Volatilisation Behaviour and Mechanism of Lead-Containing Slag during Physical Property Tests
Abstract
:1. Introduction
2. Experimental Setup and Procedure
2.1. Experimental Materials
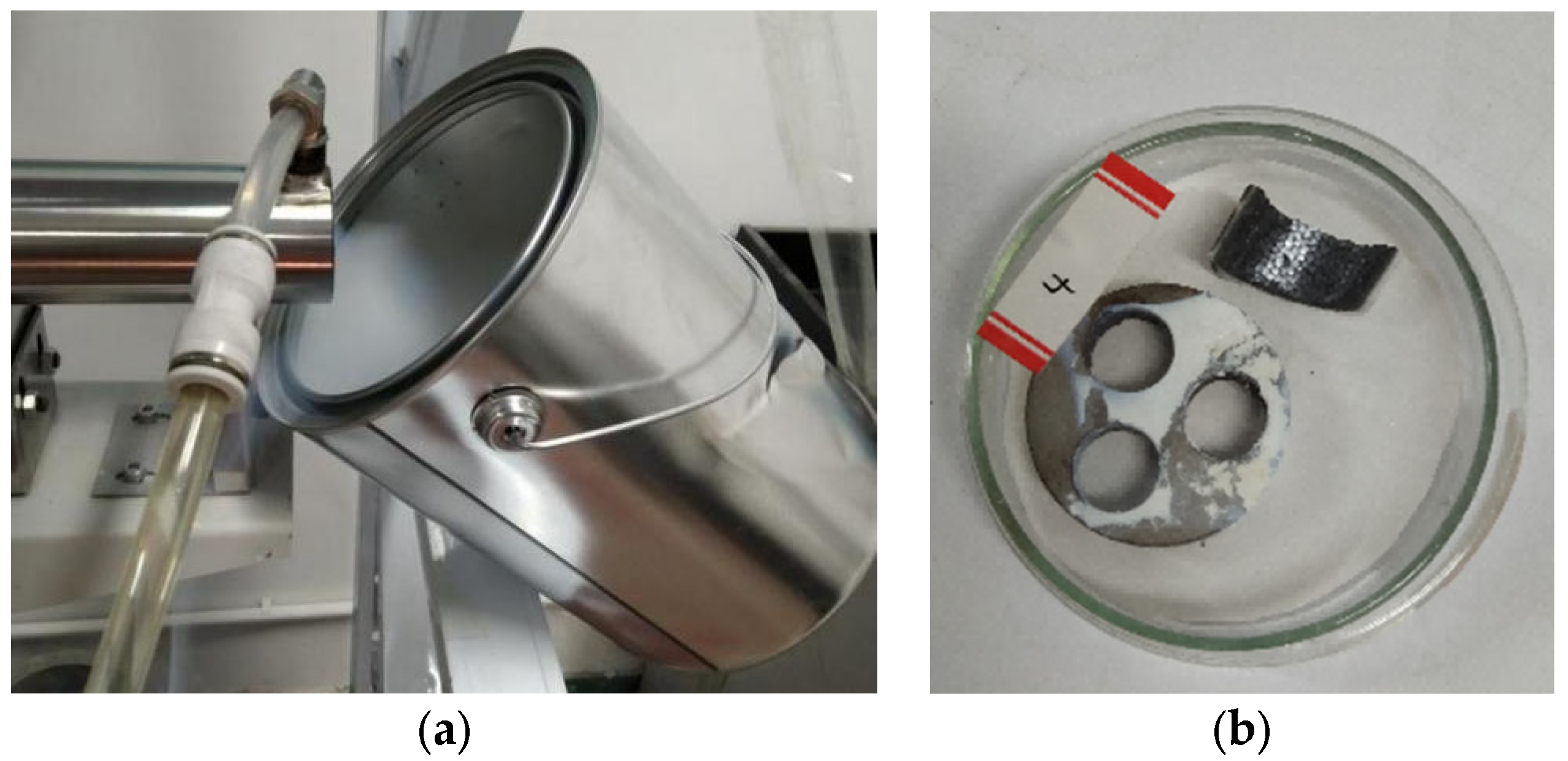
2.2. Experimental Apparatus and Procedure
3. Results and Discussion
