Effective Carbon Dioxide Mitigation and Improvement of Compost Nutrients with the Use of Composts’ Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock and Biochar Characteristics
2.2. Experiments Configuration
2.3. Analyses of Process Gas Emissions
- Conversion to volume, for H2S, CO and NH3 (ppm):
- V—gas volume;
- M—molar mas;
- a—accumulated concentration, ppm.
- Conversion to volume, applies to CO2 (%) only:
- V—gas volume;
- M—molar mas;
- a—accumulated concentration, ppm.
- Specific emissions, applies to H2S, CO, NH3 (ppm):
- E—emissions, µg∙g d.m.;
- V—gas volume;
- DTS—dry total solids.
- Specific emissions, applies to CO2 (%) only:
- E—emissions, µg∙g d.m.;
- V—gas volume;
- DTS—dry total solids.
2.4. Statistical Analysis
3. Results and Discussion
3.1. Compost, Feedstock and Compost Biochars’ Characteristics
FTIR-ATR Spectroscopy
3.2. Effect of Biochars on Composting
3.2.1. Effect of Biochars on pH and LOI during the Initial Stage of Composting
3.2.2. The Emission Change
- CO2
- ○
- 429.1–565.0 mg∙g−1 d.m. for incubation at 50 °C;
- ○
- 410.8–522.5 mg∙g−1 d.m. for incubation at 60 °C;
- ○
- 153.4–270.9 mg∙g−1 d.m. for incubation at 70 °C.
- CO
- ○
- 477.7–653.5 µg∙g−1 d.m. for incubation at 50 °C;
- ○
- 503.5–861.9 µg∙g−1 d.m. for incubation at 60 °C;
- ○
- 508.8–784.1 µg∙g−1 d.m. for incubation at 70°C.
- H2S
- ○
- 60.4–85.5 µg∙g−1 d.m. for incubation at 50 °C;
- ○
- 129.4–191.5 µg∙g−1 d.m. for incubation at 60 °C;
- ○
- 52.8–85.3 µg∙g−1 d.m. for incubation at 70 °C.
- NH3
- ○
- 0.0–34.1 µg∙g−1 d.m. for incubation at 50 °C;
- ○
- 59.0–188.5 µg∙g−1 d.m. for incubation at 60 °C;
- ○
- 0.0–119.1 µg∙g−1 d.m. for incubation at 70 °C.
CO2 Emissions
CO Emissions
H2S Emissions
NH3 Emissions
3.2.3. Effect of Biochars on Nutrients and Heavy Metal Content during Initial Stages of Composting
C, H, N, S Changes
Nutrients and Heavy Metal Changes
4. Conclusions
- The use of compost biochars was the most effective in CO2 mitigation—total reduction in emissions > 80% at 60 °C; lower reduction < 20% at 50 and 70 °C.
- The effect for CO, H2S, and NH3 mitigation was unclear; surprisingly, the reduction in CO emissions was observed at a temperature of 50 °C, with 30–60% effectiveness for B550, and 20–30% for B600; however, at 70 °C, an increase of >300% in CO emissions was observed; for H2S, the most effective was incubation at 50 °C–a reduction > 50%, with no positive effect for 60 and 70 °C.
- No positive effect for NH3 mitigation was observed, probably due to the high pH content of biochar.
- The addition of small doses (3–6% d.m.) of compost biochars reduced the observed emissions and significantly improved (the best treatment was B650, incubation at 60 °C, with 3% of the addition of biochar) the content of nutrients in composting matrix (P, K, Mg, Ca). In the same treatments, the content of trace metals was safe for the future use of compost in agriculture.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, X.; Yin, H.; Han, L.; Cui, R.; Fang, C.; Huang, G. Effects of Biochar Size and Type on Gaseous Emissions during Pig Manure/Wheat Straw Aerobic Composting: Insights into Multivariate-Microscale Characterization and Microbial Mechanism. Bioresour. Technol. 2019, 271, 375–382. [Google Scholar] [CrossRef]
- Kweku, D.W.; Bismark, O.; Maxwell, A.; Desmond, K.A.; Danso, K.B.; Asante Oti-Mensah, E.; Quachie, A.T.; Adormaa, B.B.; Dopico, E. Greenhouse Effect: Greenhouse Gases and Their Impact on Global Warming. J. Sci. Res. Rep. 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Bai, Z.; Chadwick, D.; Misselbrook, T.; Sommer, S.G.; Qin, W.; Ma, L. Mitigation of Ammonia, Nitrous Oxide and Methane Emissions during Solid Waste Composting with Different Additives: A Meta-Analysis. J. Clean. Prod. 2019, 235, 626–635. [Google Scholar] [CrossRef]
- Guan, P.; Prasher, S.O.; Afzal, M.T.; George, S.; Ronholm, J.; Dhiman, J.; Patel, R.M. Removal of Escherichia Coli from Lake Water in a Biochar-Amended Biosand Filtering System. Ecol. Eng. 2020, 150, 105819. [Google Scholar] [CrossRef]
- Ermolaev, E.; Jarvis, Å.; Sundberg, C.; Smårs, S.; Pell, M.; Jönsson, H. Nitrous Oxide and Methane Emissions from Food Waste Composting at Different Temperatures. Waste Manag. 2015, 46, 113–119. [Google Scholar] [CrossRef]
- Yang, Y.; Kumar Awasthi, M.; Du, W.; Ren, X.; Lei, T.; Lv, J. Compost Supplementation with Nitrogen Loss and Greenhouse Gas Emissions during Pig Manure Composting. Bioresour. Technol. 2020, 297, 122435. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Zeng, C.; Li, Y.; Zhu, L.; Wu, J.; Chen, J.; Wei, Z. Assessment Contributions of Physicochemical Properties and Bacterial Community to Mitigate the Bioavailability of Heavy Metals during Composting Based on Structural Equation Models. Bioresour. Technol. 2019, 289, 121657. [Google Scholar] [CrossRef] [PubMed]
