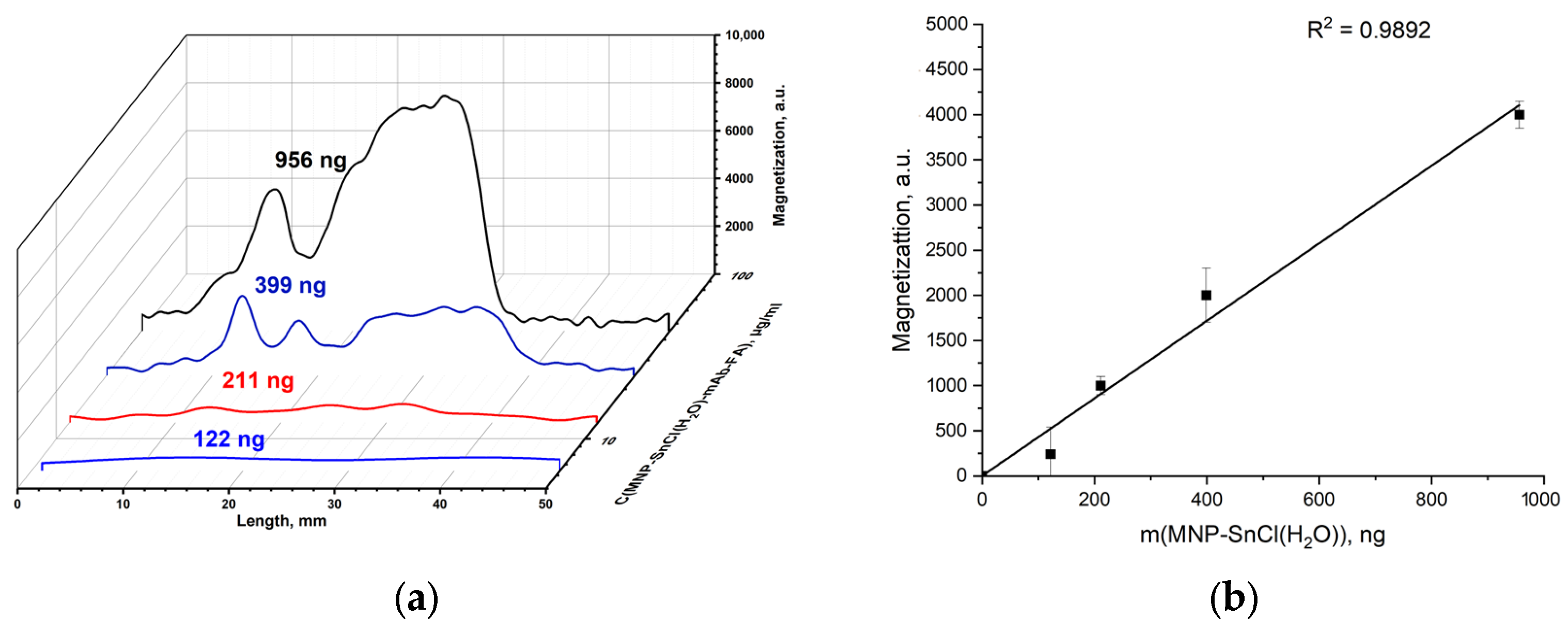
3.2.3. Conjugation of MNP-SnCl(H2O) with mAb-FA
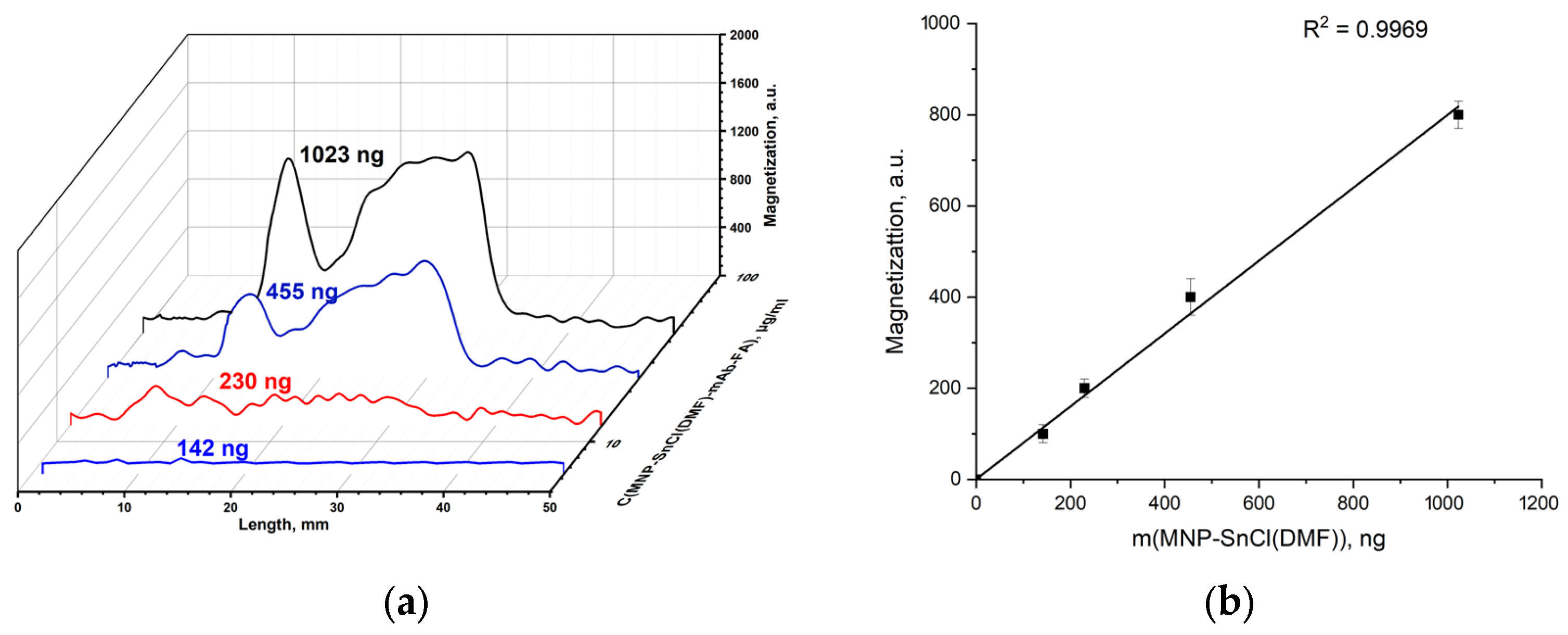
3.2.4. Conjugation of MNP-SnCl(DMF) with mAb-FA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niculescu, A.G.; Chircov, C.; Grumezescu, A.M. Magnetite Nanoparticles: Synthesis Methods—A Comparative Review. Methods 2022, 199, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Al-Madhagi, H.; Yazbik, V.; Abdelwahed, W.; Alchab, L. Magnetite Nanoparticle Co-Precipitation Synthesis, Characterization, and Applications: Mini Review. Bionanoscience 2023, 13, 853–859. [Google Scholar] [CrossRef]
- Wang, L.; Bao, J.; Wang, L.; Zhang, F.; Li, Y. One-Pot Synthesis and Bioapplication of Amine-Functionalized Magnetite Nanoparticles and Hollow Nanospheres. Chem.—Eur. J. 2006, 12, 6341–6347. [Google Scholar] [CrossRef]
- Nikitin, P.I.; Vetoshko, P.M.; Ksenevich, T.I. Magnetic Immunoassays. Sens. Lett. 2007, 5, 296–299. [Google Scholar] [CrossRef]
- Sperling, R.A.; Parak, W.J. Surface Modification, Functionalization and Bioconjugation of Colloidal Inorganic Nanoparticles. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 1333–1383. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Goud, K.Y. Magnetic Particle Bioconjugates: A Versatile Sensor Approach. Magnetochemistry 2019, 5, 64. [Google Scholar] [CrossRef]
- Bruce, I.J.; Sen, T. Surface Modification of Magnetic Nanoparticles with Alkoxysilanes and Their Application in Magnetic Bioseparations. Langmuir 2005, 21, 7029–7035. [Google Scholar] [CrossRef]
- Rashid, Z.; Shokri, F.; Abbasi, A.; Khoobi, M.; Zarnani, A.-H. Surface Modification and Bioconjugation of Anti-CD4 Monoclonal Antibody to Magnetic Nanoparticles as a Highly Efficient Affinity Adsorbent for Positive Selection of Peripheral Blood T CD4+ Lymphocytes. Int. J. Biol. Macromol. 2020, 161, 729–737. [Google Scholar] [CrossRef]
- Spoială, A.; Ilie, C.-I.; Crăciun, L.N.; Ficai, D.; Ficai, A.; Andronescu, E. Magnetite-Silica Core/Shell Nanostructures: From Surface Functionalization towards Biomedical Applications—A Review. Appl. Sci. 2021, 11, 11075. [Google Scholar] [CrossRef]
