A Preliminary Investigation on a Water- and Acetone-Based Solvolysis Recycling Process for CFRPs
Abstract
:1. Introduction
2. Experimental
2.1. Materials
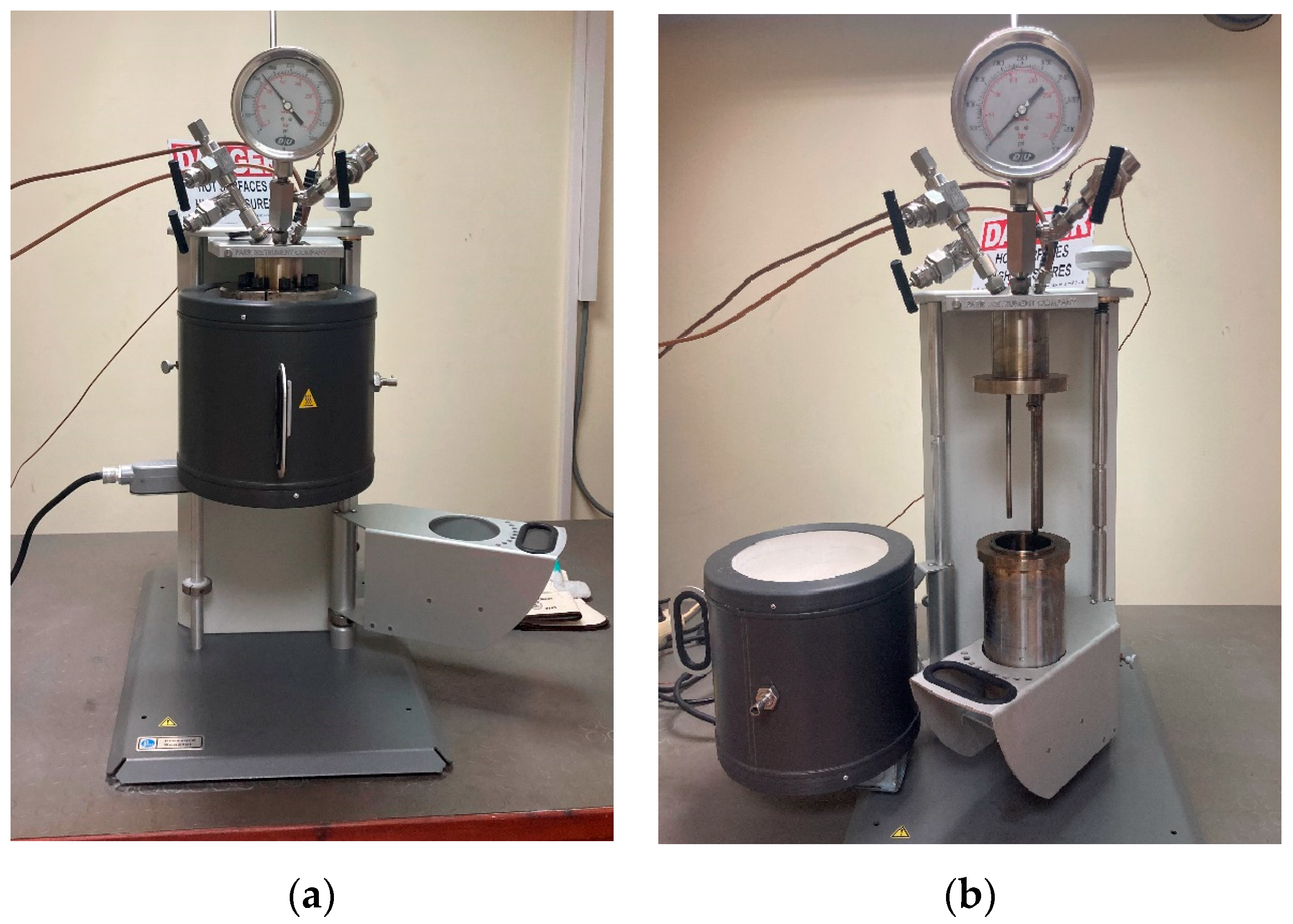
2.2. Solvolysis Tests
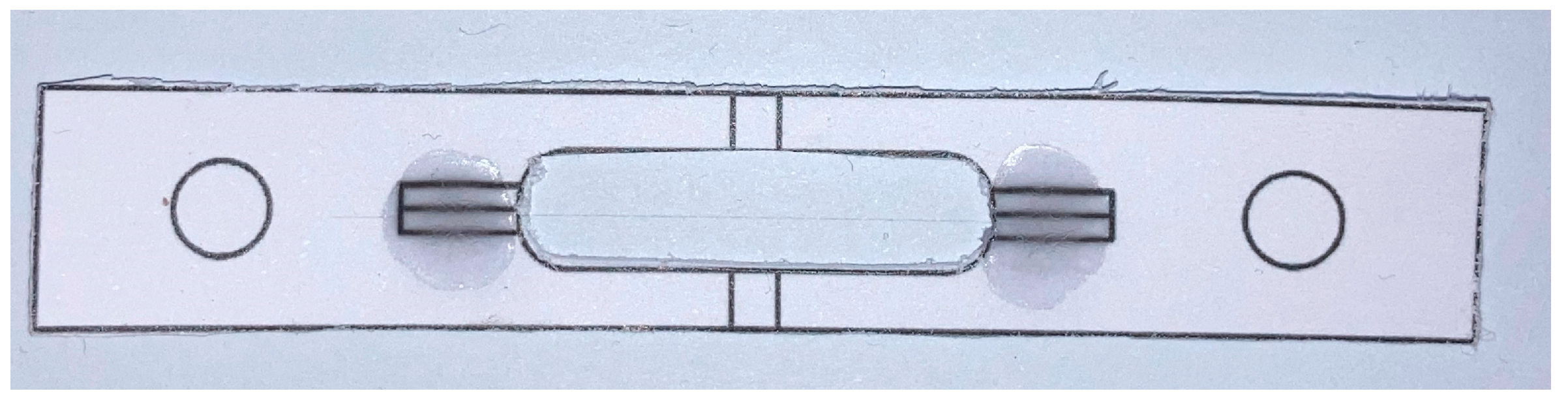
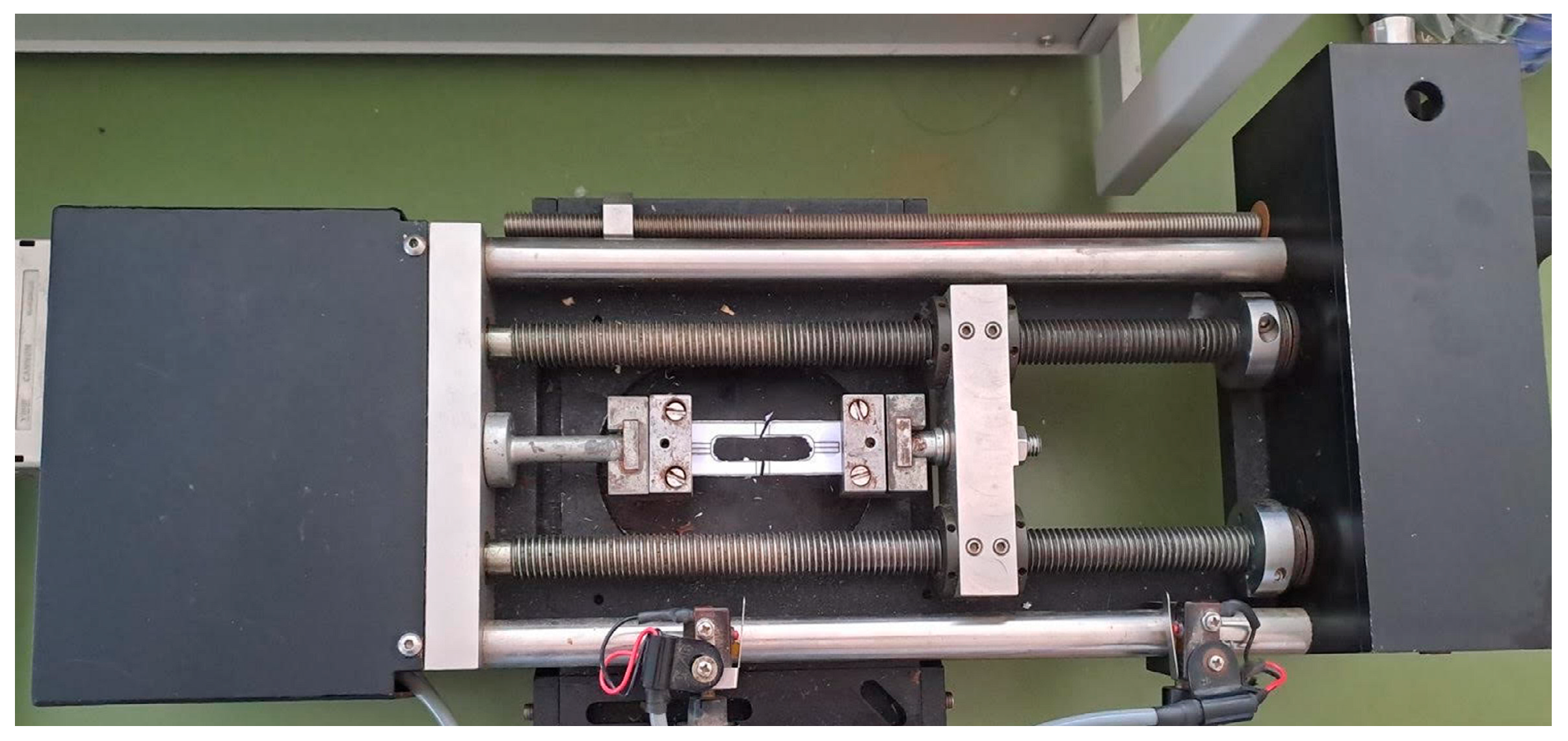
2.3. Characterization of the Recovered Fibers
3. Results and Discussion
3.1. Resin Decomposition Efficiency
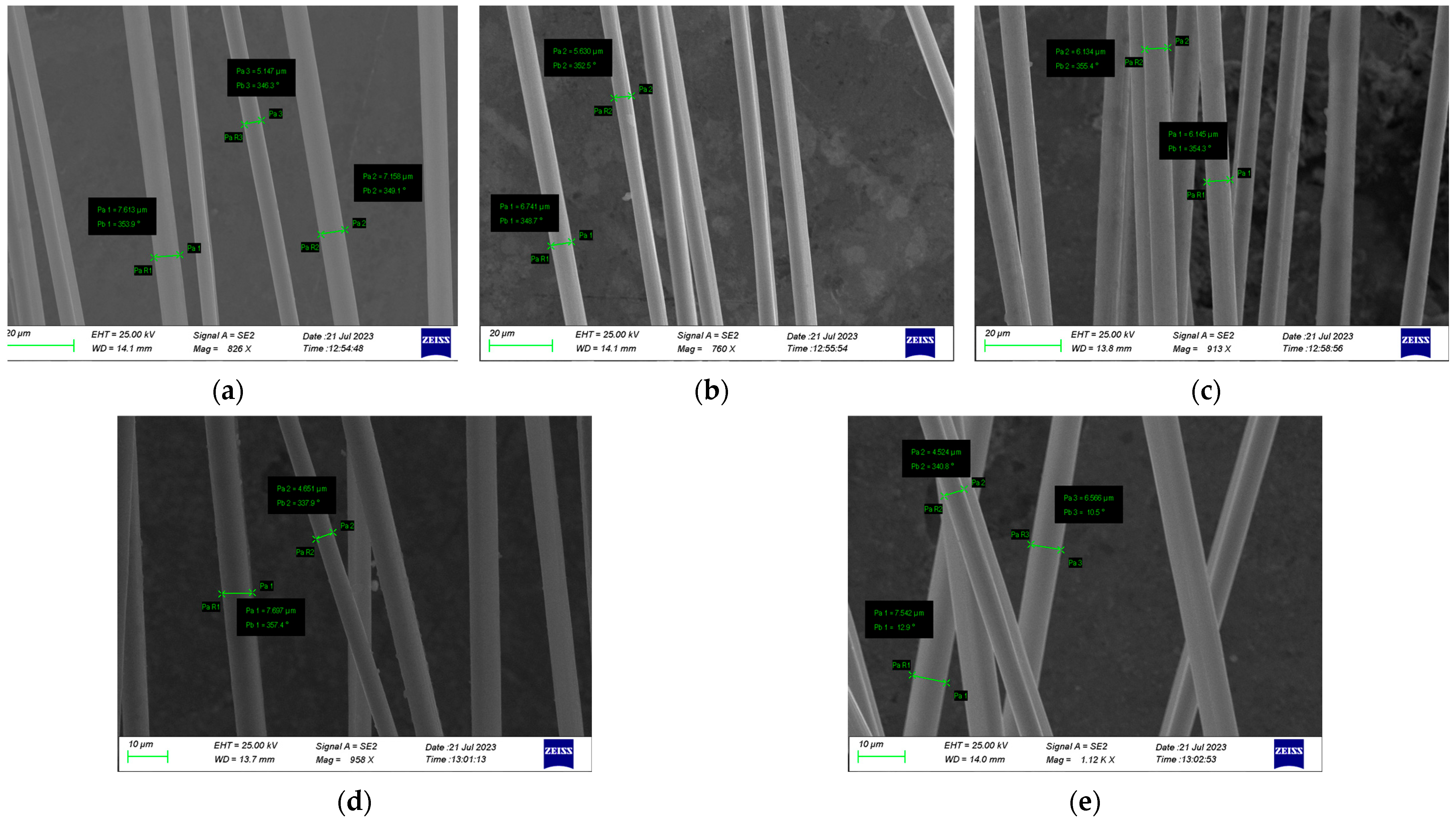
3.2. SEM Images
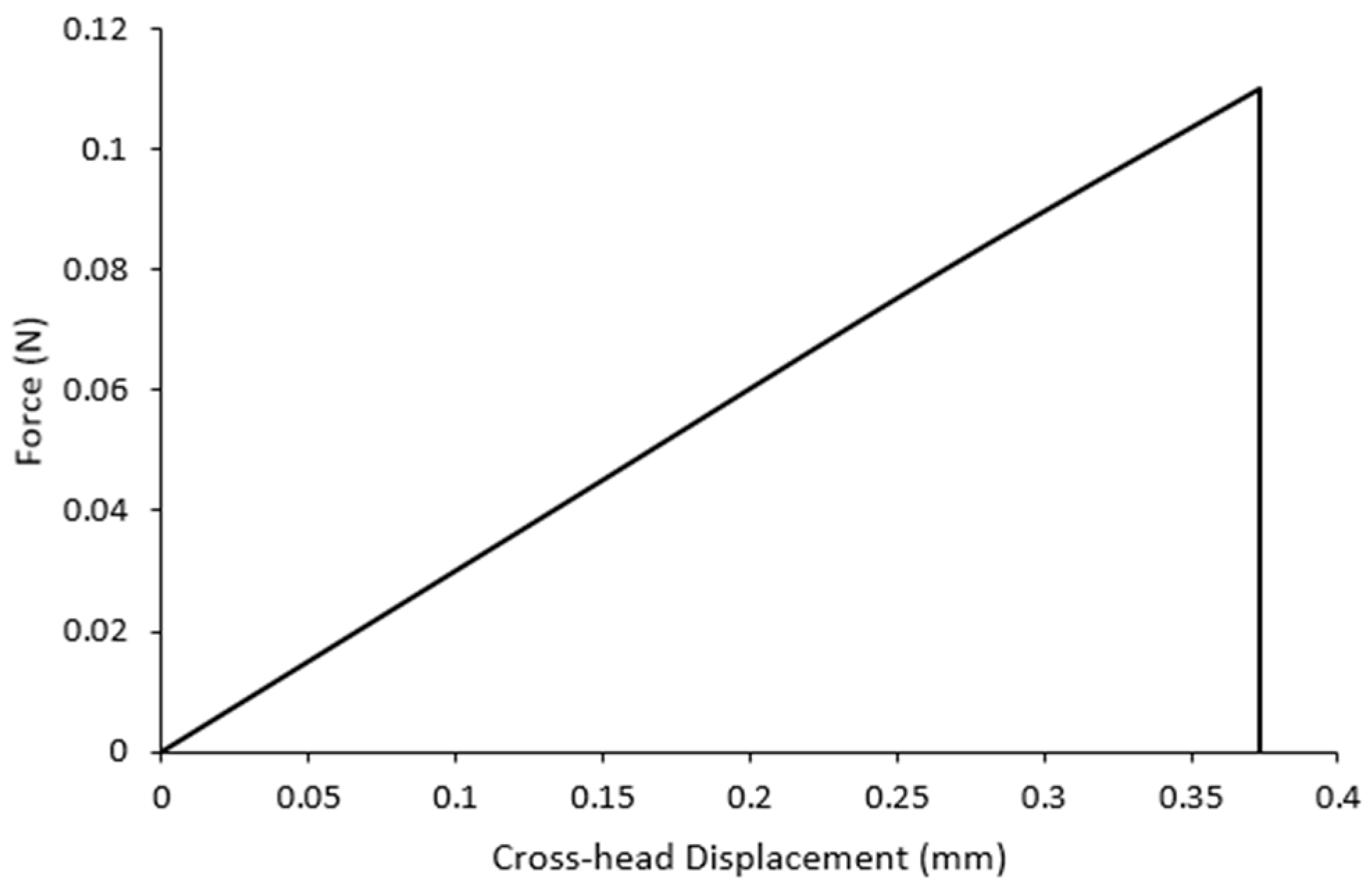
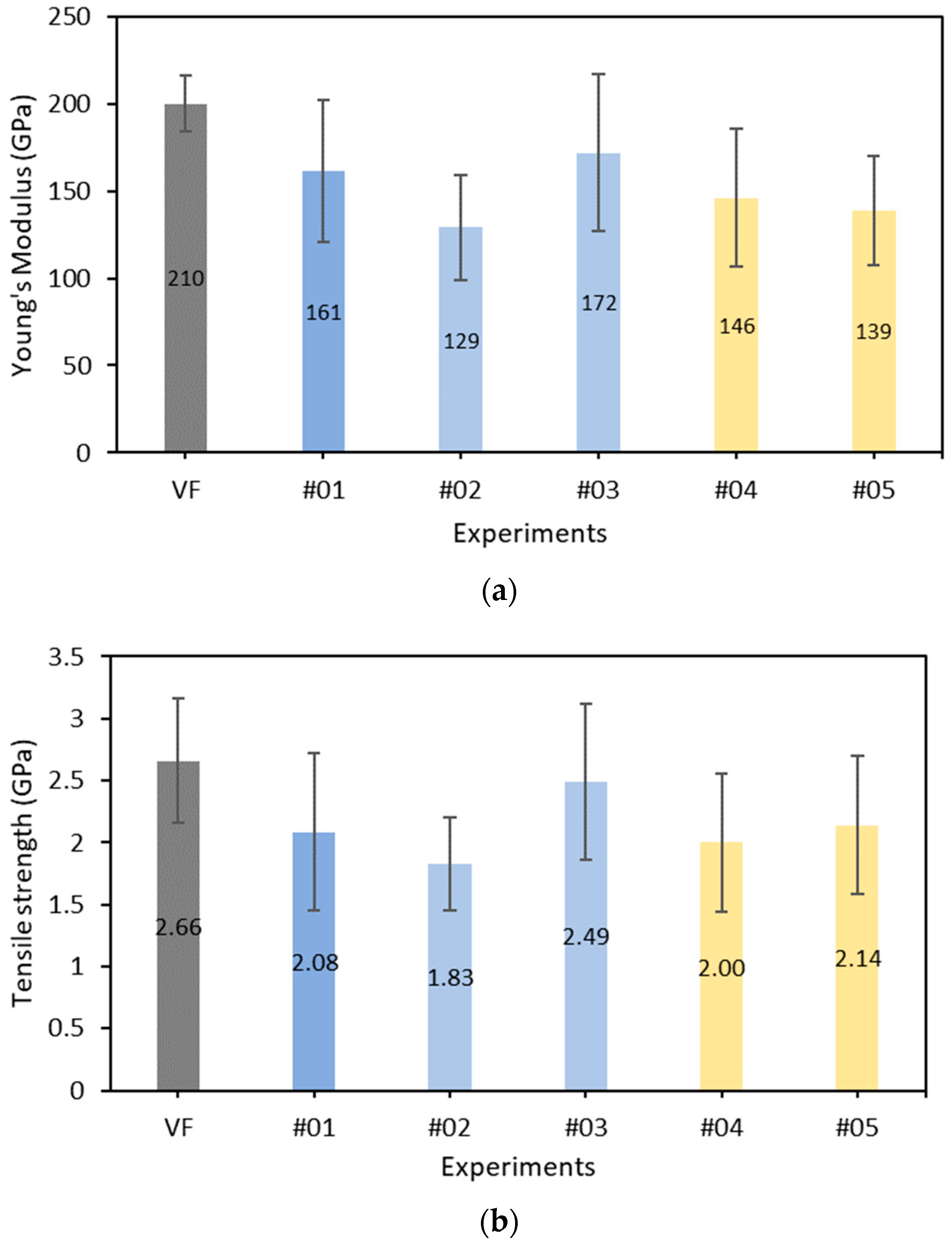
3.3. Mechanical Properties and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witten, M.E.; Sauer, M. Kühnel Market Developments, Trends, Outlook and Challenges. Available online: https://composites-united.com/media/3988/eng_ccev_market-report_2019_short-version.pdf (accessed on 13 September 2023).
- Pantelakis, S.; Tserpes, K. (Eds.) Revolutionizing Aircraft Materials and Processes; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-35345-2. [Google Scholar]