- Keiluweit, M.; Nico, P.S.; Johnson, M.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cao, X.; Zhao, L.; Zhou, H.; Luo, Q. Interaction of Organic and Inorganic Fractions of Biochar with Pb(II) Ion: Further Elucidation of Mechanisms for Pb(II) Removal by Biochar. RSC Adv. 2014, 4, 44930–44937. [Google Scholar] [CrossRef]
- Wang, H.; He, Z.; Lu, Z.; Zhou, J.; Van Nostrand, J.D.; Xu, X.; Zhang, Z. Genetic Linkage of Soil Carbon Pools and Microbial Functions in Subtropical Freshwater Wetlands in Response to Experimental Warming. Appl. Environ. Microbiol. 2012, 78, 7652–7661. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; De Yang, G.; Zeng, G.M.; Cai, Y.; Li, S.S.; Zhou, Y.Y.; Pang, Y.; Liu, Y.Y.; Zhang, Y.; Luna, B. Synergistic Effect of Iron Doped Ordered Mesoporous Carbon on Adsorption-Coupled Reduction of Hexavalent Chromium and the Relative Mechanism Study. Chem. Eng. J. 2014, 239, 114–122. [Google Scholar] [CrossRef]
- Albert, H.A.; Li, X.; Jeyakumar, P.; Wei, L.; Huang, L.; Huang, Q.; Kamran, M.; Shaheen, S.M.; Hou, D.; Rinklebe, J.; et al. Influence of Biochar and Soil Properties on Soil and Plant Tissue Concentrations of Cd and Pb: A Meta-Analysis. Sci. Total Environ. 2021, 755, 142582. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W.; Low, C.Y. Use of Biochar as Carbon Sequestering Additive in Cement Mortar. Cem. Concr. Compos. 2018, 87, 110–129. [Google Scholar] [CrossRef]
- Nan, H.; Yang, F.; Zhao, L.; Mašek, O.; Cao, X.; Xiao, Z. Interaction of Inherent Minerals with Carbon during Biomass Pyrolysis Weakens Biochar Carbon Sequestration Potential. ACS Sustain. Chem. Eng. 2019, 7, 1591–1599. [Google Scholar] [CrossRef]
- Ghorbani, M.; Neugschwandtner, R.W.; Konvalina, P.; Asadi, H.; Kopecký, M.; Amirahmadi, E. Comparative Effects of Biochar and Compost Applications on Water Holding Capacity and Crop Yield of Rice under Evaporation Stress: A Two-Years Field Study. Paddy Water Environ. 2023, 21, 47–58. [Google Scholar] [CrossRef]
- Li, Y.; Kumar Awasthi, M.; Sindhu, R.; Binod, P.; Zhang, Z.; Taherzadeh, M.J. Biochar Preparation and Evaluation of Its Effect in Composting Mechanism: A Review. Bioresour. Technol. 2023, 384, 129329. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Wang, M.; Chen, H.; Wang, Q.; Zhao, J.; Ren, X.; Li, D.S.; Awasthi, S.K.; Shen, F.; Li, R.; et al. Heterogeneity of Biochar Amendment to Improve the Carbon and Nitrogen Sequestration through Reduce the Greenhouse Gases Emissions during Sewage Sludge Composting. Bioresour. Technol. 2017, 224, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Duan, P.; Cao, Y.; Wang, K.; Li, D. Mechanisms of Mitigating Nitrous Oxide Emission during Composting by Biochar and Calcium Carbonate Addition. Bioresour. Technol. 2023, 388, 129772. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Samal, K. Sustainable Approach to Manage Solid Waste through Biochar Assisted Composting. Energy Nexus 2022, 7, 100121. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of Biochar and Greenwaste Compost Amendments on Mobility, Bioavailability and Toxicity of Inorganic and Organic Contaminants in a Multi-Element Polluted Soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef]
- Tang, W.W.; Zeng, G.M.; Gong, J.L.; Liang, J.; Xu, P.; Zhang, C.; Huang, B. Bin Impact of Humic/Fulvic Acid on the Removal of Heavy Metals from Aqueous Solutions Using Nanomaterials: A Review. Sci. Total Environ. 2014, 468–469, 1014–1027. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A Review of Biochars’ Potential Role in the Remediation, Revegetation and Restoration of Contaminated Soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef]
- Castro-Herrera, D.; Prost, K.; Kim, D.G.; Yimer, F.; Tadesse, M.; Gebrehiwot, M.; Brüggemann, N. Biochar Addition Reduces Non-CO2 Greenhouse Gas Emissions during Composting of Human Excreta and Cattle Manure. J. Environ. Qual. 2023, 52, 814–828. [Google Scholar] [CrossRef]
- Świechowski, K.; Liszewski, M.; Babelewski, P.; Koziel, J.A.; Białowiec, A. Oxytree Pruned Biomass Torrefaction: Mathematical Models of the Influence of Temperature and Residence Time on Fuel Properties Improvement. Materials 2019, 12, 2228. [Google Scholar] [CrossRef]
- Rosik, J.; Łyczko, J.; Marzec, Ł.; Stegenta-Dąbrowska, S. Application of Composts’ Biochar as Potential Sorbent to Reduce VOCs Emission during Kitchen Waste Storage. Materials 2023, 16, 6413. [Google Scholar] [CrossRef]
- PN-EN 14346:2011; Waste Characteristics. Calculation of Dry Mass on the Basis of Dry Residue or Water Content. Polski Komitet Normalizacji: Warsaw, Poland, 2011.
