Synthesis of rGO/CoFe2O4 Composite and Its Magnetorheological Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Graphene Oxide (GO)
2.2. Fabrication of Reduced GO/Cobalt Ferrite (rGO/CoFe2O4)
2.3. Preparation of MR Fluid
3. Results and Discussion
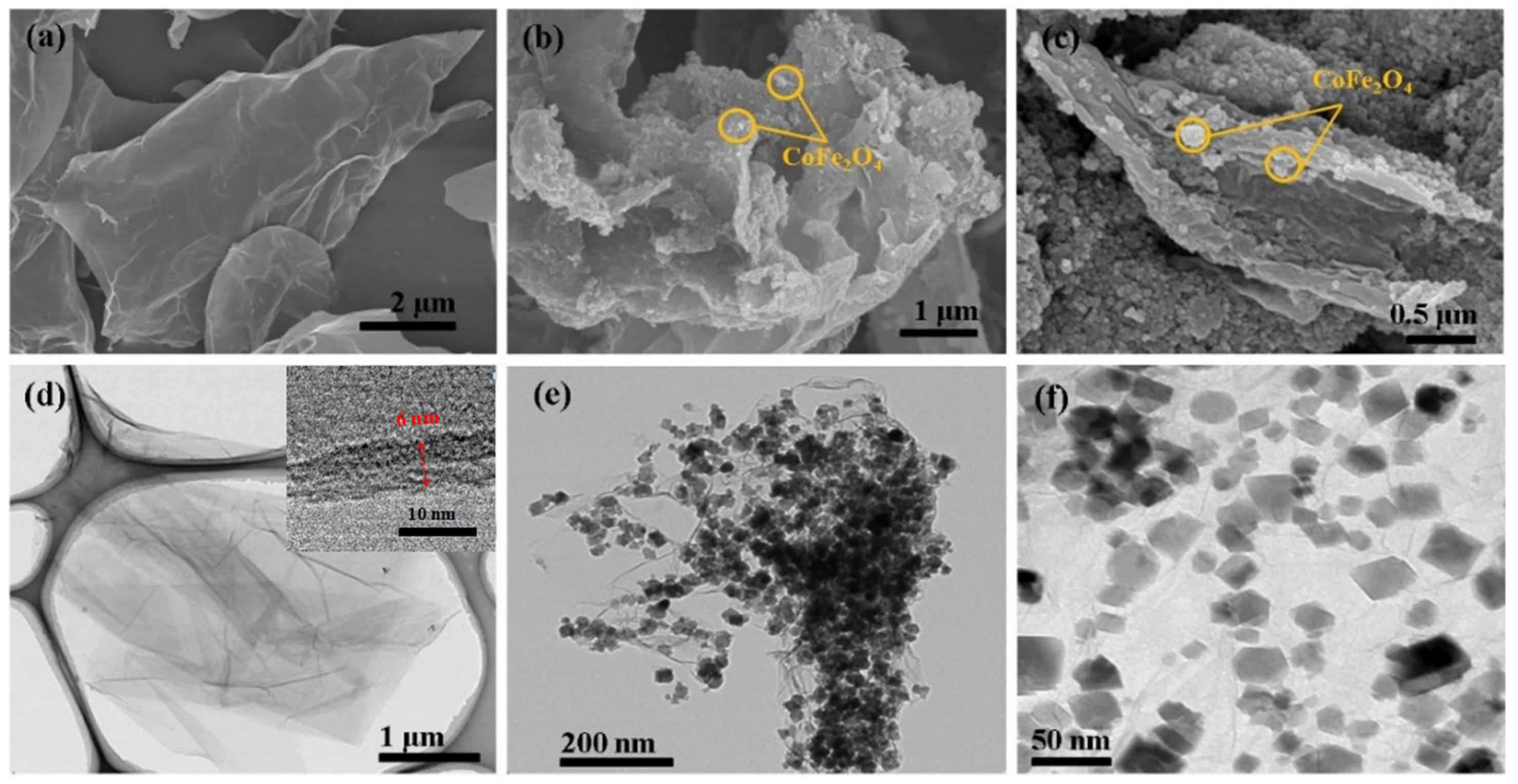
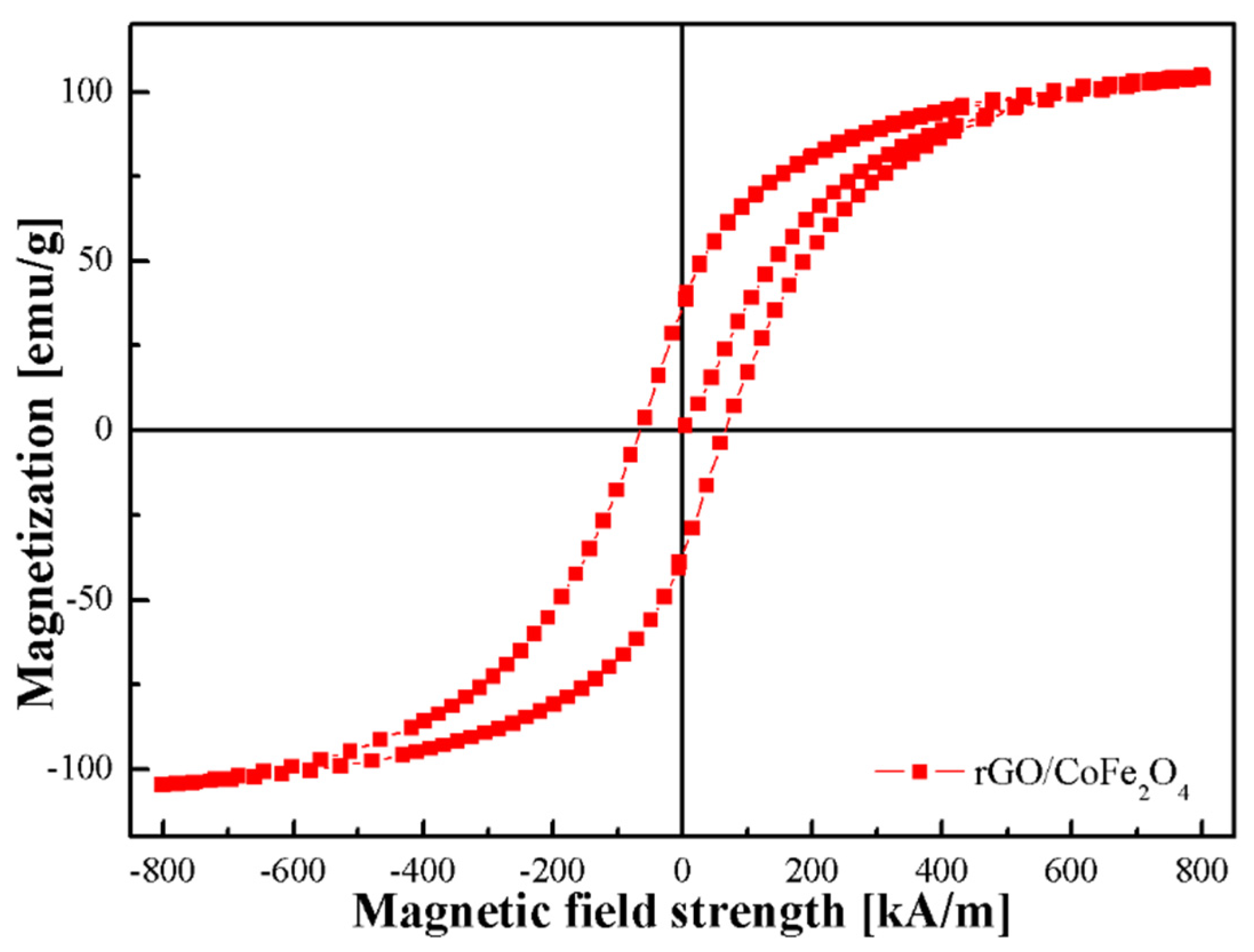
3.1. Characterization of Synthesized Materials
3.2. Magnetorheological Effect
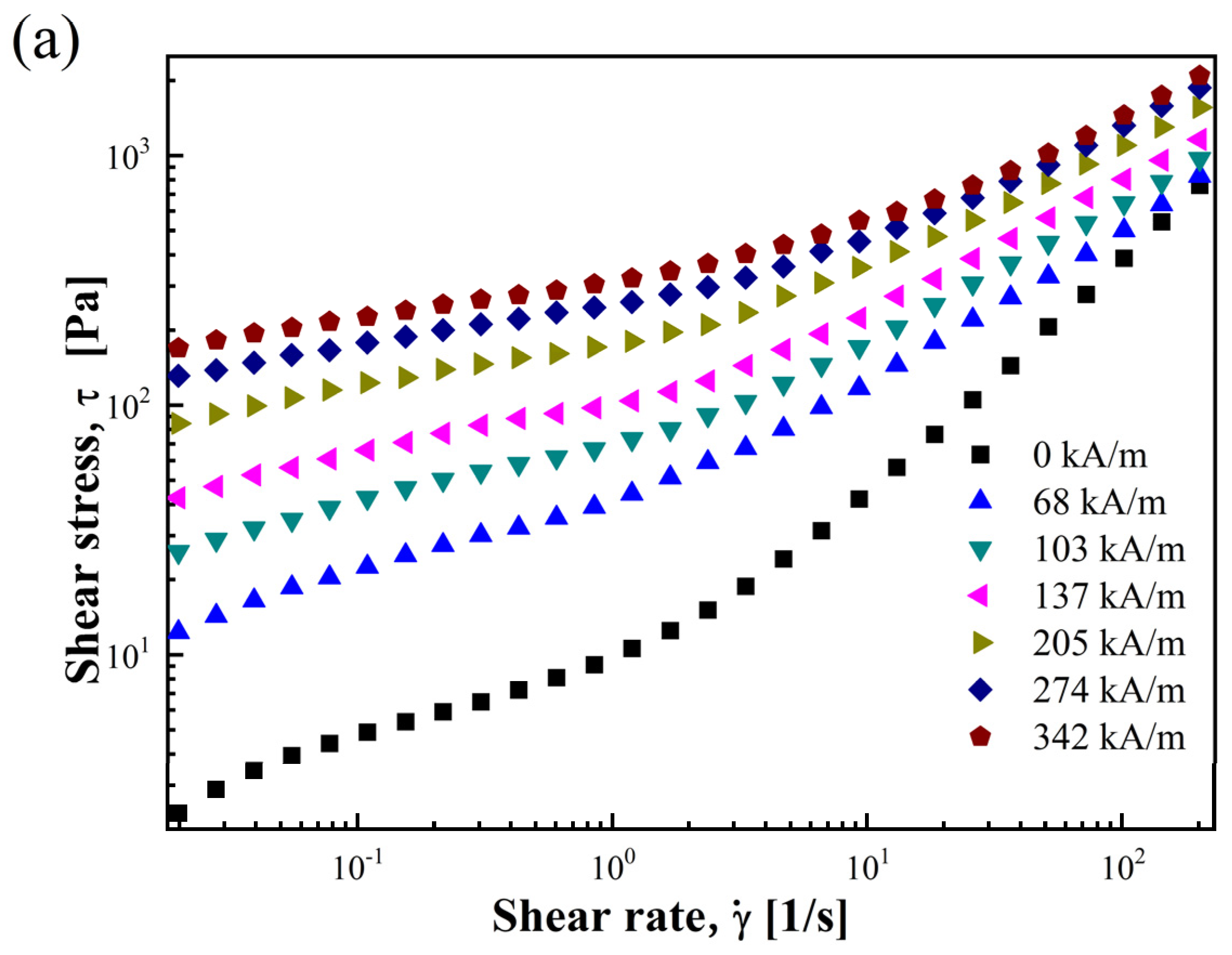