- Tregubov, A.A.; Sokolov, I.L.; Babenyshev, A.V.; Nikitin, P.I.; Cherkasov, V.R.; Nikitin, M.P. Magnetic Hybrid Magnetite/Metal Organic Framework Nanoparticles: Facile Preparation, Post-Synthetic Biofunctionalization and Tracking in Vivo with Magnetic Methods. J. Magn. Magn. Mater. 2018, 449, 590–596. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, X.; Liao, H.; Liang, Z.; Li, F.; Tian, J.; Ling, D. Artificially Engineered Cubic Iron Oxide Nanoparticle as a High-Performance Magnetic Particle Imaging Tracer for Stem Cell Tracking. ACS Nano 2020, 14, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Marcuello, C.; Chambel, L.; Rodrigues, M.S.; Ferreira, L.P.; Cruz, M.M. Magnetotactic Bacteria: Magnetism beyond Magnetosomes. IEEE Trans. Nanobiosci. 2018, 17, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Demortière, A.; Panissod, P.; Pichon, B.P.; Pourroy, G.; Guillon, D.; Donnio, B.; Bégin-Colin, S. Size-Dependent Properties of Magnetic Iron Oxide Nanocrystals. Nanoscale 2011, 3, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Škrátek, M.; Dvurečenskij, A.; Kluknavský, M.; Barta, A.; Bališ, P.; Mičurová, A.; Cigáň, A.; Eckstein-Andicsová, A.; Maňka, J.; Bernátová, I. Sensitive SQUID Bio-Magnetometry for Determination and Differentiation of Biogenic Iron and Iron Oxide Nanoparticles in the Biological Samples. Nanomaterials 2020, 10, 1993. [Google Scholar] [CrossRef] [PubMed]
- Winkler, R.; Ciria, M.; Ahmad, M.; Plank, H.; Marcuello, C. A Review of the Current State of Magnetic Force Microscopy to Unravel the Magnetic Properties of Nanomaterials Applied in Biological Systems and Future Directions for Quantum Technologies. Nanomaterials 2023, 13, 2585. [Google Scholar] [CrossRef]
- Franco, V.; Dodrill, B. Magnetic Measurement Techniques for Materials Characterization; Springer: Cham, Switzerland, 2021; ISBN 9783030704438. [Google Scholar]
- Villa, S.; Riani, P.; Locardi, F.; Canepa, F. Functionalization of Fe3O4 NPs by Silanization: Use of Amine (APTES) and Thiol (MPTMS) Silanes and Their Physical Characterization. Materials 2016, 9, 826. [Google Scholar] [CrossRef]