- Chen, L.; Liang, K.; Shan, Z. Experimental and Theoretical Studies on Bond Behavior between Concrete and FRP Bars with Different Surface Conditions. Compos. Struct. 2023, 309, 116721. [Google Scholar] [CrossRef]
- Shan, Z.; Liang, K.; Chen, L. Bond Behavior of Helically Wound FRP Bars with Different Surface Characteristics in Fiber-Reinforced Concrete. J. Build. Eng. 2023, 65, 105504. [Google Scholar] [CrossRef]
- Ji, X.-L.; Chen, L.-J.; Liang, K.; Pan, W.; Kai-Leung Su, R. A Review on FRP Bars and Supplementary Cementitious Materials for the next Generation of Sustainable and Durable Construction Materials. Constr. Build. Mater. 2023, 383, 131403. [Google Scholar] [CrossRef]
- Borjan, D.; Knez, Ž.; Knez, M. Recycling of Carbon Fiber-Reinforced Composites—Difficulties and Future Perspectives. Materials 2021, 14, 4191. [Google Scholar] [CrossRef]
- Vogiantzi, C.; Tserpes, K. On the Definition, Assessment, and Enhancement of Circular Economy across Various Industrial Sectors: A Literature Review and Recent Findings. Sustainability 2023, 15, 16532. [Google Scholar] [CrossRef]
