Current Trends in Metallic Materials for Body Panels and Structural Members Used in the Automotive Industry
Abstract
:1. Introduction
2. Steels
2.1. Background
- be characterised by high specific strength, defined as the ratio of the material’s strength to its density,
- show high energy absorption capacity in the event of a collision,
- have properties that minimise technological problems in production (including springback) and ensure high efficiency,
- have good weldability,
- show high corrosion resistance.
- soft steels, with the ultimate tensile strength (UTS) Rm below 300 MPa and elongation A80 above 30% (IF),
- conventional steels, with the UTS from 300 to 700 MPa and elongation A80 from 10% to 30% (HS, BH, HSLA),
- advanced steels, with very high UTS above 700 MPa and elongation A80 in the range of 5–30% (TRIP, DP, CP, MS).
- low-carbon and conventional HS steels: LC, BH, HSLA, solid solution strengthened (SSS),
- first-generation AHSS: DP, stretch flangeable SF, TRIP, CP and MS,
- second-generation AHSS: TWIP, lightweight steel with induced plasticity (LS-IP).
2.2. Conventional Low-Carbon Steels
2.3. Complex-Phase Steels
2.4. Interstitial Free Steels
2.5. Bake Hardenable Steels
2.6. Dual-Phase Steels
2.7. Transformation-Induced Plasticity Steels
2.8. Twinning-Induced Plasticity Steels
2.9. Triplex Steels
2.10. Martensitic Steels
2.11. Press-Hardened Steels
2.12. Quenching and Partitioning Steels
2.13. Stainless Steels
3. Aluminium and Aluminium Alloys
3.1. Characterisation of Aluminium and Aluminium Alloys
- F—as fabricated
- O—annealed
- H—strain hardened (cold worked)
- W—solution heat treated
- T—thermally treated
- H1—strain hardened only
- H2—strain hardened and partially annealed
- H3—strain hardened and stabilised
- H4—strain hardened and painted
3.2. Aluminium Alloy Families
3.3. 1xxx-Series Aluminium Alloys
3.4. 2xxx-Series Aluminium Alloys
3.5. 3xxx-Series Aluminium Alloys
3.6. 4xxx-Series Aluminium Alloys
3.7. 5xxx-Series Aluminium Alloys
3.8. 6xxx-Series Aluminium Alloys
3.9. 7xxx-Series Aluminium Alloys
3.10. 8xxx-Series Aluminium Alloys
3.11. 9xxx-Series Aluminium Alloys
4. Titanium and Titanium Alloys
4.1. Characterisation of Titanium and Titanium Alloys
4.1.1. Titanium
4.1.2. Titanium Alloys
4.2. Application of Titanium and Titanium Alloys
5. Magnesium and Magnesium Alloys
5.1. Characterisation of Magnesium and Magnesium Alloys
- alloys with the addition of aluminium, zinc and manganese: Mg–Mn, Mg–Al–Zn, Mg–Zn– (Mn, Cu),
- alloys containing mainly zinc, yttrium, zirconium, thorium and RE elements: Mg–Zn–Zr, Mg–Zn–RE, Mg–Y–RE–Zr, Mg–Th,
- alloys containing lithium, for example Mg–Li–Al.
5.2. Application of Magnesium Alloys in Car Body Components
6. Future Developments Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Samek, L.; Krizan, D. Steel—Material of choice for automotive lightweight applications. In Proceedings of the International Conference Metal’2012, Brno, Czech Republic, 23–25 May 2012; pp. 1–6. [Google Scholar]
- Ultra Light Steel Auto Body; Final Report; American Iron and Steel Institute: Washington, DC, USA, 1998; Available online: https://www.yumpu.com/en/document/view/5349987/ultralight-steel-auto-body-final-report-american-iron-steel- (accessed on 17 November 2023).
- Perka, A.K.; John, M.; Kuruveri, U.B.; Menezes, P.L. Advanced high-strength steels for automotive applications: Arc and laser welding process, properties, and challenges. Metals 2022, 12, 1051. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A. Mechanical properties of nanostructured bainitic steels. Materialia 2021, 15, 101034. [Google Scholar] [CrossRef]
- All-New 2021 Jeep® Grand Cherokee Breaks New Ground in the Full-Size SUV Segment. Available online: https://www.media.stellantis.com/uk-en/jeep/press/all-new-2021-jeep-grand-cherokee-breaks-new-ground-in-the-full-size-suv-segment-uk (accessed on 17 November 2023).
- Galán, J.; Samek, L.; Verleysen, P.; Verbeken, K.; Houbaert, Y. Advanced high strength steels for automotive industry. Rev. Metal. 2012, 48, 118–131. [Google Scholar] [CrossRef]
- Kuziak, R.; Kawalla, R.; Waengler, S. Advanced high strength steels for automotive industry. Arch. Civ. Mech. Eng. 2008, 8, 103–117. [Google Scholar] [CrossRef]
- Siczek, K.; Siczek, K. Lekkie rozwiązania w przemyśle samochodowym. Autobusy 2017, 14, 1311–1314. [Google Scholar]
- According to the Automotive and Transportation Market Research Report. Available online: https://mobilityforesights.com/product/automotive-ahss-market/ (accessed on 15 December 2023).
- Schneider, R.; Heine, B.; Grant, R.J. Mechanical Behaviour of Commercial Aluminium Wrought Alloys at Low Temperatures, Light Metal Alloys Applications; Waldemar, A.M., Ed.; InTech: Houston, TX, USA, 2014; Available online: https://www.intechopen.com/chapters/46602 (accessed on 16 November 2023).
- Bielefeldt, K.; Papacz, W.; Walkowiak, J. Environmentally friendly car plastics in automotive engineering. Arch. Motoryz. 2011, 2, 5–19. [Google Scholar]
- This Aluminum-Bodied 1953 Porsche 356 1500 Pre-A Cabriolet Is Shrouded in Mystery. Available online: https://www.hagerty.com/media/automotive-history/this-aluminum-bodied-1953-porsche-356-1500-pre-a-cabriolet-is-shrouded-in-mystery/ (accessed on 16 November 2023).
- Choi, C.H.; Park, S.S.; Hwang, T.W. Development of composite body panels for a lightweight vehicle. SAE Trans. 2001, 110, 143–149. [Google Scholar]
- Fantuzzi, N.; Bacciocchi, M.; Benedetti, D.; Agnelli, J. The use of sustainable composites for the manufacturing of electric cars. Compos. Part C Open Access 2021, 4, 100096. [Google Scholar] [CrossRef]
- Cieniek, Ł. Anizotropia i Tekstura Krystalograficzna. Starzenie po Odkształceniu. Available online: https://docplayer.pl/56885357-Cwiczenie-nr-4-anizotropia-i-tekstura-krystalograficzna-starzenie-po-odksztalceniu.html (accessed on 15 November 2023).
- Sherman, A.M.; Allison, J.E. Potential for Automotive Applications of Titanium Alloys; SAE Technical Paper 860608; SAE International: Warrendale, PA, USA, 1986. [Google Scholar] [CrossRef]
- Titanium for Automotive Applications. Available online: https://www.azom.com/article.aspx?ArticleID=553 (accessed on 16 November 2023).
- Schauerte, O. Titanium in automotive production. Adv. Eng. Mater. 2003, 5, 411–418. [Google Scholar] [CrossRef]
- Blicharski, M. Inżynieria Materiałowa, Stal.; WNT: Warszawa, Poland, 2004. [Google Scholar]
- Evaluation Technologies on High Strength Steel Sheet for Automobiles. Available online: https://www.kobelcokaken.co.jp/en/example/c/index.html (accessed on 16 November 2023).
- Senkara, J. Współczesne stale karoseryjne dla przemysłu motoryzacyjnego i wytyczne technologiczne ich zgrzewania. Prz. Spaw. 2009, 11, 3–7. [Google Scholar]
- Best Surfaces for Cat Body Panels. Available online: https://www.thyssenkrupp-steel.com/en/industries/automotivetrucks/surfaces-for-car-body-panels/best-surfaces.html (accessed on 16 December 2023).
- Kubińska-Jabcoń, E.; Niekurzak, M. Wykorzystanie nowoczesnych materiałów stosowanych w motoryzacji w celu poprawy jakości i bezpieczeństwa użytkowania pojazdów mechanicznych. Autobusy 2019, 10–11, 47–52. [Google Scholar]
- Close, D.; Lallement, R.; Feuser, P.; Bold, J. Challenges in Corrosion Protection for Press-Hardened Steels. In Tagungsband Zum 9. Erlanger Workshop Warmblechumformung; Meisenbach Verlag: Erlangen, Germany, 2014; pp. 31–52. [Google Scholar]
- Abotani, K.; Hirohata, K.; Kiyasu, T. Hot-Dip Galvanized Sheet Steel with Excellent Press Formability and Surface Quality for the Automotive Panels. Kawasaki Steel Tech. Rep. 2003, 48, 17–22. [Google Scholar]
- EN ISO 1461:2009; Hot Dip Galvanized Coatings On Fabricated Iron And Steel Articles. Specifications and Test Methods. Comite Europeen de Normalisation: Brussels, Belgium, 1994.
- Close, D. Alternative Protective Coatings for Hot Stamped Automotive Body Parts. Ph.D. Thesis, Universite de Lorraine, Lorraine, France, 22 March 2018. [Google Scholar]
- Ulbrich, D.; Kowalczyk, J.; Stachowiak, A.; Sawczuk, W.; Selech, J. The Influence of Surface Preparation of the Steel during the Renovation of the Car Body on Its Corrosion Resistance. Coatings 2021, 11, 384. [Google Scholar] [CrossRef]
- Santos, D.; Raminhos, H.; Costa, M.R.; Diamantino, T.; Goodwin, F. Performance of finish coated galvanized steel sheets for automotive bodies. Prog. Org. Coat. 2008, 62, 265–273. [Google Scholar] [CrossRef]
- Wyrobek, K. Modelowanie procesu tłoczenia części nadwozia samochodu ze stali superwysoko wytrzymałej. Inż. Masz. 2017, 22, 76–84. [Google Scholar]
- Horvath, C.D. Advanced steels for lightweight automotive structures. In Materials, Design and Manufacturing for Lightweight Vehicles; Mallick, P.K., Ed.; Woodhead Publishing: Sawston, UK, 2010; pp. 35–78. [Google Scholar]
- Grosman, F.; Piela, A. Zastosowanie nowej metody badań do wstępnej oceny właściwości użytkowych blach dla motoryzacji. Inż. Mater. 1991, 12, 84–87. [Google Scholar]
- Trzepieciński, T.; Najm, S.M. Application of artificial neural networks to the analysis of friction behaviour in a drawbead profile in sheet metal forming. Materials 2022, 15, 9022. [Google Scholar] [CrossRef] [PubMed]
- Trzepieciński, T.; Szwajka, K.; Szewczyk, M. Pressure-assisted lubrication of DC01 steel sheets to reduce friction in sheet-metal-forming processes. Lubricants 2023, 11, 169. [Google Scholar] [CrossRef]
- Kajal, G.; Tyagi, M.R.; Kumar, G. A review on the effect of residual stresses in incremental sheet metal forming used in automotive and medical sectors. Mater. Today Proc. 2023, 78, 524–534. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Z. The effects of through-thickness shear stress on the formability of sheet metal—A review. J. Manuf. Proc. 2021, 71, 269–289. [Google Scholar] [CrossRef]
- Trzepieciński, T.; Lemu, H.G. Improving prediction of springback in sheet metal forming using multilayer perceptron-based genetic algorithm. Materials 2020, 13, 3129. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, M.; Szwajka, K. Assessment of the tribological performance of bio-based lubricants using analysis of variance. Adv. Mech. Mater. Eng. 2023, 40, 31–38. [Google Scholar] [CrossRef]
- Trzepieciński, T.; Najm, S.M.; Sbayti, M.; Belhadjsalah, H.; Szpunar, M.; Lemu, H.G. New advances and future possibilities in forming technology of hybrid metal-polymer composites used in aerospace applications. J. Compos. Sci. 2021, 5, 217. [Google Scholar] [CrossRef]
- Trzepieciński, T.; Najm, S.M.; Oleksik, V.; Vasilca, D.; Paniti, I.; Szpunar, M. Recent developments and future challenges in incremental sheet forming of aluminium and aluminium alloy sheets. Metals 2022, 12, 124. [Google Scholar] [CrossRef]
- Żaba, K.; Puchlerska, S.; Kuczek, Ł.; Trzepieciński, T.; Maj, P. Effect of step size on the formability of Al/Cu bimetallic sheets in single point incremental sheet forming. Materials 2023, 16, 367. [Google Scholar] [CrossRef] [PubMed]
- Rajarajan, C.; Sivaraj, P.; Sonar, T.; Raja, S.; Mathiazhagan, N. Resistance spot welding of advanced high strength steel for fabrication of thin-walled automotive structural frames. Forces Mech. 2022, 7, 100084. [Google Scholar] [CrossRef]
- Ahmed, M.M.Z.; El-Sayed Selaman, M.M.; Fydrych, D.; Cam, G. Review on friction stir welding of dissimilar magnesium and aluminum alloys: Scientometric analysis and strategies for achieving high-quality joints. J. Magnes. Alloys 2023, 11, 4082–4127. [Google Scholar] [CrossRef]
- Tomków, J.; Fydrych, D.; Rogalski, G. Dissimilar underwater wet welding of HSLA steels. Int. J. Adv. Manuf. Technol. 2020, 109, 717–725. [Google Scholar] [CrossRef]
- André, V.; Costas, M.; Langseth, M.; Morin, D. Behavior and Large-Scale Modeling of Multi-Sheet Aluminum Connections With Self-Piercing Rivets. J. Manuf. Sci. Eng. 2023, 145, 101010. [Google Scholar] [CrossRef]
- Çavuşoğlu, O.; Bakırcı, A.; Dinkçi, H.; Yılmazoğlu, A.G. Triple joining of different sheets with self-pierce riveting method. Sci. Technol. Weld. Join. 2022, 27, 579–585. [Google Scholar] [CrossRef]
- Zhou, Z.-J.; Huang, Z.-C.; Jiang, Y.-Q.; Tang, N.-L. Joining Properties of SPFC440/AA5052 Multi-Material Self-Piercing Riveting Joints. Materials 2022, 15, 2962. [Google Scholar] [CrossRef]
- Cmorej, D.; Kaščák, Ľ. Numerical simulation of mechanical joining of three DP600 and DC06 steel sheets. Adv. Mech. Mater. Eng. 2023, 40, 23–29. [Google Scholar] [CrossRef]
- Huang, Z.C.; Huang, G.H.; Shan, F.W.; Jiang, Y.Q.; Zou, Y.Q.; Nie, X.Y. Forming quality and microstructure evolution of AA6061-T6 aluminum alloy joint during flow drill screwing process. Adv. Eng. Mater. 2023, 25, 2300054. [Google Scholar] [CrossRef]
- Kinsley, B.L. 7—Tailor welded blanks for the automotive industry. In Tailor Welded Blanks for Advanced Manufacturing; Kinsley, B.L., Wu, X., Eds.; Woodhead Publishing: Sawston, UK, 2011; pp. 164–180. [Google Scholar]
- Keeler, S.; Kimchi, M. Advanced High-Strength Steels. Application Guidelines Version 5.0, World Auto Steel. 2014. Available online: https://www.worldautosteel.org/projects/advanced-high-strength-steel-application-guidelines/ (accessed on 16 November 2023).
- ULSAB-AVC. Available online: https://www.worldautosteel.org/projects/ulsab-avc-2/ (accessed on 16 November 2022).
- ULSAC. Available online: https://www.worldautosteel.org/projects/ulsac-2/ (accessed on 16 November 2022).
- ULSAS-UltraLight Steel Auto Suspensions Report. Available online: https://www.worldautosteel.org/ulsas-ultralight-steel-auto-suspensions-report/ (accessed on 16 November 2022).
- FutureSteelVehicle. Available online: https://www.worldautosteel.org/projects/future-steel-vehicle/ (accessed on 16 November 2022).
- Richter, J.; Kuhtz, M.; Hornig, A.; Harhash, M.; Palkowski, H.; Gude, M. A mixed numerical-experimental method to characterize metal-polymer interfaces for crash applications. Metals 2021, 11, 818. [Google Scholar] [CrossRef]
- Structure-Borne-Damping Composite Material with Customized Properties. Available online: https://www.thyssenkrupp-steel.com/en/products/composite-material/overview-composite-material.html (accessed on 16 November 2023).
- Kustroń, P.; Korzeniowski, M.; Piwowarczyk, T.; Sokołowski, P. Development of resistance spot welding processes of metal–plastic composites. Materials 2021, 14, 3233. [Google Scholar] [CrossRef] [PubMed]
- Hybrix™ = ∑[214Lightweight, Formable, Strong, Eco-Friendly]. Available online: https://www.lamera.se/ (accessed on 16 November 2023).
- Hammarberg, S.; Kajberg, J.; Larsson, S.; Moshfegh, R.; Jonsén, P. Novel methodology for experimental characterization of micro-sandwich materials. Materials 2021, 14, 4396. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, O.A.; Kühn, M.; Palkowski, H. Deep drawing properties of lightweight steel/polymer/steel sandwich composites. Arch. Civ. Mech. Eng. 2012, 12, 105–112. [Google Scholar] [CrossRef]
- EN 10130; Cold Rolled Low Carbon Steel Flat Products for Cold Forming—Technical Delivery Conditions. European Committee for Standardization: Brussels, Belgium, 2009.
- Trzepieciński, T.; Szwajka, K.; Szewczyk, M. An investigation into the friction of cold-rolled low-carbon DC06 steel sheets in sheet metal forming using radial basis function neural networks. Appl. Sci. 2023, 13, 9572. [Google Scholar] [CrossRef]
- Sudo, M.; Hashimoto, S.I.; Kambe, S. Niobium bearing ferrite-bainite high strength hot-rolled sheet steel with improved formability. Trans. Iron Steel Inst. Jpn. 1983, 23, 303–311. [Google Scholar] [CrossRef]
- Complex Phase Steels. Available online: https://automotive.arcelormittal.com/products/flat/first_gen_AHSS/CP (accessed on 16 November 2023).
- Dias, E.; Horimoto, L.; dos Santos Pereira, M. Microstructural characterization of CP steel used in automotive industry. Mater. Sci. Forum 2014, 775–776, 141–145. [Google Scholar] [CrossRef]
- Santofimia, M.J.; van Bohemen, S.M.C.; Sietsma, J. Combining bainite and martensite in steel microstructures for light weight applications. J. S. Afr. Inst. Min. Metall. 2013, 113, 143–148. [Google Scholar]
- Advanced High-Strength Steels—A Collision Repair Perspective. Available online: https://i-car.co.nz/wp-content/uploads/2018/10/general-technical-info-advanced-high-strength-steels-july-aug-2006.pdf (accessed on 16 November 2022).