- Das, M.; Mishra, D.; Maiti, T.K.; Basak, A.; Pramanik, P. Bio-Functionalization of Magnetite Nanoparticles Using an Aminophosphonic Acid Coupling Agent: New, Ultradispersed, Iron-Oxide Folate Nanoconjugates for Cancer-Specific Targeting. Nanotechnology 2008, 19, 415101. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Teng, Z.; Huang, Y.; Chen, X.; Wang, Q. Size-Controlled Synthesis of Carboxyl-Functionalized Magnetite Particles: Effects of Molecular Weight of the Polymer and Aging. ACS Omega 2018, 3, 17904–17913. [Google Scholar] [CrossRef]
- Mylkie, K.; Nowak, P.; Rybczynski, P.; Ziegler-Borowska, M. Polymer-Coated Magnetite Nanoparticles for Protein Immobilization. Materials 2021, 14, 248. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Academic Press: London, UK, 2013; ISBN 9780123822390. [Google Scholar]
- Carnerero, J.M.; Jimenez-Ruiz, A.; Castillo, P.M.; Prado-Gotor, R. Covalent and Non-Covalent DNA–Gold-Nanoparticle Interactions: New Avenues of Research. ChemPhysChem 2017, 18, 17–33. [Google Scholar] [CrossRef]
- Mendizabal, E.; Ríos-Donato, N.; Jasso-Gastinel, C.F.; Verduzco-Navarro, I.P. Removal of Arsenate by Fixed-Bed Columns Using Chitosan-Magnetite Hydrogel Beads and Chitosan Hydrogel Beads: Effect of the Operating Conditions on Column Efficiency. Gels 2023, 9, 825. [Google Scholar] [CrossRef] [PubMed]
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored Functionalization of Iron Oxide Nanoparticles for MRI, Drug Delivery, Magnetic Separation and Immobilization of Biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Yeh, Y.C.; Rotello, V.M. Engineering the Nanoparticle-Protein Interface: Applications and Possibilities. Curr. Opin. Chem. Biol. 2010, 14, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Hassanin, I.A.; Elzoghby, A.O. Self-Assembled Non-Covalent Protein-Drug Nanoparticles: An Emerging Delivery Platform for Anti-Cancer Drugs. Expert Opin. Drug Deliv. 2020, 17, 1437–1458. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1997; ISBN 978-0-08-037941-8. [Google Scholar]
- Novikova, G.V.; Petrov, A.I.; Staloverova, N.A.; Shubin, A.A.; Dergachev, I.D. Complex Formation of Sn(II) with l-Cysteine: An IR, DTA/TGA and DFT Investigation. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2014, 122, 565–570. [Google Scholar] [CrossRef]