- Kao, C.C.; Ghita, O.R.; Hallam, K.R.; Heard, P.J.; Evans, K.E. Mechanical Studies of Single Glass Fibres Recycled from Hydrolysis Process Using Sub-Critical Water. Compos. Part A Appl. Sci. Manuf. 2012, 43, 398–406. [Google Scholar] [CrossRef]
- Butenegro, J.A.; Bahrami, M.; Abenojar, J.; Martínez, M.Á. Recent Progress in Carbon Fiber Reinforced Polymers Recycling: A Review of Recycling Methods and Reuse of Carbon Fibers. Materials 2021, 14, 6401. [Google Scholar] [CrossRef] [PubMed]
- Pietroluongo, M.; Padovano, E.; Frache, A.; Badini, C. Mechanical Recycling of an End-of-Life Automotive Composite Component. Sustain. Mater. Technol. 2020, 23, e00143. [Google Scholar] [CrossRef]
- Nunes, A.O. Composites Renforcés à Fibres de Carbone: Récupération des Fibres par Vapo-Thermolyse, Optimisation du Procédé. Ph.D. Thesis, Ecole des Mines d’Albi-Carmaux, Albi, France, 2015. [Google Scholar]
- Rabe, D.; Häntzsche, E.; Cherif, C. Recycling of Carbon Fibres and Subsequent Upcycling for the Production of 3D-CFRP Parts. Materials 2022, 15, 5052. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Varughese, S. A Novel Sonochemical Approach for Enhanced Recovery of Carbon Fiber from CFRP Waste Using Mild Acid–Peroxide Mixture. ACS Sustain. Chem. Eng. 2016, 4, 2080–2087. [Google Scholar] [CrossRef]