- Chaurasiya, R.; Maji, P.; Mukhopadhyay, G. High cycle fatigue behaviour of advanced high strength steel sheet (HS1000) in automotive application. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Fonstein, N. Complex phase steels. In Advanced High Strength Sheet Steels; Springer: Berlin, Germany, 2015; pp. 241–258. [Google Scholar]
- Graux, A.; Cazottes, S.; Castro, D.D.; San-Martín, D.; Capdevila, C.; Cabrera, J.M.; Molas, S.; Schreiber, S.; Mirković, D.; Danoix, F.; et al. Design and development of complex phase steels with improved combination of strength and stretch-flangeability. Metals 2020, 10, 824. [Google Scholar] [CrossRef]
- Chu, X.; Zhao, Y.; Yang, Y.; Zhou, F.; Liu, L.; Zhao, Z. Effect of recrystallization on bainite transformation and mechanical properties of complex phase steel with high formability (CH steel). J. Mater. Res. Technol. 2023, 26, 7674–7693. [Google Scholar] [CrossRef]
- Hoile, S. Processing and properties of mild interstitial free steel. Mater. Sci. Technol. 2000, 16, 1079–1093. [Google Scholar] [CrossRef]
- Rana, R.; Bleck, W.; Singh, S.B.; Mohanty, O.N. Development of high strength interstitial free steel by copper precipitation hardening. Mater. Lett. 2007, 61, 2919–2922. [Google Scholar] [CrossRef]
- Da Rocha Santos, A.P.; Da Mata, T.C.; Segundo, H.V.G.; de Almeida, L.H.; Araújo, L.S.; da Cunha Rocha, A. Texture, microstructure and anisotropic properties of IF-steels with different additions of titanium, niobium and phosphorus. J. Mater. Res. Technol. 2018, 7, 331–336. [Google Scholar] [CrossRef]
- Zhang, L.; Zhi, J.; Mei, F.; Zhu, L.; Jiang, X.; Shen, J.; Cui, J.; Cai, K.; Thomas, B.G. Basic oxygen furnace based steelmaking processes and cleanliness control at Baosteel. Ironmak. Steelmak. 2006, 33, 129–139. [Google Scholar] [CrossRef]
- Zaitsev, A.I.; Rodionova, I.G.; Koldaev, A.V. Study of methods for increasing ductility and formability of cold-rolled Ti-stabilized IF steels. IOP Conf. Ser. Mater. Sci. Eng. 2020, 969, 012018. [Google Scholar] [CrossRef]
- Ramazani, A.; Bruehl, S.; Gerber, T.; Bleck, W.; Prahl, U. Quantification of bake hardening effect in DP600 and TRIP700 steels. Mater. Des. 2014, 57, 479–486. [Google Scholar] [CrossRef]
- Oliaei, M.; Jamaati, R. Improvement of the strength-ductility-toughness balance in interstitial-free steel by gradient microstructure. Mater. Sci. Eng. A 2022, 845, 143237. [Google Scholar] [CrossRef]
- Tsuji, N.; Ito, Y.; Saito, Y.; Minamino, Y. Strength and ductility of ultrafine grained aluminum and iron produced by ARB and annealing. Scr. Mater. 2002, 57, 893–899. [Google Scholar] [CrossRef]
- Kuziak, R. Technologia ciągłego wyżarzania blach cienkich. Pr. Inst. Metal. Żelaza 2011, 3, 1–6. [Google Scholar]
- Pouranvari, M.; Marashi, S.P.H. Key factors influencing mechanical performance of dual phase steel resistance spot welds. Sci. Technol. Weld. Join. 2010, 15, 149–155. [Google Scholar] [CrossRef]
- Kuang, S.; Kang, Y.L.; Yu, H.; Liu, R.D. Effect of continuous annealing parameters on the mechanical properties and microstructures of a cold rolled dual phase steel. Int. J. Miner. Metall. Mater. 2009, 16, 159–164. [Google Scholar] [CrossRef]
- Dulucheanu, C.; Severin, T.L.; Cerlinca, D.A.; Irimescu, L. Structures and Mechanical Properties of Some Dual-Phase Steels with Low Manganese Content. Metals 2022, 12, 189. [Google Scholar] [CrossRef]
- Shaw, J.; Zuidema, B. New High Strength Steels Help Automakers Reach Future Goals for Safety, Affordability, Fuel Efficiency and Environmental Responsibility; SAE Publication 2001-01-3041; SAE International: Warrendale, PA, USA, 2001. [Google Scholar]
- Paul, S.K. Effect of forming strain on low cycle, high cycle and notch fatigue performance of automotive grade dual phase steels: A review. Forces Mech. 2023, 11, 100184. [Google Scholar] [CrossRef]
- Llewellyn, D.T.; Hudd, R.C. Steels—Metallurgy and Applications, 3rd ed.; Butterworth Heinemann: Oxford, UK, 1998. [Google Scholar]
- Ding, C.; Liu, J.; Ning, B.; Huang, M.; Wu, H. Enhanced strength-plasticity matching of lamellar 1 GPa-grade dual-phase steels via cyclic intercritical quenching. J. Mater. Res. Technol. 2023, 22, 3115–3131. [Google Scholar] [CrossRef]
- Hilditch, T.B.; de Souza, T.; Hodgson, P.D. 2—Properties and automotive applications of advanced high-strength steels (AHSS). In Welding and Joining of Advanced High Strength Steels (AHSS); Shome, M., Tumuluru, M.M., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 9–28. [Google Scholar]
- Li, Z.; Chang, Y.; Rong, J.; Min, J.; Lian, J. Edge fracture of the first and third-generation high-strength steels: DP1000 and QP1000. IOP Conf. Ser. Mater. Sci. Eng. 2023, 1284, 012021. [Google Scholar] [CrossRef]
- Papadioti, I.; Bellas, I.; Tzini, M.-I.T.; Christodoulou, P.I.; Aravas, N. TRIP Steels: A multiscale computational simulation and experimental study of heat treatment and mechanical behavior. Materials 2020, 13, 458. [Google Scholar] [CrossRef] [PubMed]
- Spišák, E.; Majerníková, J.; Kaščák, Ľ.; Mulidrán, P.; Rohaľ, V.; Bidulský, R. Experimental and numerical thickness analysis of TRIP steel under various degrees of deformation in bulge test. Materials 2022, 15, 2299. [Google Scholar] [CrossRef] [PubMed]
- Pantilimon, M.C.; Berbecaru, A.C.; Gherghescu, I.A.; Coman, G.; Ciucă, S.; Grecu, A.; Sohaciu, M.G.; Dumitrescu, R.E.; Predescu, C. Comparative Evaluation of the TRIP Effect in Steels with Different Contents of Mn and Al. Metals 2022, 12, 443. [Google Scholar] [CrossRef]
- Campbell, J. The Hot Ductility of TWIP and TRIP Steels—An Alternative Interpretation. Metals 2022, 12, 2134. [Google Scholar] [CrossRef]
- Krizan, D. TRIP steels: Advanced high strength multiphase steels for automotive applications. In Proceedings of the 14th International Scientific Conference COM-MATTECH, Trnava, Slovakia, 19–20 October 2006; pp. 659–668. [Google Scholar]
- Hu, X.; Feng, Z. Advanced High-Strength Steel—Basics and Applications in the Automotive Industry; National Technical Information Service: Springfield, VA, USA, 2021. [Google Scholar]
- Chen, L.; Zhao, Y.; Qin, X. Some aspects of high manganese twinning-induced plasticity (TWIP) steel: A review. Acta Metall. 2013, 26, 1–15. [Google Scholar] [CrossRef]
- Dobrzański, L.A. Materiały Inżynierskie i Projektowanie Materiałowe; WNT: Warszawa, Poland, 2006. [Google Scholar]
- Imandoust, A.; Hanzaki, A.Z.; Heshmati-Manesh, S.; MoMoemeni, S.; Changizian, M.P. Effects of ferrite volume fraction on the tensile deformation characteristics of dual phase twinning induced plasticity steel. Mater. Des. 2014, 53, 99–105. [Google Scholar] [CrossRef]
- Kliber, J.; Kursa, T.; Schindler, I. Hot rolling of steel with TWIP effect. Hut.—Wiad. Hut. 2008, 75, 481–483. [Google Scholar]
- Gołaszewski, A. Nowa generacja stali ultradrobnoziarnistych z efektem TRIP. Stal Met. Nowe Technol. 2015, 7–8, 38–40. [Google Scholar]
- Park, J.; Kang, M.; Sohn, S.S.; Kim, J.S.; Kim, H.S.; Cho, W.T.; Lee, S. Tensile properties of cold-rolled TWIP-cored three-layer steel sheets. Mater. Sci. Eng. A 2017, 686, 160–167. [Google Scholar] [CrossRef]
- Gronostajski, Z.; Kuziak, R. Metalurgiczne, technologiczne i funkcjonalne podstawy zaawansowanych wysokowytrzymałych stali dla przemysłu motoryzacyjnego. Pr. Inst. Metal. Żelaza 2010, 1, 22–26. [Google Scholar]
- Ghosh, M.; Ghosh, A.; Roy, A. Renewable and sustainable materials in automotive industry. In Encyclopedia of Renewable and Sustainable Materials; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 1–18. [Google Scholar]
- Song, H.; Kwon, Y.; Sohn, S.S.; Koo, M.; Kim, N.J.; Lee, B.J.; Lee, S. Improvement of tensile properties in (austenite+ferrite+κ-carbide) triplex hot-rolled lightweight steels. Mater. Sci. Eng. A 2018, 730, 177–186. [Google Scholar] [CrossRef]
- Sozańska-Jędrasik, L.; Mazurkiewicz, J.; Borek, W.; Dobrzański, L.A. Structure and mechanical properties of newly-developed high-strength TRIPLEX type steel. Inż. Mater. 2018, 5, 184–191. [Google Scholar]
- Frommeyer, G.; Brüx, G. Microstructure and mechanical properties of high-strength Fe-Mn-Al-C TRIPLEX steels. Steel Res. Int. 2006, 77, 627–633. [Google Scholar] [CrossRef]
- Moon, J.; Ha, H.Y.; Kim, K.W.; Park, S.J.; Lee, T.H.; Kim, S.D.; Jang, J.H.; Jo, H.H.; Hng, H.U.; Lee, B.H.; et al. A new class of lightweight, stainless steels with ultra-high strength and large ductility. Sci. Rep. 2020, 10, 12140. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, A.R.; Zarei-Hanzaki, A.; Abedi, H.R.; Jalali, M.S.; Park, S.J.; Park, J.Y. The high temperature deformation behavior of a triplex (ferrite+ austenite+ martensite) low density steel. J. Mater. Res. Technol. 2021, 13, 1388–1401. [Google Scholar] [CrossRef]
- Sohn, S.S.; Choi, K.; Kwak, J.H.; Kim, N.J.; Lee, S. Novel ferriteeaustenite duplex lightweight steel with 77% ductility by transformation induced plasticity and twinning induced plasticity mechanisms. Acta Mater. 2014, 78, 181–189. [Google Scholar] [CrossRef]
- Abedi, H.R.; Hanzaki, A.Z.; Ou, K.L.; Yu, C.H. Substructure hardening in duplex low density steel. Mater. Des. 2017, 116, 472–480. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, C.; Liu, Y.; Zhang, Y.; Song, C.; Zhai, Q. Microstructure, mechanical properties and deformation behavior of Cr-containing triplex low-density steels with different C content. J. Mater. Res. Technol. 2023, 23, 6075–6089. [Google Scholar] [CrossRef]
- Black, J.T.; Kohser, R.A. Materials and Processes in Manufacturing; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Venezuela, J.; Lim, F.Y.; Liu, L.; James, S.; Zhou, Q.; Knibbe, R.; Zhang, M.; Li, H.; Dong, F.; Dargusch, M.S.; et al. Hydrogen embrittlement of an automotive 1700 MPa martensitic advanced high-strength steel. Corros. Sci. 2020, 171, 108726. [Google Scholar] [CrossRef]
- Liu, Q.; Atrens, A. A critical review of the influence of hydrogen on the mechanical properties of medium strength steels. Corros. Rev. 2023, 31, 85–104. [Google Scholar] [CrossRef]
- Venezuela, J.; Blanch, J.; Zulkiply, A.; Liu, Q.; Zhou, Q.; Zhang, M.; Atrens, A. Further study of the hydrogen embrittlement of martensitic advanced high-strength steel in simulated auto service conditions. Corros. Sci. 2018, 135, 120–135. [Google Scholar] [CrossRef]
- Nagumo, M.; Takai, K. The predominant role of strain-induced vacancies in hydrogen embrittlement of steels: Overview. Acta Mater. 2019, 165, 722–733. [Google Scholar] [CrossRef]
- Venezuela, J.; Hill, T.; Zhou, Q.; Li, H.; Shi, Z.; Dong, F.; Knibbe, R.; Zhang, M.; Dargusch, M.S.; Atrens, A. Hydrogen-induced fast fracture in notched 1500 and 1700 MPa class automotive martensitic advanced high-strength steel. Corros. Sci. 2021, 188, 109550. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Zeravcic, V.S.; Sedmak, A.; Rajicic, B. The synergistic action and interplay of hydrogen embrittlement mechanisms in steels and iron: Localized plasticity and decohesion. Eng. Fract. Mech. 2019, 216, 106528. [Google Scholar] [CrossRef]
- Lynch, S. Hydrogen embrittlement phenomena and mechanisms. Corros. Rev. 2012, 30, 105–123. [Google Scholar] [CrossRef]
- Birnbaum, H.K.; Sofronis, P. Hydrogen-enhanced localized plasticity—A mechanism for hydrogen related fracture. Mater. Sci. Eng. A 1994, 176, 191–202. [Google Scholar] [CrossRef]
- Tong, Y.; Li, W.; Zhou, Q.; Li, J. Rapid evaluation of the critical condition for hydrogen-induced cracking in ultrahigh-strength automotive steel sheets: A semiquantitative investigation based on U-bend specimens. Eng. Fail. Anal. 2023, 154, 107726. [Google Scholar] [CrossRef]
- Hall, J.N.; Fekete, J.R. Steels for auto bodies: A general overview. In Automotive Steels; Rana, R., Singh, S.B., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 19–45. [Google Scholar]
- Press Hardening Steels (PHS) for Complex Shapes. Available online: https://www.ssab.com/en/brands-and-products/docol/automotive-steel-grades/press-hardening-steel (accessed on 16 November 2022).
- Press Hardened Steels (PHS). Available online: https://www.totalmateria.com/page.aspx?ID=CheckArticle&site=kts&LN=PL&NM=565 (accessed on 16 November 2022).
- Fonstein, N. Advanced high strength sheet steels. In Advanced High Strength Sheet Steels; Springer: Berlin/Heidelberg, Germany, 2015; pp. 12–16. [Google Scholar]
- Speer, J.G.; De Moor, E.; Findley, K.; Matlock, D.K.; De Cooman, B.C.; Edmonds, D.V. Analysis of microstructure evolution in quenching and partitioning automotive sheet steel. Met. Mater. Trans. A 2011, 42, 3591–3601. [Google Scholar] [CrossRef]
- Schmitt, J.H.; Iung, T. New developments of advanced high-strength steels for automotive applications. C. R. Phys. 2018, 19, 641–656. [Google Scholar] [CrossRef]
- Speer, J.G.; Assunção, F.C.R.; Matlock, D.K.; Edmonds, D.V. The “quenching and partitioning” process: Background and recent progress. Mater. Res. 2005, 8, 417–423. [Google Scholar] [CrossRef]
- Hidalgo, J.; Celada-Casero, C.; Santofimia, M. Fracture mechanisms and microstructure in a medium Mn quenching and partitioning steel exhibiting macrosegregation. Mater. Sci. Eng. A 2019, 754, 766–777. [Google Scholar] [CrossRef]
- Wang, L.; Speer, J.G. Quenching and partitioning steel heat treatment. Metallogr. Microstruct. Anal. 2013, 2, 268–281. [Google Scholar] [CrossRef]
- Pierce, D.T.; Coughlin, D.R.; Clarke, K.D.; De Moor, E.; Poplawsky, J.; Williamson, D.L.; Mazumder, B.; Speer, J.G.; Hood, A.; Clarke, A.J. Microstructural evolution during quenching and partitioning of 0.2C-1.5Mn-1.3Si steels with Cr or Ni additions. Acta Mater. 2018, 151, 454–469. [Google Scholar] [CrossRef]
- Carpio, M.; Calvo, J.; García, O.; Pedraza, J.P.; Cabrera, J.M. Heat treatment design for a QP steel: Effect of partitioning temperature. Metals 2021, 11, 1136. [Google Scholar] [CrossRef]
- Horvath, C.D.; Enloe, C.M. Opportunities and Challenges for 3rd Generation Advanced High-Strength Steels in Automotive Body Structures. Presented at 2017 Great Designs in Steel, Sponsored by American Iron and Steel Institute. Available online: http://www.sawchina.cn/en/Automobilemanufacturing/708.mhtml (accessed on 17 November 2023).
- Ford’s Hot New Bronco Built with Alabama Steel. Available online: https://www.al.com/news/mobile/2020/11/fords-hot-new-bronco-built-with-alabama-steel.html (accessed on 17 November 2023).
- EN 10088-1; Stainless Steels—Part 1: List of Stainless Steel. European Committee for Standardization: Brussels, Belgium, 2014.
- Huang, G.L.; Matlock, D.K.; Krauss, G. Martensite formation, strain rate sensitivity, and deformation behavior of type 304 stainless steel sheet. Metall. Trans. A Phys. Metall. Mater. Sci. 1989, 20A, 1239–1246. [Google Scholar] [CrossRef]
- Xu, L.; Barlat, F.; Ahn, D.C.; Bressan, J.D. Forming limit and fracture mechanism of ferritic stainless steel sheets. Mat. Sci. Eng. A-Struct. 2011, 528, 3113–3121. [Google Scholar] [CrossRef]
- Arrayago, I.; Real, E.; Gardner, L. Description of stress–strain curves for stainless steel alloys. Mater. Des. 2015, 87, 540–552. [Google Scholar] [CrossRef]
- Podatność Stali Nierdzewnych na Przeróbkę Plastyczną. Available online: http://www.stalenierdzewne.pl/84/podatnosc-stali-nierdzewnych-na-przerobke-plastyczna (accessed on 16 November 2023).
- Zastosowanie Stali Nierdzewnej w Produkcji Samochodów Osobowych. Available online: https://www.stalenierdzewne.pl/1076/zastosowanie-stali-nierdzewnej-w-produkcji-samochodow-osobowych (accessed on 16 November 2023).
- Nam, Y.H.; Park, J.S.; Baek, U.B.; Suh, J.Y.; Nahm, S.H. Low-temperature tensile and impact properties of hydrogen-charged high-manganese steel. Int. J. Hydrogen Energy 2019, 44, 7000–7013. [Google Scholar] [CrossRef]
- Mishnev, R.; Borisova, Y.; Kniaziuk, T.; Gaidar, S.; Kaibyshev, R. Quench and Tempered Embrittlement of Ultra-High-Strength Steels with Transition Carbides. Metals 2023, 13, 1399. [Google Scholar] [CrossRef]
- Tsuboi, M.; Shibata, A.; Terada, D.; Tsuji, N. Role of Different Kinds of Boundaries Against Cleavage Crack Propagation in Low-Temperature Embrittlement of Low-Carbon Martensitic Steel. Met. Mater. Trans. A 2017, 48, 3261–3268. [Google Scholar] [CrossRef]
- Song, R.; Ponge, D.; Raabe, D. Mechanical Properties of an Ultrafine Grained C-Mn Steel Processed by Warm Deformation and Annealing. Acta Mater. 2005, 53, 4881–4892. [Google Scholar] [CrossRef]
- Tsuji, N.; Okuno, S.; Koizumi, Y.; Minamino, Y. Toughness of Ultrafine Grained Ferritic Steels Fabricated by ARB and Annealing Process. Mater. Trans. 2004, 45, 2272–2281. [Google Scholar] [CrossRef]
- Fahlblöm, P. Analys för val av Emballagesystem: En Studie Gjord för att Underlätta Valet av Emballagesystem (Analysis for Choice of Packaging: A Study Done on Choosing of Packaging Methods, in Swedish). Master’s Thesis, Luleå University of Technology, Luleå, Sweden, 1997. [Google Scholar]
- Bano, X.; Laurent, J.P. Heat treated boron steels in the automotive industry. In Proceedings of the 39th Mechanical Working and Steel Processing Conference, Indianapolis, Indiana, 19–22 October 1997; Paper PR-310-066. pp. 673–677. [Google Scholar]
- Reinhardt, A. Development of hot stamped Ultra High Strength Steel parts on the Peugeot 307 and the Citroën C5. In Proceedings of the EuroCarBody 2001—3rd Global Car Body Benchmarking Conference, Bad Nauheim, Germany, 12 June 2001. [Google Scholar]
- Weigert, P. Challenges in mass production of press hardened components focusing CO2 reduction. In Proceedings of the Insight Edition Conference, Gothenburg, Sweden, 21 September 2011. [Google Scholar]
- Fidorra, A.; Baur, J. The Art of Progress: Audi—The New A8. In Proceedings of the EuroCarBody 2010, Bad Nauheim, Germany, 18–20 October 2010. [Google Scholar]
- Wuhan Economic and Technological Development Zone, Dongfeng Moves Up-Market in Electric Vehicle Segment. Available online: http://en.whkfq.gov.cn/2020-11/11/c_566509.htm (accessed on 16 December 2023).
- Lanzerath, H.; Bach, A.; Oberhofer, G.; Gese, H. Failure Prediction of Boron Steels in Crash; SAE Technical Paper 2007-01-0989; SAE International: Warrendale, PA, USA, 2007. [Google Scholar]
- Lapsien, R. Hot Forming at Benteler—Current applications and trends for the future. In Proceedings of the Materials in Car Body Engineering 2014, Bad Nauheim, Germany, 13–14 May 2014. [Google Scholar]
- Taylor, T.; Clough, A. Critical review of automotive hot-stamped sheet steel from an industrial perspective. Mater. Sci. Technol. 2018, 34, 809–861. [Google Scholar] [CrossRef]
- Staeves, J. Höherfeste Stähle für die Karosserie (High strength steels for car body—In German). In Proceedings of the Technical University of Munich, Munich, Germany, 28 June 2004. [Google Scholar]
- Gier, A. Hot Forming and Hardening of Rollformed Sections. In Aluminum and Steel Forming in Automotive Engineering; Springer: Cham, Switzerland, 2005; pp. 172–237. [Google Scholar]
- Lindberg, H. Advanced High Strength Steel Technologies in the 2016 Volvo XC90. Presented at 2016 Great Designs in Steel, Sponsored by American Iron and Steel Institute. Available online: https://docplayer.net/42205170-Advanced-high-strength-steel-technologies-in-the-2016-volvo-xc90.html (accessed on 16 December 2023).