- Krebs, B.; Henkel, G. Transition-Metal Thiolates: From Molecular Fragments of Sulfidic Solids to Models for Active Centers in Biomolecules. Angew. Chem. Int. Ed. Engl. 1991, 30, 769–788. [Google Scholar] [CrossRef]
- Messing, R.A. A Stannous Bridge for Coupling Urease to Controlled Pore Titanium. Biotechnol. Bioeng. 1974, 16, 1419–1423. [Google Scholar] [CrossRef]
- Weetall, H.H. Preparation of Immobilized Proteins Covalently Coupled through Silane Coupling Agents to Inorganic Supports. Appl. Biochem. Biotechnol. 1993, 41, 157–188. [Google Scholar] [CrossRef]
- Parmegiani Marcucci, S.M.; Zanin, G.M.; Arroyo, P.A. Synthesis of SBA-15 and Pore-Expanded SBA-15 and Surface Modification with Tin for Covalent Lipase Immobilization. Microporous Mesoporous Mater. 2022, 337, 111951. [Google Scholar] [CrossRef]
- Zucca, P.; Sanjust, E. Inorganic Materials as Supports for Covalent Enzyme Immobilization: Methods and Mechanisms. Molecules 2014, 19, 14139–14194. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, M.; Li, S.; Liu, Y.; Liu, Z.; Ying, A. One-Pot Synthesis of 3,4-Dihydropyrimidine-2-One Derivatives via Biginelli Reactions Catalyzed by SnCl2@MNPs. Chin. J. Org. Chem. 2021, 41, 2743–2749. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z.; Liu, T.; Yu, F.; Zeng, J.; Zhang, Y.; Yin, L.; Liu, X.; Jiang, H.; Wang, X. Folic Acid-Modified Cerium-Doped Carbon Dots as Photoluminescence Sensors for Cancer Cells Identification and Fe(III) Detection. Chemosensors 2022, 10, 219. [Google Scholar] [CrossRef]
- Devendiran, R.M.; kumar Chinnaiyan, S.; Yadav, N.K.; Moorthy, G.K.; Ramanathan, G.; Singaravelu, S.; Sivagnanam, U.T.; Perumal, P.T. Green Synthesis of Folic Acid-Conjugated Gold Nanoparticles with Pectin as Reducing/Stabilizing Agent for Cancer Theranostics. RSC Adv. 2016, 6, 29757–29768. [Google Scholar] [CrossRef]
- Novichikhin, D.O.; Orlov, A.V.; Antopolsky, M.L.; Znoyko, S.L.; Nikitin, P.I. Specific and Sensitive Determination of Folic Acid by Label-Free Chemosensors with Microscope Glass Slips as Single-Use Consumables. Chemosensors 2023, 11, 17. [Google Scholar] [CrossRef]