- Yang, Y.; Boom, R.; Irion, B.; Van Heerden, D.-J.; Kuiper, P.; De Wit, H. Recycling of Composite Materials. Chem. Eng. Process. Process Intensif. 2012, 51, 53–68. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.O.; Leeke, G.A. Current Status of Recycling of Fibre Reinforced Polymers: Review of Technologies, Reuse and Resulting Properties. Prog. Mater. Sci. 2015, 72, 61–99. [Google Scholar] [CrossRef]
- van Oudheusden, A.A. Recycling of Composite Materials. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2019. Available online: http://resolver.tudelft.nl/uuid:0749ed5c-7aeb-4275-abee-0f904a08ea4d (accessed on 20 January 2024).
- Knight, C.C.; Zeng, C.; Zhang, C.; Wang, B. Recycling of Woven Carbon-Fibre-Reinforced Polymer Composites Using Supercritical Water. Environ. Technol. 2012, 33, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Yuyan, L.; Guohua, S.; Linghui, M. Recycling of Carbon Fibre Reinforced Composites Using Water in Subcritical Conditions. Mater. Sci. Eng. A 2009, 520, 179–183. [Google Scholar] [CrossRef]
- Okajima, I.; Sako, T. Recycling Fiber-Reinforced Plastic Using Supercritical Acetone. Polym. Degrad. Stab. 2019, 163, 1–6. [Google Scholar] [CrossRef]
- Kim, Y.N.; Kim, Y.-O.; Kim, S.Y.; Park, M.; Yang, B.; Kim, J.; Jung, Y.C. Application of Supercritical Water for Green Recycling of Epoxy-Based Carbon Fiber Reinforced Plastic. Compos. Sci. Technol. 2019, 173, 66–72. [Google Scholar] [CrossRef]
- Sokoli, H.U.; Beauson, J.; Simonsen, M.E.; Fraisse, A.; Brøndsted, P.; Søgaard, E.G. Optimized Process for Recovery of Glass- and Carbon Fibers with Retained Mechanical Properties by Means of near- and Supercritical Fluids. J. Supercrit. Fluids 2017, 124, 80–89. [Google Scholar] [CrossRef]
- Okajima, I.; Watanabe, K.; Haramiishi, S.; Nakamura, M.; Shimamura, Y.; Sako, T. Recycling of Carbon Fiber Reinforced Plastic Containing Amine-Cured Epoxy Resin Using Supercritical and Subcritical Fluids. J. Supercrit. Fluids 2017, 119, 44–51. [Google Scholar] [CrossRef]