- Kozłowski, K.; Dach, J.; Czekała, W.; Malińska, K.; Świechowski, K.; Pulka, J.; Lewicki, A. Influence of the Parameters of Used Biochar on the Dark Fermentation Process. Energies 2023, 16, 7484. [Google Scholar] [CrossRef]
- PN-EN ISO 16948:2015-07; Solid Biofuels. Total Carbon, Ammonia and Hydrogen Determination. Polski Komitet Normalizacji: Warsaw, Poland, 2015.
- Zhao, S.X.; Ta, N.; Wang, X.D. Effect of Temperature on the Structural and Physicochemical Properties of Biochar with Apple Tree Branches as Feedstock Material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef]
- Bednik, M.; Medyńska-Juraszek, A.; Ćwieląg-Piasecka, I. Effect of Six Different Feedstocks on Biochar’s Properties and Expected Stability. Agronomy 2022, 12, 1525. [Google Scholar] [CrossRef]
- Karczewska, A. Metal Species Distribution in Top- and Sub-Soil in an Area Affected by Copper Smelter Emissions. Appl. Geochem. 1996, 11, 35–42. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Marcinkowska, K.; Gruszka, D.; Kluczek, K. The Effects of Rabbit-Manure-Derived Biochar Co-Application with Compost on the Availability and Heavy Metal Uptake by Green Leafy Vegetables. Agronomy 2022, 12, 2552. [Google Scholar] [CrossRef]
- PN-EN ISO 11885:2009; Water Quality: Determination of Selected Elements by Inductively Excited Plasma Optical Emission Spectrometry. Polski Komitet Normalizacji: Warsaw, Poland, 2009.
- Shin, Y.; Iwabuchi, K.; Itoh, T. Low-Temperature Biochars Are More Effective in Reducing Ammonia Emissions through Various Mechanisms during Manure Composting. J. Mater. Cycles Waste Manag. 2023, 26, 138–148. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Zhang, Z.; Duan, C.; Liu, Y.; Li, A.; Hu, X.; Chen, J.; Zhang, S.; Li, X.; Che, R.; Li, S.; et al. Green Waste and Sewage Sludge Feeding Ratio Alters Co-Composting Performance: Emphasis on the Role of Bacterial Community during Humification. Bioresour. Technol. 2023, 380, 129014. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Chen, M.; Zhuo, G.; Ji, R.; Wang, S.; Zhang, L.; Zhang, M.; Li, H. Comparison of Biochar Materials Derived from Coconut Husks and Various Types of Livestock Manure, and Their Potential for Use in Removal of H2s from Biogas. Sustainability 2021, 13, 6262. [Google Scholar] [CrossRef]
- Darby, I.; Xu, C.Y.; Wallace, H.M.; Joseph, S.; Pace, B.; Bai, S.H. Short-Term Dynamics of Carbon and Nitrogen Using Compost, Compost-Biochar Mixture and Organo-Mineral Biochar. Environ. Sci. Pollut. Res. 2016, 23, 11267–11278. [Google Scholar] [CrossRef] [PubMed]
- Białowiec, A.; Pulka, J.; Styczyńska, M.; Koziel, J.A.; Kalka, J.; Jureczko, M.; Felis, E.; Manczarski, P. Is Biochar from the Torrefaction of Sewage Sludge Hazardous Waste? Materials 2020, 13, 3544. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, L.; Nan, H.; Cao, Y.; Wang, H.; Kumar, T.V.; Wang, C. Phosphorus Adsorption by Functionalized Biochar: A Review. Environ. Chem. Lett. 2022, 21, 497–524. [Google Scholar] [CrossRef]
- EUR-Lex—32019R1009—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/?qid=1561534804127&uri=CELEX:32019R1009 (accessed on 16 November 2023).