- Dhar, M.; Bellevue, R.; Carmel, R. Pernicious Anemia with Neuropsychiatric Dysfunction in a Patient with Sickle Cell Anemia Treated with Folate Supplementation. N. Engl. J. Med. 2003, 348, 2204–2207. [Google Scholar] [CrossRef] [PubMed]
- Orlov, A.V.; Burenin, A.G.; Skirda, A.M.; Nikitin, P.I. Kinetic Analysis of Prostate-Specific Antigen Interaction with Monoclonal Antibodies for Development of a Magnetic Immunoassay Based on Nontransparent Fiber Structures. Molecules 2022, 27, 8077. [Google Scholar] [CrossRef]
- Znoyko, S.L.; Orlov, A.V.; Bragina, V.A.; Nikitin, M.P.; Nikitin, P.I. Nanomagnetic Lateral Flow Assay for High-Precision Quantification of Diagnostically Relevant Concentrations of Serum TSH. Talanta 2020, 216, 120961. [Google Scholar] [CrossRef]
- Morozov, V.N.; Kanev, I.L.; Mikheev, A.Y.; Shlyapnikova, E.A.; Shlyapnikov, Y.M.; Nikitin, M.P.; Nikitin, P.I.; Nwabueze, A.O.; van Hoek, M.L. Generation and Delivery of Nanoaerosols from Biological and Biologically Active Substances. J. Aerosol Sci. 2014, 69, 48–61. [Google Scholar] [CrossRef]
- Gabashvili, A.N.; Chmelyuk, N.S.; Oda, V.V.; Leonova, M.K.; Sarkisova, V.A.; Lazareva, P.A.; Semkina, A.S.; Belyakov, N.A.; Nizamov, T.R.; Nikitin, P.I. Magnetic and Fluorescent Dual-Labeled Genetically Encoded Targeted Nanoparticles for Malignant Glioma Cell Tracking and Drug Delivery. Pharmaceutics 2023, 15, 2422. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Ortega-Muñoz, M.; Plesselova, S.; Delgado, A.V.; Santoyo-Gonzalez, F.; Salto-Gonzalez, R.; Giron-Gonzalez, M.D.; Iglesias, G.R.; López-Jaramillo, F.J. Poly(Ethylene-Imine)-Functionalized Magnetite Nanoparticles Derivatized with Folic Acid: Heating and Targeting Properties. Polymers 2021, 13, 1599. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Z.; Ma, J.; Zhou, T.; Wu, Z.; Ding, P.; Sun, N.; Liu, L.; Pei, R.; Zhu, W. Folic Acid-Modified Fluorescent-Magnetic Nanoparticles for Efficient Isolation and Identification of Circulating Tumor Cells in Ovarian Cancer. Biosensors 2022, 12, 184. [Google Scholar] [CrossRef]