- Yildirir, E.; Onwudili, J.A.; Williams, P.T. Recovery of Carbon Fibres and Production of High Quality Fuel Gas from the Chemical Recycling of Carbon Fibre Reinforced Plastic Wastes. J. Supercrit. Fluids 2014, 92, 107–114. [Google Scholar] [CrossRef]
- C28 Committee. Test Method for Tensile Strength and Youngs Modulus of Fibers; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- Yang, P.; Zhou, Q.; Li, X.-Y.; Yang, K.-K.; Wang, Y.-Z. Chemical Recycling of Fiber-Reinforced Epoxy Resin Using a Polyethylene Glycol/NaOH System. J. Reinf. Plast. Compos. 2014, 33, 2106–2114. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Ding, J. Chemical Recycling of Carbon Fibre/Epoxy Composites in a Mixed Solution of Peroxide Hydrogen and N,N-Dimethylformamide. Compos. Sci. Technol. 2013, 82, 54–59. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Jiang, Z.; Tang, T. Chemical Recycling of Carbon Fibre Reinforced Epoxy Resin Composites in Subcritical Water: Synergistic Effect of Phenol and KOH on the Decomposition Efficiency. Polym. Degrad. Stab. 2012, 97, 214–220. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, Y.; Hou, X. Review of Chemical Recycling and Reuse of Carbon Fiber Reinforced Epoxy Resin Composites. New Carbon Mater. 2022, 37, 1021–1041. [Google Scholar] [CrossRef]