- Wang, H.; Wang, X.; Cui, Y.; Xue, Z.; Ba, Y. Slow Pyrolysis Polygeneration of Bamboo (Phyllostachys Pubescens): Product Yield Prediction and Biochar Formation Mechanism. Bioresour. Technol. 2018, 263, 444–449. [Google Scholar] [CrossRef]
- Silva, A.C.; Rocha, P.; Antelo, J.; Valderrama, P.; López, R.; Geraldo, D.; Proença, M.F.; Pinheiro, J.P.; Fiol, S.; Bento, F. Comparison of a Variety of Physico-Chemical Techniques in the Chronological Characterization of a Compost from Municipal Wastes. Process Saf. Environ. Prot. 2022, 164, 781–793. [Google Scholar] [CrossRef]
- Cole, E.J.; Zandvakili, O.R.; Xing, B.; Hashemi, M.; Herbert, S.; Mashayekhi, H.H. Dataset on the Effect of Hardwood Biochar on Soil Gravimetric Moisture Content and Nitrate Dynamics at Different Soil Depths with FTIR Analysis of Fresh and Aged Biochar. Data Brief. 2019, 25, 104073. [Google Scholar] [CrossRef]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; De Melo, I.C.N.A.; Melo, L.C.A.; Magriotis, Z.M.; Sánchez-Monedero, M.A. Properties of Biochar Derived from Wood and High-Nutrient Biomasses with the Aim of Agronomic and Environmental Benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef]
- Adegoke, T.O.; Ku, H.H. Temperature Response of Ammonia Emission from Sandy Loam Soil Amended with Manure Compost and Urea. Environ. Technol. Innov. 2023, 31, 103226. [Google Scholar] [CrossRef]
- Sundberg, C.; Yu, D.; Franke-Whittle, I.; Kauppi, S.; Smårs, S.; Insam, H.; Romantschuk, M.; Jönsson, H. Effects of PH and Microbial Composition on Odour in Food Waste Composting. Waste Manag. 2013, 33, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Nizami, A.S.; Aburiazaiza, A.S.; Barakat, M.A.; Ismail, I.M.I.; Rashid, M.I. Optimization of Food Waste Compost with the Use of Biochar. J. Environ. Manag. 2018, 216, 70–81. [Google Scholar] [CrossRef]
- Czekała, W.; Malińska, K.; Cáceres, R.; Janczak, D.; Dach, J.; Lewicki, A. Co-Composting of Poultry Manure Mixtures Amended with Biochar—The Effect of Biochar on Temperature and C-CO2 Emission. Bioresour. Technol. 2016, 200, 921–927. [Google Scholar] [CrossRef]
- Jia, X.; Wang, M.; Yuan, W.; Ju, X.; Yang, B. The Influence of Biochar Addition on Chicken Manure Composting and Associated Methane and Carbon Dioxide Emissions. Bioresources 2016, 11, 5255–5264. [Google Scholar] [CrossRef]
- Jindo, K.; Sonoki, T.; Matsumoto, K.; Canellas, L.; Roig, A.; Sanchez-Monedero, M.A. Influence of Biochar Addition on the Humic Substances of Composting Manures. Waste Manag. 2016, 49, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, G.; Sun, H.; Zhou, S.; Zou, G. Straw Biochar Hastens Organic Matter Degradation and Produces Nutrient-Rich Compost. Bioresour. Technol. 2016, 200, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Raclavská, H.; Růžičková, J.; Raclavský, K.; Juchelková, D.; Kucbel, M.; Švédová, B.; Slamová, K.; Kacprzak, M. Effect of Biochar Addition on the Improvement of the Quality Parameters of Compost Used for Land Reclamation. Environ. Sci. Pollut. Res. 2023, 30, 8563–8581. [Google Scholar] [CrossRef]
