Improving the Corrosion Performance of Organically Coated Steel Using a Sol–Gel Overcoat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sol–Gel Preparation and Application
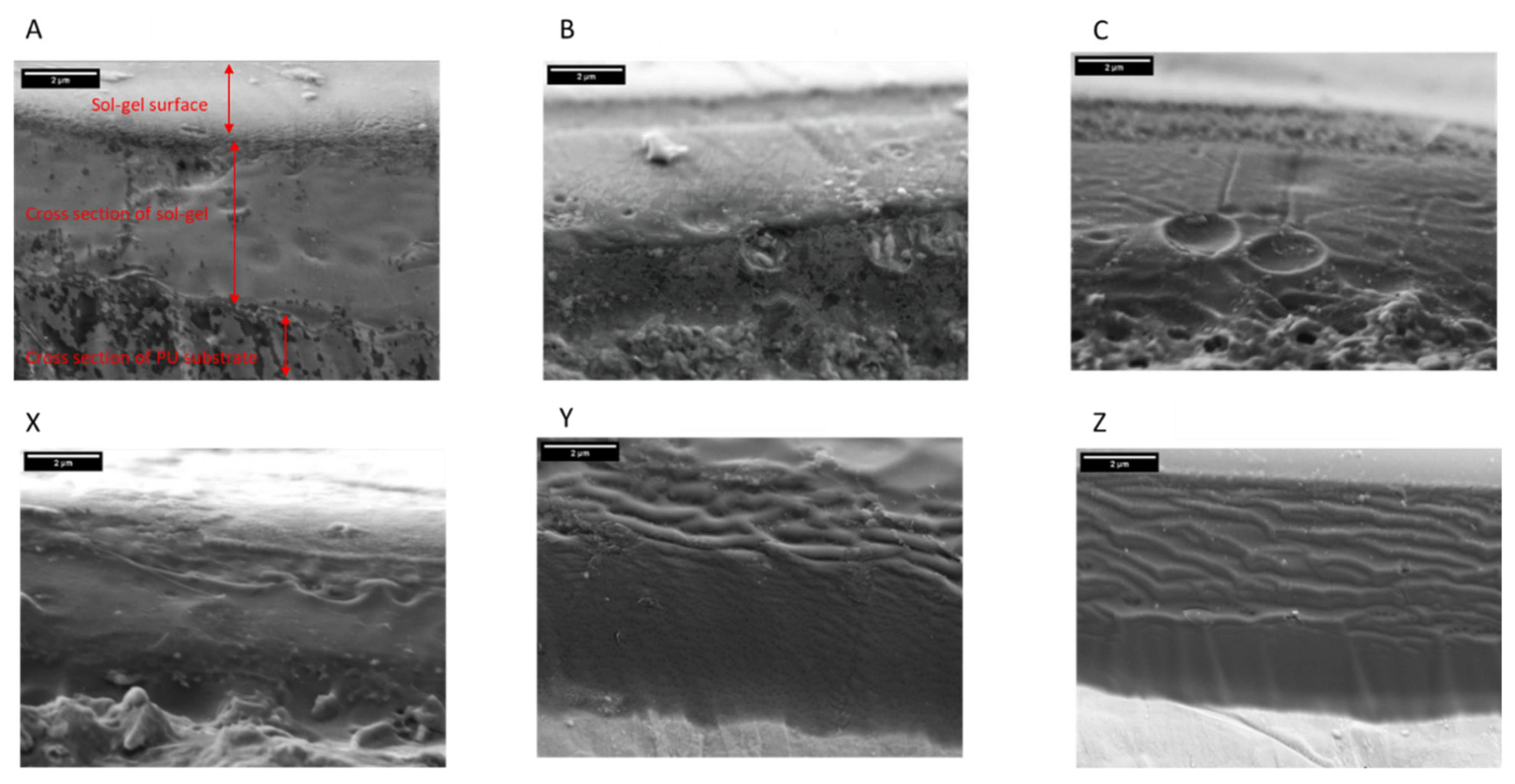
2.2. Scanning Electron Microscopy (SEM)
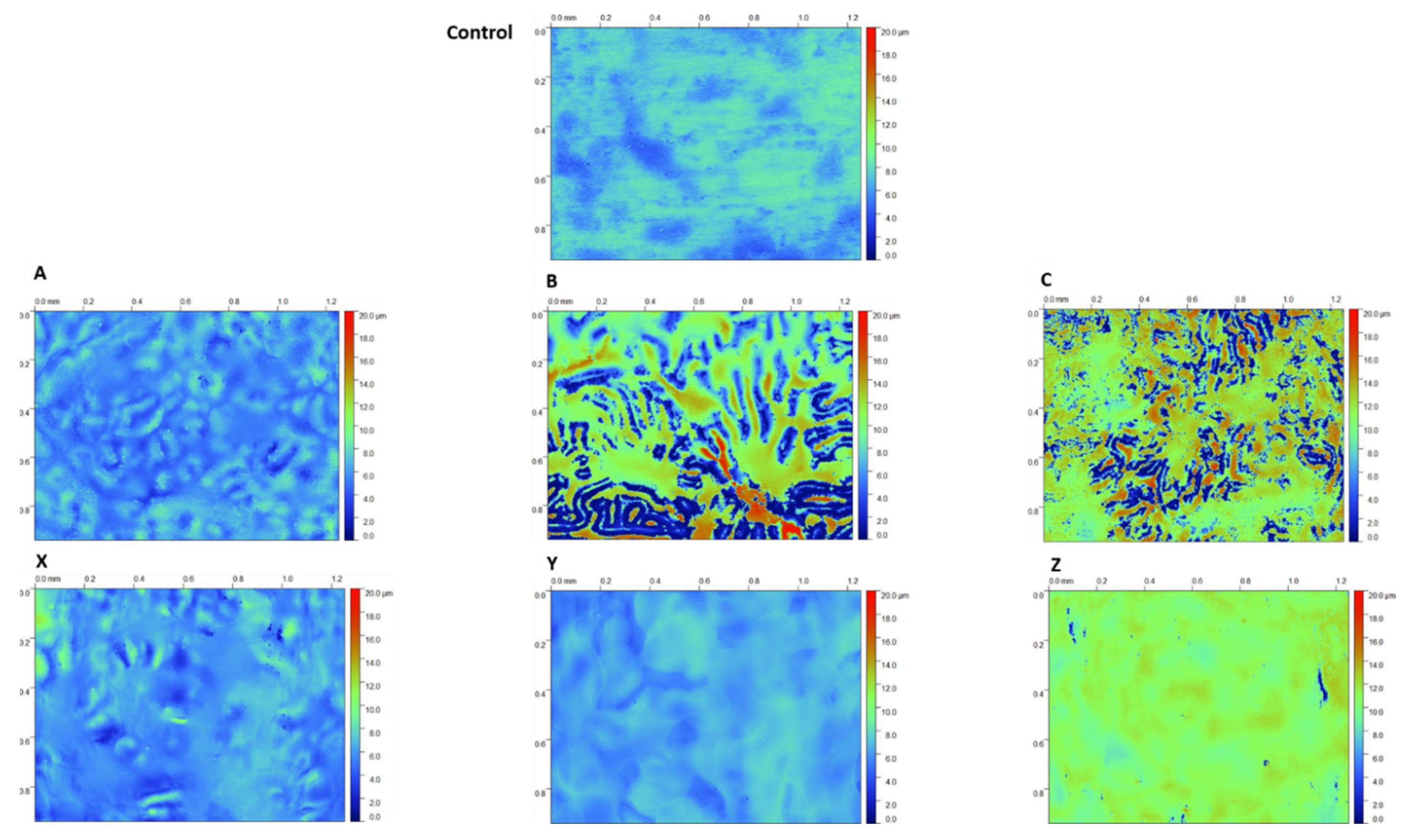