- Kröning, A. Lightweight solutions for body applications. In Proceedings of the Insight Edition Conference, Gothenburg, Sweden, 20–21 September 2011. [Google Scholar]
- Pfestorf, M.; Rensburg, J. Functional Properties of High Strength Steel in Body in White; Sponsored by American Iron and Steel Institute; Great Design in Steel: Livonia, MI, USA, 2006. [Google Scholar]
- Hilfrich, E.; Seidner, D. Crash safety with high strength steels. In Proceedings of the International Automotive Congress, Shengyang, China, 30 October 2008. [Google Scholar]
- Pfestorf, M. Multimaterial lightweight design for the body in white of the new BMW 7 series. In Proceedings of the International Conference of Innovative Developments for Lightweight Vehicle Structures, Wolfsburg, Germany, 26–27 May 2009. [Google Scholar]
- Billur, E. Hot Stamping of Ultra High-Strength Steels: From a Technological and Business Perspective; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Ludlow, D. Ford B-Max preview. Expert Reviews, 23 February 2012. [Google Scholar]
- PHS Automotive Applications and Usage. Available online: https://ahssinsights.org/forming/press-hardened-steels/phs-automotive-applications-and-usage/ (accessed on 16 December 2023).
- Horner, K. Strategic Steel Application in the Acura NSX Space Frame; Sponsored by American Iron and Steel Institute; Great Design in Steel: Livonia, MI, USA, 2018. [Google Scholar]
- Büchner, J.; Oberlander, T.; Benneker, B. Ford Focus. In Proceedings of the EuroCarBody 2018, Bad Nauheim, Germany, 16–18 October 2018. [Google Scholar]
- Vaissiere, L.; Laurent, J.P.; Reinhardt, A. Development of Pre-Coated Boron Steel for Applications on PSA Peugeot Citroën and RENAULT Bodies in White; SAE Technical Paper 2002-01-2048; SAE International: Warrendale, PA, USA, 2002. [Google Scholar]
- Plassart, G.; Philip, G. Materials Criteria Selection and Certification Process for the Body in White in PSA PEUGEOT CITROËN; SAE Technical Paper 2002-01-2051; SAE International: Warrendale, PA, USA, 2002. [Google Scholar]
- Decker, L.; Truskin, J. The All-New 2017 Chrysler Pacifica. In Proceedings of the Strategies in Car Body Engineering 2017, Bad Nauheim, Germany, 22–23 March 2017. [Google Scholar]
- Matsuoka, H.; Fujihara, K. Mazda CX-5. In Proceedings of the EuroCarBody 2011, Bad Nauheim, Germany, 18–20 October 2011. [Google Scholar]
- VAMA China. When Usibor®2000 Meets China’s Legendary SUV model Haval H6. Available online: http://www.vamachina.com/en/usibor2000-meets-haval-h6/ (accessed on 8 December 2023).
- Bernquist, J. Safety Cage Design in the Volvo XC90. Available online: http://worldautosteel.wpengine.com/wp-content/uploads/GDIS-Prez/B-44_22%20-%20Safety%20Cage%20Design%20in%20the%20Volvo%20XC90-2004.pdf (accessed on 16 December 2023).
- Lüken, I.; Tenneberg, N. Volkswagen ID.3. In Proceedings of the Aachener Karosserietage, Aachen, Germany, 17–18 September 2019. [Google Scholar]
- Press Hardened Steels. Available online: https://ahssinsights.org/metallurgy/steel-grades/phs-grades/ (accessed on 16 December 2023).
- D’Aiuto, F.; Tedesco, M.M. Development of New Structural Components with Innovative Materials and Technological Solutions. In Proceedings of the Materials in Car Body Engineering 2015, Bad Nauheim, Germany, 22–23 April 2015. [Google Scholar]
- Tobita, S.; Shinmiya, T.; Yamasaki, Y.; Hiramoto, J. Development of Forming Technology to Reduce Dimensional Scattering of Automotive Parts with Cambers by Using Bauschinger Effect. Mater. Trans. 2021, 62, 1750–1756. [Google Scholar] [CrossRef]
- Reed, D.; Belanger, P. Hot Stamped Steel One-Piece Door Ring in the All-New 2019 Ram 1500. Available online: https://www.steel.org/wp-content/uploads/2021/02/Track-2-Belanger-and-Reed.pdf (accessed on 16 December 2023).
- Fossati, B.; Machado-Baglietto, A.; Cappelaere, M. Hot Stamping Industrialization at Renault. In Proceedings of the Forming in Car Body Engineering 2014, Bad Nauheim, Germany, 24–25 September 2014. [Google Scholar]
- Black, S.; Rowlings, S.; Dietrich, C. Jaguar I-PACE. In Proceedings of the EuroCarBody 2018, Bad Nauheim, Germany, 16–18 October 2018. [Google Scholar]
- Bilur, E. Hot formed Steels. In Automotive Steels Design, Metallurgy, Processing and Applications; Rana, R., Singh, S.B., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 387–411. [Google Scholar]
- Transformation Induced Plasticity (TRIP). Available online: https://ahssinsights.org/metallurgy/steel-grades/3rdgen-ahss/transformation-induced-plasticity-trip/ (accessed on 16 December 2023).
- Christodoulou, P.I. Effect of Retained Austenite Transformation on the Fatigue Behaviour of Aluminum Containing TRIP Steels. Ph.D. Thesis, University of Thessaly, Volos, Greece, September 2017. [Google Scholar]
- Martensite. Available online: https://ahssinsights.org/metallurgy/steel-grades/1stgen-ahss/martensite/ (accessed on 16 December 2023).
- JFE Steel Corporation. Toyota Lexus Adopts 1.5 GPa High-Strength Cold-Rolled Steel Sheet to Structural Part by Unique Cold Press Forming Technology; JFE Steel Corporation: Tokyo, Japan, 2021. [Google Scholar]
- Krajewski, S.; Nowacki, J. Mikrostruktura i właściwości stali o wysokiej wytrzymałości AHSS. Prz. Spaw. 2011, 7, 22–27. [Google Scholar] [CrossRef]
- Dual Phase. Available online: https://ahssinsights.org/metallurgy/steel-grades/ahss/dual-phase/ (accessed on 16 December 2023).
- Complex Phase. Available online: https://ahssinsights.org/metallurgy/steel-grades/complex-phase-steel/ (accessed on 16 December 2023).
- Ferrite-Bainite. Available online: https://ahssinsights.org/metallurgy/steel-grades/ferrite-bainite-steel/ (accessed on 16 December 2023).
- Nissan Japan Motors. Infiniti QX50. In Proceedings of the EuroCarBody 2001—20th Global Car Body Benchmarking Conference, Bad Neucheim, Germany, 16–18 October 2018. [Google Scholar]
- 3rd Generation Steels. Available online: https://ahssinsights.org/metallurgy/steel-grades/3rd-generation-steels/ (accessed on 16 December 2023).
- Coakley, D. 2015 Nissan Murano; Sponsored by American Iron and Steel Institute; Great Design in Steel: Livonia, MI, USA, 2015. [Google Scholar]
- Coakley, D.; Zischke, J. The 2016 Nissan Maxima; Sponsored by American Iron and Steel Institute; Great Design in Steel: Livonia, MI, USA, 2015. [Google Scholar]
- Wang, L.; Bian, J.; Wang, J.; Ye, Y. Development and Application of New Generation AHSS Based on Q&P Process. In Proceedings of the Materials in Car Body Engineering 2019, Bad Nauheim, Germany, 14–19 May 2019. [Google Scholar]
- Twinning Induced Plasticity. Available online: https://ahssinsights.org/metallurgy/steel-grades/ahss/twinning-induced-plasticity/ (accessed on 16 December 2023).
- Nam, J.B. Development of New Auto Steels and Application Technology. Available online: https://docplayer.net/48872013-Development-of-new-auto-steels-and-application-technology.html (accessed on 16 December 2023).
- D’Aiuto, F. Innovative materials and solutions for automotive components. In Proceedings of the ANFIA—Associazione Nazionale Fra Industrie Automobilistiche (National Association of the Automobile Industry), Torino, Italy, 28 April 2016. [Google Scholar]
- Renault Press Release. EOLAB Concept Showcases Renault’s Pursuit of Ultra-Low Fuel Consumption. Available online: https://www.press.renault.co.uk/en-gb/releases/1707 (accessed on 16 December 2023).
- Capelli, F.; Boneschi, V.; Viganò, P.; Inox, C. Stainless Steel: A new structural automotive material. In Proceedings of the 9th International Conference & Exhibition, FLORENCE ATA 2005, Florence, Italy, 11–13 May 2005. [Google Scholar]
- Applications and Uses of Stainless Steel in Automotive Industry. Available online: https://www.vishwastainless.com/stainless-steel-applications-and-uses-in-automotive-industry/ (accessed on 16 December 2023).
- Snelgrove, P. Stainless Steel Automotive and Transport Developments. Available online: https://www.worldstainless.org/Files/issf/non-image-files/PDF/Stainlesssteelautomotiveandtransportdevelopments.pdf (accessed on 16 December 2023).
- Stainless Steel Applications—Automotive. Available online: https://www.worldstainless.org/Files/issf/non-image-files/PDF/Automotiveapplications.pdf (accessed on 16 December 2023).
- Application of Stainless Steel in Automobile Industry. Available online: https://www.ronscopipe.com/infodetail/application-of-stainless-steel-in-automobile-industry.html (accessed on 16 December 2023).
- Podder, A.S.; Bhanja, A. Applications of stainless steel in automobile industry. Adv. Mater. Res. 2013, 794, 731–740. [Google Scholar] [CrossRef]
- Emmons, J.E.; Blessing, L.J. Ultra-light Stainless Steel Urban Bus Concept; SAE Technical Paper 2001-01-2073; SAE International: Warrendale, PA, USA, 2001. [Google Scholar]
- Kwiatkowski, L. Podatność na korozję i skuteczność aktualnych metod ochrony przed korozją stopów aluminium stosowanych w budownictwie. Inż. Powierz. 2009, 4, 24–33. [Google Scholar]
- Callister, W.D., Jr.; Rethwisch, D.G. Materials Science and Engineering: An Introduction, 10th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Kondratiuk, J.; Kuhn, P. Tribological investigation on friction and wear behaviour of coatings for hot sheet metal forming. Wear 2011, 270, 839–849. [Google Scholar] [CrossRef]
- Davies, J.R. ASM Specialty Handbook: Aluminum and Aluminum Alloys; ASM International: Materials Park, OH, USA, 2014. [Google Scholar]
- EN 573-3; Aluminium and Aluminium Alloys—Chemical Composition and Form of Wrought Products—Part 3: Chemical Composition and Form of Products. European Committee for Standardization: Brussels, Belgium, 2005.
- Davis, J.R. Aluminium and aluminium alloys. In Alloying: Understanding the Basics; Davis, J.R., Ed.; ASM International: Materials Park, OH, USA, 2001; pp. 351–416. [Google Scholar]
- Djukanovic, G. Aluminium Alloys in the Automotive Industry: A Handy Guide. Available online: https://aluminiuminsider.com/aluminium-alloys-automotive-industry-handy-guide/ (accessed on 19 November 2023).
- Huber, G.; Djurdjevic, M.B.; Manasijevic, S. Determination some thermo-physical and metallurgical properties of aluminum alloys using their known chemical composition. Int. J. Heat Mass Transf. 2019, 139, 548–553. [Google Scholar] [CrossRef]
- Vijayakumar, M.D.; Dhinakaran, V.; Sathish, T.; Muthu, G.; Bupathiram, P.M. Experimental study of chemical composition of aluminium alloys. Mater. Today Proc. 2021, 37, 1790–1793. [Google Scholar] [CrossRef]
- Hattori, C.S.; Almeida, G.F.C.; Gonçalves, R.L.P.; Santos, R.G.; Souza, R.C.; da Silva, W.C., Jr.; Cunali, J.R.C., Jr.; Couto, A.A. Microstructure and fatigue properties of extruded aluminum alloys 7046 and 7108 for automotive applications. J. Mater. Res. Technol. 2021, 14, 2970–2981. [Google Scholar] [CrossRef]
- Puga, H. Casting and forming of advanced aluminum alloys. Metals 2020, 10, 494. [Google Scholar] [CrossRef]
- Stojanovic, B.; Bukvic, M.; Epler, I. Application of aluminum and aluminum alloys in engineering. Appl. Eng. Lett. J. Eng. Appl. Sci. 2018, 3, 52–62. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Z.; Xu, G.; Zeng, X.; Hu, W.; Matsubae, K. Wrought and cast aluminum flows in China in the context of electric vehicle diffusion and automotive lightweighting. Resour. Conserv. Recycl. 2023, 191, 106877. [Google Scholar] [CrossRef]
- Baser, T.A.; Umay, E.; Akinci, V. New Trends in Aluminum Die Casting Alloys for Automotive Applications. Eurasia Proc. Sci. Technol. Eng. Math. 2022, 21, 79–87. [Google Scholar] [CrossRef]
- Ducker, C. Mega-Casting Trends for Automotive Manufacturers in 2022. Available online: https://www.linkedin.com/pulse/mega-casting-trends-automotive-manufacturers-2022-ducker-worldwide (accessed on 17 December 2023).
- Aluminium in Transport. Available online: https://www.aluminiumleader.com/application/transport/ (accessed on 17 December 2023).
- Szczucka-Lasota, B.; Węgrzyn, T.; Jurek, A. Aluminum alloy welding in automotive industry. Transp. Probl. 2020, 15, 67–78. [Google Scholar] [CrossRef]
- Kaufman, J.G. Applications for aluminum alloys and tempers. Introd. Alum. Alloys Tempers 2000, 1100, 242. [Google Scholar]
- Understanding the Alloys of Aluminium. Available online: http://www.alcotec.com/us/en/education/knowledge/techknowledge/understanding-the-alloys-of-aluminum.cfm (accessed on 18 November 2023).
- Benedyk, J.C. Aluminum alloys for lightweight automotive structures. In Materials, Design and Manufacturing for Lightweight Vehicles Materials, Design and Manufacturing for Lightweight Vehicles; Mallick, P.K., Ed.; Woodhead Publishing: Sawston, UK, 2010; pp. 79–113. [Google Scholar]
- The Aluminum Association Auto & Light Truck Group (ALTG). Available online: https://www.southadams.k12.in.us/site/Default.aspx?PageType=3&DomainID=101&PageID=531&ViewID=5c8b25c6-c8f8-4bd5-923b-8a7c70a93dda&FlexDataID=1671 (accessed on 24 May 2020).
- Fridlyander, I.N.; Sister, V.G.; Grushko, O.E.; Berstenev, V.V.; Sheveleva, L.M.; Ivanova, L.A. Aluminum alloys: Promising materials in the automotive industry. Met. Sci. Heat Treat. 2002, 44, 365–370. [Google Scholar] [CrossRef]
- Why Are Aluminum Alloys Used in the Auto Industry? Available online: https://www.howardprecision.com/why-are-aluminum-alloys-used-in-the-auto-industry/ (accessed on 15 December 2023).
- Rooy, E.L. Introduction to Aluminum and Aluminum Alloys. In ASM Handbook Committee. Volume 2: Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM International: Materials Park, OH, USA, 1990; pp. 3–14. [Google Scholar] [CrossRef]
- Mukhopadhyay, P. Alloy designation, processing, and use of AA6XXX series aluminium alloys. Int. Sch. Res. Not. 2012, 2012, 165082. [Google Scholar] [CrossRef]
- Aluminum Alloys Used in the Automotive Industry! Available online: https://www.metalsupermarkets.com/aluminum-alloys-used-in-the-automotive-industry/ (accessed on 15 December 2023).
- What Is Aluminum Used for: Automotive Edition. Available online: https://www.kloecknermetals.com/blog/what-is-aluminum-used-for-automotive-edition/ (accessed on 15 December 2023).
- Polmear, I.J. Light Alloys: From Traditional Alloys to Nanocrystals, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Constellium Supplying Aluminum Solutions for Audi E-Tron GT EV. Constellium Supplying Aluminum Solutions for Audi E-Tron GT EV. Retrieved 2023-3-31.
- Applications—Car Body—Body Structures. Available online: https://european-aluminium.eu/wp-content/uploads/2022/11/1_aam_body-structures.pdf (accessed on 15 December 2023).
- Koganti, R.; Weishaar, J. Aluminum Vehicle Body Construction and Enabling Manufacturing Technologies. SAE Int. J. Mater. Manuf. 2009, 1, 491–502. [Google Scholar] [CrossRef]
- Applications—Car Body—Body Components. Available online: https://european-aluminium.eu/wp-content/uploads/2022/11/2_aam_body-components.pdf (accessed on 15 December 2023).
- Hofer-Hauser, P.; Gschwandtner, R. Influence of Die Evacuation on Mechanical Properties and Heat Treatability of HPD-Castings. Available online: https://www.fondarex.com/media/fdx_hofer_gschwandtner_en.pdf (accessed on 15 December 2023).
- Luo, A.A.; Sachdev, A.K.; Apelian, D. Alloy Development and Process Innovations for Light Metals Casting. J. Mater. Process. Technol. 2022, 306, 117606. [Google Scholar] [CrossRef]
- Hartlieb, M. Aluminum Alloys for Structural Die Casting. Die Cast. Eng. 2013, 57, 40–43. [Google Scholar]
- Pezda, J.; Jezierski, J. Non-Standard T6 Heat Treatment of the Casting of the Combustion Engine Cylinder Head. Materials 2020, 13, 4114. [Google Scholar] [CrossRef]
- Aluminium in Cars: Unlocking the Lightweighting Potential; European Aluminium Association: Brussels, Belgium, 2021; Available online: https://european-aluminium.eu/wp-content/uploads/2022/10/aluminium-in-cars-unlocking-the-lightweighting-potential.pdf (accessed on 31 March 2023).
- Applications—Car Body—Roof and Trim. Available online: https://european-aluminium.eu/wp-content/uploads/2022/11/5_6_aam_roof-and-trim.pdf (accessed on 15 December 2023).
- Applications—Car Body—Crash Management Systems. Available online: https://european-aluminium.eu/wp-content/uploads/2022/11/4_aam_crash-management-systems1.pdf (accessed on 15 December 2023).
- Rheinfelden Alloys GmbH & Co. KG. Alloys Alloys for High Pressure Die Casting; Rheinfelden Alloys GmbH & Co. KG: Rheinfelden, Germany, 2015; pp. 1–60. [Google Scholar]
- Burger, G.B.; Gupta, A.K.; Jeffrey, P.W.; Lloyd, D.J. Microstructural control of aluminum sheet used in automotive applications. Mater. Charact. 1995, 35, 23–39. [Google Scholar] [CrossRef]
- Application of Aluminum Alloy in Automobile Manufacturing. Available online: https://hw-alu.com/blog/application-of-aluminum-alloy-in-automobile-manufacturing.html (accessed on 15 December 2023).
- Uno, T.; Baba, Y. Neue Aluminiumlegierung für Karosseriebleche. Aluminium 1987, 63, 1243–1246. [Google Scholar]
- Applications—Car Body—Hang-On Parts. Available online: https://european-aluminium.eu/wp-content/uploads/2022/11/3_aam_hang-on-parts.pdf (accessed on 15 December 2023).
- Types and Applications of Aluminum Alloys for Vehicles. Available online: https://uacj-automobile.com/types_and_applications.html (accessed on 15 December 2023).
- Miller, W.S.; Zhuang, L.; Bottema, J.; Wittebroad, A.J.; De Smet, P.; Haszler, A.; Vieregge, A. Recent development in aluminium alloys for the automotive industry. Mater. Sci. Eng. A 2000, 280, 37–49. [Google Scholar] [CrossRef]
- What Are the Aluminum Alloys Used in Cars. Available online: https://www.autoaluminumsheet.com/a/what-are-the-aluminum-alloys-used-in-cars.html (accessed on 15 December 2023).
- Olandersson, H. Novelis—Overview of Flat Rolled Aluminium Products. Available online: https://swedsoft.se/wp-content/uploads/sites/24/2016/12/161108_Novelis-presentation-SAMS.pdf (accessed on 15 December 2023).
- Panda, S.S. Aluminum Alloys in Automotive Application; Technical Report; Gdansk University of Technology: Gdańsk, Poland, 2015. [Google Scholar]
- Baruah, M.; Borah, A. Processing and precipitation strengthening of 6xxx series aluminium alloys: A review. Int. J. Mater. Sci. 2020, 1, 40–48. [Google Scholar] [CrossRef]
- Couper, M.J.; Edwards, G.A. 6xxx Series Aluminium Alloy. Canadian Patent No CA2259322C, 12 February 2013. Available online: https://patentimages.storage.googleapis.com/42/0c/d7/c9cec2c1012038/CA2259322C.pdf (accessed on 24 January 2024).