3.1. Weight Loss of Lead Slag at High Temperatures
3.2. Tubular Furnace Experiment at High-Experiment
3.2.1. The Maximum Volatilisation Ratio of Lead Slag
3.2.2. Analysis of Volatiles
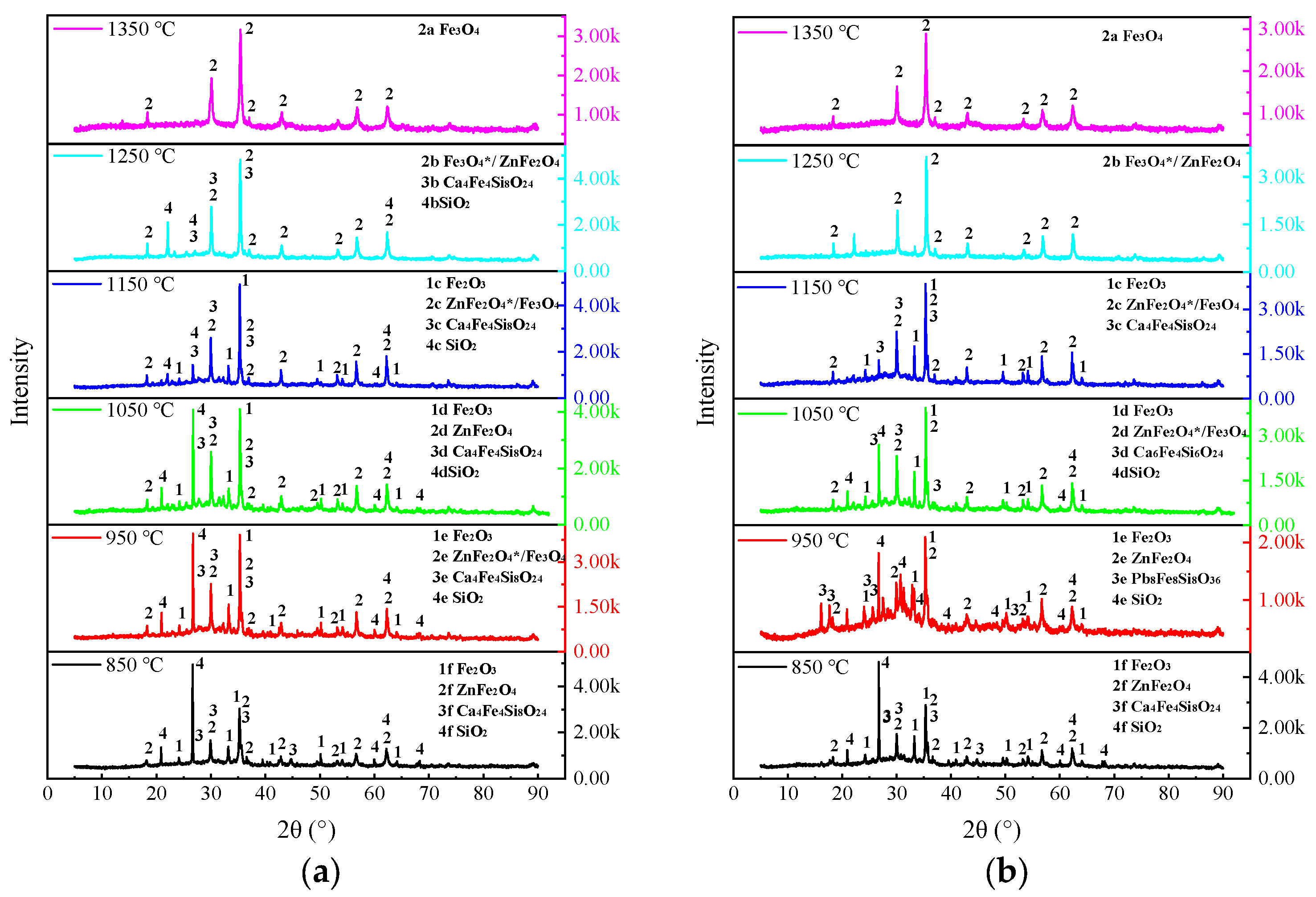
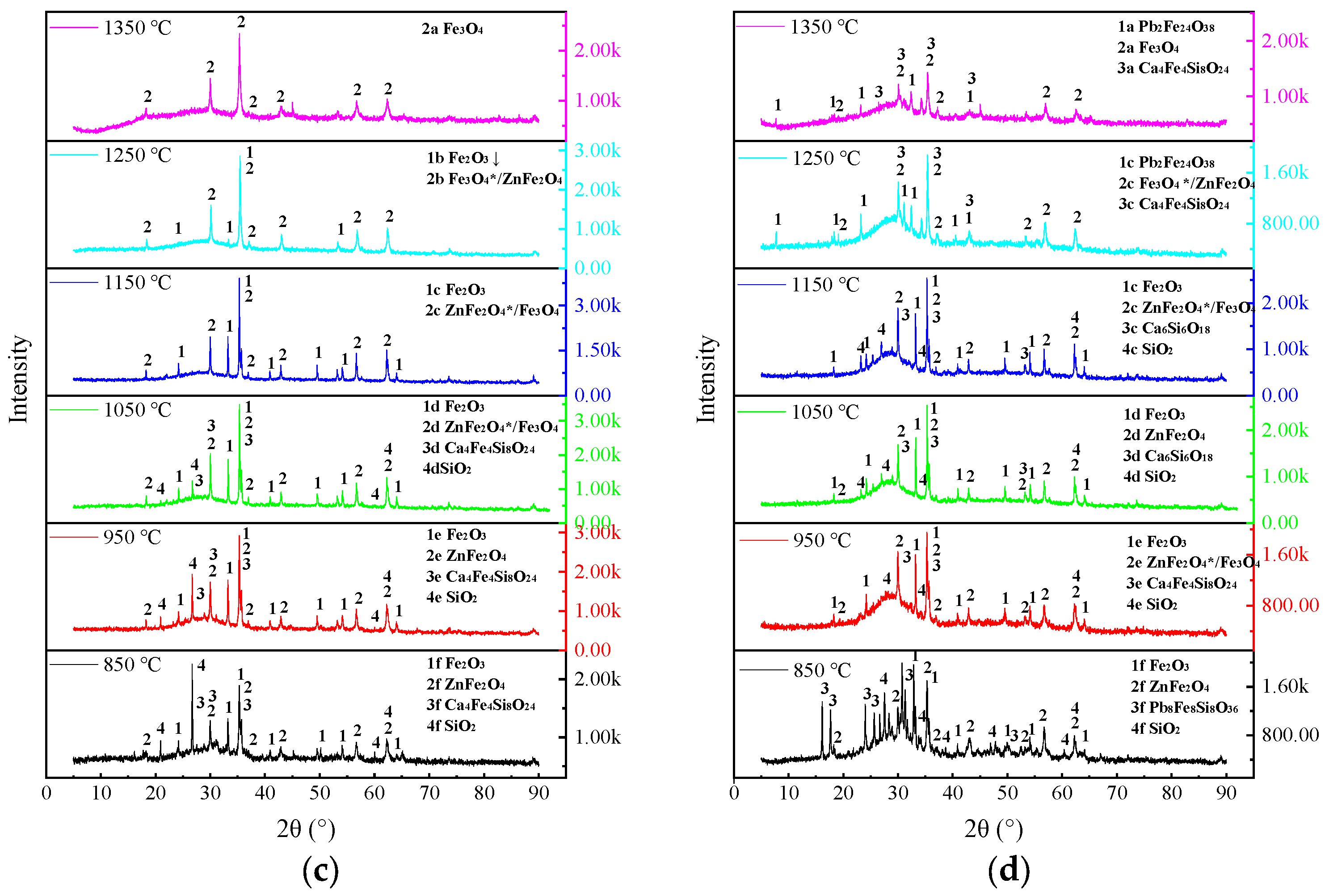
3.2.3. Analysis of the Residual Slag Physical Phases
4. Conclusions
- The weight loss the slags is at the level of about 3.68% to 10.39%. when the temperature below 720°C, the weight loss caused by CO2 and crystalline water release exists. Above 900 °C, the weight loss can be considered the volatilisation of slag compononts and increases with the PbO content increase.
