Foaming and Physico-Mechanical Properties of Geopolymer Pastes Manufactured from Post-Metallurgical Recycled Slag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Properties of Post-Metallurgical Recycled Slag
2.2. Preparation of Foamed Geopolymer
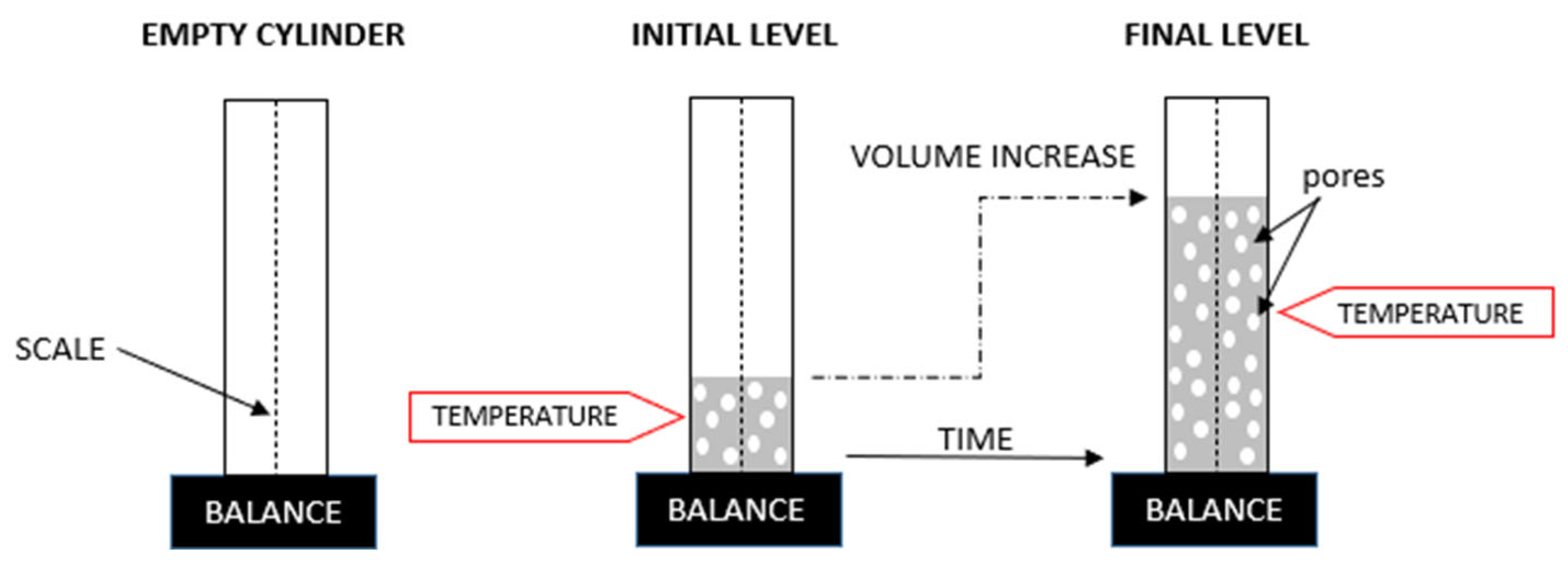
2.3. Methods of Testing and Analysis
- Total porosity ;
- The specific surface area of pores ;
- Number of pores per 1 cm2 n
3. Results and Analysis
3.1. Properties of Foamed Geopolymer Pastes
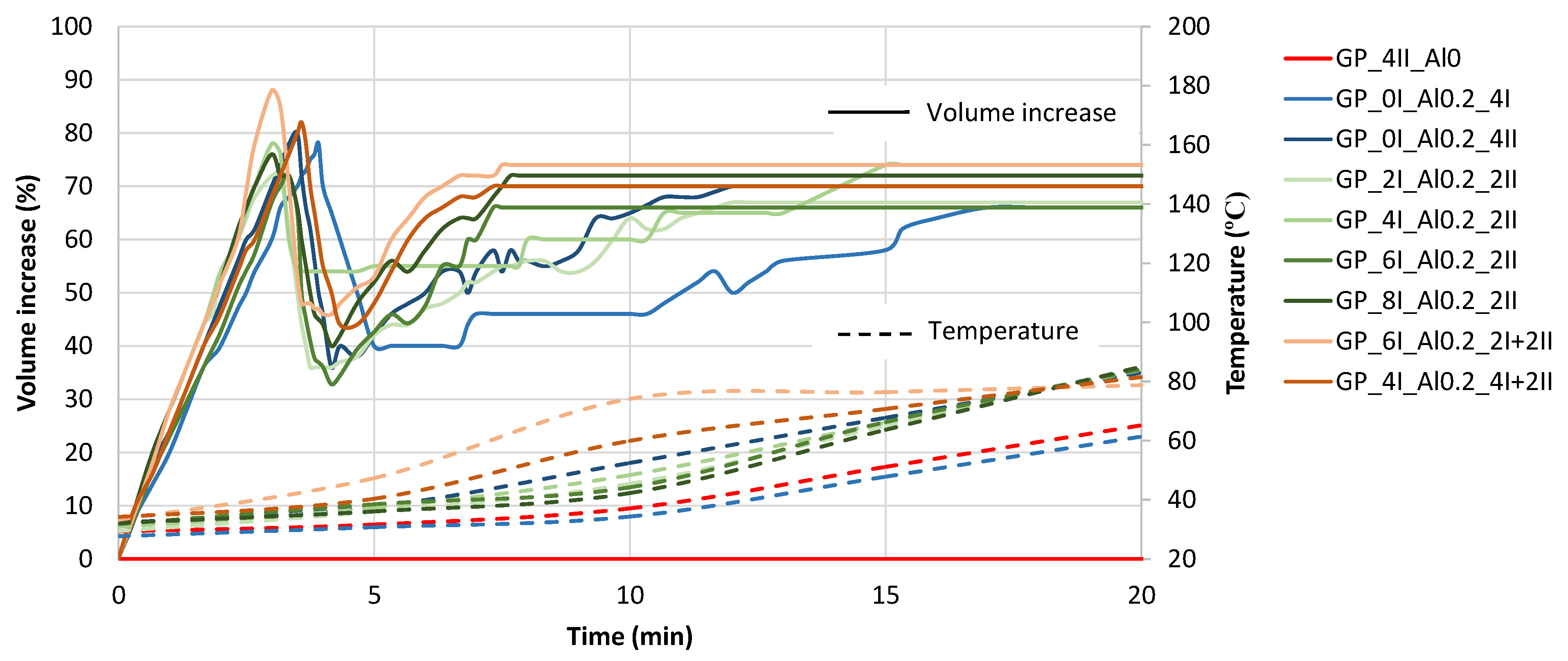
3.1.1. Initial Volume and Temperature Development of Fresh Paste
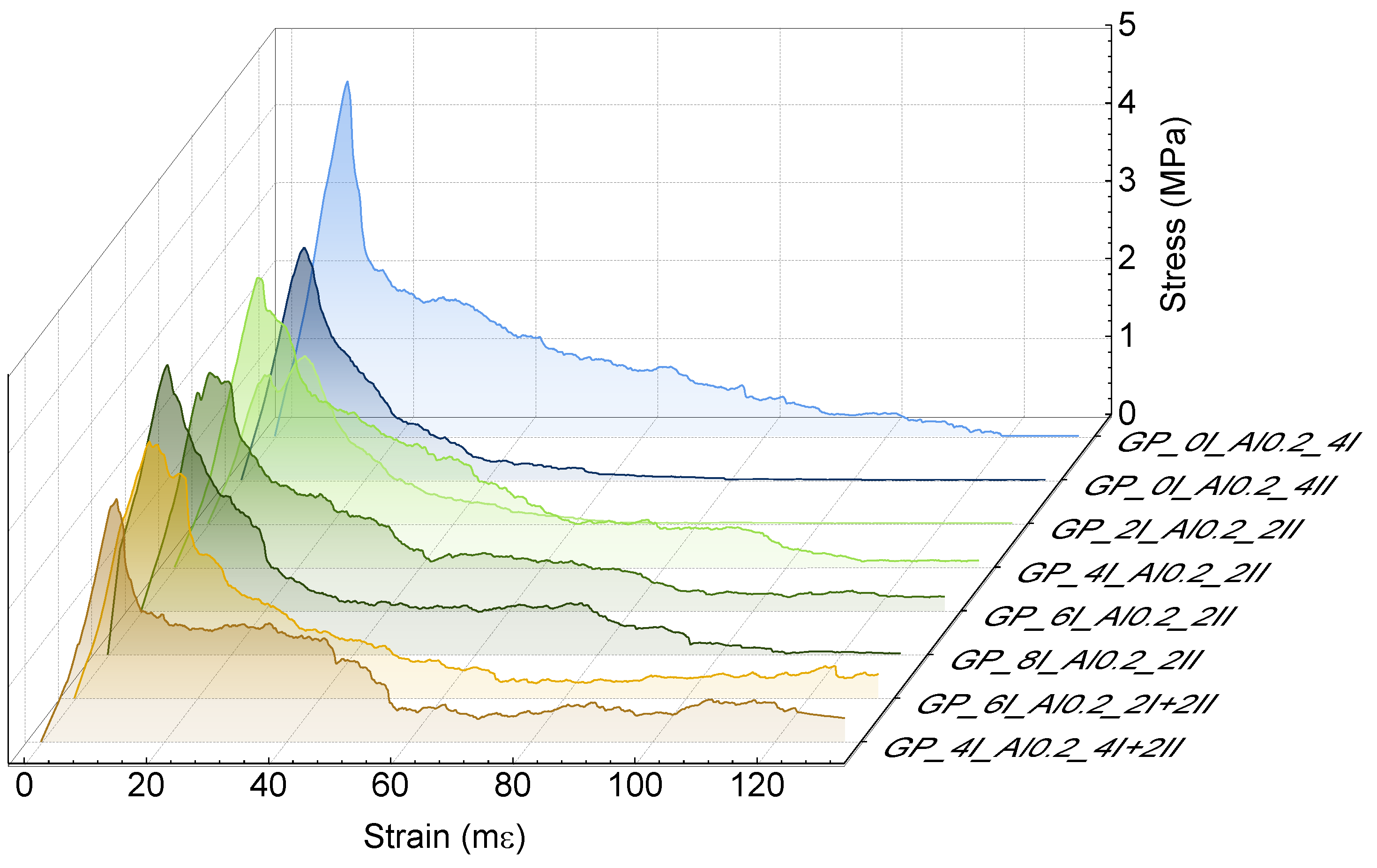
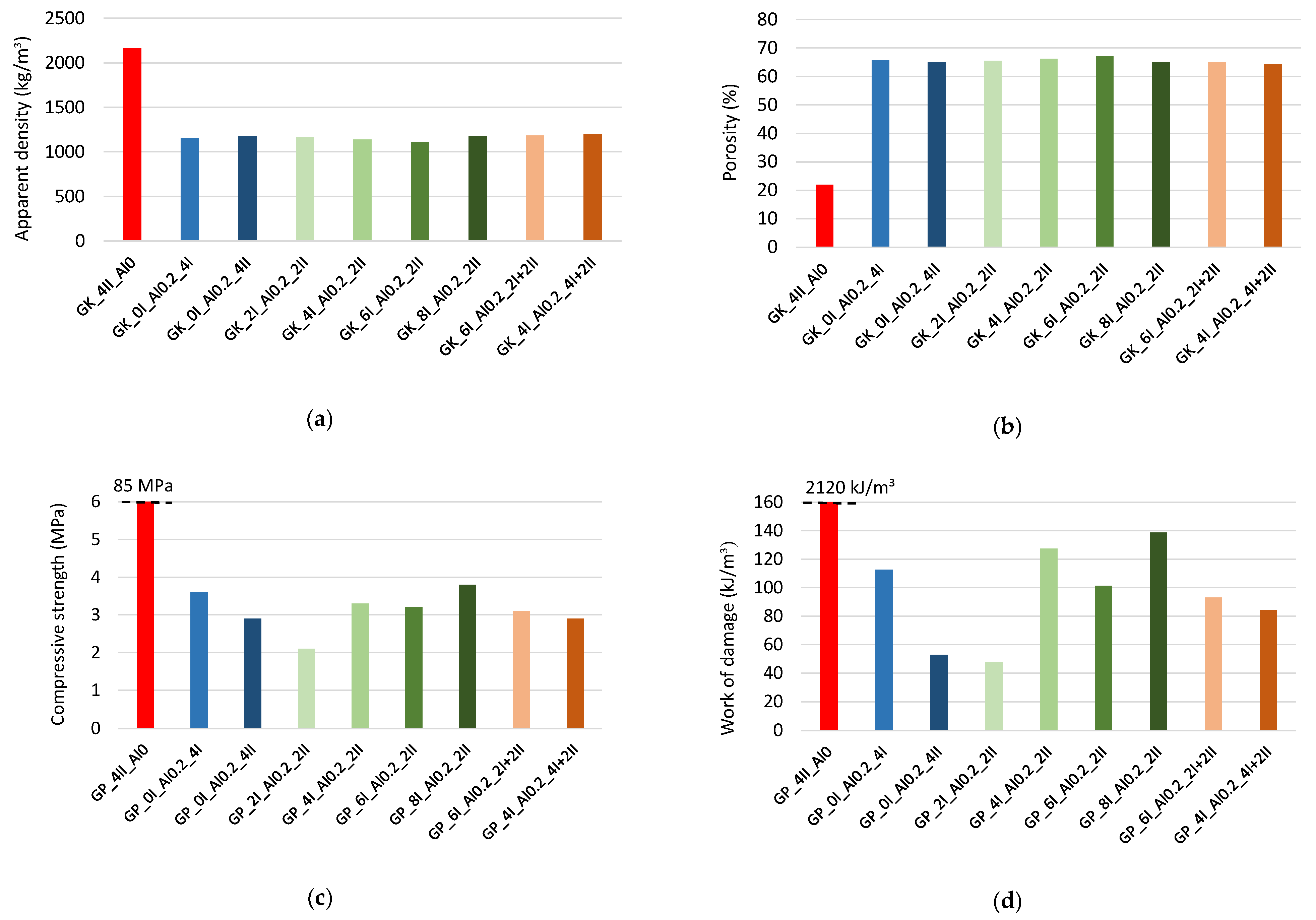
3.1.2. Physical and Mechanical Properties of Hardened Pastes
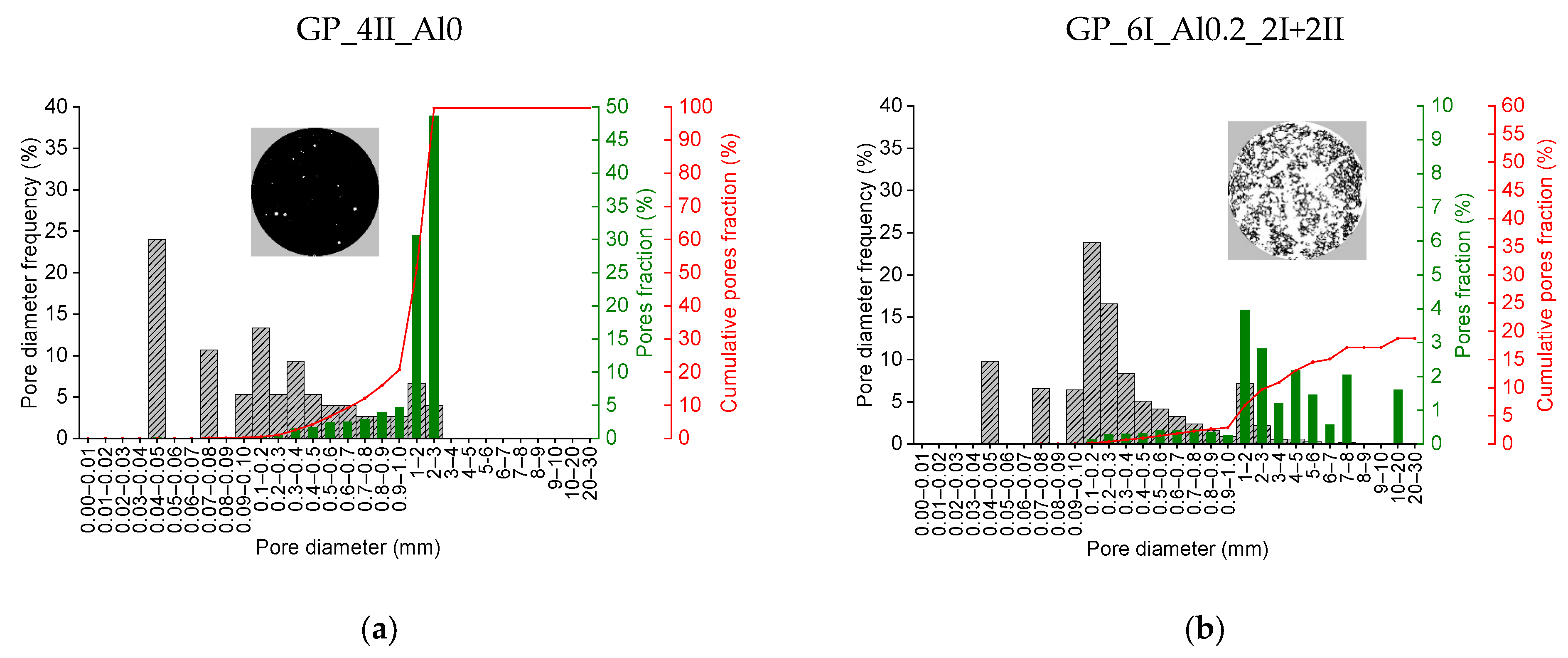
3.1.3. Detailed Analysis of Porous Structure in Hardened Geopolymer Pastes