- Zupanič, F.; Klemenc, J.; Steinacher, M.; Glodež, S. Microstructure, mechanical properties and fatigue behaviour of a new high-strength aluminium alloy AA 6086. J. Alloys Compd. 2023, 941, 168976. [Google Scholar] [CrossRef]
- Lin, C.W.; Hung, F.Y.; Lui, T.S. Microstructural characteristics and mechanical behaviors of new type SIMA processed aluminum alloy. In Aluminium Alloys—Recent Trends in Processing, Characterization, Mechanical Behavior and Applications; InTechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Xu, H.; Zhang, G. Decreased dislocation density as an origin for the quench sensitivity of the Al-Si-Mg alloys with high Si kontent. J. Alloys Compd. 2022, 910, 165011. [Google Scholar] [CrossRef]
- Chakrabarti, D.; Laughlin, D.E. Phase relations and precipitation in Al–Mg–Si alloys with Cu additions. Prog. Mater. Sci. 2004, 49, 389–410. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Ban, C. Effect of Fe content on the microstructure and properties of hot-extruded 6061 aluminum alloy. J. Phys. Conf. Ser. 2021, 1986, 012011. [Google Scholar] [CrossRef]
- Lin, X.Z.; Yin, F.; Sun, B.D. lnfluence of Fe on the properties of Al Si alloy and methods of neutralizing the effect of Fe. Foundry Technol. 1999, 5, 29–32. [Google Scholar]
- Xu, Z.; Zhang, X.Y.; Wang, H.B.; Gao, A.; Ma, T.; Song, H. Effect of Mn/Fe ratio on the microstructure and properties of 6061 sheets obtained by twin-roll cast. Mater. Charact. 2020, 168, 110536. [Google Scholar] [CrossRef]
- Kaufman, J.G. Corrosion of aluminum and aluminum alloys. In Properties and Selection of Aluminum Alloys; ASM International: Materials Park, OH, USA, 2019. [Google Scholar] [CrossRef]
- Muzykiewicz, W.; Rękas, A.; Kosmalski, G. Badania walidacyjne blachy w gatunku 6082 w stanie ‘0’ pod kątem jej zastosowań do procesów tłoczenia. Rudy Met. Nieżelazne 2006, 51, 422–427. [Google Scholar]
- Rochet, C.; Veron, M.; Raucvh, E.F.; Lowe, T.C.; Arfaei, B.; Laurino, A.; Harouard, J.P.; Blanc, C. Influence of equal-channel angular pressing on the microstructure and corrosion behaviour of a 6xxx aluminium alloy for automotive conductors. Corros. Sci. 2020, 166, 108453. [Google Scholar] [CrossRef]
- Oana, S.A.; Karancsi, O.; Mitelea, I.; Uţu, I.D.; Craciunescu, C.M. The role of filler material selection in the laser welding process of deformable 6xxx series aluminum alloys. Mater. Today Proc. 2023, 78, 287–294. [Google Scholar] [CrossRef]
- Hirsch, J. Aluminium alloys for automotove application. Mater. Sci. Forum 1997, 242, 33–50. [Google Scholar] [CrossRef]
- Novelis and Jaguar Land Rover to Supply Lightweight SUVs. Available online: https://www.ai-online.com/2012/11/novelis-and-jaguar-land-rover-to-supply-lightweight-suvs/ (accessed on 15 December 2023).
- Sun, J. Research on Situation and Application Prospect of Automotive Body Sheets Al-Mg-Si Based (6000series) Alloy. IOP Conf. Ser. Mater. Sci. Eng. 2018, 452, 022082. [Google Scholar] [CrossRef]
- Swapna, D.; Rao, C.S.; Kumar, D.S.; Radhika, S. AHP and TOPSIS based selection of aluminium alloy for automobile panels. J. Mech. Energy Eng. 2019, 3, 43–50. [Google Scholar] [CrossRef]
- Tisza, M.; Lukács, Z. High strength aluminum alloys in car manufacturing. IOP Conf. Ser. Mater. Sci. Eng. 2018, 418, 012033. [Google Scholar] [CrossRef]
- Li, Y. Effect of Alloy Elements on Microstructure and Hot Tearing Susceptibility in Direct-Chill Casting of 7xxx Series Aluminum Alloys. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2019. [Google Scholar]
- Dai, Y.; Yan, L.; Hao, J. Review on micro-alloying and preparation method of 7xxx series aluminum alloys: Progresses and prospects. Materials 2022, 15, 1216. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.; Toda, H.; Uesugi, K.; Takeuchi, A.; Watanabe, Y. Damage micromechanisms in high Mn and Zn content 7xxx series aluminum alloys. Mater. Sci. Eng. A 2020, 793, 139423. [Google Scholar] [CrossRef]
- Valeev, I.S.; Barykin, N.P.; Trifonov, V.G.; Valeeva, A.K. Effect of powerful current pulses on the structure and mechanical properties of the aluminum alloy Al-6%Mg-0.6%Mn. J. Mater. Eng. Perform. 2014, 14, 236–240. [Google Scholar] [CrossRef]
- Fang, H.C.; Yang, H.L.; Zhu, J.M.; Xiao, P.; Chen, Z.; Liu, T. Effect of minor Cr, Mn, Zr or Ti on recrystallization, secondary phases and fracture behaviour of Al-Zn-Mg-Cu-Yb Alloys. Rare Met. Mater. Eng. 2020, 49, 0797–0810. [Google Scholar]
- Li, G.F.; Zhang, X.M.; Zhu, H.F. Effect of minor Er and Y additions to Al-Zn-Mg-Cu-Zr alloy on homogenizing behavior. Hangkong Cailiao Xuebao/J. Aeronaut. Mater. 2010, 30, 1–6. [Google Scholar]
- Long, R.S.; Boettcher, E.; Crawford, D. Current and future uses of aluminum in the automotive industry. JOM 2017, 69, 2635–2639. [Google Scholar] [CrossRef]
- Kumar, M.; Poletti, C.; Degischer, H.P. Precipitation kinetics in warm forming of AW-7020 alloy. Mater. Sci. Eng. A 2013, 561, 362–370. [Google Scholar] [CrossRef]
- Polak, S.; Kaczyński, P.; Gronostajski, Z.; Jaśkiewicz, K.; Krawczyk, J.; Skwarski, M.; Zwierzchowski, M.; Chorzępa, W. Warm forming of 7075 aluminum alloys. Procedia Eng. 2017, 207, 2399–2404. [Google Scholar] [CrossRef]
- Jaśkiewicz, K.; Skwarski, M.; Kaczyński, P.; Gronostajski, Z.; Polak, S.; Trzpis, P. Warm sheet metal forming of en-ergy-absorbing elements made 7075 aluminum alloy in the hardened state T6. Int. J. Adv. Manuf. Technol. 2022, 119, 3157–3179. [Google Scholar] [CrossRef]
- Shin, J.; Kim, T.; Kim, D.; Kim, D.; Kim, K. Castability and mechanical properties of new 7xxx aluminum alloys for automotive chassis/body applications. J. Alloys Compd. 2017, 698, 577–590. [Google Scholar] [CrossRef]
- Svendsen, A. Aluminum Continues Unprecedented Growth in Automotive Applications—Light Metal Age Magazine. Light Metal Age Magazine 2020. Available online: https://www.lightmetalage.com/news/industry-news/automotive/aluminum-continues-unprecedented-growth-in-automotive-applications/ (accessed on 19 November 2023).
- The Application of Aluminum Alloy in Automotive Industry. Available online: https://www.aluminiummanufacturer.com/blog/the-application-of-aluminum-alloy-in-automotive-industry/ (accessed on 15 December 2023).
- Gray Square 9000 Series Aluminum, Grade: Good. Available online: https://www.indiamart.com/proddetail/9000-series-aluminum-25920719997.html (accessed on 19 November 2023).
- Xu, C.; Xiao, W.; Hanada, S.; Yamagata, H.; Ma, C. The effect of scandium addition on microstructure and mechanical properties of Al–Si–Mg alloy: A multi-refinement modifier. Mater. Charact. 2015, 110, 160–169. [Google Scholar] [CrossRef]
- Liu, G.; Blake, P.; Ji, S. Effect of Zr on the high cycle fatigue and mechanical properties of Al–Si–Cu–Mg alloys at elevated temperatures. J. Alloys Compd. 2019, 809, 151795. [Google Scholar] [CrossRef]
- Rahimian, M.; Amirkhanlou, S.; Blake, P.; Ji, S. Nanoscale Zr-containing precipitates; a solution for significant improvement of high-temperature strength in Al-Si-Cu-Mg alloys. Mater. Sci. Eng. A 2018, 721, 328–338. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Samuel, F.H.; Al Kahtani, S. Microstructure, tensile properties and fracture behavior of high temperature Al–Si–Mg–Cu cast alloys. Mater. Sci. Eng. A 2013, 577, 64–72. [Google Scholar] [CrossRef]
- Pushp, P.; Dasharath, S.M.; Arati, C. Classification and applications of titanium and its alloys. Mater. Today Proc. 2022, 54, 537–542. [Google Scholar] [CrossRef]
- Boyer, R.R.; Briggs, R.D. The use of β titanium alloys in the aerospace industry. J. Mater. Eng. Perform. 2005, 14, 681–685. [Google Scholar] [CrossRef]
- Donachie, J.; Matthew, J. Titanium: A Technical Guide; ASM International: Materials Part, OH, USA, 2000. [Google Scholar]
- Wang, K.; Kopec, M.; Chang, S.; Qu, B.; Liu, J.; Politis, D.J.; Wang, L.; Liu, G. Enhanced formability and forming efficiency for two-phase titanium alloys by fast light alloys stamping technology (FAST). Mater. Des. 2020, 194, 108948. [Google Scholar] [CrossRef]
- Elias, C.N.; Meyers, M.A.; Valiev, R.Z.; Monteiro, S.N. Ultrafine grained titanium for biomedical applications: An overview of performance. J. Mater. Res. Technol. 2013, 2, 340–350. [Google Scholar] [CrossRef]
- Shahmir, H.; Langdon, T. An evaluation of the hexagonal close-packed to face-centered cubic phase transformation in a Ti-6Al-4V alloy during high-pressure torsion. Mater. Sci. Eng. A 2017, 704, 212–217. [Google Scholar] [CrossRef]
- Kolli, R.P.; Devaraj, A. A review of metastable beta titanium alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef]
- Wood, J.R.; Russo, P.A.; Welter, M.F.; Crist, E.M. Thermomechanical processing and heat treatment of Ti–6Al–2Sn–2Zr–2Cr–2Mo–Si for structural application. Mater. Sci. Eng. A 1998, 243, 109–118. [Google Scholar] [CrossRef]
- Huang, S.; Zhao, Q.; Wu, C.; Lin, C.; Zhao, Y.; Jia, W.; Mao, C. Effects of β-stabilizer elements on microstructure formation and mechanical properties of titanium alloys. J. Alloys Compd. 2021, 867, 160085. [Google Scholar] [CrossRef]
- Muraca, R.F.; Whittick, J.S. Materials Data Handbook. Titanium 6Al-4V; National Aeronautics and Space Administration: Washington, DC, USA, 1972; Volume 2. [Google Scholar]
- Salihu, S.A.; Suleiman, Y.I.; Eyinavi, A.I. Classification, Properties and Applications of titanium and its alloys used in automotive industry—A Review. Am. J. Eng. Res. 2019, 4, 92–98. [Google Scholar]
- Wang, Z.; Liu, L.; Zhang, L.; Sheng, J.; Wu, D.; Yuan, M. Effect of heat treatment on the microstructure and mechanical properties of high-strength Ti–6Al–4V–5Fe alloy. Mater. Trans. 2019, 60, 269–276. [Google Scholar] [CrossRef]
- Brice, D.A.; Samimi, P.; Ghamarian, I.; Kiu, Y.; Brice, R.M.; Reidy, R.F.; Cotton, J.D.; Kaufman, M.J.; Collins, P.C. Oxidation behavior and microstructural decomposition of Ti-6Al-4V and Ti-6Al-4V-1B sheet. Corros. Sci. 2016, 112, 338–346. [Google Scholar] [CrossRef]
- Fan, Y.; Tian, W.; Guo, Y.; Sun, Z.; Xu, J. Relationships among the microstructure, mechanical properties, and fatigue behavior in thin Ti6Al4V. Adv. Mater. Sci. Eng. 2016, 2016, 7278267. [Google Scholar] [CrossRef]
- Loier, C.; Thauvin Hazotte, G.A.; Simon, A. Influence of deformation on the β→α+β transformation kinetics of Ti-6 wt.%Al-4 wt.%V alloy. J. Less Common Met. 1985, 108, 295–312. [Google Scholar] [CrossRef]
- Leyens, C.; Peter, M. Titanium and Titanium Alloys: Fundamentals and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Kang, L.M.; Yang, C. A review on high-strength titanium alloys: Microstructure, strengthening, and properties. Adv. Eng. Mater. 2019, 2019, 1801359. [Google Scholar] [CrossRef]
- Yamashita, Y.; Takayama, I.; Fujii, H.; Yamazaki, T. Applications and features of titanium for automotive industry. Nippon Steel Tech. Rep. 2002, 85, 11–14. [Google Scholar]
- Bieler, T.R.; Trevino, R.M.; Zeng, L. Alloys: Titanium. Encyclopedia of Condensed Matter Physics; Elsevier: Amsterdam, The Netherlands, 2005; pp. 65–76. [Google Scholar]
- Sbayti, M.; Ghiotti, A.; Bahloul, R.; Belhadjsalah, H.; Bruschi, S. Finite element analysis of hot single point incremental forming of hip prostheses. MATEC Web Conf. 2016, 80, 14006. [Google Scholar] [CrossRef]
- Sornsuwit, N.; Sittisakuljaroen, S.; Sangsai, N.; Suwankan, P. Effect of heat treatment on single point incremental forming for titanium Grade 2 sheet. In Proceedings of the 2018 Third International Conference on Engineering Science and Innovative Technology (ESIT), North Bangkok, Thailand, 19–22 April 2018; p. 4. [Google Scholar]
- Mechanical Properties of Titanium Alloy. Available online: https://www.kobelco.co.jp/english/titan/files/details.pdf (accessed on 5 January 2022).
- You, S.H.; Lee, J.H.; Oh, S.H. A Study on Cutting Characteristics in Turning Operations of Titanium Alloy used in Automobile. Int. J. Precis. Eng. Manuf. 2019, 20, 209–216. [Google Scholar] [CrossRef]
- Gialanella, S.; Malandruccolo, A. Titanium and Titanium Alloys. In Aerospace Alloys; Gialanella, S., Malandruccolo, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 129–189. [Google Scholar]
- Drossou-Agakidou, V.; Kanakoudi-Tsakalidou, F.; Sarafidis, K.; Taparkou, A.; Tzimouli, V.; Tsandali, H.; Kremenopoulos, G. Administration of recombinant human granulocytecolony stimulating factor to septic neonates induces neutrophilia and enhances the neutrophil respiratory burst and β2 integrin expression results of a randomized controlled trial. Eur. J. Pediatr. 1998, 157, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Takahashi, K.; Mori, K.; Takebe, H. Application of Titanium and its Alloys for Automobile Parts. MATEC Web Conf. 2020, 321, 02003. [Google Scholar] [CrossRef]
- Wollmann, M.; Kiese, J.; Wagner, L. Properties and applications of titanium alloys in transport. In Proceedings of the 12th World Conference on Titanium, China National Convention Center (CNCC), Beijing, China, 19–24 June 2011; Volume 2, pp. 837–844. [Google Scholar]
- Nyamekye, P.; Rahimpour Golroudbary, S.; Piili, H.; Luukka, P.; Kraslawski, A. Impact of additive manufacturing on titanium supply chain: Case of titanium alloys in automotive and aerospace industries. Adv. Ind. Manuf. Eng. 2023, 6, 100112. [Google Scholar] [CrossRef]
- Froes, F.H. Advanced metals for aerospace and automotive use. Mater. Sci. Eng. A 1994, 184, 119–133. [Google Scholar] [CrossRef]
- Fujii, H.; Takahashi, K.; Yamashita, Y. Application of titanium and its alloys for automobile parts. Nippon Steel Tech. Rep. 2003, 88, 70–75. [Google Scholar]
- Saito, T. The automotive application of discontinuously reinforced TiB-Ti composites. JOM 2004, 56, 33–36. [Google Scholar] [CrossRef]
- Kosaka, Y.; Fox, S.P.; Faller, K. Newly developed titanium alloy sheets for the exhaust systems of motorcycles and automobiles. JOM 2004, 56, 32–34. [Google Scholar] [CrossRef]
- Oldenberger, E.L.; Oldenburg, M.; Thilderkvist, P.; Stoehr, T.; Lechler, J.; Merklein, M. Tool development based on modelling and simulation of hot sheet metal forming of Ti–6Al–4V titanium alloy. J. Mater. Process. Technol. 2011, 211, 1324–1335. [Google Scholar] [CrossRef]
- Göttmann, A.; Diettrich, J.; Bergweiler, G.; Bambach, M.; Hirt, G.; Loosen, P.; Poprawe, R. Laser-assisted asymmetric incremental sheet forming of titanium sheet metal Parts. Prod. Eng. Res. Dev. 2011, 5, 263–271. [Google Scholar] [CrossRef]
- Gagliardi, F.; Ambrogio, G.; Filice, L. Incremental forming with local induction heating on materials with magnetic and non-magnetic properties. Procedia Eng. 2017, 183, 143–148. [Google Scholar]
- Oleksik, V.; Trzepieciński, T.; Szpunar, M.; Chodoła, Ł.; Ficek, D.; Szczęsny, I. Single-point incremental forming of titanium and titanium alloy sheets. Materials 2021, 14, 6372. [Google Scholar] [CrossRef]
- Trzepieciński, T.; Oleksik, V.; Pepelnjak, T.; Najm, S.M.; Paniti, I.; Maji, K. Emerging trends in single point incremental sheet forming of lightweight metals. Metals 2021, 11, 1188. [Google Scholar] [CrossRef]
- Application of Titanium Alloy in Automobile. Available online: https://titanium.net/application-of-titanium-alloy-in-automobile/ (accessed on 19 December 2023).
- Titanium and Its Alloy Used for Automotive Applications. Available online: https://energy-ti.com/titanium-and-its-alloy-used-for-automotive-applications/ (accessed on 19 December 2023).
- Furuta, T. Automobile applications of titanium. In Titanium for Consumer Applications Real World Use of Titanium; Froes, F., Qian, M., Niinomi, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 77–90. [Google Scholar]
- Applications of Titanium Alloy in the Automobile Industry. Available online: https://www.refractorymetal.org/applications-of-titanium-alloy-in-automobile-industry/ (accessed on 19 December 2023).
- Application of Titanium in the Automotive Industry. Available online: https://www.yunchtitanium.com/news/application-of-titanium-in-the-automotive-indu-34966228.html (accessed on 19 December 2023).
- Isaka, M.; Takebe, H.; Kawakami, A.; Takahashi, K. Applications of Titanium for the Automotive Sector. Nippon Steel Tech. Rep. 2022, 128, 34–37. [Google Scholar]
- Bloodhound Car Begins to Take Shape. Available online: https://www.bbc.com/news/science-environment-31694204 (accessed on 19 December 2023).