- Cai, G.; Yin, G.; Wada, M.; Kitaoka, S.; Wei, H.; Ohsawa, I.; Takahashi, J. Influence of recycling process on the tensile property of carbon fiber. In Proceedings of the 21st International Conference on Composite Materials, Xi’an, China, 20–25 August 2017. [Google Scholar]
- Jiang, G.; Pickering, S.; Lester, E.; Turner, T.; Wong, K.; Warrior, N. Characterisation of Carbon Fibres Recycled from Carbon Fibre/Epoxy Resin Composites Using Supercritical n-Propanol. Compos. Sci. Technol. 2009, 69, 192–198. [Google Scholar] [CrossRef]

| Experiment | Solvent | Temperature (°C) | Pressure (bar) | Reaction Time (min) |
|---|---|---|---|---|
| #01 | Water | 320 | 115 | 90 |
| #02 | Water | 380 | 270 | 15 |
| #03 | Water | 400 | 300 | 5 |
| #04 | Acetone | 300 | 85 | 60 |
| #05 | Acetone | 350 | 230 | 40 |
| Experiment | Wc (g) | Wsr (g) | RDF (%) |
|---|---|---|---|
| #01 | 10.72 | 7.48 | 91.58 |
| #02 | 11.45 | 7.83 | 95.81 |
| #03 | 11.05 | 7.41 | 99.82 |
| #04 | 10.23 | 8.01 | 65.76 |