- The volatile corresponding to the weight loss above 900 °C is mainly PbO (more than 90%) and less ZnO. The content of PbO in the collected volatile increases with the content of PbO in the slag. The content of ZnO in the collected volatile decreases with the content of PbO in the slag.
- The higher the temperature is, the stronger the volatilisation is. With the increase in temperature and keeping time, most of the PbO can be evaporated and leaves little PbO in the residual slag. This will has great effect to physico-chemical property measurement of the slag with higher PbO content, especially to the property measurement that be kept at high temperature for a long time.
- Volatile in-situ examination with pyrolytic time-of-flight (ISP-TOF) mass spectrometer is limited by the condensation of volatiles, i.e., PbO, Zn and so on, in the connection pipeline. The device should be modified for these use.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Şahin, M.; Erdem, M. Cleaning of high lead-bearing zinc leaching residue by recovery of lead with alkaline leaching. Hydrometallurgy 2015, 153, 170–178. [Google Scholar] [CrossRef]
- Turebekova, G.Z.; Akhmetova, S.O.; Bagova, Z.I. Ways of the lead-bearing slag waste utilization. In Proceedings of the 1st International Scientific and Practical Conference “lnnovative Technologies in Environmental Engineering and Agroecosystems”, Denver, CO, USA, 24–27 March 2021; p. 4003. [Google Scholar]
- Kicińska, A. Physical and chemical characteristics of slag produced during Pb refining and the environmental risk associated with the storage of slag. Environ. Geochem. Health 2021, 43, 2723–2741. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J.; Barron, A.R. Pyrometallurgical removal of zinc from basic oxygen steelmaking dust—A review of best available technology. Resour. Conserv. Recycl. 2020, 157, 104746. [Google Scholar] [CrossRef]
- Mustafa, S.; Luo, L.; Zheng, B.T.; Wei, C.X.; Christophe, N. Effect of Lead and Zinc Impurities in Iron-making and the Corresponding Removal Methods: A Review. Metals 2021, 11, 407. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, R.; Kasai, E.; Li, S. Vaporization behavior of lead from the FeO-CaO-SiO2-Al2O3 slag system. Trans. Nonferrous Met. Soc. China 2015, 25, 2772–2780. [Google Scholar] [CrossRef]
- Kurashima, K.; Kumagai, S.; Kameda, T.; Saito, Y.; Yoshioka, T. Heavy metal removal from municipal solid waste fly ash through chloride volatilization using poly(vinyl chloride) as chlorinating agent. J. Mater. Cycles Waste Manag. 2020, 22, 1270–1283. [Google Scholar] [CrossRef]