- Ghanbarpour Mamaghani, Z.; Hawboldt, K.A.; MacQuarrie, S. Adsorption of CO2 Using Biochar—Review of the Impact of Gas Mixtures and Water on Adsorption. J. Environ. Chem. Eng. 2023, 11, 109643. [Google Scholar] [CrossRef]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and Trends in CO2 Capture/Separation Technologies: A Review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Choi, S.W.; Igalavithana, A.D.; Yang, X.; Tsang, D.C.W.; Wang, C.H.; Kua, H.W.; Lee, K.B.; Ok, Y.S. Sustainable Gasification Biochar as a High Efficiency Adsorbent for CO2 Capture: A Facile Method to Designer Biochar Fabrication. Renew. Sustain. Energy Rev. 2020, 124, 109785. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Lin, C.; Hoang, H.G.; Sanderson, P.; Dang, B.T.; Bui, X.T.; Nguyen, N.S.H.; Vo, D.V.N.; Tran, H.T. Evaluate the Role of Biochar during the Organic Waste Composting Process: A Critical Review. Chemosphere 2022, 299, 134488. [Google Scholar] [CrossRef]
- Yan, H.; Yang, H.; Li, K.; Zhu, P.; Li, X.; Li, Q. Biochar Addition Modified Carbon Flux and Related Microbiota in Cow Manure Composting. Waste Biomass Valorization 2023, 14, 847–858. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Y.; Xu, J.; Liu, X.; Sheng, L. Effects of Additives on Nitrogen Transformation and Greenhouse Gases Emission of Co-Composting for Deer Manure and Corn Straw. Environ. Sci. Pollut. Res. 2021, 28, 13000–13020. [Google Scholar] [CrossRef] [PubMed]
- Ottani, F.; Parenti, M.; Santunione, G.; Moscatelli, G.; Kahn, R.; Pedrazzi, S.; Allesina, G. Effects of Different Gasification Biochar Grain Size on Greenhouse Gases and Ammonia Emissions in Municipal Aerated Composting Processes. J. Environ. Manag. 2023, 331, 117257. [Google Scholar] [CrossRef] [PubMed]
- Sobieraj, K.; Stegenta-Dąbrowska, S.; Zafiu, C.; Binner, E.; Białowiec, A. Carbon Monoxide Production during Bio-Waste Composting under Different Temperature and Aeration Regimes. Materials 2023, 16, 4551. [Google Scholar] [CrossRef]
- Sánchez-García, M.; Alburquerque, J.A.; Sánchez-Monedero, M.A.; Roig, A.; Cayuela, M.L. Biochar Accelerates Organic Matter Degradation and Enhances N Mineralisation during Composting of Poultry Manure without a Relevant Impact on Gas Emissions. Bioresour. Technol. 2015, 192, 272–279. [Google Scholar] [CrossRef]
- Stegenta-Dabrowska, S.; Sobieraj, K.; Koziel, J.A.; Bieniek, J.; Bialowiec, A. Kinetics of Biotic and Abiotic CO Production during the Initial Phase of Biowaste Composting. Energies 2020, 13, 5451. [Google Scholar] [CrossRef]
- Hellebrand, H.J.; Schade, G.W. Carbon Monoxide from Composting Due to Thermal Oxidation of Biomass. J. Environ. Qual. 2008, 37, 592–598. [Google Scholar] [CrossRef]
- Sobieraj, K.; Stegenta-Dąbrowska, S.; Luo, G.; Koziel, J.A.; Białowiec, A. Biological Treatment of Biowaste as an Innovative Source of CO—The Role of Composting Process. Front. Bioeng. Biotechnol. 2023, 11, 1126737. [Google Scholar] [CrossRef]