| #05 | 10.62 | 7.22 | 97.01 |
| Carbon Fibers | Average Diameter Values (μm) |
|---|---|
| Virgin CF | 7.00 |
| Experiment #01 | 6.65 |
| Experiment #02 | 6.88 |
| Experiment #03 | 6.19 |
| Experiment #04 | 6.90 |
| Experiment #05 | 6.22 |
| Experiment | Mean Maximum Load (N) | Displacement at Failure (mm) |
|---|---|---|
| #01 | 0.072 | 0.34 |
| #02 | 0.068 | 0.37 |
| #03 | 0.075 | 0.38 |
| #04 | 0.075 | 0.36 |
| #05 | 0.065 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogiantzi, C.; Tserpes, K. A Preliminary Investigation on a Water- and Acetone-Based Solvolysis Recycling Process for CFRPs. Materials 2024, 17, 1102. https://doi.org/10.3390/ma17051102
Vogiantzi C, Tserpes K. A Preliminary Investigation on a Water- and Acetone-Based Solvolysis Recycling Process for CFRPs. Materials. 2024; 17(5):1102. https://doi.org/10.3390/ma17051102
Chicago/Turabian StyleVogiantzi, Christina, and Konstantinos Tserpes. 2024. "A Preliminary Investigation on a Water- and Acetone-Based Solvolysis Recycling Process for CFRPs" Materials 17, no. 5: 1102. https://doi.org/10.3390/ma17051102