2.3. White Light Interferometry
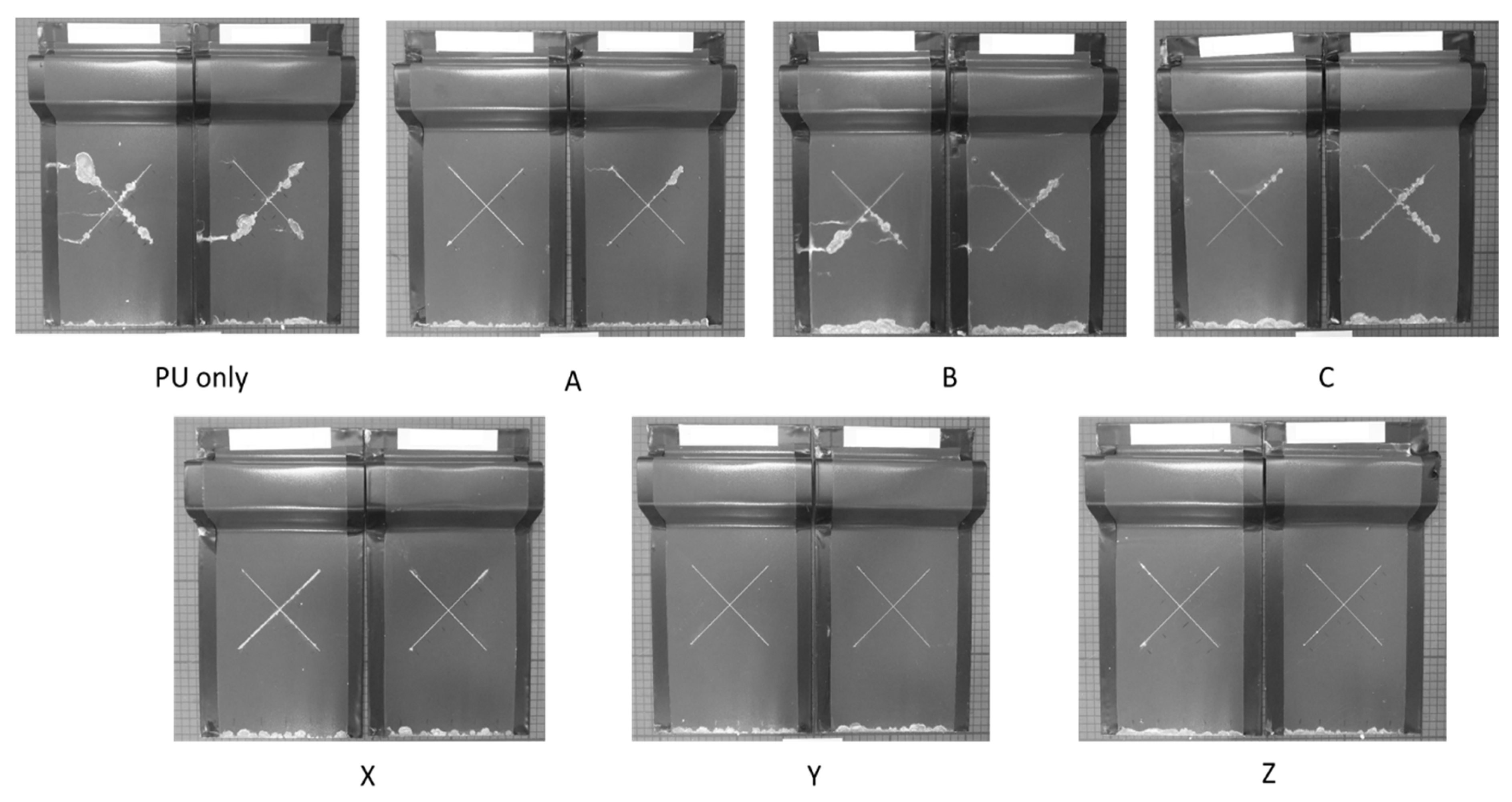
2.4. Salt Spray Testing
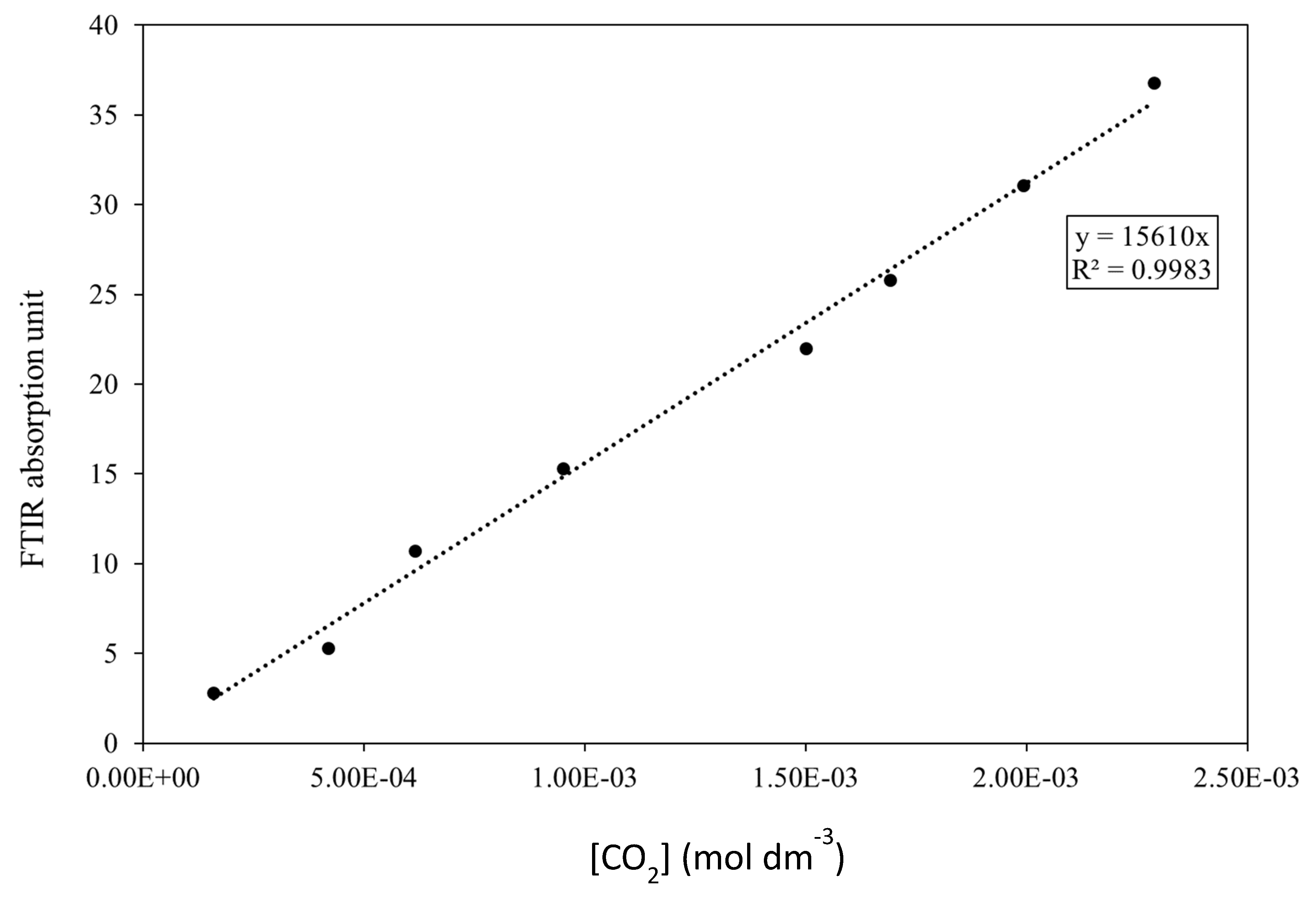
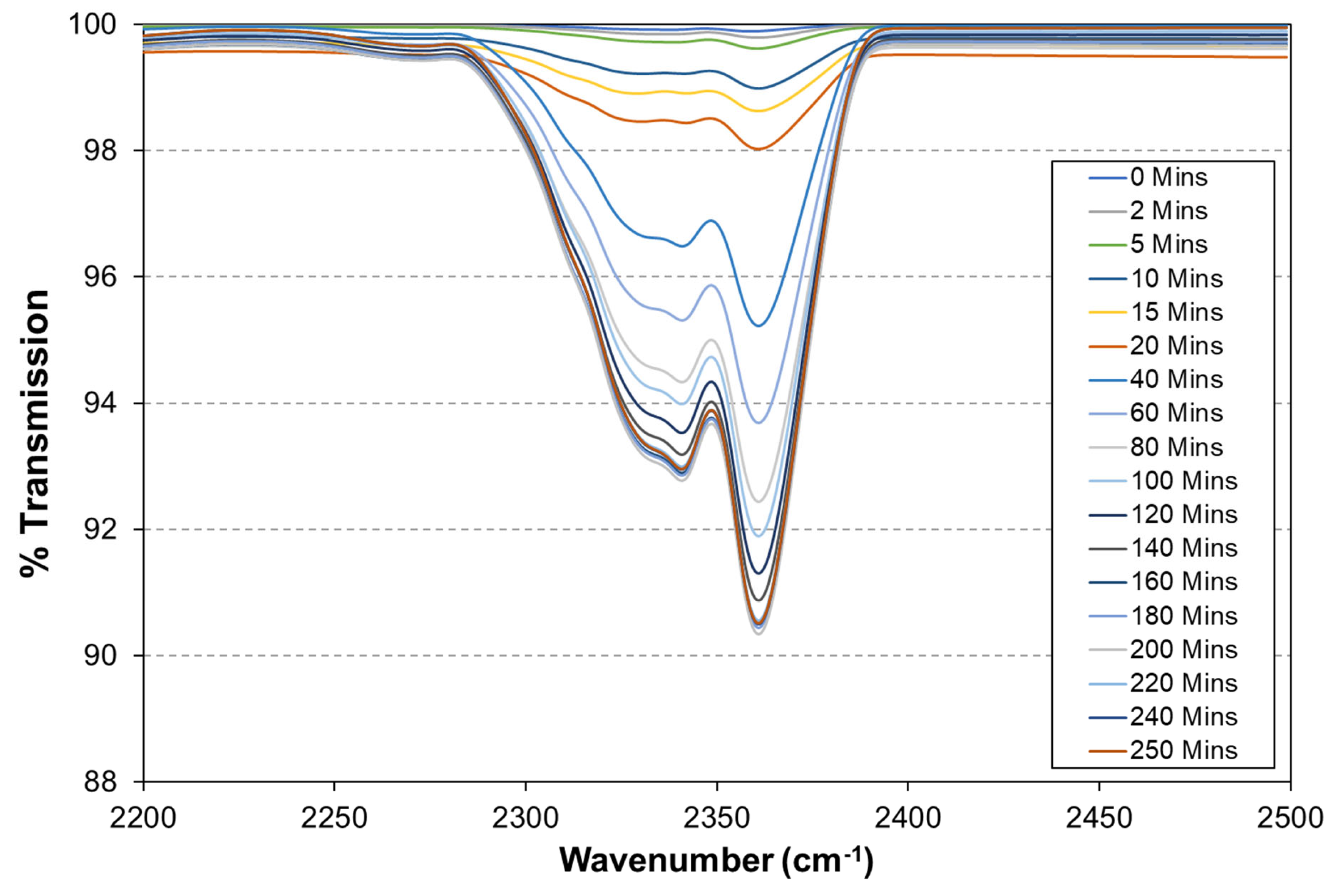
2.5. O2 Permeation Measurements Using FTIR in a Closed-Loop Flow Reactor
2.6. ATR-FTIR
3. Results
3.1. Coating Morphology and Thickness