- Chung, W.J.; Chang, S.W.; Chaudhary, D.K.; Shin, J.D.; Kim, H.; Karmegam, N.; Govarthanan, M.; Chandrasekaran, M.; Ravindran, B. Effect of Biochar Amendment on Compost Quality, Gaseous Emissions and Pathogen Reduction during in-Vessel Composting of Chicken Manure. Chemosphere 2021, 283, 131129. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The Forms of Alkalis in the Biochar Produced from Crop Residues at Different Temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, R.; Li, D.; Qi, C.; Han, L.; Chen, M.; Fu, F.; Yuan, J.; Li, G. Effects of Calcium Magnesium Phosphate Fertilizer, Biochar and Spent Mushroom Substrate on Compost Maturity and Gaseous Emissions during Pig Manure Composting. J. Environ. Manag. 2020, 267, 110649. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Lin, H.; Hu, Z.; Zheng, Y.; Li, P.; Huang, W. Effect of Biochar Structure on H2S Emissions during Sludge Aerobic Composting: Insights into Microscale Characterization and Microbial Mechanism. Biomass Convers. Biorefin. 2022, 1, 1–14. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Lin, C.; Hoang, H.G.; Bui, X.T.; Ngo, H.H.; Le, V.G.; Tran, H.T. Investigation of Biochar Amendments on Odor Reduction and Their Characteristics during Food Waste Co-Composting. Sci. Total Environ. 2023, 865, 161128. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Zhang, Z.; Wang, Q.; Shen, F.; Li, R.; Li, D.S.; Ren, X.; Wang, M.; Chen, H.; Zhao, J. New Insight with the Effects of Biochar Amendment on Bacterial Diversity as Indicators of Biomarkers Support the Thermophilic Phase during Sewage Sludge Composting. Bioresour. Technol. 2017, 238, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, J.; Han, L.; Ma, S.; Sun, X.; Huang, G. Role and Multi-Scale Characterization of Bamboo Biochar during Poultry Manure Aerobic Composting. Bioresour. Technol. 2017, 241, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huo, R.; Xu, J.; Liang, S.; Li, J.; Zhao, T.; Wang, S. Effects of Biochar on Nitrogen Transformation and Heavy Metals in Sludge Composting. Bioresour. Technol. 2017, 235, 43–49. [Google Scholar] [CrossRef]
- Agyarko-Mintah, E.; Cowie, A.; Singh, B.P.; Joseph, S.; Van Zwieten, L.; Cowie, A.; Harden, S.; Smillie, R. Biochar Increases Nitrogen Retention and Lowers Greenhouse Gas Emissions When Added to Composting Poultry Litter. Waste Manag. 2017, 61, 138–149. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, M.; Wan, J.; Sun, Y.; Lan, H.; Deng, X. Effects of PH, Dissolved Humic Acid and Cu2+ on the Adsorption of Norfloxacin on Montmorillonite-Biochar Composite Derived from Wheat Straw. Biochem. Eng. J. 2018, 130, 104–112. [Google Scholar] [CrossRef]
- Vijayanand, M.; Ramakrishnan, A.; Subramanian, R.; Issac, P.K.; Nasr, M.; Khoo, K.S.; Rajagopal, R.; Greff, B.; Wan Azelee, N.I.; Jeon, B.H.; et al. Polyaromatic Hydrocarbons (PAHs) in the Water Environment: A Review on Toxicity, Microbial Biodegradation, Systematic Biological Advancements, and Environmental Fate. Environ. Res. 2023, 227, 115716. [Google Scholar] [CrossRef]
- Neeli, S.T.; Ramsurn, H. Synthesis and Formation Mechanism of Iron Nanoparticles in Graphitized Carbon Matrices Using Biochar from Biomass Model Compounds as a Support. Carbon 2018, 134, 480–490. [Google Scholar] [CrossRef]
- Oldfield, T.L.; Sikirica, N.; Mondini, C.; López, G.; Kuikman, P.J.; Holden, N.M. Biochar, Compost and Biochar-Compost Blend as Options to Recover Nutrients and Sequester Carbon. J. Environ. Manag. 2018, 218, 465–476. [Google Scholar] [CrossRef]