- Wagner, L.; Schauerte, O. Status of Titanium and Titanium Alloys in Automotive Applications. In Ti-2007 Science and Technology; Ninomi, M., Akiyama, S., Ikeda, M., Hagiwara, M., Maruyama, K., Eds.; The Japan Institute of Metals: Sendai, Japan, 2007; pp. 1371–1378. [Google Scholar]
- Veiga, C.; Davim, J.P.; Loureiro, A.J.R. Properties and applications of titanium alloys: A brief review. Rev. Adv. Mater. Sci. 2012, 32, 133–148. [Google Scholar]
- Czerwinski, F. Current Trends in Automotive Lightweighting Strategies and Materials. Materials 2021, 14, 6631. [Google Scholar] [CrossRef]
- Dziadoń, A.; Mola, R. Magnez. Kierunki kształtowania własności mechanicznych. Obróbka Plast. Met. 2023, 24, 253–277. [Google Scholar]
- Dziadoń, A. Magnez i Jego Stopy; Politechnika Świętokrzyska: Kielce, Poland, 2012. [Google Scholar]
- Roberts, C.S. Magnesium and Its Alloy; J. Wiley & Sons Inc.: Hoboken, NJ, USA, 1960; p. 8. [Google Scholar]
- Ghali, E. Magnesium and magnesium alloys. In Uhlig’s Corrosion Handbook, 3rd ed.; Revie, R.W., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 809–836. [Google Scholar]
- Kuczmaszewski, J.; Zagórski, I. Magnez i jego stopy. In Obróbka Skrawaniem Stopów Magnezu; Kuczmaszewski, J., Zaleski, K., Eds.; Politechnika Lubelska: Lublin, Poland, 2015; pp. 27–38. [Google Scholar]
- Hadasik, E.; Kuc, D.; Szuła, A. Kształtowanie plastyczne stopu magnezu AZ31. Rudy Met. Nieżelazne 2010, 6, 313–317. [Google Scholar]
- Powell, B.R.; Luo, A.A.; Krajewski, P.E. Magnesium alloys for lightweight powertrains and automotive bodies. In Advanced Materials in Automotive Engineering; Rowe, J., Ed.; Woodhead Publishing: Oxford, UK, 2012; pp. 150–209. [Google Scholar]
- Neite, G.; Kubota, K.; Higashi, K.; Hehmann, F. Magnesium-based alloys. In Materials Science and Technology; Cahn, R.W., Haasen, P., Kramer, E.J., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2005; pp. 113–212. [Google Scholar]
- Gray, J.E.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Zhao, D.B. The FEA Comparison of the Front Sub Frame in a Car between JDM2 Magnesium and steel. Appl. Mech. Mater. 2014, 538, 87–90. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Zhanmg, X.; Yang, Q.; Zhang, J.; Li, X. Development and application of magnesium alloy parts for automotive OEMs: A review. J. Magn. Alloys 2023, 11, 15–47. [Google Scholar] [CrossRef]
- Powell, B.R.; Krajewski, P.E.; Luo, A.A. Magnesium Alloys for Lightweight Powertrains and Automotive Structures. In Materials, Design and Manufacturing for Lightweight Vehicles; Elsevier: Amsterdam, The Netherlands, 2021; pp. 125–186. [Google Scholar]
- Luo, A.A. Applications: Aerospace, Automotive and Other Structural Applications of Magnesium. In Fundamentals of Magnesium Alloy Metallurgy; Elsevier: Amsterdam, The Netherlands, 2013; pp. 266–316. [Google Scholar]
- Joost, W.J.; Krajewski, P.E. Towards magnesium alloys for high-volume automotive applications. Scr. Mater. 2007, 128, 107–112. [Google Scholar] [CrossRef]
- Luo, A.A.; Quinn, J.F.; Wang, Y.M.; Lee, T.M.; Verma, R.; Wagner, D.A.; Forsmark, J.H.; Su, X.; Zindel, J.; Li, M.; et al. The USAMP magnesium front end research and development project: Focusing on a demonstration structure. Light Met. Age 2012, 70, 54–58. [Google Scholar]
- Luo, A.A.; Shi, R.; Miao, J.; Avey, T. Review: Magnesium sheet alloy development for room temperature forming. JOM 2021, 73, 1403–1418. [Google Scholar] [CrossRef]
- Tan, J.; Ramakrishna, S. Applications of Magnesium and Its Alloys: A Review. Appl. Sci. 2021, 11, 6861. [Google Scholar] [CrossRef]
- Golroudbary, S.R.; Makarava, I.; Repo, E.; Kraslawski, A.; Luukka, P. Magnesium life cycle in automotive industry. Procedia CIRP 2022, 105, 589–594. [Google Scholar] [CrossRef]
- Xue, Y.; Horstemeyer, M.F.; McDowell, D.L.; El Kadiri, H.; Fan, J. Microstructure-based multistage fatigue modeling of a cast AE44 magnesium alloy. Int. J. Fatigue 2007, 29, 666–676. [Google Scholar] [CrossRef]
- Khademian, N.; Peimaei, Y. Magnesium alloys and applications in automotive industry. In Proceedings of the 5th International Conference on Science and Development of Nanotechnology, Tbilisi, Georgia, 10 August 2021; pp. 1–23. [Google Scholar]
- Hector, B.; Heiss, W. Magnesium Die-Castings as Structural Members in the Integral Seat of the New Mercedes-Benz Roadster; SAE Technical Paper 900798; SAE International: Warrendale, PA, USA, 1990. [Google Scholar] [CrossRef]
- Kumar, D.S.; Sasanka, C.T.; Ravindra, K.; Suman, K.N.S. Magnesium and Its Alloys in Automotive Applications—A Review. Am. J. Mater. Sci. Technol. 2015, 4, 12–30. [Google Scholar] [CrossRef]
- Aune, T.K.; Westengen, H.; Ruden, T. Mechanical Properties of Energy Absorbing Magnesium Alloys; SAE Technical Paper 930418; SAE International: Warrendale, PA, USA, 1993. [Google Scholar] [CrossRef]
- Alves, H.; Koster, U.; Aghion, E.; Eliezer, D. Environmental Behavior of Magnesium and Magnesium Alloys. Mater. Technol. 2001, 16, 110–126. [Google Scholar] [CrossRef]
- Magnesium Semisolid Forming Equipment. SSD-Magnesium. Available online: http://www.ssd-magnesium.com/product/I8rC1o.html (accessed on 19 December 2021).
- Blanchard, P.J.; Bretz, G.T.; Subramanian, S. The Application of Magnesium Die Casting to Vehicle Closures; SAE Technical Paper 2005-01-0338; SAE International: Warrendale, PA, USA, 2005. [Google Scholar] [CrossRef]
- Hubbert, T.; Chen, X.; Li, N.; Pineo, S. 2005 Ford GT Magnesium I/P Structure; SAE Technical Paper 2004-01-1261; SAE International: Warrendale, PA, USA, 2004. [Google Scholar] [CrossRef]
- Fan, S.; Wang, X.; Wang, G.G.; Weiller, J.P. Applications of High-Pressure Die-Casting (HPDC) Magnesium Alloys in Industry. In Magnessium Alloys—Processing, Potential and Applications; Tański, T.A., Cesarz-Andraczke, K., Jonda, E., Eds.; Intechopen: London, UK, 2023. [Google Scholar]
- Gerken, R.T.; Ghaffari, B.; Sachdev, A.K.; Mehta, M.; Carter, J.T. Low-cost magnesium alloy sheet component development and demonstration project. SAE Int. J. Adv. Curr. Prac. Mobil. 2023, 5, 15–32. [Google Scholar] [CrossRef]
- Sadayappan, K.; Vassos, M. Evaluation of a Thixomolded Magnesium Alloy Component for Automotive Application; SAE Technical Paper 2010-01-0403; SAE International: Warrendale, PA, USA, 2010. [Google Scholar] [CrossRef]
- Logan, S.; Kizyma, A.; Patterson, C.; Rama, S. Lightweight Magnesium Intensive Body Structure. SAE Trans. 2006, 115, 469–486. [Google Scholar]
- Magnesium Alloy Parts Application Case Studies (Client Provides Up to 5). Available online: https://www.yiruimetalmg.com/magnesium-alloy-parts-application-case-studies-client-provides-up-to-5.html (accessed on 19 December 2023).
- Westengen, H.; Bakke, P. Magnesium Die Casting Alloys for Use in Applications Exposed to Elevated Temperatures: Can They Compete with Aluminium? Mater. Sci. Forum 2003, 419, 35–42. [Google Scholar] [CrossRef]
- Pekguleryuz, M.O.; Baril, E. Development of Creep Resistant Mg-Al-Sr alloys. In Essential Readings in Magnesium Technology; Mathaudhu, S.N., Luo, A.A., Neelameggham, N.R., Nyberg, E.A., Sillekens, W.H., Eds.; Springer: Cham, Switzerland, 2016; pp. 283–289. [Google Scholar]
- Verma, R.; Carter, J.T. Quick Plastic Forming of a Decklid Inner Panel with Commercial AZ31 Magnesium Sheet; SAE Technical Paper 2006-01-0525; SAE International: Warrendale, PA, USA, 2006. [Google Scholar] [CrossRef]
- Carter, J.T.; Krajewski, P.E.; Verma, R. The Hot Blow Forming of AZ31 Mg Sheet: Formability Assessment and Application Development. JOM 2008, 60, 77–81. [Google Scholar] [CrossRef]
- Luo, A.A.; Forsmark, J.; Sun, X.; Shook, S. Mechanical and Thermophysical Properties of Magnesium Alloy Extrusions; SAE Technical Paper 2010-01-0410; SAE International: Warrendale, PA, USA, 2010. [Google Scholar] [CrossRef]
- Wickberg, A.; Ericsson, R. Magnesium in the Volvo LCP 2000; SAE Technical Paper 850418; SAE International: Warrendale, PA, USA, 1985. [Google Scholar] [CrossRef]
- Jekl, J.; Auld, J.; Sweet, C.; Carter, J.; Resch, S.; Klarner, A.; Brevick, J.; Luo, A. Development of a Thin-Wall Magnesium Side Door Inner Panel for Automobiles; General Motors LLC: Detroit, MI, USA, 2015. [Google Scholar]
- Hawke, D.; Gaw, K. Effects of Chemical Surface Treatments on the Performance of an Automotive Paint System on Die Cast Magnesium; SAE Technical Paper 920074; SAE International: Warrendale, PA, USA, 1992. [Google Scholar] [CrossRef]
- Grebetz, J.C. A Comparison of the Impact Characteristics of Several Magnesium Die Casting Alloys; SAE Technical Paper 930417; SAE International: Warrendale, PA, USA, 1993. [Google Scholar] [CrossRef]
- Murray, R.W.; Hillis, J.E. Magnesium Finishing: Chemical Treatment and Coating Practices; SAE Technical Paper 900791; SAE International: Warrendale, PA, USA, 1990. [Google Scholar] [CrossRef]
- Annamalai, S.; Periyakgoundar, S.; Paramasivam, K.; Selvaraj, A.B. Investigation of Bending, Sound Absorption, and Damping Properties of AZ91D-Swivel Plate. Adv. Mater. Sci. Eng. 2020, 2020, 9621921. [Google Scholar] [CrossRef]
- Aune, T.K.; Westengen, H.; Ruden, T. The effects of varying aluminum and rare-earth content on the mechanical properties of die cast magnesium alloys. SAE Trans. 1994, 103, 553–557. [Google Scholar]
- Durairaj, S.R.N.; Ganesan, T.; Rao, P.C. Vibration Analysis on Magnesium Alloy Housing and Analysis of Resonant Frequency on the Housing between Magnesium and Aluminium Alloy; SAE Technical Paper 2017-28-1969; SAE International: Warrendale, PA, USA, 2017. [Google Scholar] [CrossRef]
- Froes, F.H.; Eliezer, D.; Aghion, E. The science, technology, and applications of magnesium. JOM 1998, 50, 30–34. [Google Scholar] [CrossRef]
- Luo, A.A. Magnesium casting technology for structural applications. J. Magn. Alloys 2013, 1, 2–22. [Google Scholar] [CrossRef]
- Riopelle, L. Magnesium Applications. In Proceedings of the International Magnesium Association (IMA) Annual Magnesium in Automotive Seminar, Livonia, MI, USA, 20 April 2004. [Google Scholar]
- An Assessment of Mass Reduction Opportunities for a 2017–2020 Model Year Vehicle Program. Available online: http://theicct.org/sites/default/files/publications/Mass_reduction_final_2010.pdf (accessed on 19 December 2023).