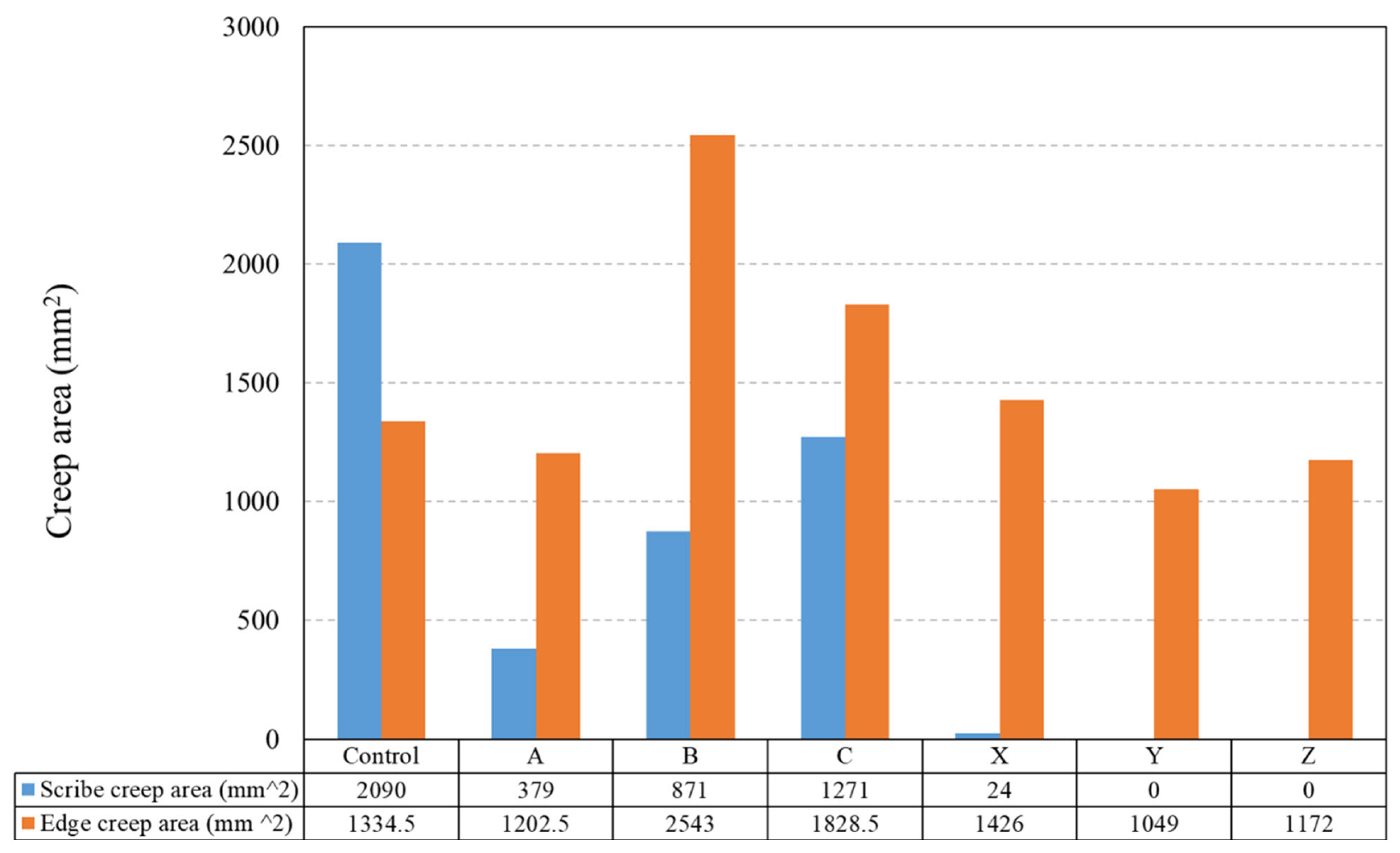
3.2. Salt Spray
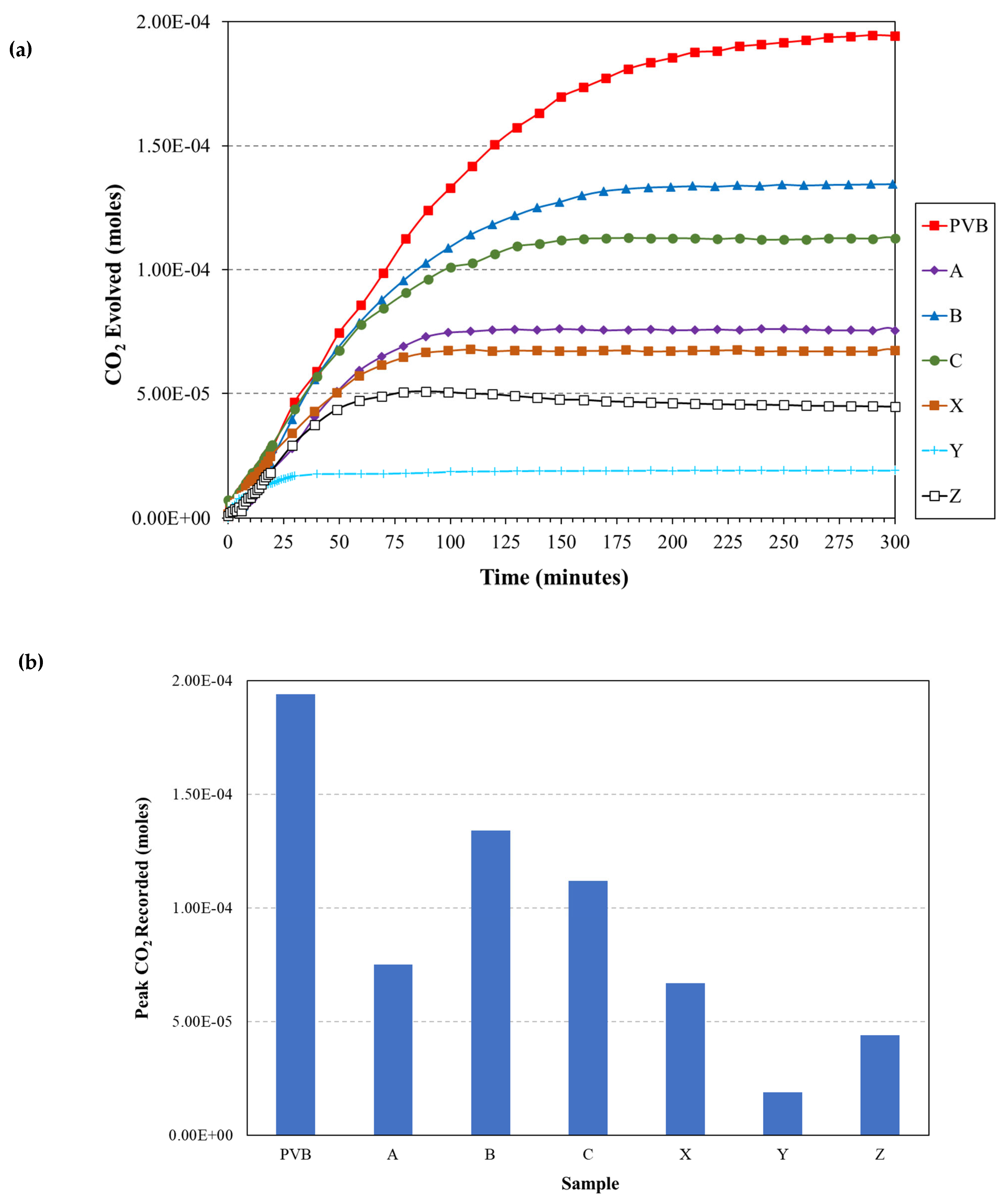
3.3. O2 Barrier Measurements
3.4. Contact Angle
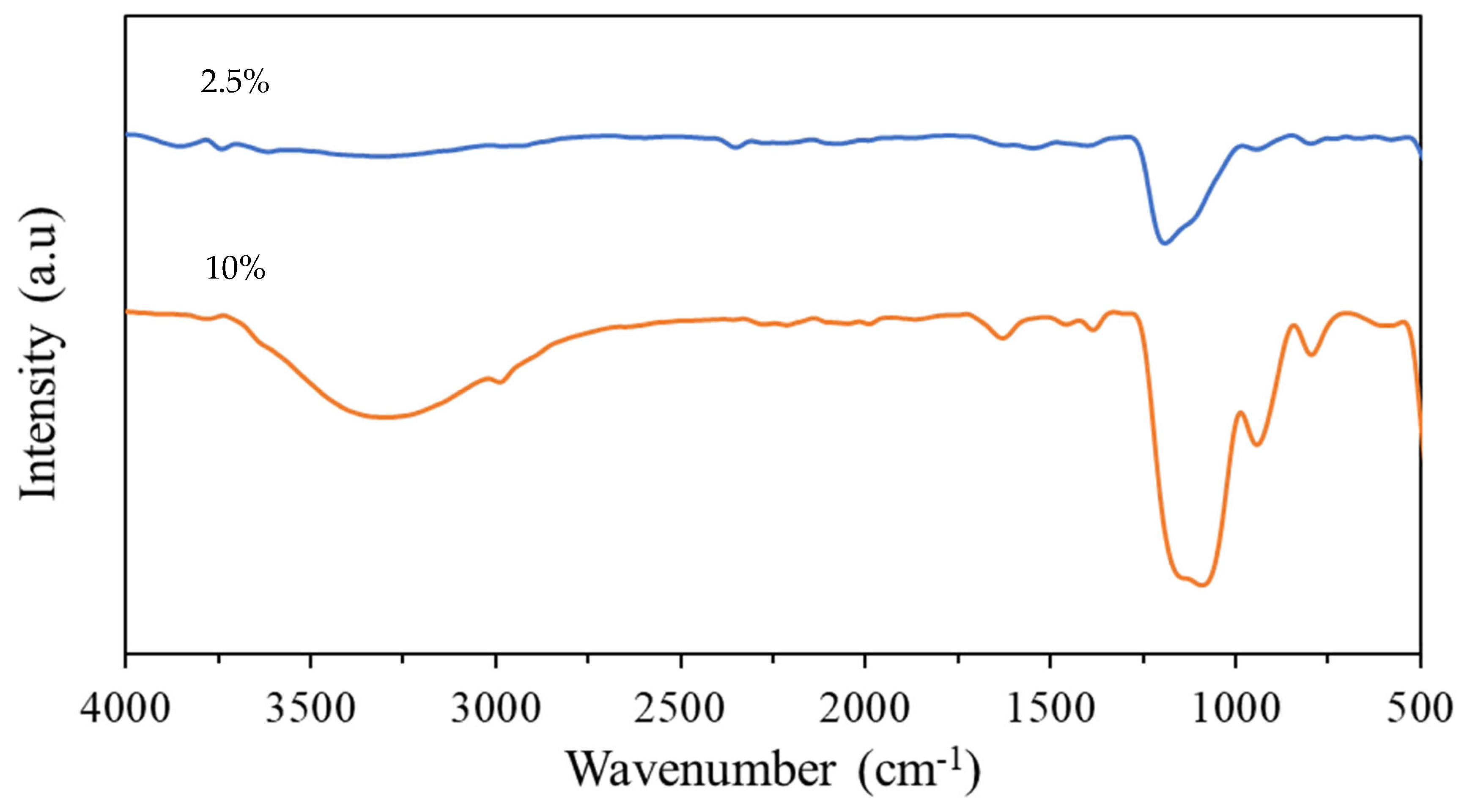
3.5. ATR-FTIR Analysis
4. Discussion
5. Conclusions
- It is possible to overcoat a PU-coated steel panel using a TEOS-based sol–gel. The optimisation of the oxide film application can facilitate the production of high-quality coatings even with a low concentration of TEOS and a short exposure time. Coatings produced using the 10% TEOS were dense, homogeneous and thicker than those produced using the 2.5% TEOS. Salt spray testing showed that these coatings provided substantial improvements in corrosion resistance with no scribe creep observed and a reduction of up to 21% edge creep compared to that of an uncoated PU substrate. This is suggested to be due to the sol–gel coating providing a barrier to oxygen and thereby reducing cathodic disbondment.