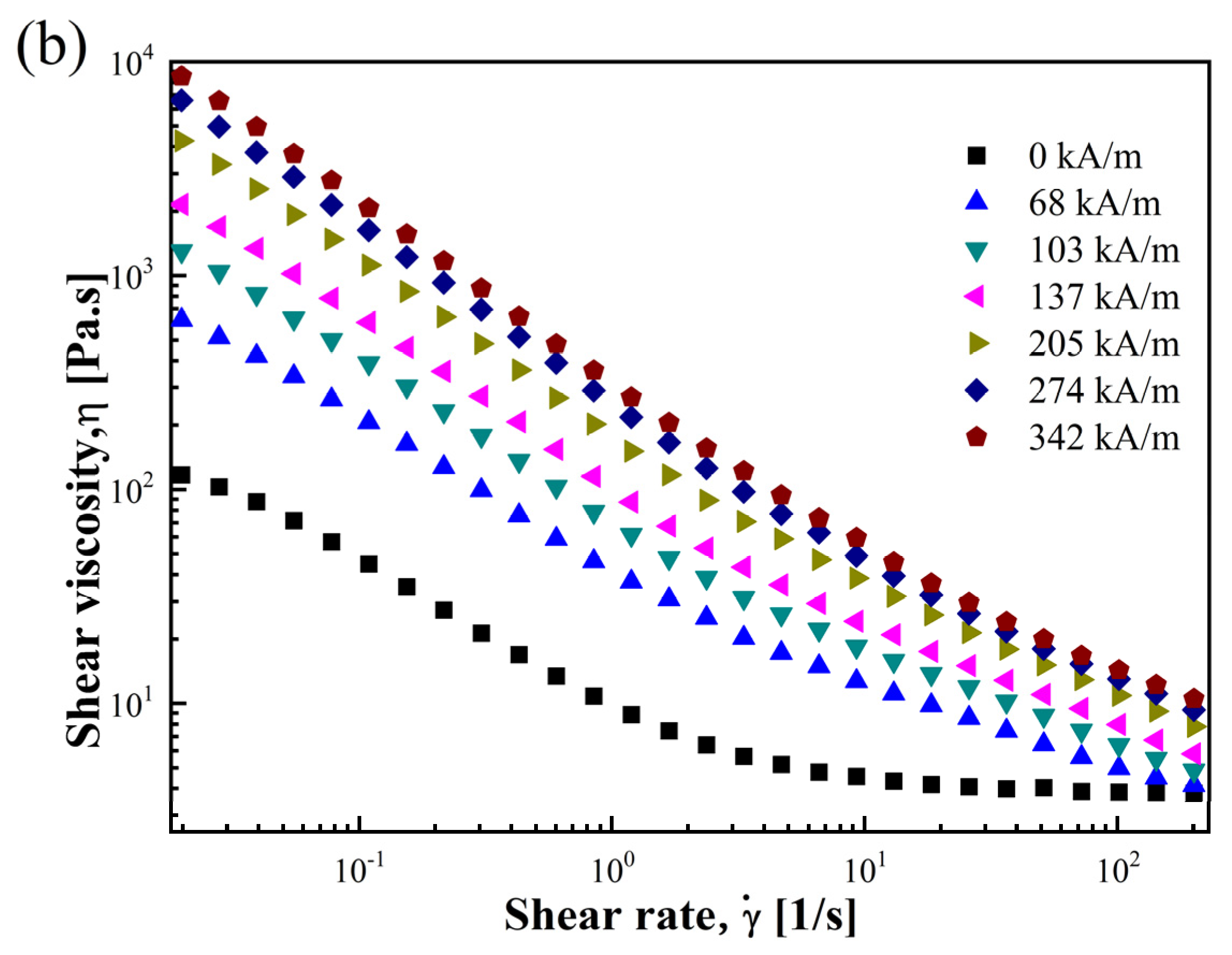
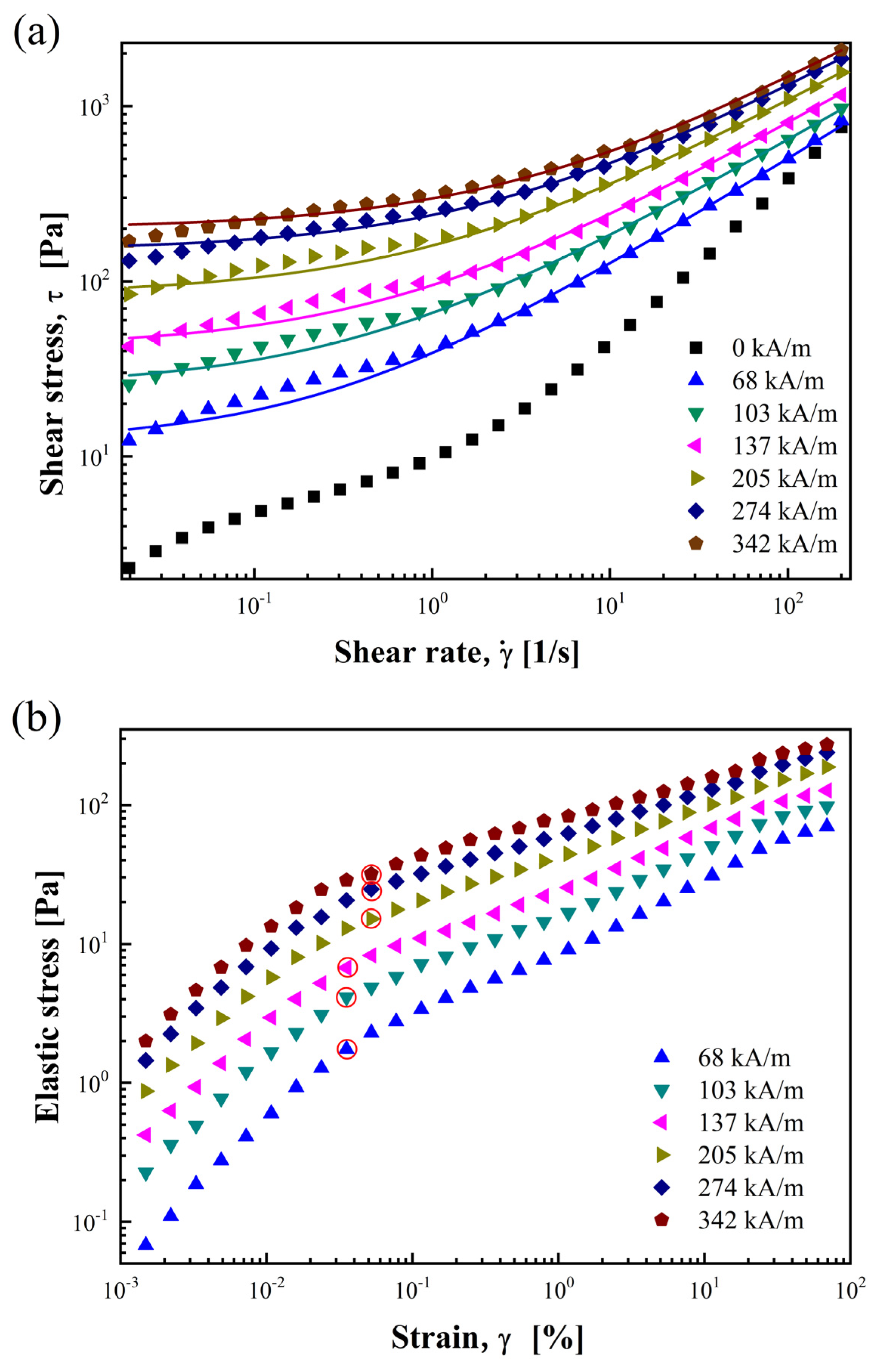
3.2.1. Shear Stress and Shear Viscosity
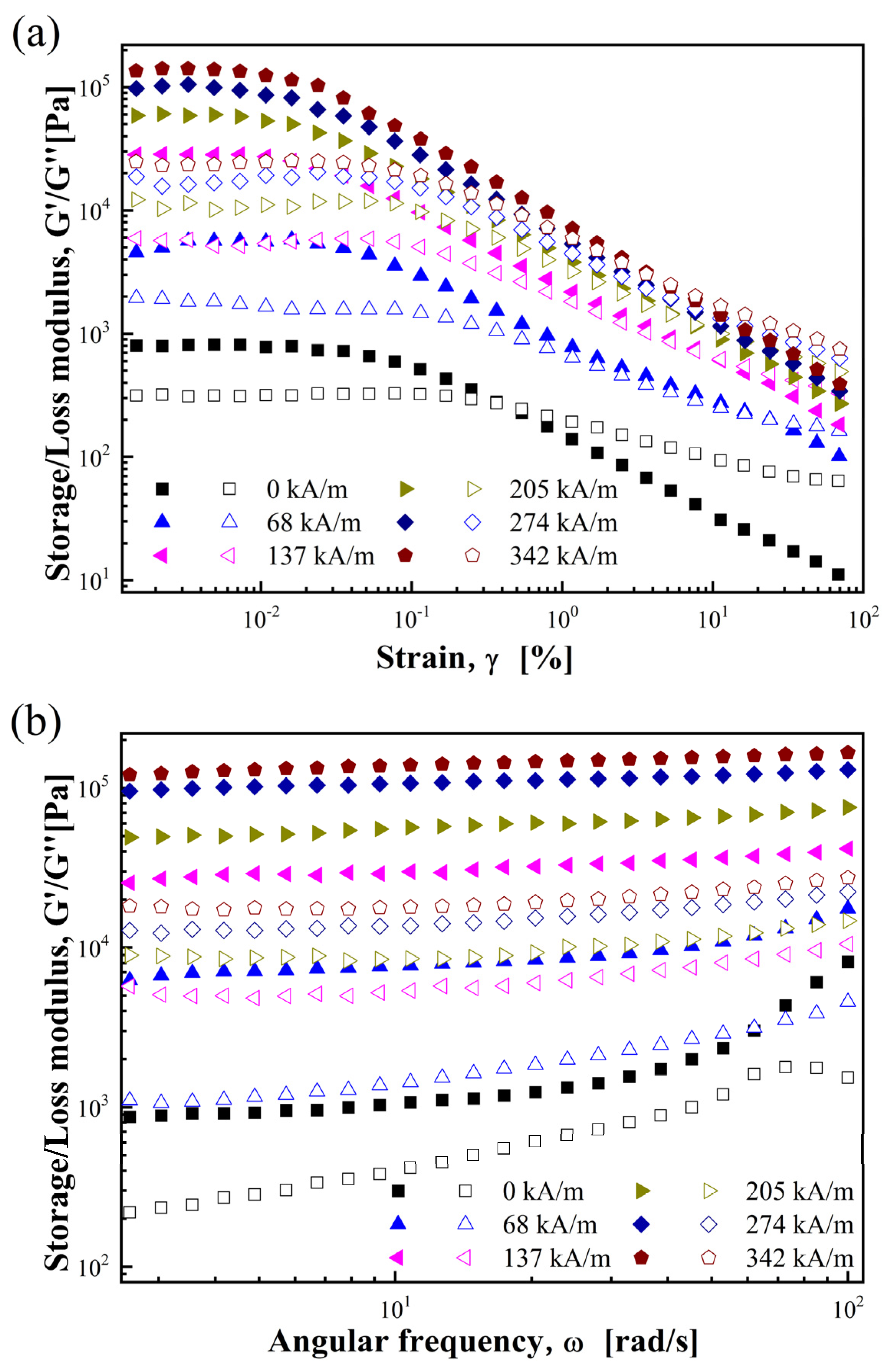
3.2.2. Storage/Loss Modulus and Relaxation Modulus
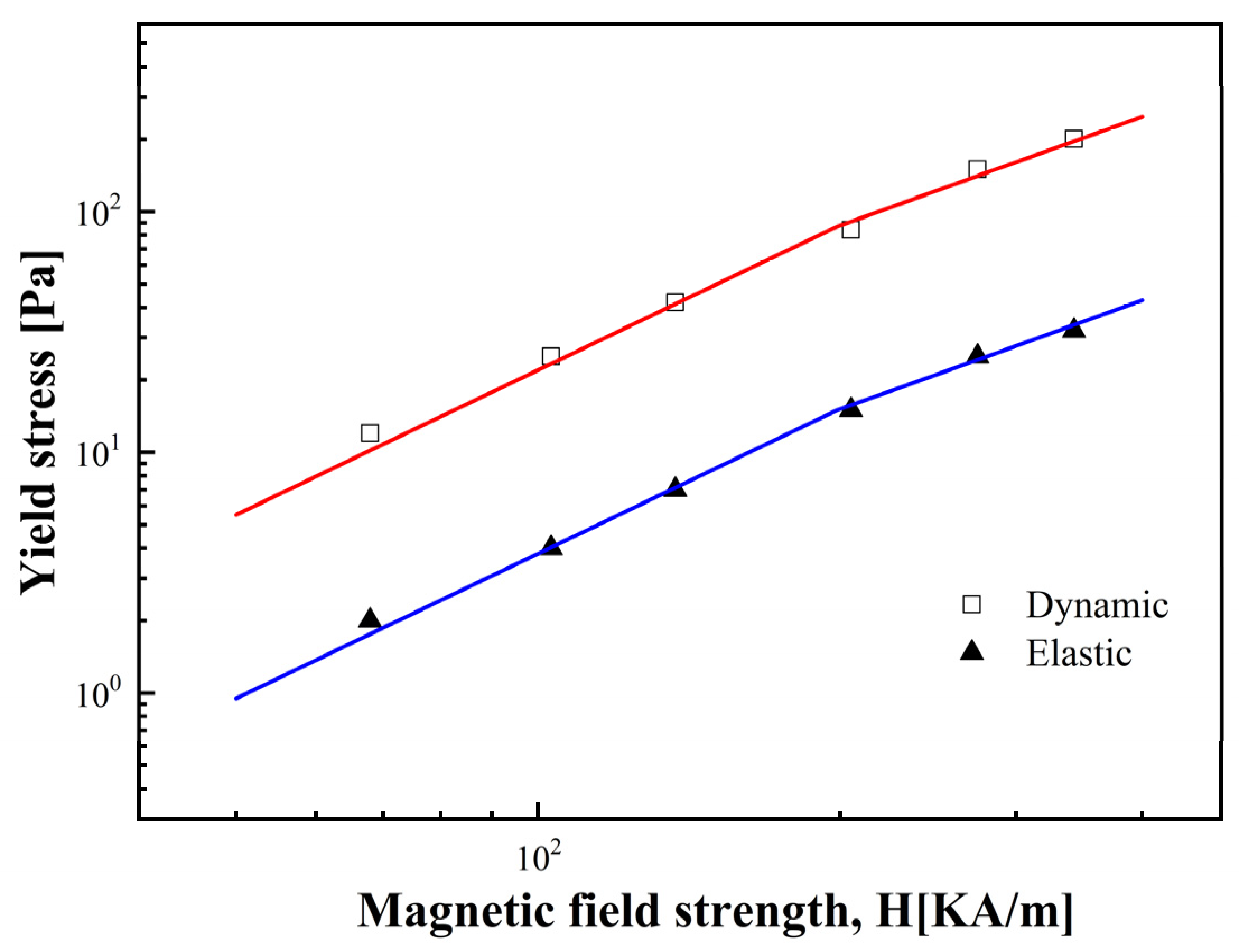
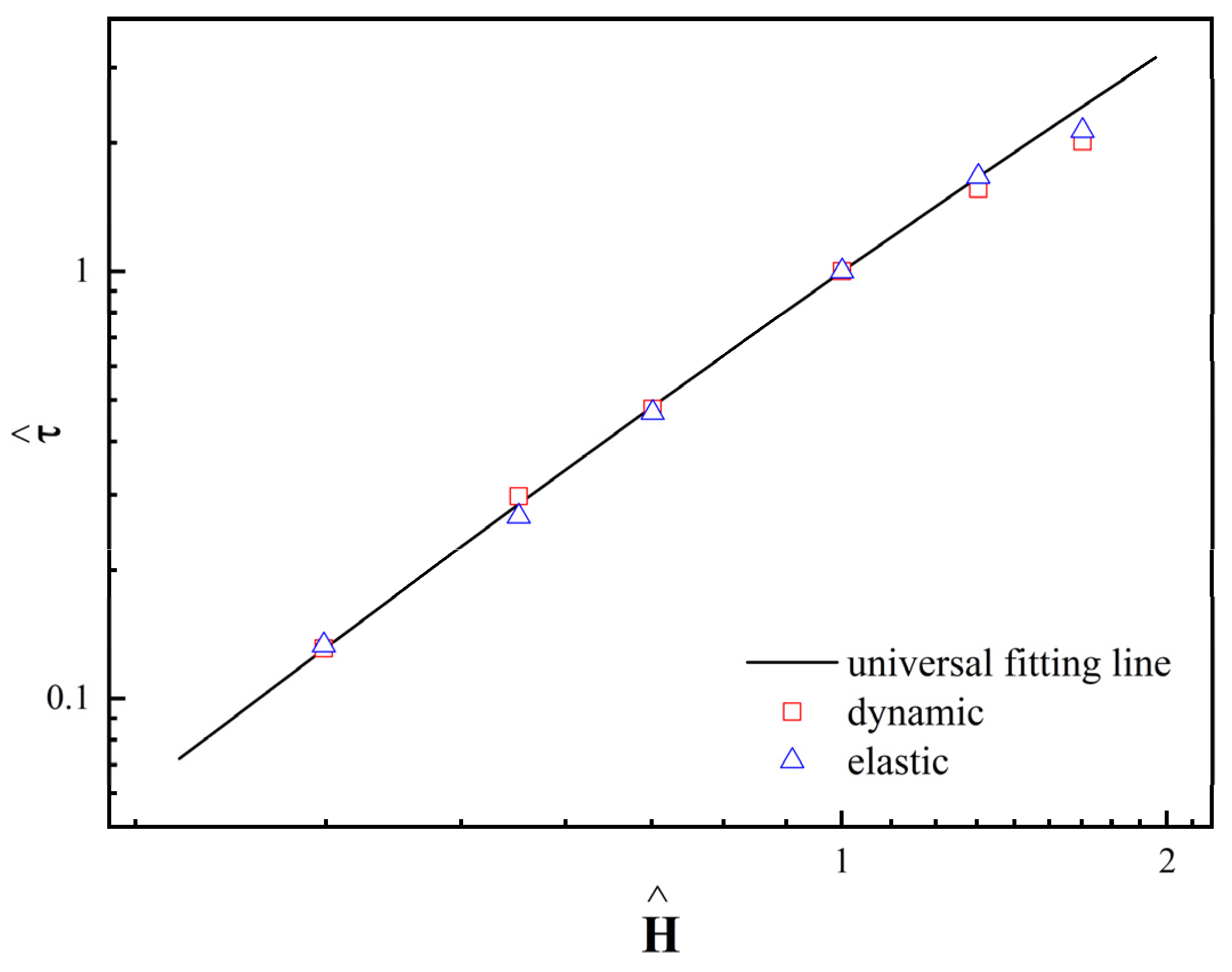
3.2.3. Dynamic and Elastic Yield Stress
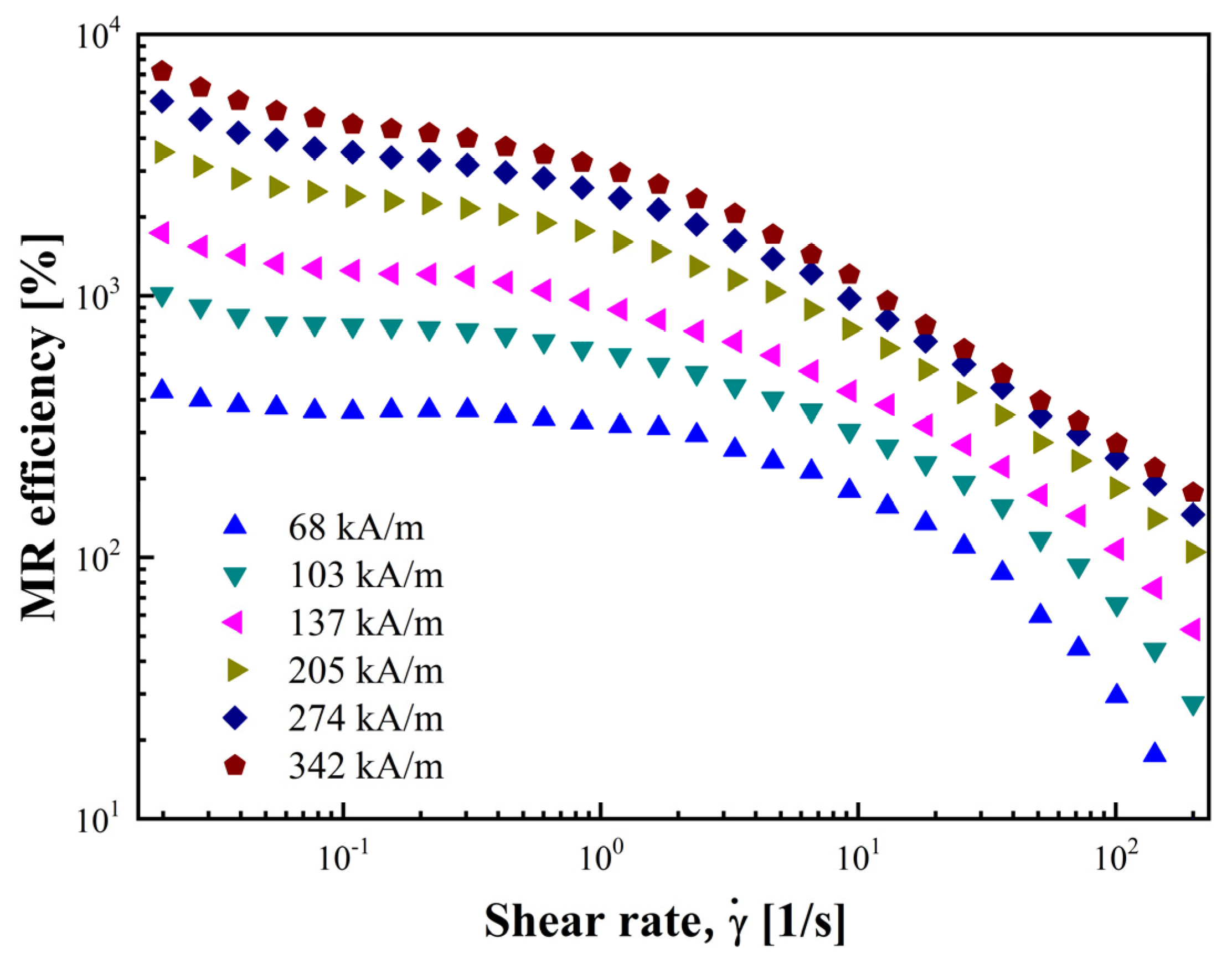
3.2.4. MR Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ginder, J.M.; Davis, L.C. Shear