- Weiler, J.P. A review of magnesium die-castings for closure applications. J. Magnes. Alloys 2019, 7, 297–304. [Google Scholar] [CrossRef]
- US, Chinese Automakers Increasingly Use Magnesium as Vehicle Body Material. Available online: https://aaa.fourin.com/reports/8fb96120-2860-11ea-8162-d9787bdc6011/us-chinese-automakers-increasingly-use-magnesium-as-vehicle-body-material (accessed on 19 December 2023).
- Wang, J.; Pang, X.; Jahed, H. Surface protection of Mg alloys in automotive applications: A review. AIMS Mater. Sci. 2019, 6, 576–600. [Google Scholar] [CrossRef]
- Ahmad, H.; Markina, A.A.; Porotnikov, M.V.; Ahmad, F. A review of carbon fiber materials in automotive industry. IOP Conf. Ser. Mater. Sci. Eng. 2020, 971, 032011. [Google Scholar] [CrossRef]
- Wazeer, A.; Das, A.; Abeykoon, C.; Sinha, A.; Karmakar, A. Composites for electric vehicles and automotive sector: A review. Green Energy Intell. Transp. 2023, 2, 100043. [Google Scholar] [CrossRef]
- Wan, Y.; Takahashi, J. Development of Carbon Fiber-Reinforced Thermoplastics for Mass-Produced Automotive Applications in Japan. J. Compos. Sci. 2021, 5, 86. [Google Scholar] [CrossRef]
- Battaglia, M.; Sellitto, A.; Giamundo, A.; Visone, M.; Riccio, A. Shape Memory Alloys Applied to Automotive Adaptive Aerodynamics. Materials 2023, 16, 4832. [Google Scholar] [CrossRef]
- Riccio, A.; Sellitto, A.; Ameduri, S.; Concilio, A.; Arena, M. Shape memory alloys (SMA) for automotive applications and challenges. In Shape Memory Alloy Engineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 785–808. [Google Scholar]
- Jani, J.M.; Leary, M.; Subic, A. Shape Memory Alloys in Automotive Applications. Appl. Mech. Mater. 2014, 663, 248–253. [Google Scholar] [CrossRef]
- Tuazon, B.J.; Custodio, N.A.V.; Basuel, R.B.; Delos Reyes, L.A.; Dizon, J.R.C. 3D Printing Technology and Materials for Automotive Application: A Mini-Review. Key Eng. Mater. 2022, 913, 3–16. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Elakkad, A.S. 3D Technology in the Automotive Industry. Int. J. Eng. Res. 2019, V8, 248–251. [Google Scholar] [CrossRef]
- Gechev, T. A short review of 3D printing methods used in the automotive industry. Bulg. J. Eng. Des. 2021, 44, 67–76. [Google Scholar]

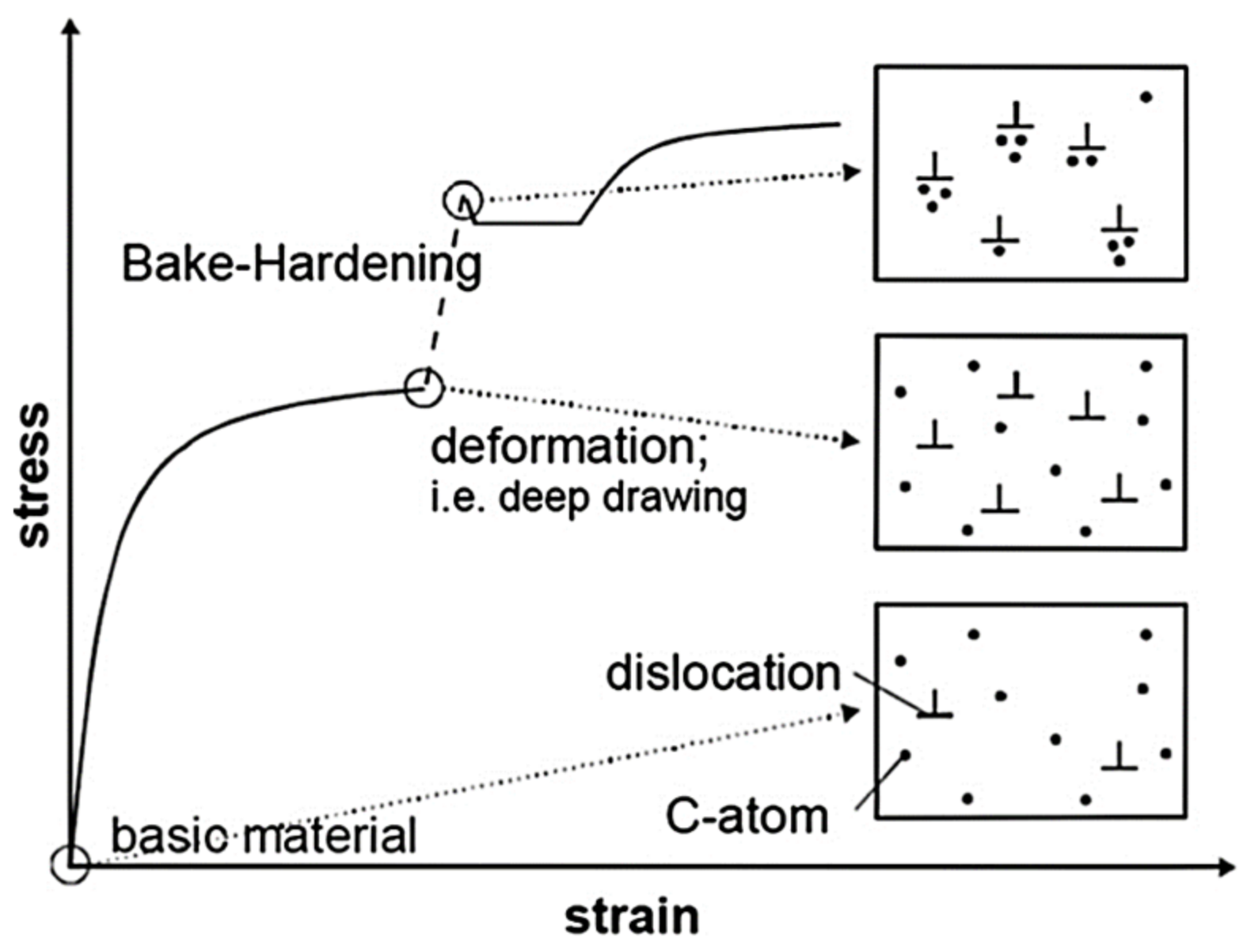
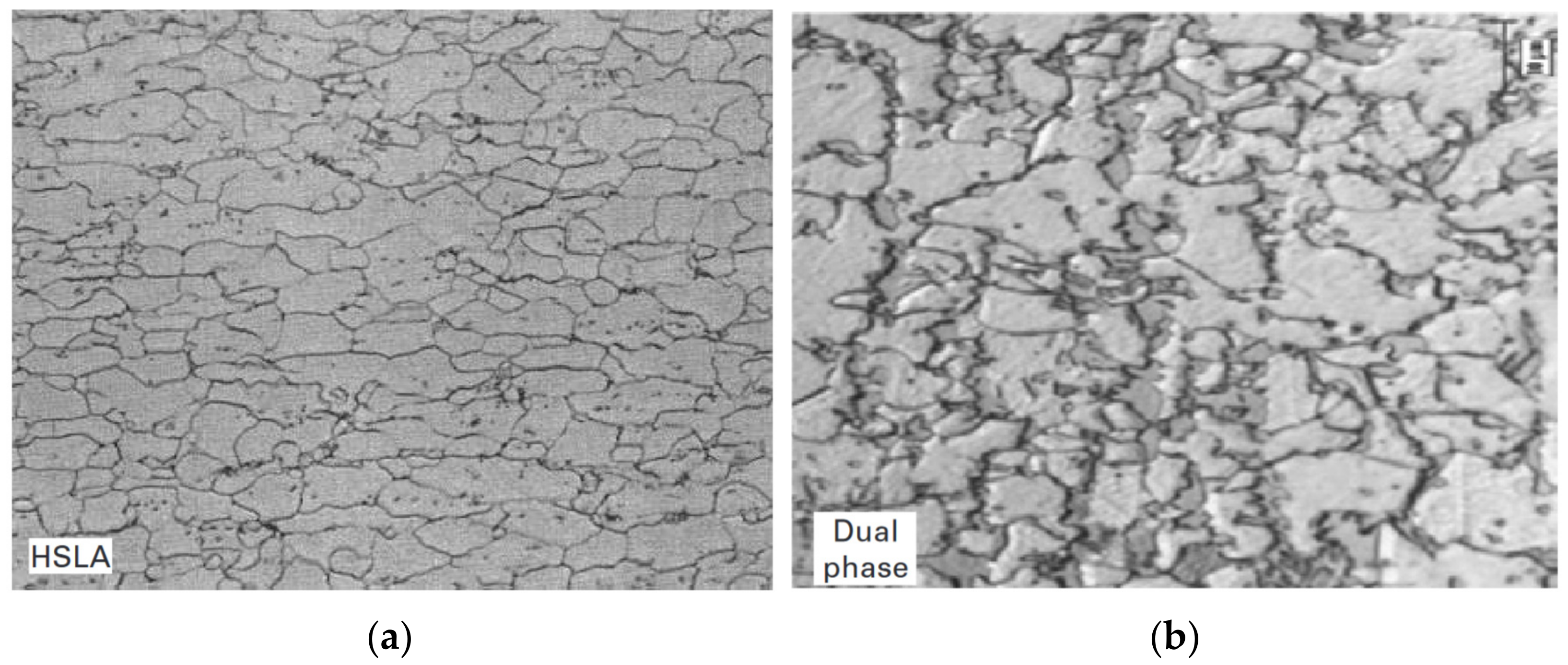
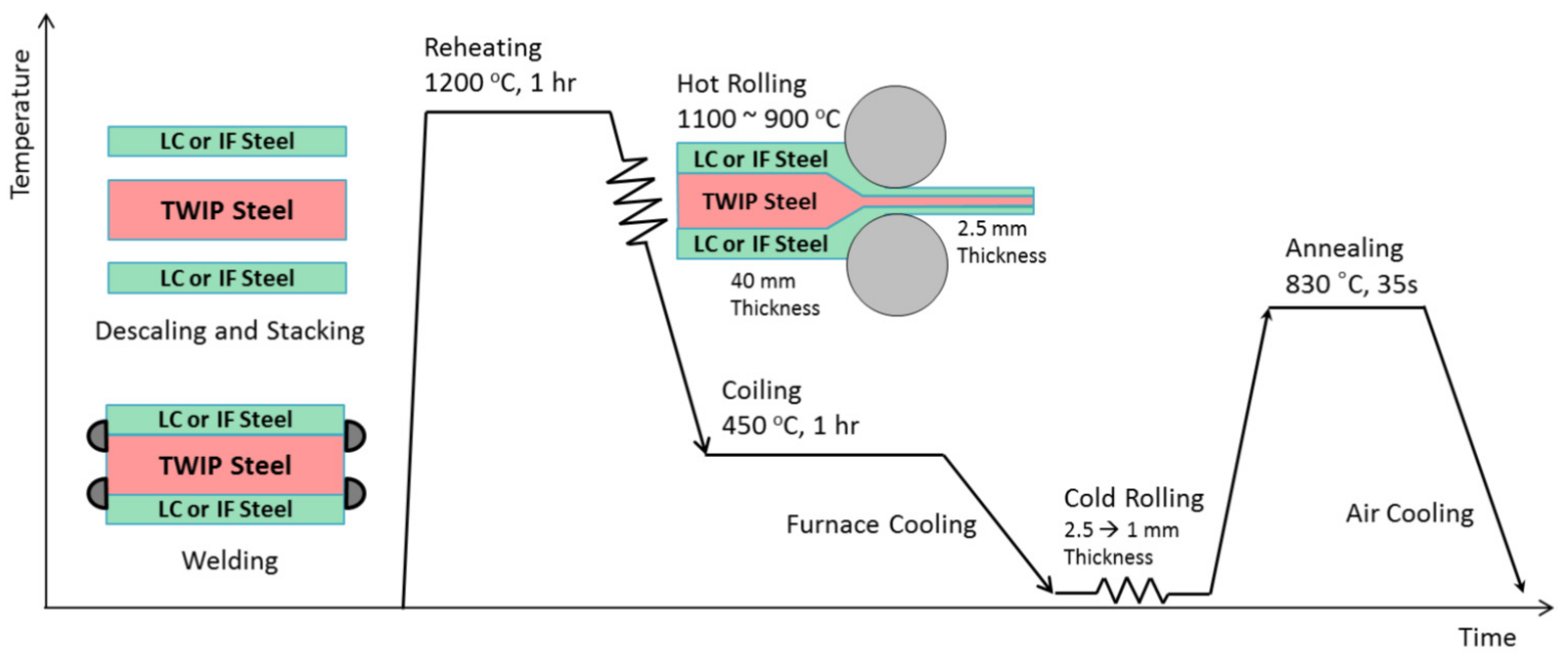

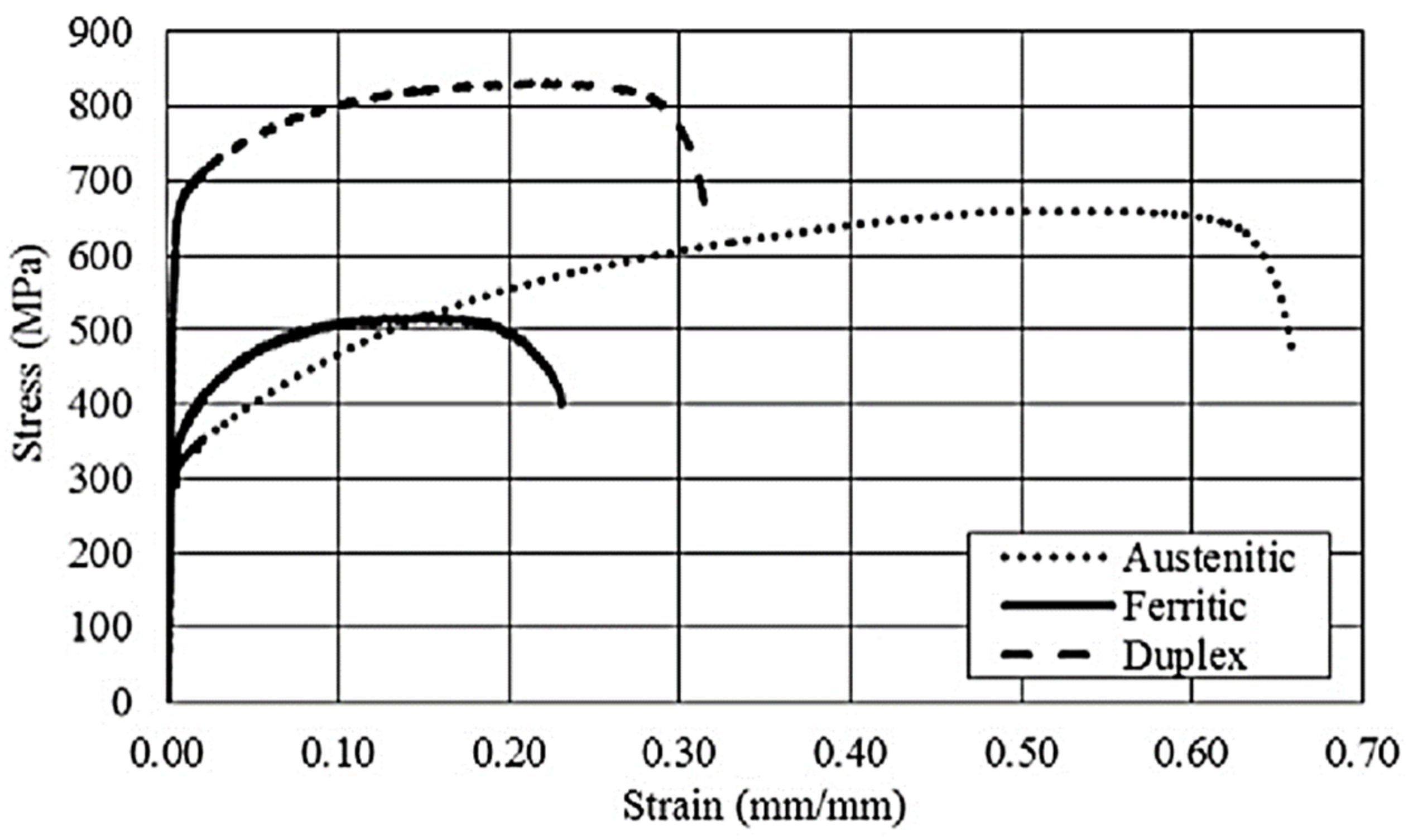
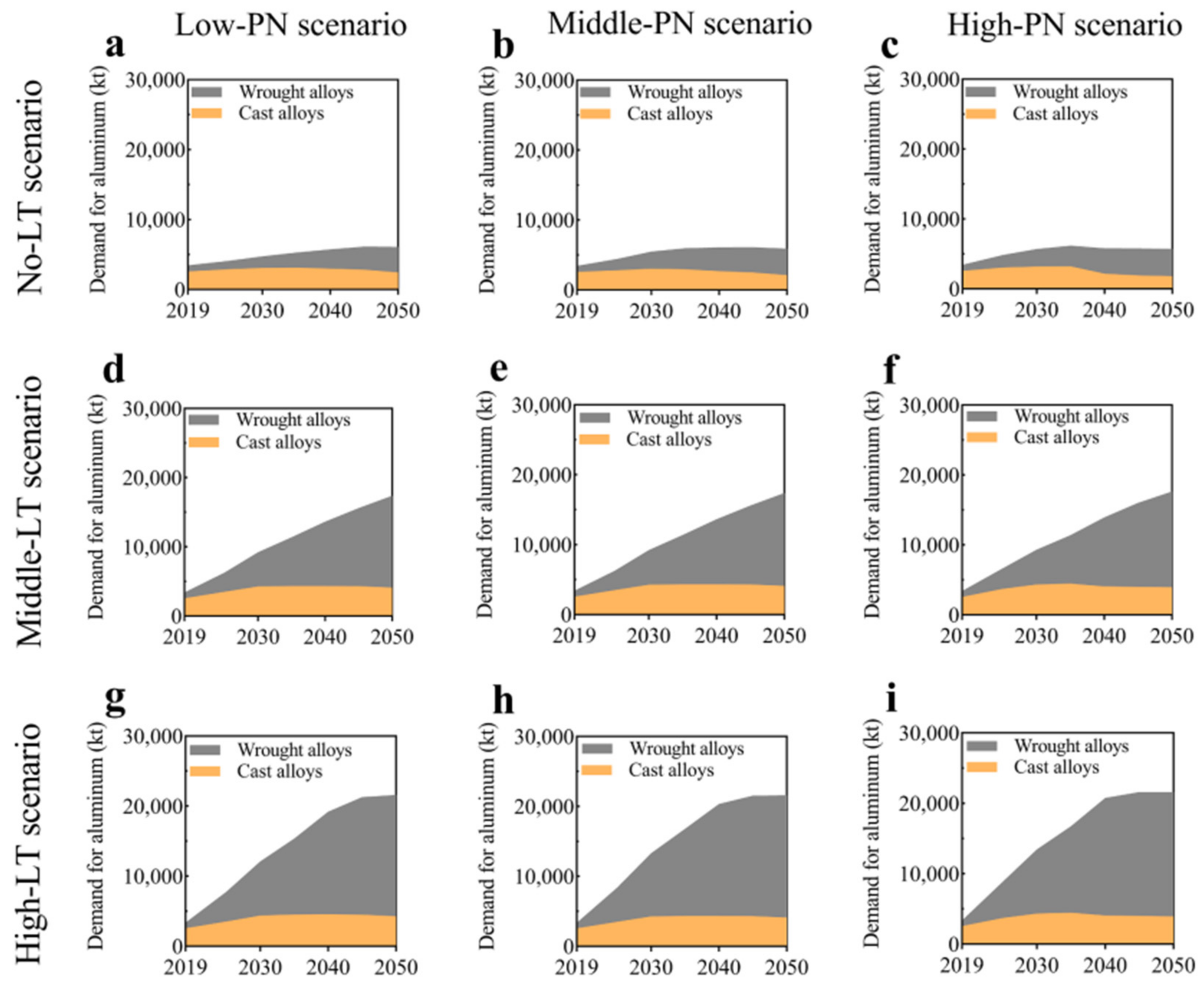

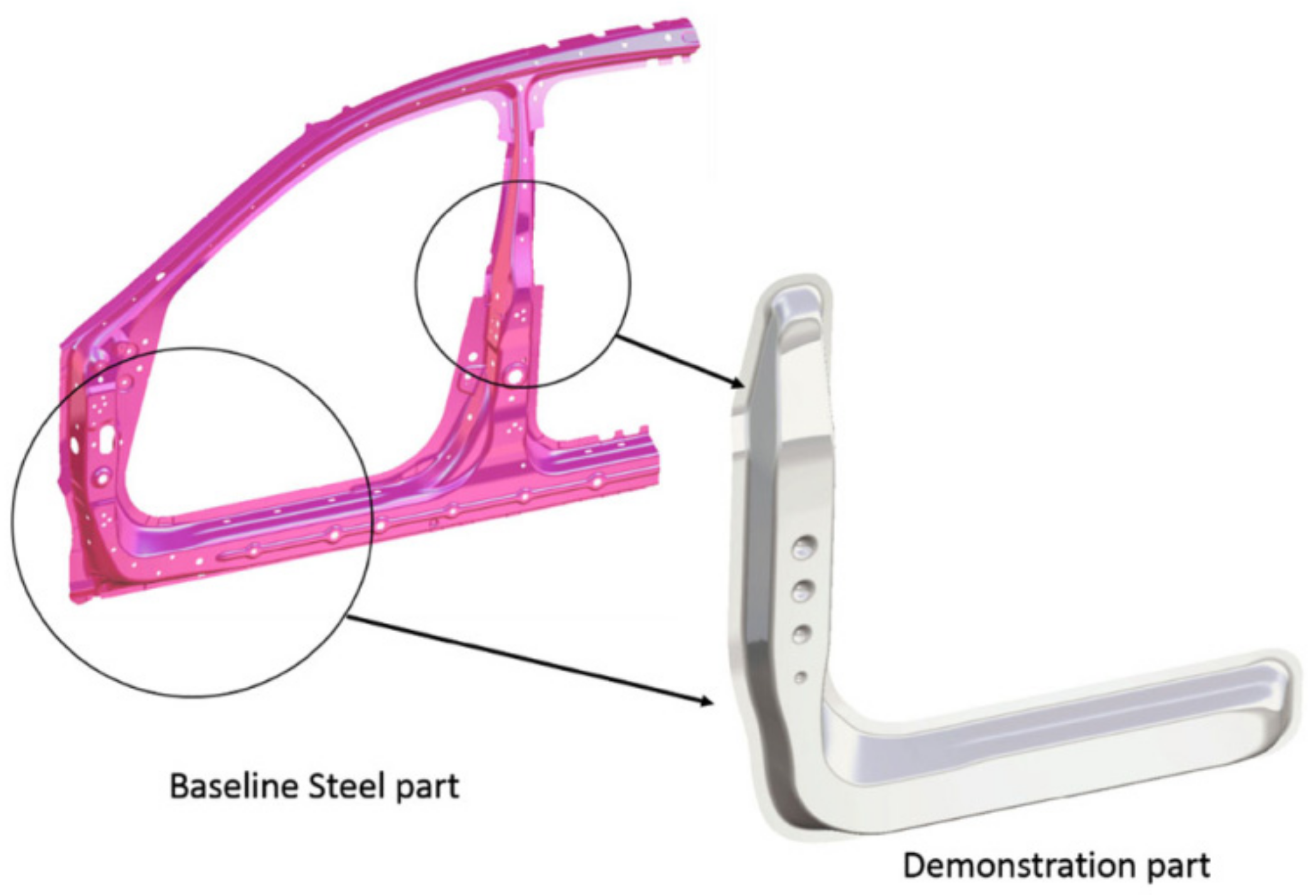
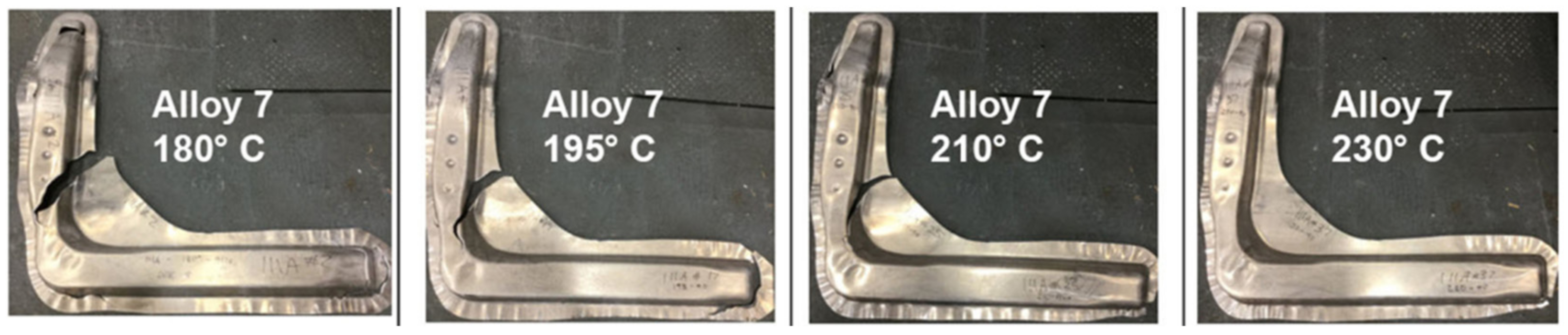
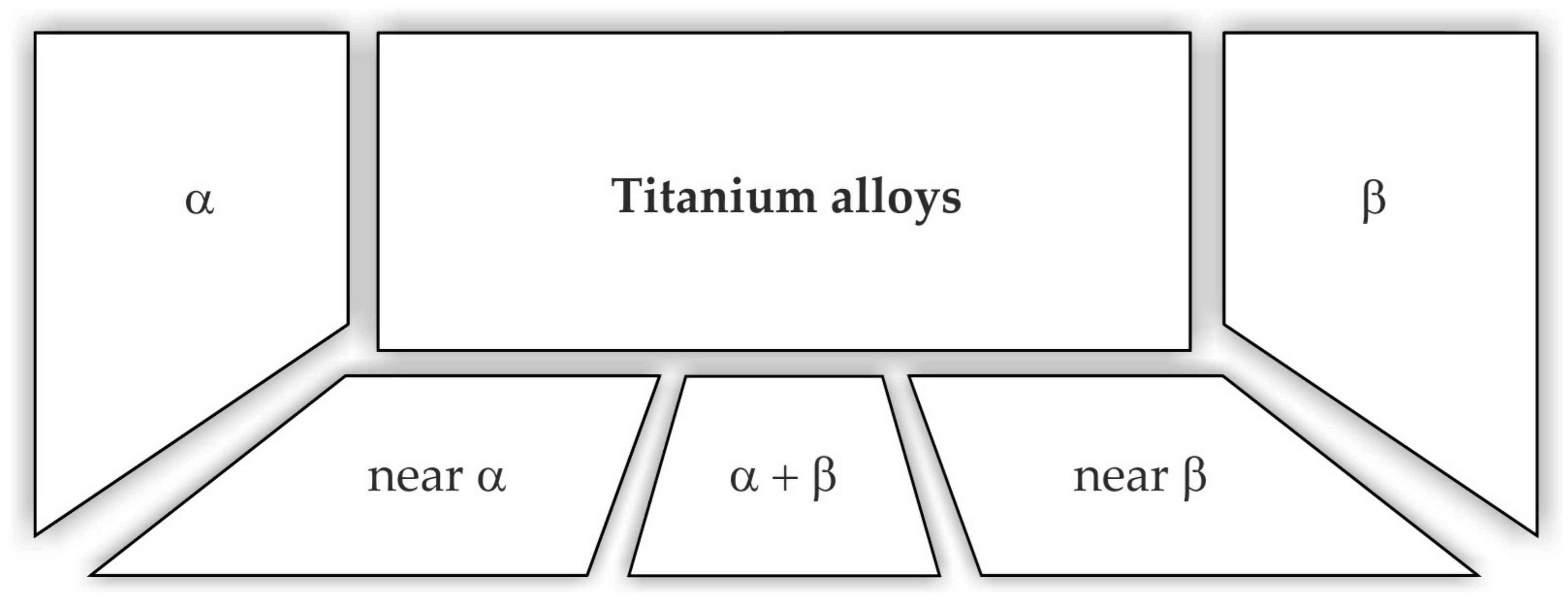
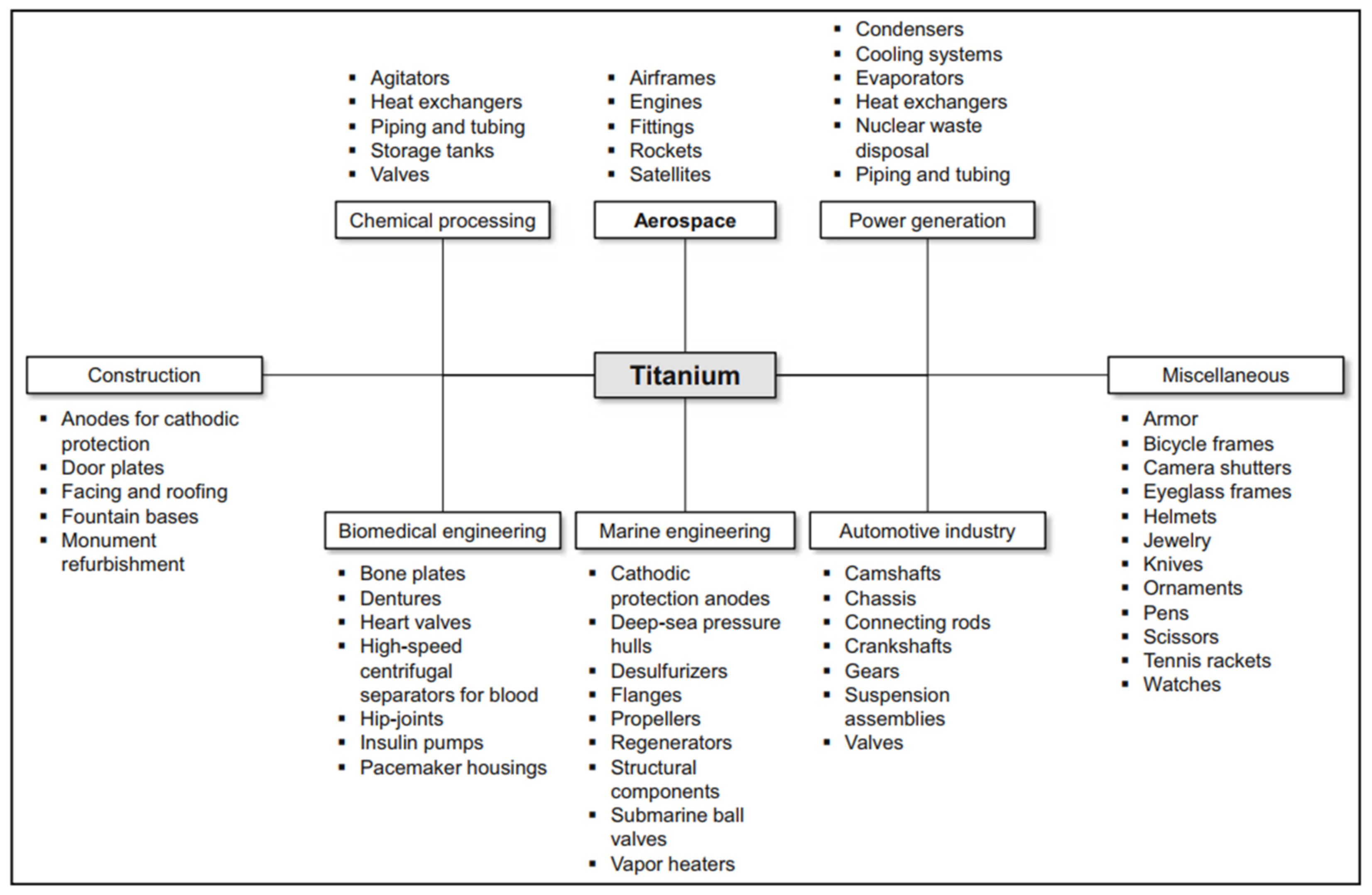


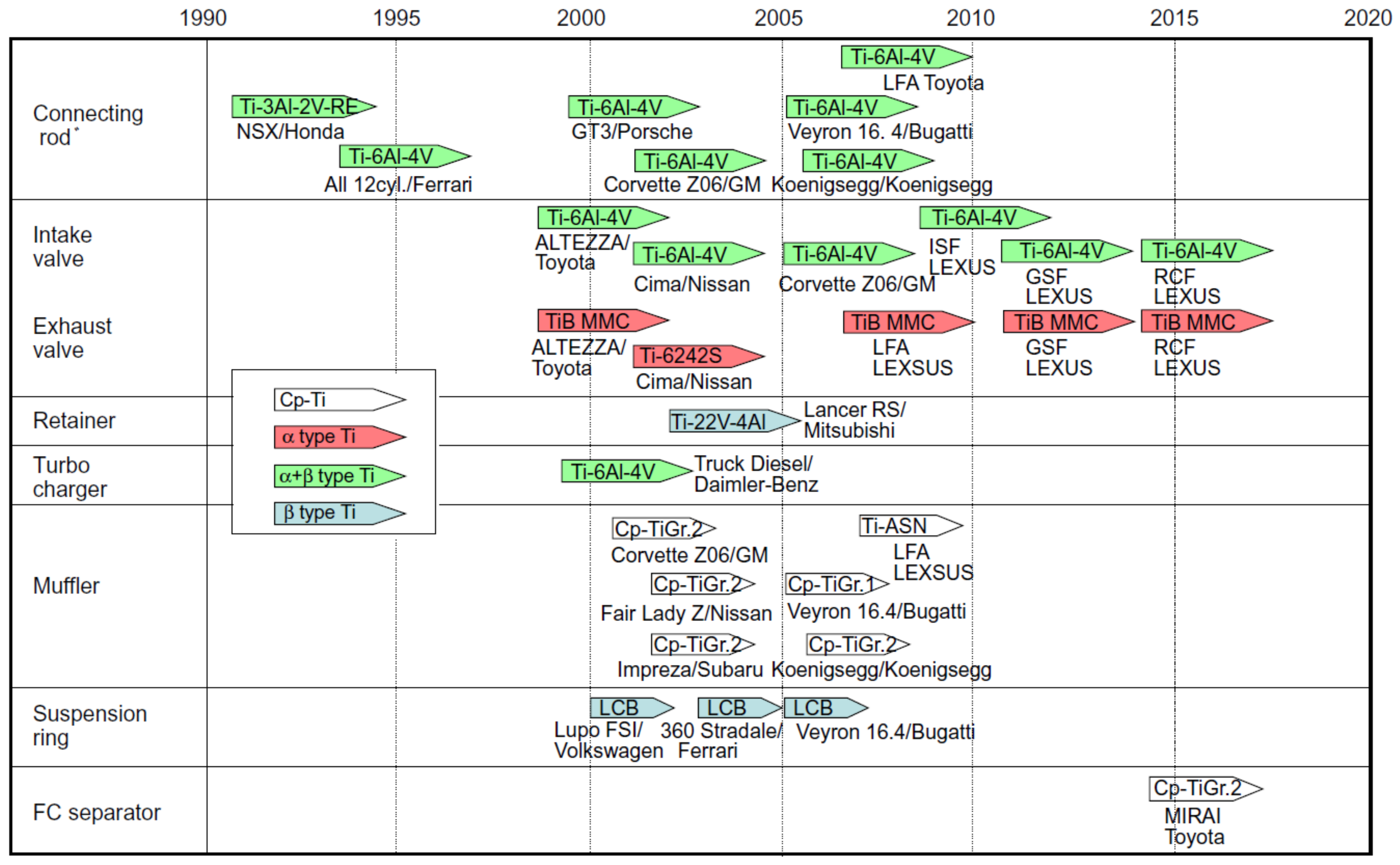
| Grade | Yield Stress Rp0.2 (max.), MPa | Ultimate Tensile Strength Rm, MPa | Elongation A50 (min.), % |
|---|---|---|---|
| DC01 | 280 | 270–410 | 28 |
| DC03 | 240 | 270–370 | 34 |
| DC04 | 210 | 270–350 | 38 |
| DC05 | 180 | 270–330 | 40 |
| DC06 | 180 | 270–350 | 38 |
| DC07 | 150 | 250–310 | 44 |
| Steel Group/Grade | Components | Reference |
|---|---|---|
| PHS | press hardened body parts | [147] |
| PHS | press hardened front and/or rear bumper beam | [148] |
| PHS | A- and B-pillars | [149] |
| PHS | transmission tunnel and the firewall (Volkswagen Passat) | [150] |
| PHS | B-pillar (Audi A8) | [151] |
| PHS | body panels (Dongfeng Voyah iFree) | [152] |
| PHS | press hardened door beams (Jaguar XJ40) | [153] |
| PHS | door beams (VW Polo) | [154] |
| PHS | press hardened bumper beam (Renault Safrane) | [148] |
| PHS | press hardened bumper beams (Volvo S80) | [155] |
| PHS | A-pillar (BMW series 3) | [156] |
| PHS | 3rd row seating support (Volvo V70) | [157] |
| PHS | front bumper beam, and the right/left A-pillars (Citroën C5) | [149] |
| PHS | 2nd row seat frame (Volvo XC90) | [158] |
| PHS | “form fixture hardened” front and rear bumpers (Ford Mustang 5th generation) | [157] |
| PHS | tailor-rolled and press hardened components (Dodge Caliper, BMW X5) | [159,160] |
| PHS | tailor welded B-pillar (Audi A4) | [161] |
| PHS | B-pillar reinforcement, A-pillar reinforcement, rear rail, tunnel reinforcement, upper B-pillar reinforcement (BMW 7 series 5th generation) | [162] |
| PHS | modular transverse platform (Volkswagen Golf, Audi A3) | [163] |
| PHS | swing doors in the front and two sliding rear doors (Ford B-Max) | [164] |
| PHS | A- and B-pillars, hinge pillar, and front portion of the rocker reinforcement (Honda Acura MDX, 3rd generation) | [165] |
| PHS | A-pillars (Honda Acura NSX) | [166] |
| PHS | a tailor rolled tube (Ford Focus fourth gen., Jeep Wrangler 4th generation) | [167] |
| PHS | A-pillar reinforcements (BMW E46 Cabrio, Citroën C5) | [156,168] |
| PHS | B-pillar reinforcements and roof rail (Peugeot 307) | [169] |
| PH1500 | B-pillar reinforcement (Chrysler Pacifica) | [170] |
| PHS1800 | bumper beam reinforcements (Mazda CX-5) | [171] |
| PHS2000 | hot stamped door ring (third generation Haval H6) | [172] |
| Boron steel (*) | B roof bow, rear seat frame (Volvo XC90) | [173] |
| MBW 1900 (PHS) | seat crossbeams (Volkswagen ID.3) | [174] |
| 22MnB5 | hot stamped B-pillars (Audi A5 Sportback, Volkswagen Tiguan) | [175] |
| PQS450 | front side members, B-pillar reinforcements, rear side members (Volvo XC90) | [158] |
| PQS450 | rear side member (Fiat Tipo) | [163] |
| PQS450 | laser-welded tailored rear side member (Fiat 500X) | [176] |
| PQS450 | B-Pillar (Jaguar I-Pace) | [176] |
| PQS550 | front door ring, B-pillar (Chrysler Pacifica, Chrysler RAM) | [177,178] |
| PQS550 | tailored B-pillar (Renault Scenic 3) | [179] |
| PQS550 + PHS1500 | patchwork B-pillar (Mercedes C-class) | [180] |
| HSLA | the energy absorbing components | [181] |
| Rephos (a phosphorus alloy high-strength steel) | A-pillar upper, W-screen member (Volvo XC90) | [173] |
| TRIP steels | front longitudinal beams, A-pillar and B-pillar reinforcements | [96] |
| TRIP 350/600 | rail reinforcements, frame rails | [182] |
| TRIP 400/700 | crash box, side rails | [182] |
| TRIP 450/800 | roof rails, dash panels | [182] |
| TRIP 600/980 | engine cradle, roof rail, front and rear rails, B-pillar upper, seat frame | [182] |
| TRIP780 | bumper cross member, B-pillar reinforcement | [183] |
| MS steels (*) | front and rear bumpers, roof cross members, door beams | [123] |
| MS 950/1200 | bumper beams, cross members, side intrusion beams | [184] |
| MS 1150/1400 | bumper reinforcements, side intrusion beams | [184] |
| MS 1250/1500 | bumper reinforcements, side intrusion beams, bumper beams | [184] |
| CR1200Y1470T-MSteel | centre roof reinforcement (Lexus NX) | [185] |
| DP steels | seat guides, child seats, windshield pillars | [186] |
| DP steels | bumper reinforcements, car body panels | [86,90] |
| DP90 | rocker, B-pillar (Volvo XC90) | [175] |
| DP 300/500 | floor panel, door outer, roof outer | [187] |
| DP 350/600 | body side outer, fender, floor panel | [187] |
| DP 500/800 | rear rails, body side inner | [187] |
| DP 600/980 | floor panels, B-pillar, front sub-frame | [187] |
| DP 700/1000 | roof rails | [187] |
| DP 800/1180 | B-pillar upper | [187] |
| CP steels | tunnel stiffeners, pillar reinforcements, bumper beams, side beams, frame rails, rocker panels | [68,188] |
| FB steels | engine sub-frames, upper and lower control arms, seat cross members, longitudinal beams | [189] |
| IF steels | complex stampings, inner wheel arches, boot lid reinforcements | [79] |
| BH steels | doors, boot lids | [81] |
| TBF980 | structural reinforcements (Infiniti QX50) | [190] |
| TBF 1180—TRIP-assisted bainitic ferrite steel | A- and B-pillar reinforcements, side reinforcement, roof rail (Infiniti Q50) | [191] |
| TBF 1180 | A-pillar inner and reinforcements (Nissan Murano) | [192] |
| TBF 1180 | A- and B-pillar reinforcements (Nissan Maxima) | [193] |
| QP980 | front and rear floor assemblies (Ford Bronco) | [135] |
| QP980 | A-pillar inner lower, A-pillar inner upper, hinge pillar inner, kick down lower (General Motors vehicles) | [194] |
| TWIP | energy-absorbing elements and structurally responsible components with complex shapes | [99] |
| TWIP | A-pillar, B-pillar, crash box, bumper beam, dash lower reinforcement, tunnel, floor cross-members, door hinge reinforcement, wheelhouse, front side member, rear side member, door impact beam, shock absorber housings (i.e., General Motors, Daewoo, Ssangyong, Renault) | [195,196] |
| TWIP1000 | bumper beam (Fiat Nuova Panda) | [196] |
| TWIP 450/950 | bumper reinforcements (Jeep Renegade BU/520) | [197] |
| TWIP980 | sill side outer, A-pillar lower (Renault EOLAB concept) | [198] |
| stainless steel (*) | buses’ frames, side members, bumper beams, self-supporting body, panels, outer covering body, body panels, structural frameworks of buses and coaches | [199,200,201] |
| stainless steel (*) | energy absorbing components (Volvo) | [199] |
| stainless steel (*) | auto body components (Audi A6), bumper system and collision boxes (Saab), frame (Hyundai Mobis), fuel tanks (Volkswagen Beetle) | [199] |
| AISI 304, AISI 304L | fuel tanks | [202] |
| AISI 304, AISI 304L, AISI 321, AISI 3167, AISI 316L, ASTM S30415 | housings for turbochargers and catalytic converters | [202] |
| AISI 304, AISI 304L, ASTM S41050 | chassis for buses and trucks, structural components | [202] |
| AISI 304, AISI 304L, AISI 430, AISI 434, AISI 436 | door scuff plates, bumpers, headlight bezels | [202] |
| AISI 304L | automobile frames, A-pillar structural tubes, | [203] |
| AISI 304 | whole frame (Pininfarina Nido) | [199] |
| H400 stainless steel | rear and front side members, lower rear axle wishbone (Porsche Carrera GT) | [199] |
| Fe-15Cr-10Mn-0.35Ni-1.6Cu-0.12 stainless steel | bumper (Ashok Leyland) | [204] |
| AISI 304 | fuel tank (Fiat Barchetta) | [204] |
| Nitronic 30 (15Cr-1.5Ni-8Mn-0.18N) stainless steel | urban bus frames (Autocinetics Inc.) | [205] |
| Alloy Series | Alloy Additions |
|---|---|
| 1xxx | Al (99% pure) |
| 3xxx | Al-Mn |
| 5xxx | Al-Mg |
| Alloy Series | Type of Alloy | Ultimate Tensile Strength, MPa | Basic Properties |
|---|---|---|---|