4. Discussion
5. Conclusions
- The tested post-metallurgical recycled slag as a precursor when reacting with the activator causes the release of a large amount of energy (exothermic reaction); therefore, it is possible to induce the geopolymerization process under ambient conditions.
- The presence of aluminum powder in the geopolymer mixture causes an additional increase in its temperature, which affects the kinetics of the setting and hardening of the geopolymer composite.
- When the geopolymer paste is mixed at a higher rate, the temperature of the mixture increases as a result of the internal friction of the components, which further promotes the activation of the foaming agent additive, i.e., the aluminum powder. This is shown by comparing the test results of the GP_0I_Al0.2_4I (140 rpm) and GP_0I_Al0.2_4II (285 rpm) mixtures. As a result, the temperature of the mixture increases more quickly. No effect of the initial mixing time (140 rpm) before adding the foaming agent on the final temperature of the geopolymer mixture is observed. However, when aluminum powder is added to a mixture, increasing the mixing time usually leads to faster temperature increase during the foaming process. This was confirmed by comparing the test results of GP_2I_Al0.2_2II (2 min of mixing), GP_0I_Al0.2_4II (4 min of mixing) and GP_4I_Al0.2_4I+2II (6 min of mixing).
- Increasing the mixing time after the addition of aluminum powder and thus the greater increase in the mix temperature while foaming causes the recovery of the volume after the collapse effect to occur earlier (about 7 min), and the structure of the composite stabilizes and maintains the highest level of foaming at about 70%. In the future, it will be necessary to optimize the amount of aluminum powder added to improve the efficiency of producing a porous material structure and to reduce the adverse effects that cause the material structure to collapse in the early stages of skeleton formation. The possibility of incorporating a surfactant into the mix should be considered, as this type of additive can have a stabilizing effect on the resulting porous structure.
- The GP_6I_Al0.2_2I+2II mix showed the highest level of volume gain, at around 90%. In addition, it showed a comparable level of collapse to the other mixes, achieving the most favorable volume recovery. Therefore, this mixing procedure seems to be the most promising for further studies related to the participation of a foaming agent and the presence of stabilizers in the form of surfactants to reduce the collapse problem.