- Rieger, J.; Colla, V.; Matino, I.; Branca, T.A.; Stubbe, G.; Panizza, A.; Brondi, C.; Falsafi, M.; Hage, J.; Wang, X.; et al. Residue Valorization in the Iron and Steel Industries: Sustainable Solutions for a Cleaner and More Competitive Future Europe. Metals 2021, 11, 1202. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, R.; Yadav, S. Distribution, pollution levels, toxicity, and health risk assessment of metals in surface dust from Bhiwadi industrial area in North India. Hum. Ecol. Risk Assess. Int. J. 2020, 26, 2091–2111. [Google Scholar] [CrossRef]
- Wang, G.; Cui, Y.; Yang, Z.; Guo, Z.; Zhao, L.; Li, X.; Zhao, J.; Tang, W. Volatilization charac-teristics of high-lead slag and its influence on measurement of physicochemical properties at high temperature. J. Min. Metall. Sect. B Metall. 2020, 56, 59–68. [Google Scholar] [CrossRef]
- Anjos, P.D.; Quaresma, L.A.; Machado, M.L. Artificial neural networks for predicting the viscosity of lead-containing glasses. arXiv 2022, arXiv:2211.07587. [Google Scholar]
- Kim, W.-Y.; Pelton, A.D.; Decterov, S.A. Modeling the Viscosity of Silicate Melts Containing Lead Oxide. Met. Mater. Trans. B 2011, 43, 325–336. [Google Scholar] [CrossRef]
- Slobodkin, L.V.; Sannikov, Y.A.; Grinin, Y.A.; Lyamina, M.A.; Shumskij, V.A.; Ushakov, N.N. The Kivcet Treatment of Polymetallic Feeds; Lead-Zinc 2000; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 687–692. [Google Scholar]
- Chen, L.; Yang, T.; Bin, S.; Liu, W.; Zhang, D.; Bin, W.; Zhang, L. An efficient reactor for high-lead slag reduction process: Oxygen-rich side blow furnace. JOM J. Miner. Met. Mater. Soc. 2014, 66, 1664–1669. [Google Scholar] [CrossRef]
- Perez-Labra, M.; Romero-Serrano, A.; Hernandez-Ramirez, A.; Almaguer-Guzman, I.; Benavides-Perez, R. Effect of CaO/SiO2 and Fe/SiO2 ratios on phase equilibria in PbO-ZnO-CaO-SiO2-“Fe2O3” system in air. Trans. Nonferrous Met. Soc. China 2012, 22, 665–674. [Google Scholar] [CrossRef]
- Wang, S.J.; He, P.J.; Shao, L.M.; Zhang, H. Multifunctional effect of Al2O3, SiO2 and CaO on the volatilization of PbO and PbCl2 during waste thermal treatment. Chemosphere 2016, 161, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Zhang, Y.; Li, S. Thermodynamic and experimental research of the vaporization of zinc and lead in CaO-SiO2-FeO-Cl system. J. Wuhan Univ. Sci. Technol. (Nat. Sci. Ed.) 2010, 33, 14–19. [Google Scholar]