- Gao, S.; Harrison, B.P.; Thao, T.; Gonzales, M.L.; An, D.; Ghezzehei, T.A.; Diaz, G.; Ryals, R.A. Biochar Co-Compost Improves Nitrogen Retention and Reduces Carbon Emissions in a Winter Wheat Cropping System. GCB Bioenergy 2023, 15, 462–477. [Google Scholar] [CrossRef]
- Schulz, H.; Dunst, G.; Glaser, B. Positive Effects of Composted Biochar on Plant Growth and Soil Fertility. Agron. Sustain. Dev. 2013, 33, 817–827. [Google Scholar] [CrossRef]
- Samudrika, K.P.D.; Ariyawansha, R.T.K.; Basnayake, B.F.A.; Siriwardana, A.N. Optimization of Biochar Additions for Enriching Nitrogen in Active Phase Low-Temperature Composting. Org. Agric. 2020, 10, 449–463. [Google Scholar] [CrossRef]
- Bello, A.; Deng, L.; Sheng, S.; Jiang, X.; Yang, W.; Meng, Q.; Wu, X.; Han, Y.; Zhu, H.; Xu, X. Biochar Reduces Nutrient Loss and Improves Microbial Biomass of Composted Cattle Manure and Maize Straw. Biotechnol. Appl. Biochem. 2020, 67, 799–811. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Spokas, K.A.; Novak, J.M.; Lentz, R.D.; Cantrell, K.B. Biochar Elemental Composition and Factors Infl Uencing Nutrient Retention. In Biochar for Environmental Management: Science, Technology and Implementation; Routledge: London, UK, 2015; Volume 139. [Google Scholar]
- Nwajiaku, I.M.; Olanrewaju, J.S.; Sato, K.; Tokunari, T.; Kitano, S.; Masunaga, T. Change in Nutrient Composition of Biochar from Rice Husk and Sugarcane Bagasse at Varying Pyrolytic Temperatures. Int. J. Recycl. Org. Waste Agric. 2018, 7, 269–276. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov Vladimir, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P.F. Influence of Pyrolysis Temperature on Production and Nutrient Properties of Wastewater Sludge Biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, N.; Subdiaga, E.; Orsetti, S.; de la Rosa, J.M.; Knicker, H.; Schmidt, H.P.; Kappler, A.; Behrens, S. Effect of Biochar Amendment on Compost Organic Matter Composition Following Aerobic Composting of Manure. Sci. Total Environ. 2018, 613–614, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, G.; Hayat, R.; Hussain, Q.; Ahmed, M. Physio-Chemical Characterization of Biochar, Compost and Co-Composted Biochar Derived from Green Waste. Sustainability 2021, 13, 4628. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, T.; Huang, H.; Zhao, D.; Kobayashi, N.; Chen, Y. Influence of Pyrolysis Temperature on Physical and Chemical Properties of Biochar Made from Sewage Sludge. J. Anal. Appl. Pyrolysis 2015, 112, 284–289. [Google Scholar] [CrossRef]
- Yoshida, T.; Antal, M.J. Sewage Sludge Carbonization for Terra Preta Applications. Energy Fuels 2009, 23, 5454–5459. [Google Scholar] [CrossRef]
- Hanc, A.; Szakova, J.; Svehla, P. Effect of Composting on the Mobility of Arsenic, Chromium and Nickel Contained in Kitchen and Garden Waste. Bioresour. Technol. 2012, 126, 444–452. [Google Scholar] [CrossRef]
- Tibu, C.; Annang, T.Y.; Solomon, N.; Yirenya-Tawiah, D. Effect of the Composting Process on Physicochemical Properties and Concentration of Heavy Metals in Market Waste with Additive Materials in the Ga West Municipality, Ghana. Int. J. Recycl. Org. Waste Agric. 2019, 8, 393–403. [Google Scholar] [CrossRef]