- The characterization of the pore distribution of foamed building materials, featuring a high porosity of several tens of percent and individual pore sizes expressed in µm or mm, is practically impossible with known methods for building materials, such as mercury intrusion porosimetry, MIP. Therefore, the method presented above for the stereological measurement of porosity and its distribution in relation to pore diameter appears to be a very promising method. The use of the ImageJ software and its algorithms for this process makes it possible not only to determine the total porosity or its distribution but also additional parameters such as the specific surface area and circularity of the pores.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Yu, J.; Nong, Y.; Yang, Y.; Zhang, H.; Tang, Y. Beyond Time: Enhancing Corrosion Resistance of Geopolymer Concrete and BFRP Bars in Seawater. Compos. Struct. 2023, 322, 117439. [Google Scholar] [CrossRef]
- Zhong, W.L.; Sun, Y.H.; Zhao, X.; Fan, L.F. Study on Synthesis and Water Stability of Geopolymer Pavement Base Material Using Waste Sludge. J. Clean. Prod. 2024, 445, 141331. [Google Scholar] [CrossRef]
- Guo, T.; Ma, F.; Shen, P.; Wang, X.; Bai, X.; An, Y.; Huang, Z.; Bai, X.; Han, P. The Setting and Hardening of Geopolymer Concrete Based on Low-Field Nuclear Magnetic Resonance and Cyclic Voltammetry Methods. Constr. Build. Mater. 2024, 418, 135471. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Abdollahnejad, Z.; Camões, A.F.; Jamshidi, M.; Ding, Y. Durability of Alkali-Activated Binders: A Clear Advantage over Portland Cement or an Unproven Issue? Constr. Build. Mater. 2012, 30, 400–405. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon Dioxide Equivalent (CO2-e) Emissions: A Comparison between Geopolymer and OPC Cement Concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Dudek, M.; Sitarz, M. Analysis of Changes in the Microstructure of Geopolymer Mortar after Exposure to High Temperatures. Materials 2020, 13, 4263. [Google Scholar] [CrossRef]
- Hager, I.; Sitarz, M.; Mróz, K. Fly-Ash Based Geopolymer Mortar for High-Temperature Application–Effect of Slag Addition. J. Clean. Prod. 2021, 316, 128168. [Google Scholar] [CrossRef]
- Moutaoukil, G.; Sobrados, I.; Alehyen, S.; Taibi, M. Understanding the Thermomechanical Behavior of Geopolymer Foams: Influence of Rate and Type of Foaming Agent and Stabilizer. Chem. Data Collect. 2024, 50, 101111. [Google Scholar] [CrossRef]
- Song, Y.; Xue, C.; Guo, W.; Bai, Y.; Shi, Y.; Zhao, Q. Foamed Geopolymer Insulation Materials: Research Progress on Insulation Performance and Durability. J. Clean. Prod. 2024, 444, 140991. [Google Scholar] [CrossRef]
- Kaddami, A.; Pitois, O. A Physical Approach towards Controlling the Microstructure of Metakaolin-Based Geopolymer Foams. Cem. Concr. Res. 2019, 124, 105807. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Hu, Y.; Li, Y.; Hu, P.; Zhao, Y. Physical and High Temperature Properties of Basalt Fiber-Reinforced Geopolymer Foam with Hollow Microspheres. Constr. Build. Mater. 2024, 411, 134698. [Google Scholar] [CrossRef]
- Yan, D.; Shi, Y.; Zhang, Y.; Wang, W.; Qian, H.; Chen, S.; Liu, Y.; Ruan, S. A Comparative Study of Porous Geopolymers Synthesized by Pre-Foaming and H2O2 Foaming Methods: Strength and Pore Structure Characteristics. Ceram. Int. 2024, in press. [Google Scholar] [CrossRef]