- Bao, X.; Mao, Y.; Si, G.; Kang, L.; Xu, B.; Gu, N. Iron Oxide Nanoparticles: A Promising Approach for Diagnosis and Treatment of Cardiovascular Diseases. Nano Res. 2023, 16, 12453–12470. [Google Scholar] [CrossRef]
- Garello, F.; Svenskaya, Y.; Parakhonskiy, B.; Filippi, M. On the Road to Precision Medicine: Magnetic Systems for Tissue Regeneration, Drug Delivery, Imaging, and Theranostics. Pharmaceutics 2023, 15, 1812. [Google Scholar] [CrossRef] [PubMed]
- Marasini, S.; Yue, H.; Ho, S.-L.; Park, J.-A.; Kim, S.; Yang, J.-U.; Cha, H.; Liu, S.; Tegafaw, T.; Ahmad, M.Y.; et al. In Vivo Positive Magnetic Resonance Imaging of Brain Cancer (U87MG) Using Folic Acid-Conjugated Polyacrylic Acid-Coated Ultrasmall Manganese Oxide Nanoparticles. Appl. Sci. 2021, 11, 2596. [Google Scholar] [CrossRef]
- Movendane, Y.; Sipalo, M.G.; Chan, L.C.Z. Advances in Folic Acid Biosensors and Their Significance in Maternal, Perinatal, and Paediatric Preventive Medicine. Biosensors 2023, 13, 912. [Google Scholar] [CrossRef]
- Almethen, A.A.; Alotaibi, K.M.; Alhumud, H.S.; Alswieleh, A.M. Highly Efficient and Rapid Removal of Methylene Blue from Aqueous Solution Using Folic Acid-Conjugated Dendritic Mesoporous Silica Nanoparticles. Processes 2022, 10, 705. [Google Scholar] [CrossRef]
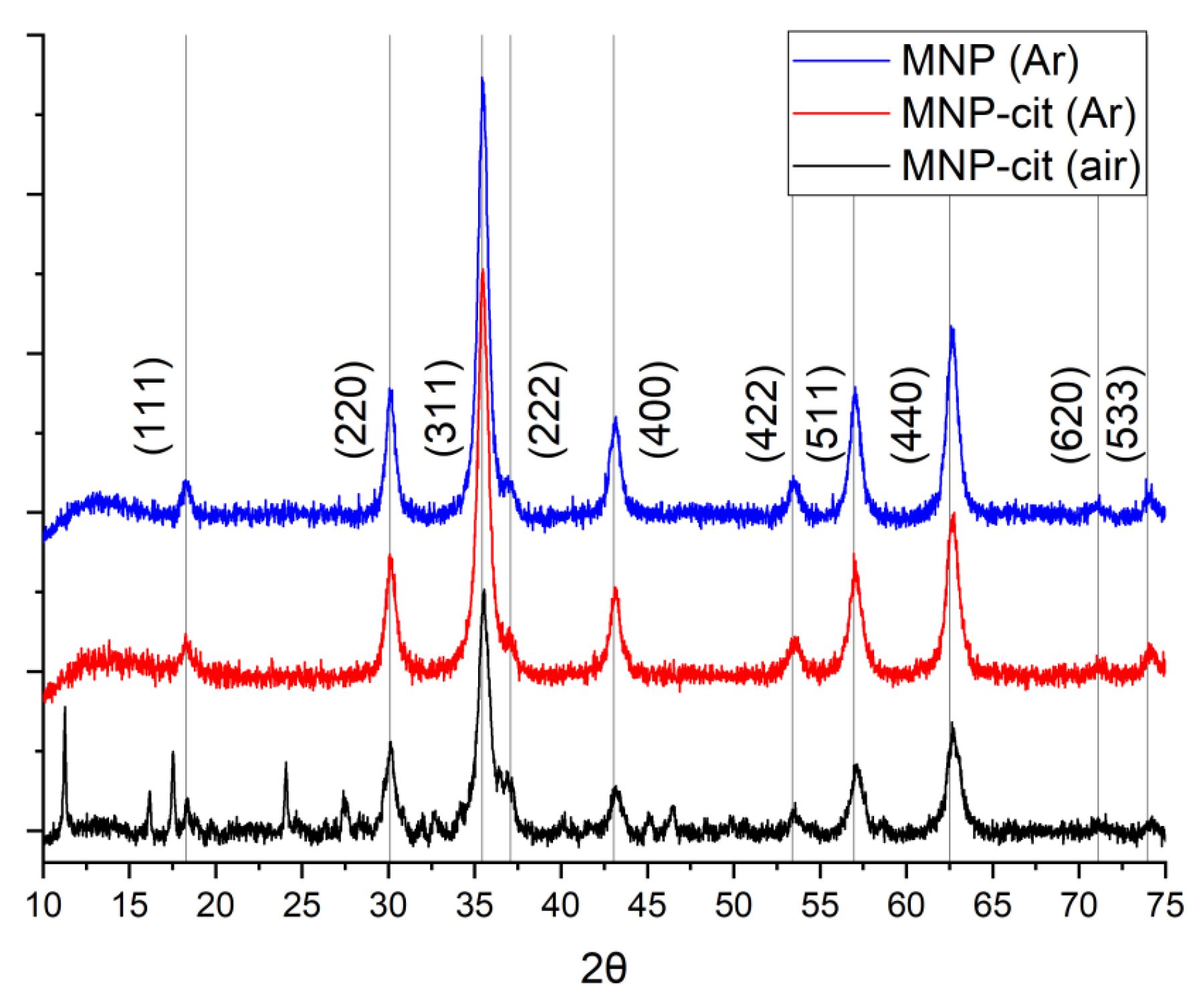



| MNP (Ar) | MNP-Cit (Ar) | MNP (Air) | |
|---|---|---|---|
| d, nm | 12.32 | 11.93 | 11.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zolotova, M.O.; Znoyko, S.L.; Orlov, A.V.; Nikitin, P.I.; Sinolits, A.V. Efficient Chlorostannate Modification of Magnetite Nanoparticles for Their Biofunctionalization. Materials 2024, 17, 349. https://doi.org/10.3390/ma17020349
Zolotova MO, Znoyko SL, Orlov AV, Nikitin PI, Sinolits AV. Efficient Chlorostannate Modification of Magnetite Nanoparticles for Their Biofunctionalization. Materials. 2024; 17(2):349. https://doi.org/10.3390/ma17020349
Chicago/Turabian StyleZolotova, Maria O., Sergey L. Znoyko, Alexey V. Orlov, Petr I. Nikitin, and Artem V. Sinolits. 2024. "Efficient Chlorostannate Modification of Magnetite Nanoparticles for Their Biofunctionalization" Materials 17, no. 2: 349. https://doi.org/10.3390/ma17020349