- Coatings produced using the 2.5% TEOS were inhomogeneous and had a rough morphology. The low concentration of Si species available to undergo hydrolysis and condensation at the PU surface limits the ability to form a consistent coating. Island-like formation, similar to that of the coffee-ring effect, was observed. The poor coating formation reduced the effectiveness of the coating’s corrosion resistance when subjected to salt spray testing.
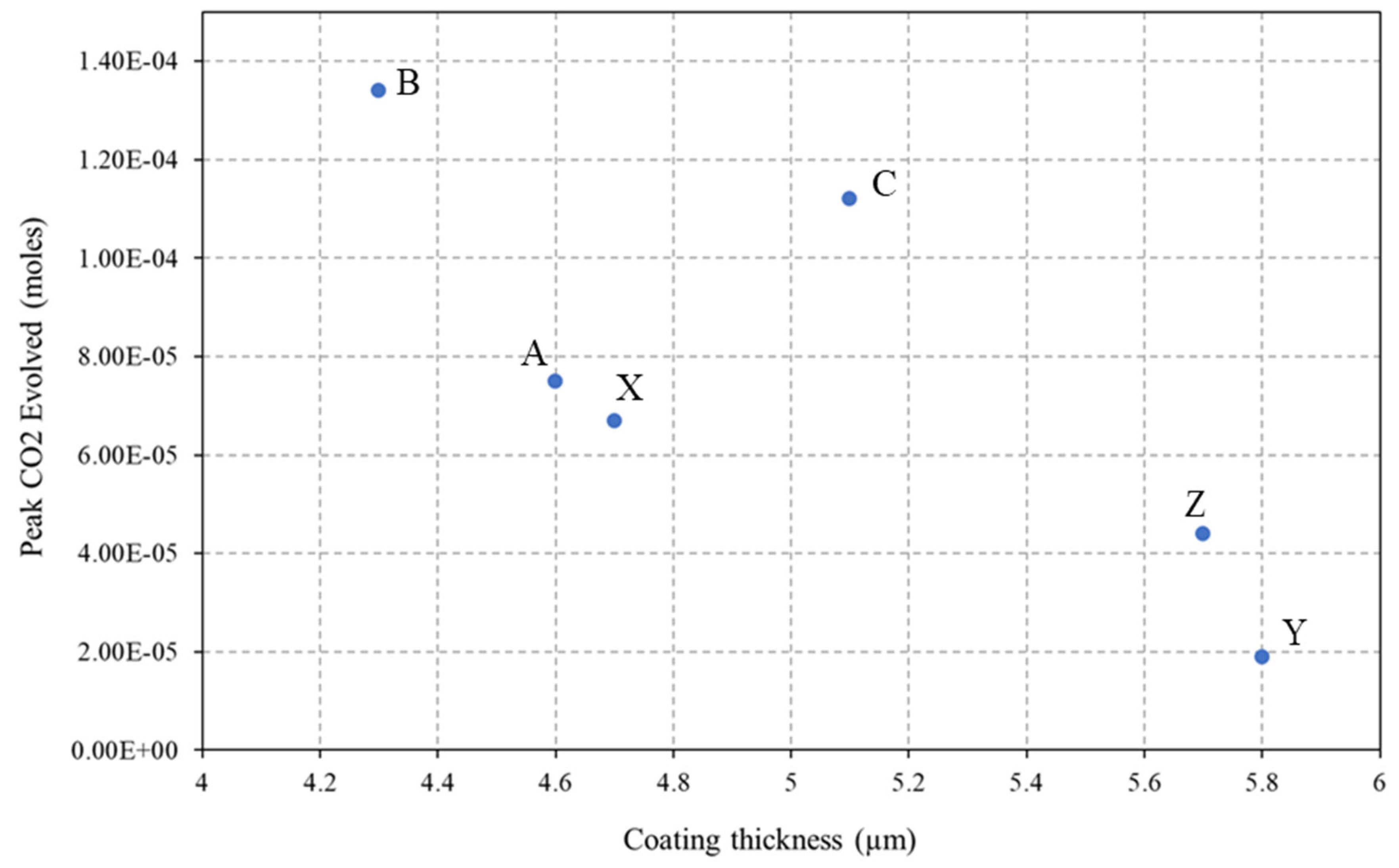
- A novel closed-loop reactor was used to determine the effect of the sol–gel coating to limit the diffusion of O2, which reduces the rate at which CO2 evolves from the UV degradation of a PVB coating containing TiO2. This technique gave further evidence that the 2.5% TEOS afforded a poor barrier performance compared to that of the 10% TEOS, which can be attributed to inhomogeneous coating formation.