- Bai, C.; Colombo, P. Processing, Properties and Applications of Highly Porous Geopolymers: A Review. Ceram. Int. 2018, 44, 16103–16118. [Google Scholar] [CrossRef]
- Ding, P.; Bakalis, S.; Zhang, Z. Foamability in High Viscous Non-Newtonian Aqueous Two-Phase Systems Composed of Surfactant and Polymer. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123817. [Google Scholar] [CrossRef]
- Arellano Aguilar, R.; Burciaga Díaz, O.; Escalante García, J.I. Lightweight Concretes of Activated Metakaolin-Fly Ash Binders, with Blast Furnace Slag Aggregates. Constr. Build. Mater. 2010, 24, 1166–1175. [Google Scholar] [CrossRef]
- Novais, R.M.; Ascensão, G.; Ferreira, N.; Seabra, M.P.; Labrincha, J.A. Influence of Water and Aluminium Powder Content on the Properties of Waste-Containing Geopolymer Foams. Ceram. Int. 2018, 44, 6242–6249. [Google Scholar] [CrossRef]
- Bell, J.L.; Kriven, W.M. Preparation of Ceramic Foams from Metakaolin-Based Geopolymer Gels. In Developments in Strategic Materials: Ceramic Engineering and Science Proceedings; Wiley and Sons: Hoboken, NJ, USA, 2009; Volume 29, pp. 97–112. [Google Scholar]
- Kioupis, D.; Zisimopoulou, A.; Tsivilis, S.; Kakali, G. Development of Porous Geopolymers Foamed by Aluminum and Zinc Powders. Ceram. Int. 2021, 47, 26280–26292. [Google Scholar] [CrossRef]
- Kränzlein, E.; Pöllmann, H.; Krcmar, W. Metal Powders as Foaming Agents in Fly Ash Based Geopolymer Synthesis and Their Impact on the Structure Depending on the Na /Al Ratio. Cem. Concr. Compos. 2018, 90, 161–168. [Google Scholar] [CrossRef]
- Ariffin, N.; Abdullah, M.M.; Postawa, P.; Zamree Abd Rahim, S.; Mohd Arif Zainol, M.R.; Putra Jaya, R.; Śliwa, A.; Omar, M.F.; Wysłocki, J.J.; Błoch, K.; et al. Effect of Aluminium Powder on Kaolin-Based Geopolymer Characteristic and Removal of Cu2+. Materials 2021, 14, 814. [Google Scholar] [CrossRef] [PubMed]
- Keawpapasson, P.; Tippayasam, C.; Ruangjan, S.; Thavorniti, P.; Panyathanmaporn, T.; Fontaine, A.; Leonelli, C.; Chaysuwan, D. Metakaolin-Based Porous Geopolymer with Aluminium Powder. Key Eng. Mater. 2014, 608, 132–138. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P. How Does Aluminium Foaming Agent Impact the Geopolymer Formation Mechanism? Cem. Concr. Compos. 2017, 80, 277–286. [Google Scholar] [CrossRef]
- Alghamdi, H.; Dakhane, A.; Alum, A.; Abbaszadegan, M.; Mobasher, B.; Neithalath, N. Synthesis and Characterization of Economical, Multi-Functional Porous Ceramics Based on Abundant Aluminosilicates. Mater. Des. 2018, 152, 10–21. [Google Scholar] [CrossRef]
- Medri, V.; Papa, E.; Dedecek, J.; Jirglova, H.; Benito, P.; Vaccari, A.; Landi, E. Effect of Metallic Si Addition on Polymerization Degree of in Situ Foamed Alkali-Aluminosilicates. Ceram. Int. 2013, 39, 7657–7668. [Google Scholar] [CrossRef]
- Gualtieri, M.L.; Cavallini, A.; Romagnoli, M. Interactive Powder Mixture Concept for the Preparation of Geopolymers with Fine Porosity. J. Eur. Ceram. Soc. 2016, 36, 2641–2646. [Google Scholar] [CrossRef]
- Skvara, F.; Šulc, R.; Tišler, Z.; Skricik, P.; Šmilauer, V.; Zlámalová Cílová, Z. Preparation and Properties of Fly Ash-Based Geopolymer Foams. Ceram. Silik. 2014, 58, 188–197. [Google Scholar]