- Zhang, Y.; Shibata, E.; Kasai, E. Vapor Pressure of Zinc and Zinc Chloride in the FetO-CaO-SiO2-Al2O3 Slag System. Mater. Trans. 2006, 47, 1341–1346. [Google Scholar] [CrossRef]
- Nakada, H.; Mihara, N.; Kawaguchi, Y.; Osada, S.; Kuchar, D.; Matsuda, H. Volatilization behavior of lead from molten slag under conditions simulating municipal solid waste melting. J. Mater. Cycles Waste Manag. 2008, 10, 19–23. [Google Scholar] [CrossRef]
- Mijatović, N.; Terzić, A.; Miličić, L.; Živojinović, D. Immobilization of heavy metal ions Zn2+, Ni2+, Pb2+ and Cu2+ in the structure of cement-based materials. Zast. Mater. 2020, 61, 116–127. [Google Scholar] [CrossRef]
- Simoni, M.; Reiter, W.; Suer, J.; Di Sante, L.; Cirilli, F.; Praolini, F.; Mosconi, M.; Guzzon, M.; Malfa, E.; Algermissen, D.; et al. Towards the Circularity of the EU Steel Industry: Modern Technologies for the Recycling of the Dusts and Recovery of Resources. Metals 2024, 14, 233. [Google Scholar] [CrossRef]
- Pan, D.; Li, L.; Tian, X.; Wu, Y.; Cheng, N.; Yu, H. A review on lead slag generation, characteristics, and utilization. Resour. Conserv. Recycl. 2019, 146, 140–155. [Google Scholar] [CrossRef]
- Nowińska, K.; Adamczyk, Z. Zinc and Lead Metallurgical Slags as a Potential Source of Metal Recovery: A Review. Materials 2023, 16, 7295. [Google Scholar] [CrossRef] [PubMed]
- Khasraw, D.; Yan, Z.; Hage, J.L.; Meijer, K.; Li, Z. Reduction of FeO in Molten Slag by Solid Car-bonaceous Materials for HIsarna Alternative Ironmaking Process. Metall. Mater. Trans. B Process Met. Lurgy Mater. Process. Sci. 2022, 53, 3246–3261. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Zhao, Z.-W.; Yang, W.; Luo, Y.-G. Study on oxygen side-blown reduction smelting of liquid lead-rich slag. Technol. Dev. Enterp. 2013, 32, 16. [Google Scholar]
- Chen, L.; Yang, T.-Z.; Liu, W.-F.; Zhang, D.-C.; Bin, S.; Bin, W.-D. Distribution of valuable metals in liquid high lead slag during reduction process. Chin. J. Nonferrous Met. 2014, 24, 1056–1062. [Google Scholar]
- Shi, L.; Wang, X.; Zhang, S.; Wu, X.; Yuan, L.; Tang, Z. A new in-situ pyrolytic time-of-flight mass spec-trometer instrument for study on coal pyrolysis. J. Anal. Appl. Pyrolysis 2016, 117, 347–353. [Google Scholar] [CrossRef]
- Jak, E.; Hayes, P.C. Experimental study of phase equilibria in the PbO-ZnO-”Fe2O3”-CaO-SiO2 system in air for high lead smelting slags (CaO/SiO2 = 0.35 and PbO/(CaO+ SiO2)= 5.0 by weight). Metall. Mater. Trans. B 2002, 33, 817–825. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, B.; Jak, E.; Hayes, P.C. Experimental study of phase equilibria in the PbO-MgO-SiO2 system. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2001, 32, 11–16. [Google Scholar] [CrossRef]