- ATR-FTIR analysis of the coatings showed that there was a significant reduction in Si–O–Si vibrations recorded for a coating produced using the 2.5% TEOS compared to those of the 10% TEOS, suggesting the reduced development of the sol–gel network.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sander, J. Coil Coating; Vincentz Network: Hanover, Germany, 2014. [Google Scholar]
- Knudsen, O.O.; Forsgren, A. Corrosion Control Through Organic Coatings, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Morcillo, M.; Rodríguez, F.; Bastidas, J. The influence of chlorides, sulphates and nitrates at the coating-steel interface on underfilm corrosion. Prog. Org. Coat. 1997, 31, 245–253. [Google Scholar] [CrossRef]
- Zhang, J.T.; Hu, J.M.; Zhang, J.Q.; Cao, C.N. Studies of impedance models and water transport behaviors of polypropylene coated metals in NaCl solution. Prog. Org. Coat. 2004, 49, 293–301. [Google Scholar] [CrossRef]
- Fredj, N.; Cohendoz, S.; Mallarino, S.; Feaugas, X.; Touzain, S. Evidencing antagonist effects of water uptake and leaching processes in marine organic coatings by gravimetry and EIS. Prog. Org. Coat. 2010, 67, 287–295. [Google Scholar] [CrossRef]
- Park, J.; Lee, G.; Ooshige, H.; Nishikata, A.; Tsuru, T. Monitoring of water uptake in organic coatings under cyclic wet–dry condition. Corros. Sci. 2003, 45, 1881–1894. [Google Scholar] [CrossRef]
- Ji, W.-G.; Hu, J.-M.; Zhang, J.-Q.; Cao, C.-N. Reducing the water absorption in epoxy coatings by silane monomer incorporation. Corros. Sci. 2006, 48, 3731–3739. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y. Comparisons of clear coating degradation in NaCl solution and pure water. Prog. Org. Coat. 2013, 76, 1674–1682. [Google Scholar] [CrossRef]
- Inone, P.C.; Garcia, C.M.; Rúvolo-Filho, A. Evaluating barrier properties of organic coatings by water permeation and electrochemical methods. J. Coat. Technol. 2003, 75, 29–36. [Google Scholar] [CrossRef]
- Jalilian, E.; Terryn, H.; Van Assche, G. Water permeation in coatings. J. Coat. Technol. Res. 2020, 17, 1437–1445. [Google Scholar] [CrossRef]
- Marques, A.C. Sol–Gel Process: An Overview; Lehigh University: Bethlehem, PA, USA, 2007. [Google Scholar]
- Hench, L.L.; West, J. The Sol-Gel Process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Figueira, R.B.; Fontinha, I.R.; Silva, C.J.R.; Pereira, E.V. Hybrid sol-gel coatings: Smart and green materials for corrosion mitigation. Coatings 2016, 6, 12. [Google Scholar] [CrossRef]
- Suleiman, R.K. Improved mechanical and anticorrosion properties of metal oxide-loaded hybrid sol–gel coatings for mild steel in a saline medium. J. Adhes. Sci. Technol. 2020, 34, 1315–1330. [Google Scholar] [CrossRef]
- Esposito, S. “Traditional” sol-gel chemistry as a powerful tool for the preparation of supported metal and metal oxide catalysts. Materials 2019, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, G.; Mishra, N.K.; Chaudhary, P. Thin Film Coating through Sol-Gel Gel Technique. Res. J. Chem. Sci. 2016, 6, 65–72. [Google Scholar]
- Imai, H.; Hirashima, H.; Awazu, K.; Onuki, H. Modification and Densification of Sol-Gel-Derived Silica Films at Low Temperatures; Dunn, B.S., Mackenzie, J.D., Pope, E.J.A., Eds.; Sol-Gel Optics IV; SPIE: Bellingham, DC, USA, 1997; pp. 144–149. [Google Scholar]
- Garcia, R.B.R.; da Silva, F.S.; Kawachi, E.Y. New sol–gel route for SiO2/ZrO2 film preparation. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 484–488. [Google Scholar] [CrossRef]
- Figueira, R.B. Hybrid sol–gel coatings for corrosion mitigation: A critical review. Polymers 2020, 12, 689. [Google Scholar] [CrossRef] [PubMed]
- Rodič, P.; Milošev, I. Corrosion Properties of UV Cured Hybrid Sol-Gel Coatings on AA7075-T6 Determined under Simulated Aircraft Conditions. J. Electrochem. Soc. 2014, 161, C412–C420. [Google Scholar] [CrossRef]
- Khramov, A.; Balbyshev, V.; Kasten, L.; Mantz, R. Sol–gel coatings with phosphonate functionalities for surface modification of magnesium alloys. Thin Solid Films 2006, 514, 174–181. [Google Scholar] [CrossRef]
- Shreya, M.K.; Indhumathi, C.; Rajarajeswari, G.R.; Ashokkumar, V.; Preethi, T. Facile green route sol–gel synthesis of nano-titania using bio-waste materials as templates. Clean Technol. Environ. Policy 2021, 23, 163–171. [Google Scholar] [CrossRef]
- Habte, L.; Shiferaw, N.; Mulatu, D.; Thenepalli, T.; Chilakala, R.; Ahn, J.W. Synthesis of Nano-Calcium Oxide from Waste Eggshell by Sol-Gel Method. Sustainability 2019, 11, 3196. [Google Scholar] [CrossRef]
- Claire, L.; Marie, G.; Julien, G.; Jean-Michel, S.; Jean, R.; Marie-Joëlle, M.; Stefano, R.; Michele, F. New architectured hybrid sol-gel coatings for wear and corrosion protection of low-carbon steel. Prog. Org. Coat. 2016, 99, 337–345. [Google Scholar] [CrossRef]
- Metroke, T.L.; Parkhill, R.L.; Knobbe, E.T. Passivation of metal alloys using sol–gel-derived materials—A review. Prog. Org. Coat. 2001, 41, 233–238. [Google Scholar] [CrossRef]
- Wang, H.; Akid, R. A room temperature cured sol–gel anticorrosion pre-treatment for Al 2024-T3 alloys. Corros. Sci. 2007, 49, 4491–4503. [Google Scholar] [CrossRef]
- Stambolova, I.; Yordanov, S.; Lakov, L.; Vassilev, S.; Blaskov, V.; Jivov, B. Preparation of sol-gel SiO2 coatings on steel and their corrosion resistance. In MATEC Web of Conferences; Institute of Mechanics: Sofia, Bulgaria, 2018. [Google Scholar]
- Cotolan, N.; Varvara, S.; Albert, E.; Szabó, G.; Hórvölgyi, Z.; Mureşan, L.-M. Evaluation of corrosion inhibition performance of silica sol–gel layers deposited on galvanised steel. Corros. Eng. Sci. Technol. 2016, 51, 373–382. [Google Scholar] [CrossRef]
- Pathak, S.S.; Khanna, A.S. Sol–Gel Nanocoatings for Corrosion Protection; Woodhead Publishing Ltd.: Sawston, UK, 2012. [Google Scholar]
- Bera, S.; Rout, T.; Udayabhanu, G.; Narayan, R. Comparative Study of Corrosion Protection of Sol–Gel Coatings with Different Organic Functionality on Al-2024 substrate. Prog. Org. Coat. 2015, 88, 293–303. [Google Scholar] [CrossRef]
- Wint, N.; Wijesinghe, S.L.; Yan, W.; Ong, W.K.; Wu, L.Y.; Williams, G.; McMurray, H.N. The Sacrificial Protection of Steel by Zinc-Containing Sol-Gel Coatings. J. Electrochem. Soc. 2019, 166, C434–C444. [Google Scholar] [CrossRef]
- Yu, S.-P.; Tai, H.-J. Sol–gel enhanced polyurethane coating for corrosion protection of 55% Al-Zn alloy-coated steel. J. Polym. Res. 2021, 28, 158. [Google Scholar] [CrossRef]
- Moghaddam, A.R.; Mohseni, M.; Hedayati, H.; Ramezanzadeh, B. Studying the rheology, optical clarity and surface tension of an acrylic/melamine automotive clearcoat loaded with different additives. Prog. Org. Coat. 2014, 77, 101–109. [Google Scholar] [CrossRef]
- Watkins, E.; Griffiths, C.; Batchelor, C.; Barker, P.; Carnie, M. Processing and Weathering of Sol-Gel Clearcoats for Coil-Coated Steel. Coatings 2023, 13, 982. [Google Scholar] [CrossRef]
- ISO 17872:2019; Paints and Varnishes—Guidelines for the Introduction of Scribe Marks through Coatings on Metallic Panels for Corrosion Testing. ISO: Geneva, Switzerland, 2019.