- Liu, X.; Hu, C.; Chu, L. Microstructure, Compressive Strength and Sound Insulation Property of Fly Ash-Based Geopolymeric Foams with Silica Fume as Foaming Agent. Materials 2020, 13, 3215. [Google Scholar] [CrossRef] [PubMed]
- Youmoue, M.; Fongang, R.T.; Gharzouni, A.; Kaze, C.; Kamseu, E.; Sglavo, V.; Kenfack, I.; Nait-Ali, B.; Rossignol, S. Effect of Silica and Lignocellulosic Additives on the Formation and the Distribution of Meso and Macropores in Foam Metakaolin-Based Geopolymer Filters for Dyes and Wastewater Filtration. SN Appl. Sci. 2020, 2, 642. [Google Scholar] [CrossRef]
- Papa, E.; Medri, V.; Kpogbemabou, D.; Morinière, V.; Laumonier, J.; Vaccari, A.; Rossignol, S. Porosity and Insulating Properties of Silica-Fume Based Foams. Energy Build. 2016, 131, 223–232. [Google Scholar] [CrossRef]
- Petlitckaia, S.; Poulesquen, A. Design of Lightweight Metakaolin Based Geopolymer Foamed with Hydrogen Peroxide. Ceram. Int. 2019, 45, 1322–1330. [Google Scholar] [CrossRef]
- Damjanovic, A.; Genshaw, M.A.; Bockris, J.O. Hydrogen Peroxide Formation in Oxygen Reduction at Gold Electrodes: II. Alkaline Solution. J. Electroanal. Chem. Interfacial Electrochem. 1967, 15, 173–180. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 4th ed.; Geopolymer Institute: Saint-Quentin, France, 2015; ISBN 9782951482050. [Google Scholar]
- Bai, C.; Ni, T.; Wang, Q.; Li, H.; Colombo, P. Porosity, Mechanical and Insulating Properties of Geopolymer Foams Using Vegetable Oil as the Stabilizing Agent. J. Eur. Ceram. Soc. 2018, 38, 799–805. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, X.; Bai, C.; Li, H.; Yan, J.; Wang, Y.; Wang, X.; Zhang, X.; Zheng, T.; Colombo, P. Effects of Surfactants/Stabilizing Agents on the Microstructure and Properties of Porous Geopolymers by Direct Foaming. J. Asian Ceram. Soc. 2021, 9, 412–423. [Google Scholar] [CrossRef]
- Masi, G.; Rickard, W.D.A.; Vickers, L.; Bignozzi, M.C.; van Riessen, A. A Comparison between Different Foaming Methods for the Synthesis of Light Weight Geopolymers. Ceram. Int. 2014, 40, 13891–13902. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Pacheco-Torgal, F.; Félix, T.; Tahri, W.; Barroso Aguiar, J. Mix Design, Properties and Cost Analysis of Fly Ash-Based Geopolymer Foam. Constr. Build. Mater. 2015, 80, 18–30. [Google Scholar] [CrossRef]
- Böke, N.; Birch, G.D.; Nyale, S.M.; Petrik, L.F. New Synthesis Method for the Production of Coal Fly Ash-Based Foamed Geopolymers. Constr. Build. Mater. 2015, 75, 189–199. [Google Scholar] [CrossRef]
- Bumanis, G.; Vitola, L.; Bajare, D.; Dembovska, L.; Pundiene, I. Impact of Reactive SiO2/Al2O3 Ratio in Precursor on Durability of Porous Alkali Activated Materials. Ceram. Int. 2017, 43, 5471–5477. [Google Scholar] [CrossRef]
- Sornlar, W.; Wannagon, A.; Supothina, S. Stabilized Homogeneous Porous Structure and Pore Type Effects on the Properties of Lightweight Kaolinite-Based Geopolymers. J. Build. Eng. 2021, 44, 103273. [Google Scholar] [CrossRef]
- Kočí, V.; Černý, R. Directly Foamed Geopolymers: A Review of Recent Studies. Cem. Concr. Compos. 2022, 130, 104530. [Google Scholar] [CrossRef]