- De Figueiredo, C.C.; Chagas, J.K.M.; da Silva, J.; Paz-Ferreiro, J. Short-Term Effects of a Sewage Sludge Biochar Amendment on Total and Available Heavy Metal Content of a Tropical Soil. Geoderma 2019, 344, 31–39. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Bednik, M.; Chohura, P. Assessing the Influence of Compost and Biochar Amendments on the Mobility and Uptake of Heavy Metals by Green Leafy Vegetables. Int. J. Environ. Res. Public Health 2020, 17, 7861. [Google Scholar] [CrossRef] [PubMed]

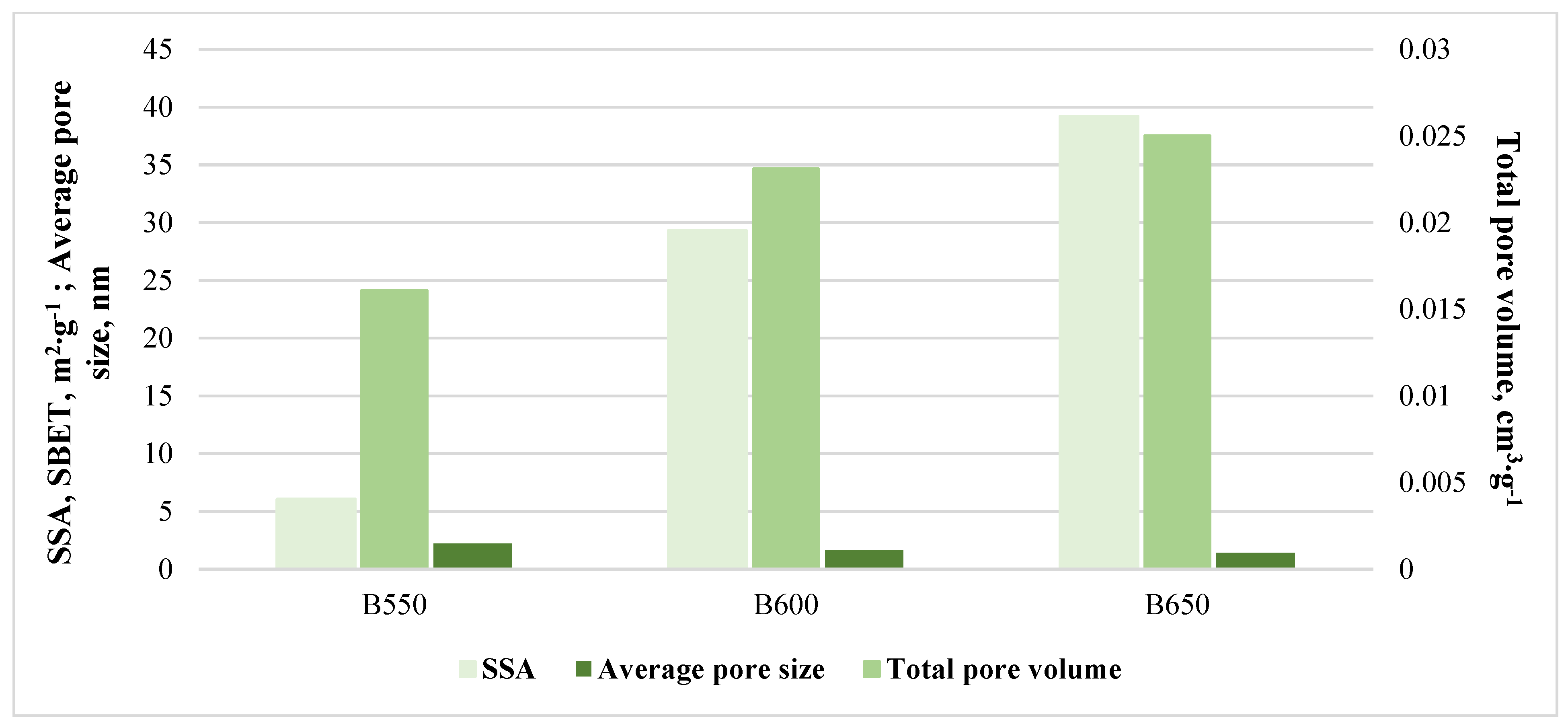
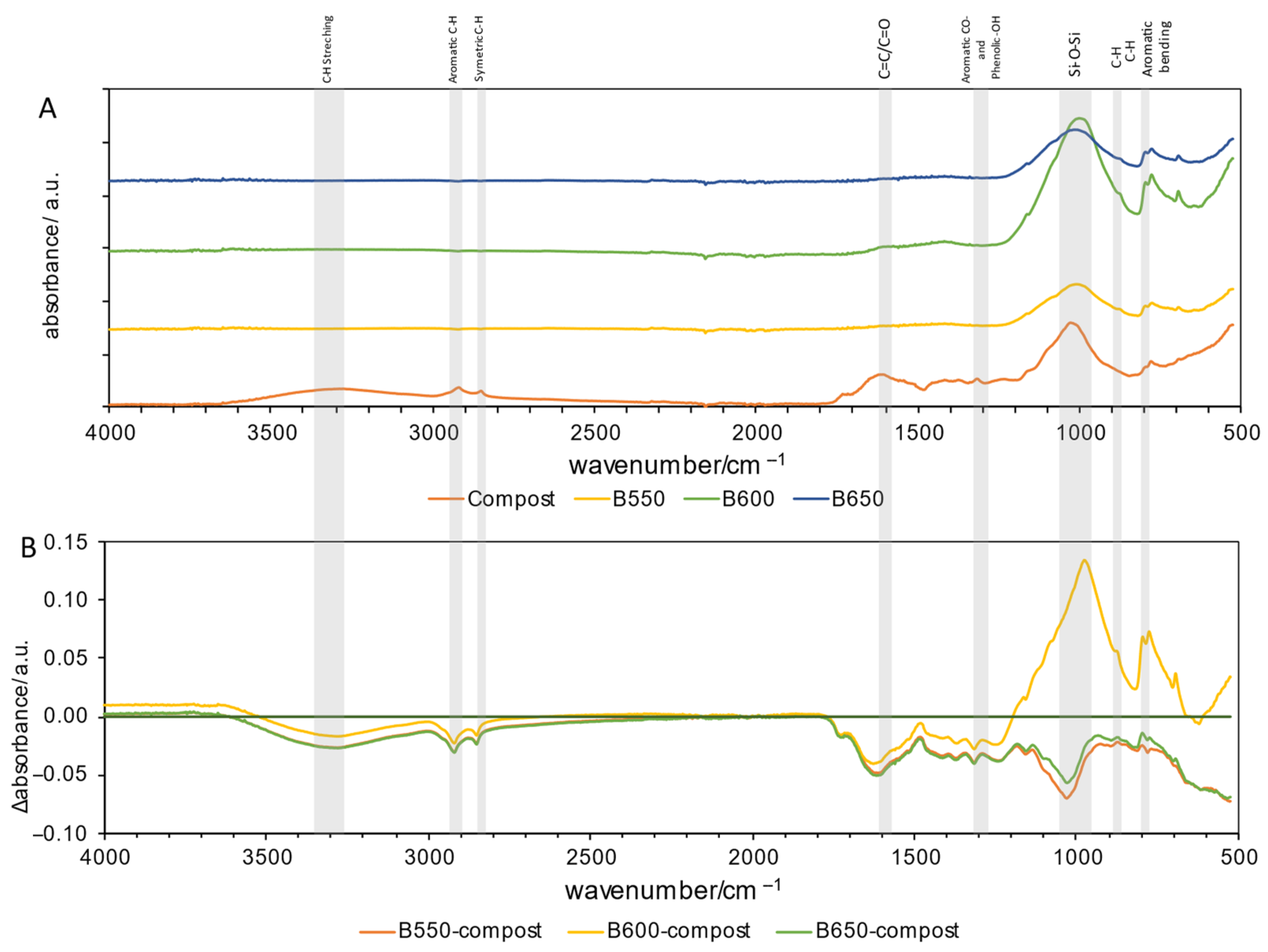


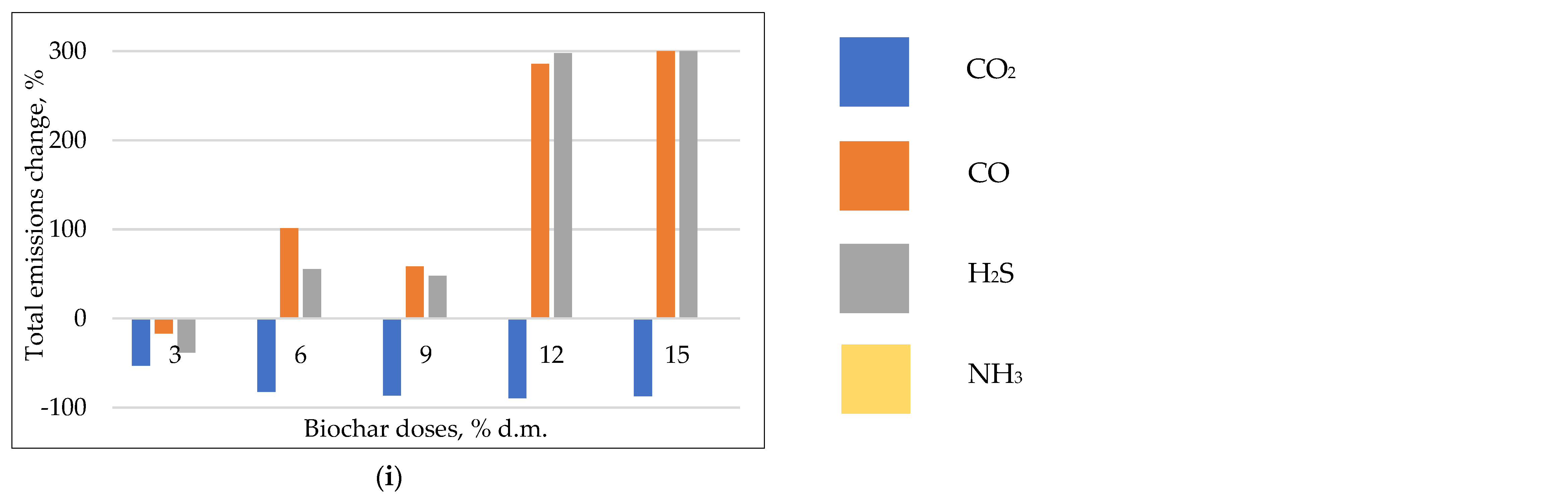



| Parameters | Compost | Feedstock | Biochars’ Variant | ||
|---|---|---|---|---|---|
| B550 | B600 | B650 | |||
| pH | 7.32 ± 0.04 | 4.77 ± 0.04 | 8.61 ± 0.03 | 9.10 ± 0.03 | 9.50 ± 0.04 |
| LOI, % d.m. | 24.80 ± 0.00 | 82.01 ± 3.70 | 11.0 ± 0.00 | 11.9 ± 0.00 | 9.30 ± 0.00 |
| Ca, mg∙kg−1 | 17,760 ± 109 | 14,878 ± 92.5 | 19,795 ± 175 | 18,460 ± 105 | 15,975 ± 80 |
| K, mg∙kg−1 | 6155 ± 65 | 8608 ± 30 | 7865 ± 65 | 7665 ± 60 | 7390 ± 65 |
| Mg mg∙kg−1 | 2697 ± 29 | 2405 ± 22 | 3417 ± 35 | 3382 ± 35 | 2967 ± 10 |
| Na, mg∙kg−1 | 1793 ± 1.1 | 773 ± 5.8 | 2263 ± 1.5 | 2168 ± 1.0 | 2143 ± 1.0 |
| P, mg∙kg−1 | 5835 ± 1167 | 6300 ± 1260 | 6865 ± 1373 | 6953 ± 1391 | 6749 ± 1350 |
| Pb, mg∙kg−1 | 35.52 ± 7.11 | 25.57 ± 0.70 | 45.81 ± 9.16 | 39.59 ± 7.92 | 31.01 ± 6.20 |
| Cd, mg∙kg−1 | 1.66 ± 0.33 | 0.800 ± 0.00 | 1.98 ± 0.40 | 1.91 ± 0.38 | 1.15 ± 0.23 |
| Zn, mg∙kg−1 | 553.8 ± 110.8 | 253.3 ± 1.5 | 585.3 ± 117.3 | 511.8 ± 102.3 | 426.8 ± 85.4 |
| Cr, mg∙kg−1 | 60.7 ± 12.2 | 33.0 ± 1.6 | 141.3 ± 28.3 | 96.3 ± 19.3 | 56.6 ± 11.3 |
| Ni, mg∙kg−1 | 11.9 ± 2.4 | 20.7 ± 0.05 | 43.2 ± 8.6 | 32.1 ± 6.4 | 10.6 ± 2.1 |
| Cu, mg∙kg−1 | 63.7 ± 12 | 44.03 ± 0.2 | 72.7 ± 13.8 | 62.1 ± 11.7 | 45.2 ± 8.3 |
| Mn, mg∙kg−1 | 362.3 ± 72.5 | 253.3 ± 1.5 | 416.8 ± 83.4 | 391.5 ± 78.3 | 341 ± 68.2 |
| Hg, mg∙kg−1 | 0.106 ± 0.03 | 0.08 ± 0.02 | <0.001 | <0.001 | <0.001 |
| C, % | 13 ± 3 | 35.89 ± 4.1 | 8.3 ± 1.7 | 9.3 ± 1.8 | 10 ± 3 |
| H, % | 1.2 ± 0.2 | 4.82 ± 0.9 | 0.21 ± 0.04 | 0.23 ± 0.05 | 0.05 ± 0.2 |
| N, % | 1.1 ± 0.2 | 2.37 ± 0.2 | 0.48 ± 0.1 | 0.62 ± 0.12 | 0.44 ± 0.2 |
| S, % | 0.38 ± 0.08 | 1.64 ± 0.05 | 0.31 ± 0.06 | 0.33 ± 0.07 | 0.31 ± 0.08 |
| O, % | 8.7 ± 0.4 | 37.29 ± 1.9 | 1.7 ± 0.3 | 0.82 ± 0.2 | 0.4 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stegenta-Dąbrowska, S.; Syguła, E.; Bednik, M.; Rosik, J. Effective Carbon Dioxide Mitigation and Improvement of Compost Nutrients with the Use of Composts’ Biochar. Materials 2024, 17, 563. https://doi.org/10.3390/ma17030563
Stegenta-Dąbrowska S, Syguła E, Bednik M, Rosik J. Effective Carbon Dioxide Mitigation and Improvement of Compost Nutrients with the Use of Composts’ Biochar. Materials. 2024; 17(3):563. https://doi.org/10.3390/ma17030563
Chicago/Turabian StyleStegenta-Dąbrowska, Sylwia, Ewa Syguła, Magdalena Bednik, and Joanna Rosik. 2024. "Effective Carbon Dioxide Mitigation and Improvement of Compost Nutrients with the Use of Composts’ Biochar" Materials 17, no. 3: 563. https://doi.org/10.3390/ma17030563