stresses in magnetorheological fluids: Role of magnetic saturation. Appl. Phys. Lett. 1994, 65, 3410–3412. [Google Scholar] [CrossRef]
- Zhu, X.; Jing, X.; Cheng, L. Magnetorheological fluid dampers: A review on structure design and analysis. J. Intell. Mater. Syst. Struct. 2012, 23, 839–873. [Google Scholar] [CrossRef]
- Goncalves, F.D.; Ahmadian, M.; Carlson, J.D. Investigating the magnetorheological effect at high flow velocities. Smart Mater. Struct. 2006, 15, 75–85. [Google Scholar] [CrossRef]
- de Vicente, J.; Klingenberg, D.J.; Hidalgo-Alvarez, R. Magnetorheological fluids: A review. Soft Matter 2011, 7, 3701–3710. [Google Scholar] [CrossRef]
- Olabi, A.G.; Grunwald, A. Design and application of magneto-rheological fluid. Mater. Des. 2007, 28, 2658–2664. [Google Scholar] [CrossRef]
- Wang, M.; Nie, M.; Liu, Y.; Guo, H. Simulation of Magnetorheological Plane Polishing Scratch Creation Process and Suppression Method. Machines 2022, 10, 812. [Google Scholar] [CrossRef]
- Masa’id, A.; Lenggana, B.W.; Ubaidillah, U.; Susilo, D.D.; Choi, S.-B. A Review on Vibration Control Strategies Using Magnetorheological Materials Actuators: Application Perspective. Actuators 2023, 12, 113. [Google Scholar] [CrossRef]
- Li, G.; Ruan, Z.; Gu, R.; Hu, G. Fuzzy Sliding Mode Control of Vehicle Magnetorheological Semi-Active Air Suspension. Appl. Sci. 2021, 11, 10925. [Google Scholar] [CrossRef]