| 1xxx | Al (impurity content < 1%) | 70–150 | good plasticity in cold and elevated temperature forming, low strength, good corrosion resistance, high electrical and thermal conductivity |
| 2xxx | Al–Cu–Mg Al–Cu–Mg–Si | 170–530 | low corrosion resistance |
| 3xxx | Al–Mn–Mg | 140–280 | good plasticity but low strength, good weldability, and corrosion resistance |
| 4xxx | Al–Si | 100–360 | high strength and corrosion resistance, good casting properties |
| 5xxx | Al–Mg Al–Mg–Mn | 140–350 | good saltwater corrosion resistance, good weldability and analysability |
| 6xxx | Al–Mg–Si | 160–370 | high corrosion resistance, good formability, very good anodising ability |
| 7xxx | Al–Zn–Mg Al–Zn–Mg–Cu | 360–610 | the highest strength of all aluminium alloys, low and medium corrosion resistance |
| 8xxx | various alloying elements, Al–Li, Al–Fe, Al–Li–Cu–Mg | 260–580 | properties depending on the chemical composition |
| 9xxx | zinc and copper | Not specified | remarkable strength and superior mechanical properties depending on the specific alloy composition |
| Aluminium Alloy Grade | Components | Reference |
|---|---|---|
| EN AW-2008, EN AW-2010 | outer and inner body panels | [225] |
| EN AW-2013 | seat shells, load floors, outer and inner body panels | [226] |
| EN AW-2016 | external body panels | [227] |
| EN AW-2024 | wheel spokes, structural components | [228] |
| Aluminium Alloy Grade | Components | Reference |
|---|---|---|
| EN AW-3003 | trailer and truck panels, awning slats, pressure vessels, piping | [231] |
| EN AW-3004 | truck trailer sheet, horse trailers, interior panels and components | [212] |
| EN AW-3105 | floor components | [232] |
| Aluminium Alloy Grade | Components | Reference |
|---|---|---|
| EN AC-43500 | suspension strut dome (Audi A7, BMW 5 Gran Turismo) | [235] |
| A356 (AlSi7Mg0.3) | suspension–cradle interface (Chevrolet Corvette Stingray C7) | [235] |
| C448 (AlSi9Mg) | hinge pillars, brackets, B-pillar reinforcements, shock towers | [236] |
| AlSi10MgMn(Sr) | front longitudinal member (Audi A8), engine torsion support | [237,238] |
| (AlSi10MgMnFe) | front sections, shock tower, engine cradle | [239,240,241,242] |
| Aural®-2 (AlSi10MgMnFe) | one-piece B-pillar, assembled structural elements (Audi A2) | [235] |
| Castasil®-37 (AlSi9MnMoZr) | rear longitudinal beam (Audi A8 D4) components of retractable roofs | [235,243] |
| Castasil®-37 (AlSi9MnMoZr) | shock tower, front sections, engine cradle | [239,240,241,242] |
| Silafont®-36 (EN AC-43500) | engine bracket (BMW N52), front end carrier (BMW 3 series), front crash management system (Audi A2) | [237,244] |
| Silafont®-36 (EN AC-43500) | shock tower, engine cradle, front sections, space-frame members, suspensions, B-pillar reinforcements, shock towers, brackets, hinge pillars | [236,240,245] |
| Aluminium Alloy Grade | Components | Reference |
|---|---|---|
| AF350 (AlMg1/AlMg5.7/AlMg1) | shell doors (BMW 5 and 7) series | [249] |
| C446 (AlMg3Mn) | B-pillar reinforcements, shock towers, brackets, hinge pillars | [236] |
| EN AC-51500 | suspension strut dome (BMW 5 series, Porsche Panamera) | [237] |
| EN AC-AlMg5Si2Mn | inner door frames (Mercedes Benz S-class) | [249] |
| EN AW-5022 | pillars, floors, roofs, doors, oil pans, rear fenders, bonnets | [250] |
| EN AW-5042, EN AW-5182 | weight-reduced front end (BMW E60) | [235] |
| EN AW-5051A-O, EN AW-5182-O | inner panels | [251] |
| EN AW-5052 | floor components, truck trailers | [231,232] |
| EN AW-5052 | bumpers, body panels, interior panels | [226] |
| EN AW-5052, EN AW-5182, EN AW-5454 | internal body parts | [227] |
| EN AW-5052, EN AW-5083 | car doors, automobile plates | [252] |
| EN AW-5083, EN AW-5456 | complex automotive components | [212] |
| EN AW-5083 | boot lid (Cadillac STS) | [250] |
| EN AW-5083 | lashing rails | [250] |
| EN AW-5110A | reflective panels | [250] |
| EN AW-5154 | underbody components, drivetrain components, suspension components | [250] |
| EN AW-5182 | dust covers, seat frames, air cleaner cases, inner panels | [212,235,250] |
| EN AW-5182 | rear fenders, car hoods, car front, and car doors | [252] |
| EN AW-5182 | reinforcement members, inner body panels | [226] |
| EN AW-5182 | structural panels (Audi A8 D2) | [235] |
| EN AW-5182 | inner panels (Saab 9-3, Renault Vel Satis, BMW 3 series) | [249] |
| EN AW-5182 | hood inner (Jaguar XE) | [253] |
| EN AW-5182 | one-piece inner panel (Jaguar XJ353) | [235] |
| EN AW-5182-O | inner panel (Chevrolet GMT 830) | [249] |
| EN AW-5182-O | roof structure (Renault Avantime) | [243] |
| EN AW-5182, EN AW-5457 | internal structural parts | [254] |
| EN AW-5754 | structural sheet applications | [212] |
| EN AW-5454 | suspension components | [250] |
| EN AW-5454, EN AW-5182 | structural panels (Rolls Royce Phantom) | [235] |
| EN AW-5454 | engine brackets and mounts | [226] |
| EN AW-5456 | armour plates | [226] |
| EN AW-5454-O, EN AW-5754-O | body structures (sheets) | [251] |
| EN AW-5754 | automobile plates, structural sheets, load floors, inner body panels | [226,235,252] |
| EN AW-5754 | extrusions (Lotus Evora) | [235] |
| EN AW-5754 | instrument panel support (Volkswagen Polo, Skoda Fabia, Seat Ibiza) | [237] |
| EN AW-5754 | cockpit carrier (Audi A6, Audi A7) | [235] |
| EN AW-5745 | B-pillar assembly, floor panels (Chevrolet Corvette Z06) | [235] |
| EN AW-5754 | door inner, hood inner, B-pillar, rocker, brackets, structural reinforcements | [236] |
| EN AW-5754, EN AW-5182 | structural sheets, inner panels (BMW Z8) | [235] |
| Formall®-545 (EN AW-5083) | inner front door (Maybach) | [249] |
| Magsimal®-59 (AlMg5Si2Mn) | doors (Range Rover L322) | [249] |
| Magsimal®-59 (AlMg5Si2Mn) | components of retractable roofs | [243] |
| Magsimal®-59 (AlMg5Si2Mn) | door frames (Rolls Royce Phantom) | [235] |
| Aluminium Alloy Grade | Components | Reference |
|---|---|---|
| A356 (EN AC-42000) | B-pillar reinforcements, lift gate outer, body side, shock towers | [236] |
| AdvanzTM C300-T61 | long members (Jaguar XE) | [253] |
| AdvanzTM E170 | fender, hood outer (Jaguar XE); doors outer (Ford F150); roof, bodyside (GM CT6) | [253] |
| AdvanzTM S600 | roof, strength parts inner (Jaguar XE), fender, roof (Ford F150) | [253] |
| Alcoa C611 Mercalloy® 367 | front sections, shock tower, engine cradle | [239,240,241,242] |
| Anticorodal®-120 (EN AW-6016-PX) | outer body panels (Audi A2, Audi A8) | [235] |
| Anticorodal®-121 (EN AW-6061) | outer panel (Audi A2) | [249] |
| Anticorodal®-170 (EN AW-6014) | one piece body side (Range Rover) | [235] |
| Anticorodal®-170 (EN AW-6014), Anticorodal®-600 (EN AW-6451-T4) | body shell (Ferrari 548) | [235] |
| Anticorodal®-300 (EN-AW 6014-T61) | structural body applications with high crash performance (Mercedes SL R231) | [237] |
| Anticorodal®-300 (EN-AW 6014-T61) | tunnel (Mercedes SL R231), front longitudinal beam (Land Rover L405) | [237] |
| Anticorodal®-300 (EN-AW 6014-T61) | long crash members or bumper beams (Land Rover) | [269] |
| Anticorodal®-600 PX (EN AW-6181A) | exterior body panels side (Range Rover) | [235] |
| Anticorodal®-600 PX (EN AW-6181A) | roof (Range Rover Evoque) | [243] |
| Anticorodal®-600 (EN AW-6451-T4) | inner and outer panels (Range Rover) | [269] |
| Ecodal®-608 (EN AW-6181A) | inner and structural panels (Audi A2, Audi A8), cover sheet for the tunnel (Audi R8), reinforcements, inner panels (Audi A2) | [235,249] |
| EN AW-6005 | seat frame components (extruded) | [226] |
| EN AW-6005C | seat frames, space frames, bumpers, shock absorbers, side sills | [250] |
| EN AW-6008, EN AW-6060 | front longitudinal beam (BMW E60) | [235] |
| EN AW-6009 | inner panels (Audi A8) | [235] |
| EN AW-6009 | bumper face bars, load floors, inner and outer body panels, bumper reinforcements | [226] |
| EN AW-6009, EN AW-6010, EN AW-6016 | inner and outer body panels | [226] |
| EN AW-6009, EN AW-6010, EN AW-6016 | outer and inner panels | [270] |
| EN AW-6009, EN AW-6016, EN AW-6061, EN AW-6111 | outer panels, frames | [227] |
| EN AW-6013 | suspension arms | [250] |
| EN AW-6014 | front longitudinal member (Jaguar XK & F) | [237] |
| EN AW-6014 | crash-sensitive components (Range Rover), hydroformed lateral roof frame (Audi A2) | [235] |
| EN AW-6016 | body-in-white applications (Audi A8), outer panels (BMW Z8, Rolls Royce Phantom) | [235,268] |
| EN AW-6016 | outer panels (Lamborghini Gallardo) | [235] |
| EN AW-6016 | external structural parts | [254] |
| EN AW-6016 | outer panel (Saab 9-3, Renault Vel Satis, BMW 3 series, Mercedes Benz S class, Mercedes Benz SL, BMW Z8) | [249] |
| EN AW-6016A-T4 | inner panels | [251] |
| EN AW-6016-T4, EN AW-6016-T4P | outer panels | [251] |
| EN AW-6022-T6, EN AW-6082-T6, EN AW-6181A-T6 | space frame (Ferrari 548) | [235] |
| EN AW-6060 | extrusions (Lotus Evora), space frame (Audi A8 D2), extruded structural components (Audi A8 D3, Lamborghini Gallardo) | [235] |
| EN AW-6060 | instrument panel support (Mercedes-Benz A class) | [237] |
| EN AW-6060 | front bumper (Opel Corsa) | [244] |
| EN AW-6060 | sunroof channels (BMW 7 series), top rear end, transverse and cant rail assembly (Ford Think) | [243] |
| EN AW-6060, EN AW-6063, EN AW-6082 | frame structure (BMW Z8) | [235] |
| EN AW-6060, EN AW-6063, EN AW-6082 (T5 or T7) | extruded components (Rolls Royce Phantom) | [235] |
| EN AW-6060-T51, EN AW-6106-T51 | roof structure (Renault Avantime) | [243] |
| EN AW-6061 | car steering knuckles | [212] |
| EN AW-6061 | door outers, roof panels, body side, hood outers | [236] |
| EN AW-6061 | brackets, suspension parts, bumper reinforcements | [226] |
| EN AW-6061 | cross members, links, arms | [250] |
| EN AW-6061-T4, EN AW-6061-T6, EN AW-6063-T4 | engine cradle (Chevrolet Monte Carlo) | [237] |
| EN AW-6061, EN AW-6063 | rear frame (Corvette ZR-1/LT-1) | [237] |
| EN AW-6061-T6 | panel applications | [271] |
| EN AW-6061-T6 | extruded seatback beam (Chevrolet Corvette Z06) | [235] |
| EN AW-6063 | seat frames, roof railings | [250] |
| EN AW-6063 | body components | [226] |
| EN AW-6063 | bumpers, hinge pillars, A-pillars, roof bows | [236] |
| EN AW-6063 | bumper reinforcements, the roof reinforcement bar, A-pillar (Chevrolet Corvette Z06) | [235] |