- ASTM B117-64; Standard Method of Salt Spray (Fog) Testing. ASTM: West Conshohocken, PA, USA, 1964.
- Searle, J.; Worsley, D. Titanium dioxide photocatalysed oxidation of plasticisers in thin poly(vinyl chloride) films. Plast. Rubber Compos. 2002, 31, 329–335. [Google Scholar] [CrossRef]
- Robinson, A.; Searle, J.; Worsley, D. Novel flat panel reactor for monitoring photodegradation. Mater. Sci. Technol. 2004, 20, 1041–1048. [Google Scholar] [CrossRef]
- Woods, R.; Searle, J.; Pursglove, A.; Worsley, D. Comparison of the photoactivity of TiO2 coatings using a flat panel reactor and FTIR to monitor the CO2 evolution rate. J. Environ. Chem. Eng. 2019, 7, 103336. [Google Scholar] [CrossRef]
- Pang, Y.X.; Hodgson, S.N.B.; Weglinski, B.; Gaworska, D. Investigations into sol-gel silica and silica hybrid coatings for dielectromagnetic soft magnetic composite applications. J. Mater. Sci. 2006, 41, 5926–5936. [Google Scholar] [CrossRef]
- Innocenzi, P. Infrared spectroscopy of sol–gel derived silica-based films: A spectra-microstructure overview. J. Non-Cryst. Solids 2003, 316, 309–319. [Google Scholar] [CrossRef]
- Post, P.; Wurlitzer, L.; Maus-Friedrichs, W.; Weber, A.P. Characterization and Applications of Nanoparticles Modified in-Flight with Silica or Silica-Organic Coatings. Nanomaterials 2018, 8, 530. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.P.; Cao, G. Adhesion of sol-gel-derived organic-inorganic hybrid coatings on polyester. J. Sol-Gel Sci. Technol. 2003, 27, 31–41. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Publisher Correction: Capillary flow as the cause of ring stains from dried liquid drops. Nature 2021, 592, E12. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, H.; Loveridge, M. Inhibition of corrosion-driven organic coating disbondment on galvanised steel by smart release group II and Zn(II)-exchanged bentonite pigments. Electrochim. Acta 2010, 55, 1740–1748. [Google Scholar] [CrossRef]
- Griffiths, C.; Wint, N.; Williams, G.; McMurray, H. The contribution of Zn(II) and phosphate anions to the inhibition of organic coating cathodic disbondment on galvanised steel by zinc phosphate pigment. Corros. Sci. 2022, 198, 110111. [Google Scholar] [CrossRef]
- Fürbeth, W.; Stratmann, M. Scanning Kelvinprobe investigations on the delamination of polymeric coatings from metallic surfaces. Prog. Org. Coat. 2000, 39, 23–29. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, H.N. Chromate Inhibition of Corrosion-Driven Organic Coating Delamination Studied Using a Scanning Kelvin Probe Technique. J. Electrochem. Soc. 2001, 148, B377. [Google Scholar] [CrossRef]
- Zin, I.M.; Lyon, S.B.; Badger, S.J.; Scantlebury, J.D.; Pokhmurskii, V.I. Chromate-free inhibiting pigments for coil-coated galvanised steel. J. Corros. Sci. Eng. 1999, 2, 15–18. [Google Scholar]
- Leng, A.; Streckel, H.; Stratmann, M. The delamination of polymeric coatings from steel. Part 2: First stage of delamination, effect of type and concentration of cations on delamination, chemical analysis of the interface. Corros. Sci. 1998, 41, 579–597. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pagliaro, M.; Palmisano, G. Sol-Gel for Environmentally Green Products. Sol-Gel Handb. 2015, 3, 1055–1070. [Google Scholar] [CrossRef]




| Sample I.D. | SiO2 Yield (wt.%) | Residence Time (s) | Dosage (µL/µm2) |
|---|---|---|---|
| A | 2.5 | 0.72 | 1.11 |
| B | 2.5 | 1.44 | 2.22 |
| C | 2.5 | 2.88 | 4.44 |
| X | 10 | 0.72 | 1.11 |
| Y | 10 | 1.44 | 2.22 |
| Z | 10 | 2.88 | 4.44 |
| Sample I.D. | Average Coating Thickness (µm) | Surface Roughness (Ra) (µm) |
|---|---|---|
| Uncoated PU | - | 0.75 ± 0.12 |
| A | 4.6 ± 0.3 | 0.7 ± 0.05 |
| B | 4.3 ± 0.6 | 3.69 ± 0.32 |
| C | 5.1 ± 0.7 | 3.1 ± 0.98 |
| X | 4.7 ± 0.3 | 0.72 ± 0.04 |
| Y | 5.8 ± 0.2 | 0.59 ± 0.04 |
| Z | 5.7 ± 0.2 | 0.72 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watkins, E.; Griffiths, C.M.; Richards, C.A.J.; Potts, S.-J.; Batchelor, C.; Barker, P.; Searle, J.; Jewell, E. Improving the Corrosion Performance of Organically Coated Steel Using a Sol–Gel Overcoat. Materials 2024, 17, 1075. https://doi.org/10.3390/ma17051075
Watkins E, Griffiths CM, Richards CAJ, Potts S-J, Batchelor C, Barker P, Searle J, Jewell E. Improving the Corrosion Performance of Organically Coated Steel Using a Sol–Gel Overcoat. Materials. 2024; 17(5):1075. https://doi.org/10.3390/ma17051075
Chicago/Turabian StyleWatkins, Evan, Chris M. Griffiths, Calvin A. J. Richards, Sarah-Jane Potts, Chris Batchelor, Peter Barker, Justin Searle, and Eifion Jewell. 2024. "Improving the Corrosion Performance of Organically Coated Steel Using a Sol–Gel Overcoat" Materials 17, no. 5: 1075. https://doi.org/10.3390/ma17051075