- OuYang, K.; Dou, Z.-H.; Zhang, T.-A.; Liu, Y. Effect of ZnO/PbO and FeOX/SiO2 ratio on the viscosity of lead smelting slags. J. Min. Met. Sect. B Met. 2020, 56, 27–33. [Google Scholar] [CrossRef]
- Gupta, S.K. Viscosity of PbO-SiO2 Melts. Metall. Mater. Trans. B 1995, 26, 281–287. [Google Scholar] [CrossRef]
- Yan, Z.; Reddy, R.G.; Lv, X.; Pang, Z.; Bai, C. Viscosity of Iron Oxide Aluminosilicate Melts. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2019, 50, 251–261. [Google Scholar] [CrossRef]



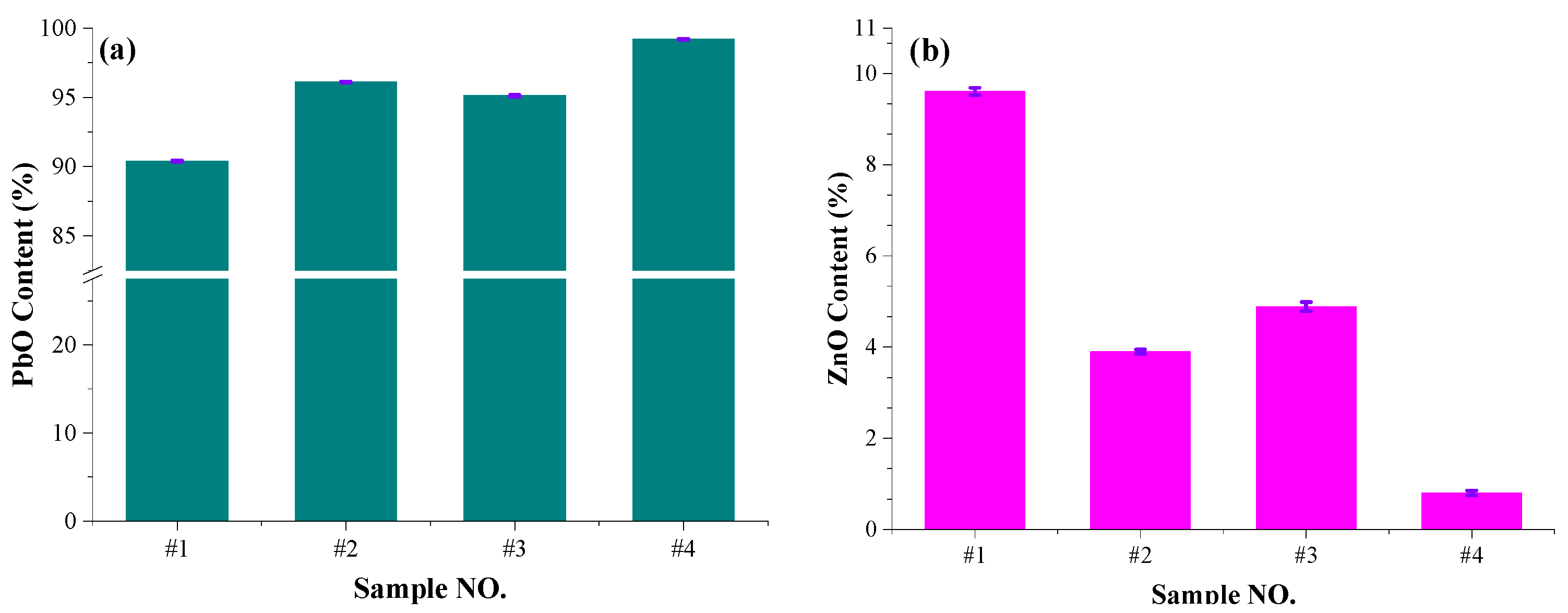

| No. | Component (%, ω) | ||||
|---|---|---|---|---|---|
| PbO | ZnO | Fe3O4 | CaO | SiO2 | |
| #1 | 2.69 | 13.00 | 44.97 | 11.24 | 28.10 |
| #2 | 10.00 | 11.00 | 42.13 | 10.53 | 26.33 |
| #3 | 20.00 | 10.00 | 37.33 | 9.33 | 23.33 |
| #4 | 40.00 | 6.00 | 28.80 | 7.20 | 18.00 |
| t/°C | T/K | PPb/Pa | PPbO/Pa | PZn/Pa_ | PZnO/Pa_ |
|---|---|---|---|---|---|
| 900 | 1173 | 4.224 × 101 | 4.037 × 102 | 0.932 × 105 | |
| 1000 | 1273 | 1.858 × 102 | 2.66 × 103 | 2.334 × 105 | |
| 1100 | 1373 | 6.552 × 102 | 1.307 × 104 | 5.078 × 105 | |
| 1200 | 1473 | 1.937 × 103 | 5.082 × 104 | 9.878 × 105 | |
| 1300 | 1573 | 4.968 × 103 | 1.637 × 105 | 1.756 × 106 | 2.0 × 102 |
| 1400 | 1673 | 1.134 × 104 | 4.515 × 105 | 2.901 × 106 | 4.0 × 102 |
| #1 | #2 | #3 | #4 | |
|---|---|---|---|---|
| Total weight loss rate (TWL) | 5.71 | 13.63 | 23.09 | 32.53 |
| Crystalline water and CO2 (WAC) | 3.00 | 3.00 | 2.70 | 3.20 |
| TWL-WAC | 2.71 | 10.63 | 20.39 | 29.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.-D.; Zhao, J.-X. Volatilisation Behaviour and Mechanism of Lead-Containing Slag during Physical Property Tests. Materials 2024, 17, 1531. https://doi.org/10.3390/ma17071531
Tang W-D, Zhao J-X. Volatilisation Behaviour and Mechanism of Lead-Containing Slag during Physical Property Tests. Materials. 2024; 17(7):1531. https://doi.org/10.3390/ma17071531
Chicago/Turabian StyleTang, Wen-Dan, and Jun-Xue Zhao. 2024. "Volatilisation Behaviour and Mechanism of Lead-Containing Slag during Physical Property Tests" Materials 17, no. 7: 1531. https://doi.org/10.3390/ma17071531
APA StyleTang, W.-D., & Zhao, J.-X. (2024). Volatilisation Behaviour and Mechanism of Lead-Containing Slag during Physical Property Tests. Materials, 17(7), 1531. https://doi.org/10.3390/ma17071531