| EN AW-6069-T7 | frame rail (Chevrolet Corvette Z06) | [235] |
| EN AW-6070 | extruded structural components | [216] |
| EN AW-6082 | structural elements (extruded) (BMW 6) series, Rolls-Royce Phantom, Jaguar XJ, Range Rover, Jaguar XK | [272] |
| EN AW-6082-T6 | rear bumper beam (Citroen C4 Picasso) | [244] |
| EN AW-6082-T6 | post/cant rail aluminium extrusion assembly, door reinforcements (Jaguar XJ350) | [235] |
| EN AW-6106 | extruded tunnel member (Porsche 9 × 1), upper A post panel (Audi R8) | [237] |
| EN AW-6111 | closure panels (Jaguar), outer skin (Jaguar XJ353) | [212,235] |
| EN AW-6111 | outer skins | [235] |
| EN AW-6111 | body panels | [226] |
| EN AW-6111-T4 | outer panels | [251] |
| EN AW-6111-T4P | reinforcements (Chevrolet GMT 830) | [249] |
| EN AW-6111-T4PD | outer panel (Chevrolet GMT 830) | [249] |
| EN AW-6181 | inner panels (Lamborghini Gallardo) | [235] |
| EN AW-6181A | Inner panels | [251] |
| EN AW-6181A, EN AW-6022 | outer panels | [212] |
| Novelis Advanz e600 (EN AW-6451-T6) | external panels, skin and structural applications | [212] |
| Novelis FusionTM AS250 | floor structure (Audi A8) | [237] |
| Aluminium Alloy Grade | Components | Reference |
|---|---|---|
| EN AW-7003 | door impact beams, frames, seat sliders, bumper reinforcement | [250] |
| EN AW-7003 | folding crash components (BMW E38), bumper systems (Renault Megane) | [244] |
| EN AW-7006, EN AW-7004 | bumper reinforcements, seat tracks | [226] |
| EN AW-7020 | bumper beams, complex automotive components | [212,254] |
| EN AW-7021 | bumper reinforcements, brackets, bumper face bars | [226] |
| EN AW-7033 | forged suspension arms | [226] |
| EN AW-7046 | impact beams, motorbike frames, bumper reinforcement | [250] |
| EN AW-7071 | car bumper brackets, bumper enhanced supports | [285] |
| EN AW-7075 | links, seatbelt hinges, bars | [212,250] |
| EN AW-7108 | rear seats for roll-over protection (Porsche 911, 966) | [244] |
| EN AW-7108 | bumper beams (BMW E38, Volkswagen Passat B5, Audi TT), rear bumper (Opel Corsa) | [244] |
| EN AW-7108-T6 | front beam (Jaguar XJ350) | [235] |
| EN AW-7204 | cross members, steering components | [250] |
| Alloy | Example |
|---|---|
| Alpha (α) alloys | Commercially pure Ti—ASTM Grades 1, 2, 3, and 4 Ti/Pd alloys—ASTM Grades 7 and 11 |
| Alpha (α) + compound | Ti-2.5%Cu—IMI 230 |
| Near Alpha alloys | Ti-8%Al-1%Mo-1%V Ti-6%Al-5%Zr-0.5%Mo-0.2%Si—IMI 685 Ti-6%Al-2%Sn-4%Zr-2%Mo-0.08%Si Ti-5.5%Al-3.5%Sn-3%Zr-1%Nb-0.3%Mo-0.3%Si—IMI 829 Ti-5.8%Al-4%Sn-3.5%Zr-0.7%Nb-0.5%Mo-0.3%Si—IMI 834 Ti-6%Al-3%Sn-4%Zr-0.5%Mo-0.5%Si—Ti 1100 |
| Alpha-Beta (α—β) alloys | Ti-6%Al-4%V Ti-4%Al-4%Mo-2%Sn-0.5%Si Ti-4%Al-4%Mo-4%Sn-0.5%Si—IMI 551 Ti-6%Al-6%V-2%Sn Ti-6%Al-2%Sn-4%Zr-%Mo |
| Metastable Beta (β) alloys | Ti-3%Al-8%V-6%Cr-4%Zr-4%Mo—Beta C Ti-15%Mo-3%Nb-3%Al-0.2%Si—Timetal 21 S Ti-15%V-3%Cr-3%Sn-3%Al |
| Systems and Parts Materials | |
|---|---|
| Frame structures | |
| Suspension springs | Ti-6.8Mo-4.5Fe-1.5Al |
| Ti-6Al-4V | |
| Armor | Ti-6Al-4V |
| Body | CP-Ti (Grade 4) |
| Ti-6Al-4V | |
| Engines | |
| Outlet valves | outlet valves γ(TiAl), Grade 2 |
| Ti-6Al-4V | |
| 2Sn-4Zr-2Mo-0.1Si | |
| Intake valves | intake valves Ti-6Al-4V |
| Turbocharger rotors | γ(TiAl) |
| Connecting rods | Ti-6Al-4V |
| Exhaust system | Grade 2 |
| Titanium and Titanium Alloys | Components | Reference |
|---|---|---|
| titanium alloys (*) | car door into the beam, car stop bracket | [332] |
| pure titanium (*) | shock absorber centre, door projecting beam, suspension systems | [333] |
| pure titanium (*) | panels (roof, hood) | [334] |
| pure titanium (*) | door beams | [329] |
| pure titanium (*) | car body frames | [330] |
| pure titanium (*) | crash elements | [301] |
| pure titanium (*) | active spoiler bracket, tailpipe trim covers (Bugatti) | [319] |
| pure titanium (*) | body panels (Thrust Super Sonic Car) | [335] |
| pure titanium (*) | muffler (Volkswagen Golf) | [330] |
| pure titanium (Grade 1) | The outer shell of the muffler (Bugatti Veyron 16.4) | [336] |
| pure titanium (Grade 2) | heat shields (Bugatti Veyron 16.4) | [336] |
| pure titanium (Grade 2) | exhaust system (Nissan Fair lady Z, Subaru Impreza, Koenigsegg) | [336] |
| pure titanium (Grade 2) | exhaust systems | [301] |
| pure titanium (Grade 2) | exhaust system (Bugatti Veyron) | [336] |
| pure titanium (Grade 4), Ti-6Al-4V | body panels | [301] |
| pure titanium (Grade 4) | car bodies | [337] |
| pure titanium (Grade 4), Ti-6Al-4V | car bodies | [337] |
| Ti-6Al-4V | bumpers | [301] |
| Ti-6Al-4V | armours | [337] |
| Ti-6Al-4V | exhaust systems (Chevrolet Corvette Z06) | [301] |
| Ti-6Al-4V | axle suspension, crash clamps (Bugatti Veyron 16.4) | [336] |
| Ti-6Al-4V, Ti-6.8Mo-4.5Fe-1.5Al | suspension springs | [337] |
| Ti-6Al-4V, Ti-6.8Mo-4.5Fe-1.5Al | suspension springs | [301] |
| Ti-5Al-2.5Sn | exhaust systems | [16] |
| Ti-6.8Mo-4.5Fe-1.5Al | suspension spring, (Bugatti Veyron 16.4, Ferrari 360 Stradale) | [336] |
| Ti-6.8Mo-4.5Fe-1.5Al | suspension springs (Volkswagen Lupo FSI) | [301] |
| Ti-1 Cu-1 Sn-0.35 Si-0.2 Nb, Ti-0.5Si-Fe (Ti-Exhaust XT), Ti-1Cu, Ti-0.1Fe-0.35Si, Ti-1.5Al, Ti-1 Cu-0.5 Nb, Ti-0.5Al-0.45Si-0.2Nb | exhaust systems | [317] |
| Ti-10V-2Fe-3Al, Ti-6Al-2Sn-4Zr-2Mo, Ti-6Al-4V, Ti-6Al-6V-2Sn, Ti- 8Al-1Mo-1V | high-strength performance components | [337] |
| Ti-1Cu-0.5Nb, Ti-0.45Si-0.25Fe, Ti-1.5Al, Ti-0.5Al-0.45Si-0.2Nb, Ti-0.9SA, Ti-1Cu-0.5Nb | mufflers | [331] |
| Magnesium Alloy | Components | Reference |
|---|---|---|
| A2401-002 | steering wheel (Shanghai GMC) | [349] |
| AE44 (Mg-4Al-4RE) | engine bracket | [357] |
| AE44 (Mg-4Al-4RE) | cradle (Chevrolet Corvette Z06) | [358] |
| AM20 (Mg-2Al-0.5Mn), AM50 (Mg5.0Al0.3Mn) | seat frames (Mercedes Benz Roadster, Mercedes Benz SL500) | [359,360] |
| AM50 (Mg5.0Al0.3Mn) | seat frame, inside plate | [361] |
| AM50 (Mg5.0Al0.3Mn) | steering wheel | [362] |
| AM50 (Mg5.0Al0.3M) | car seat backrest | [363] |
| AM50 (Mg5.0Al0.3Mn) | trunk lid (Mercedes Benz E-series) | [349] |
| AM50 (Mg5.0Al0.3Mn) | cross car beam (Voyah Free) | [349] |
| AM50A (MgAl5Mn) | central control bracket (Volvo XC60) | [349] |
| AM50A (MgAl5Mn) | front- end carrier (Porsche Panamera G2) | [349] |
| AM50 (Mg5.0Al0.3Mn) | side door (Aston Martin DB9) | [364] |
| AM60B (Mg-6Al-0.4Mn) | cross car beam | [365] |
| AM60B (Mg-6Al-0.4Mn) | seat frame | [361] |
| AM60B (Mg-6Al-0.4Mn) | front-end carrier (Tesla S) | [366] |
| AM60B (Mg-6Al-0.4Mn) | inside door panel | [367] |
| AM60B (Mg-6Al-0.4Mn) | front-end frame | [368] |
| AM60B (Mg-6Al-0.4Mn) | folding roofs (Mercedes Benz SLK) | [349] |
| AM60B (Mg-6Al-0.4Mn) | body structure (Chrysler) | [369] |
| AM60B (Mg-6Al-0.4Mn) | front- end carrier (Range Rover) | [349,370] |
| AS41B (Mg-4Al-0.3Mn-1Si) | crankcase housing | [371] |
| AS41B (Mg-4Al-0.3Mn-1Si) | transmission housing | [372] |
| AS21 (MgAl2.2Si1Mn0.3), AS41 (MgAl4.5Si1Mn0.3) | transmission housing, crank case (Volkswagen) | [358] |
| AZ31 (Mg-3Al-1Zn-0.2Mn) | inner panel | [373] |
| AZ31B (Mg-3Al-1Zn) | upper rail (USAMP project) | [354] |
| AZ31B (Mg-3Al-1Zn) | Inner panel (Cadillac STS) | [374] |
| AZ31B-H24 (Mg-3Al-1Zn) | luggage retainer for Renault-Samsung SM7), roof panel (Porsche Carrera GT) | [354] |
| AZ61 (MgAl6Zn1) | column beam, luggage rack skeleton | [375] |
| AZ91 (MgAl9Zn1) | rear suspension, subframe members, clutch housing, transmission housing (Volvo LCP 2000) | [376] |
| AZ91 (MgAl9Zn1) | rear subframe (Audi A8) | [349] |
| AZ91B (Mg-2.5Al-0.7Zn-0.2Mn) | clutch Housing (Ford Ranger) | [360] |
| AZ91D (MgAl9Zn1(A)) | inner panel (Ford F150) | [377] |
| AZ91D (MgAl9Zn1(A)) | transmission housing | [378] |
| AZ91D (MgAl9Zn1(A)) | steering column bracket | [379] |
| AZ91D (MgAl9Zn1(A)) | clutch housing | [380] |
| AZ91D (MgAl9Zn1(A)) | swivel plate | [381] |
| AZ91D (MgAl9Zn1(A)) | central control bracket | [382] |
| AZ91D (MgAl9Zn1(A)) | starter housing | [383] |
| AZ91D (MgAl9Zn1(A)) | car dashboard member | [363] |
| AZ91D (MgAl9Zn1(A)) | steering column (Ford Aerostar) | [360] |
| AZ91D (MgAl9Zn1(A)) | truck gearbox housing (Volkswagen Passat, Audi A4 and A6) | [384] |
| cast magnesium alloy (*) | cabin bracket (Audi A8) | [349] |
| cast magnesium alloy (*) | roof frame, hardtop roof (Cadillac XLR) | [349] |
| cast magnesium alloy (*) | steering wheel (Dongfeng Nissan) | [349] |
| cast magnesium alloy (*) | tubular subframe (BMW 5- and 7-series) | [385] |
| cast magnesium alloy (*) | intake manifold cover (Audi A8) | [349] |
| cast magnesium alloy (*) | one-piece inner panel (Mercedes S-class) | [386] |
| cast magnesium alloy (*) | liftgate inner (Mercedes E-Class T) | [349] |
| cast magnesium alloy (*) | lift doors, side doors (Toyota Venza) | [387] |
| cast magnesium alloy (*) | side door, inner panels (Aston Martin Vanquish S) | [349] |
| cast magnesium alloy (*) | rear back doors (Jeep Wrangler) | [349] |
| cast magnesium alloy | bezel-less doors (Volkswagen) | [388] |
| magnesium alloys (*) | cross car beams (BMW X3, BMW X5, BMW X6, Chrysler Pacifica, Honda Ridgeline, Honda Acura MDX, Jaguar F-type, Jaguar XF, Jeep Grand Cherokee, Jeep Wrangler, Land Rover Discovery, Range Rover Evoque) | [389] |
| magnesium alloys (*) | lift gate inner (Chrysler Pacifica, Lincoln MKT), front deck (Dodge Viper, Mercedes AMG), ABS mounting bracket (Chrysler), trunk lid (Cadillac SLS), front end carrier (Tesla Model S) | [390] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trzepieciński, T.; Najm, S.M. Current Trends in Metallic Materials for Body Panels and Structural Members Used in the Automotive Industry. Materials 2024, 17, 590. https://doi.org/10.3390/ma17030590
Trzepieciński T, Najm SM. Current Trends in Metallic Materials for Body Panels and Structural Members Used in the Automotive Industry. Materials. 2024; 17(3):590. https://doi.org/10.3390/ma17030590
Chicago/Turabian StyleTrzepieciński, Tomasz, and Sherwan Mohammed Najm. 2024. "Current Trends in Metallic Materials for Body Panels and Structural Members Used in the Automotive Industry" Materials 17, no. 3: 590. https://doi.org/10.3390/ma17030590