- Ducman, V.; Korat, L. Characterization of Geopolymer Fly-Ash Based Foams Obtained with the Addition of Al Powder or H2O2 as Foaming Agents. Mater. Charact. 2016, 113, 207–213. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Li, X.; Tian, D.; Ma, M.; Wang, T. Optimized Pore Structure and High Permeability of Metakaolin/Fly-Ash-Based Geopolymer Foams from Al– and H2O2–Sodium Oleate Foaming Systems. Ceram. Int. 2022, 48, 18348–18360. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer Foam Concrete: An Emerging Material for Sustainable Construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Coster, M.; Chermant, J.-L. Image Analysis and Mathematical Morphology for Civil Engineering Materials. Cem. Concr. Compos. 2001, 23, 133–151. [Google Scholar] [CrossRef]
- Stubenrauch, C.; Von Klitzing, R. Disjoining Pressure in Thin Liquid Foam and Emulsion Films—New Concepts and Perspectives. J. Phys. Condens. Matter 2003, 15, R1197–R1232. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer Concrete: A Review of Some Recent Developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of Curing Temperature on the Development of Hard Structure of Metakaolin-Based Geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]



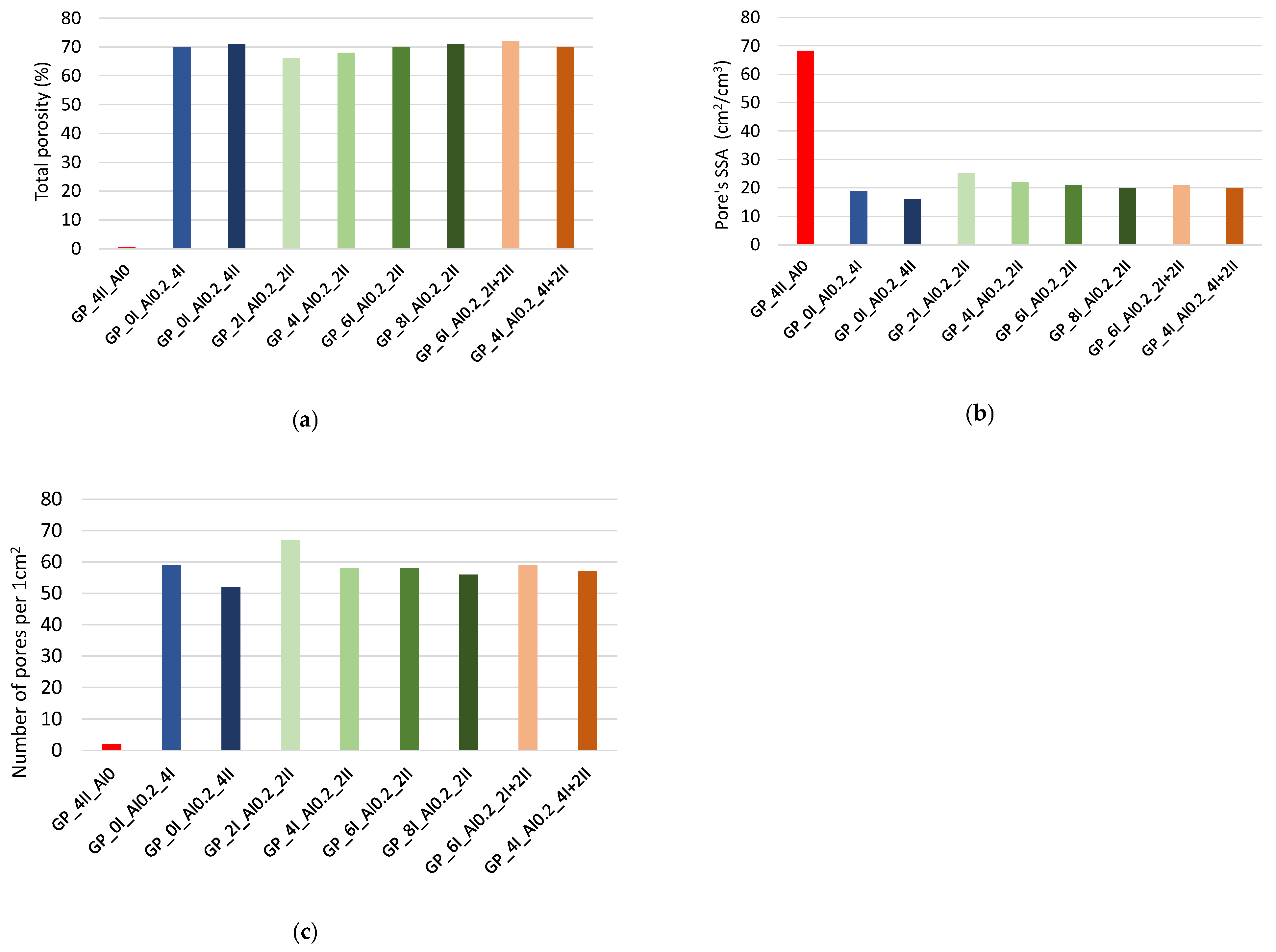
| Oxide | Content (%) | Oxide | Content (%) |
|---|---|---|---|
| Fe2O3 | 51.7 | Cr2O3 | 1.4 |
| SiO2 | 27.6 | P2O5 | 1.2 |
| Al2O3 | 6.6 | MnO | 0.82 |
| ZnO | 4.3 | MgO | 0.81 |
| CaO | 3.3 | CuO | 0.69 |
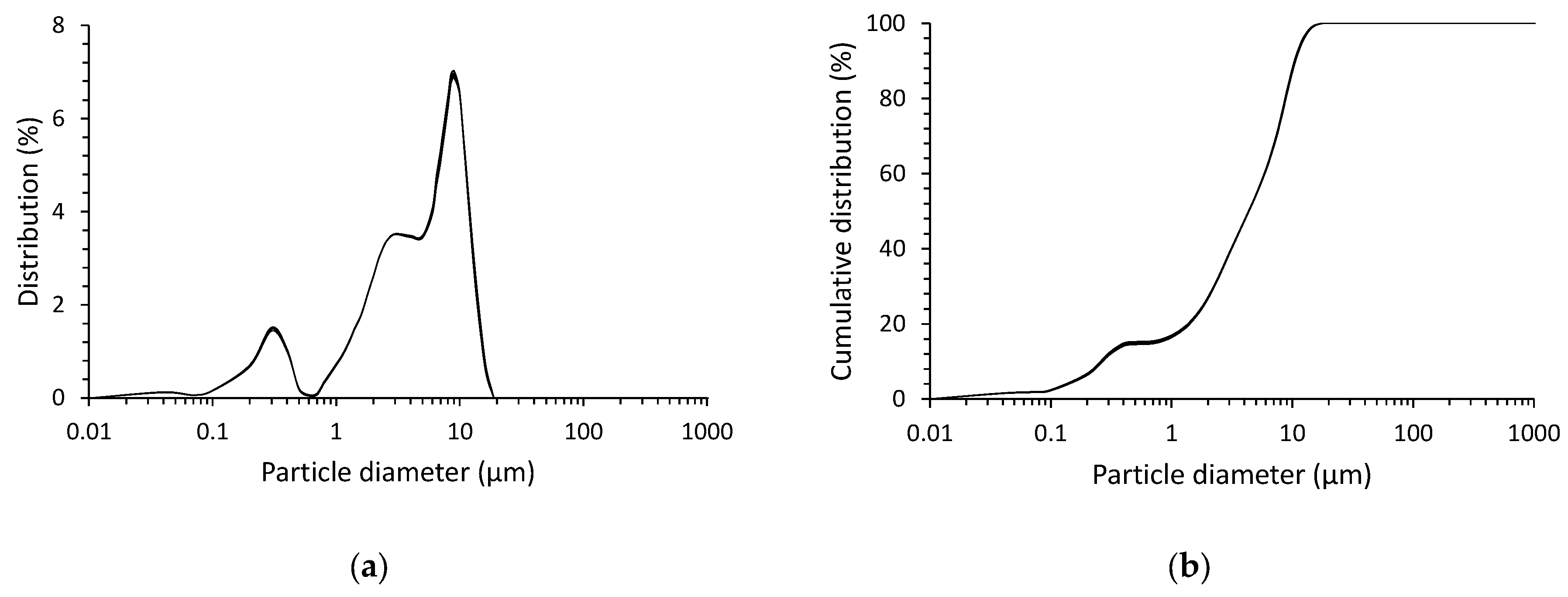
| SLAG | Mean Particle Size (μm) | d10 (μm) | d50 (μm) | d90 (μm) |
| 5.4 | 0.26 | 4.3 | 10.5 |
| Characteristic | Woellner Geosil 14517 | Unit |
|---|---|---|
| K2O content | 21.84 | wt.% |
| SiO2 content | 23.5 | wt.% |
| density | 1.512 | g/cm3 |
| viscosity | 22 | mPa·s |
| weight ratio (WR = wt.% SiO2/wt.% K2O) | 1.08 | − |
| molar ratio (MR = mol SiO2/molK2O) | 1.70 | − |
| Mix Composition | Content (kg/m3) |
|---|---|
| Slag | 1843.0 |
| Woellner Geosil 14517 (K—water glass) | 552.9 |
| Additional water | 129.0 |
| Al powder—0.2% by weight of slag | 3.7 |
| GP_6I_Al0.2_2I+2II | PARAMETER | VALUE |
|---|---|---|
 | Total porosity [%]: | 72.0 |
| Pores SSA (S/V) [cm−1]: | 15.4 | |
| Number of pores per 1 cm2: | 45 | |
| Average Feret’s diameter [mm]: | 0.50 | |
| Circularity C > 0.90 [%]: | 61.9 | |
| GP_4II_Al0 | PARAMETER | VALUE |
 | Total porosity [%]: | 0.4 |
| Pores SSA (S/V) [cm−1]: | 68.3 | |
| Number of pores per 1 cm2: | 2 | |
| Average Feret’s diameter [mm]: | 0.41 | |
| Circularity C > 0.90 [%]: | 46.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitarz, M.; Zdeb, T.; Mróz, K.; Hager, I.; Setlak, K. Foaming and Physico-Mechanical Properties of Geopolymer Pastes Manufactured from Post-Metallurgical Recycled Slag. Materials 2024, 17, 1449. https://doi.org/10.3390/ma17061449
Sitarz M, Zdeb T, Mróz K, Hager I, Setlak K. Foaming and Physico-Mechanical Properties of Geopolymer Pastes Manufactured from Post-Metallurgical Recycled Slag. Materials. 2024; 17(6):1449. https://doi.org/10.3390/ma17061449
Chicago/Turabian StyleSitarz, Mateusz, Tomasz Zdeb, Katarzyna Mróz, Izabela Hager, and Kinga Setlak. 2024. "Foaming and Physico-Mechanical Properties of Geopolymer Pastes Manufactured from Post-Metallurgical Recycled Slag" Materials 17, no. 6: 1449. https://doi.org/10.3390/ma17061449