- Yang, J.; Sun, S.; Yang, X.; Ma, Y.; Yun, G.; Chang, R.; Tang, S.-Y.; Nakano, M.; Li, Z.; Du, H.; et al. Equipping New SMA Artificial Muscles With Controllable MRF Exoskeletons for Robotic Manipulators and Grippers. IEEE/ASME Trans. Mechatron. 2022, 27, 4585–4596. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, D.; Yang, W.; Wu, S.; Luo, X. Reduced graphene oxide enhanced magnetic nanocomposites for removal of carbamazepine. J. Mater. Sci. 2018, 53, 15474–15486. [Google Scholar] [CrossRef]
- Eshgarf, H.; Ahmadi Nadooshan, A.; Raisi, A. An overview on properties and applications of magnetorheological fluids: Dampers, batteries, valves and brakes. J. Energy Storage 2022, 50, 104648. [Google Scholar] [CrossRef]
- Ashtiani, M.; Hashemabadi, S.H.; Ghaffari, A. A review on the magnetorheological fluid preparation and stabilization. J. Magn. Magn. Mater. 2015, 374, 716–730. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kwon, S.H.; Choi, H.J. Magnetorheological characteristics of carbonyl iron microparticles with different shapes. Korea-Aust. Rheol. J. 2019, 31, 41–47. [Google Scholar] [CrossRef]
- Zhu, W.; Dong, X.; Huang, H.; Qi, M. Iron nanoparticles-based magnetorheological fluids: A balance between MR effect and sedimentation stability. J. Magn. Magn. Mater. 2019, 491, 165556. [Google Scholar] [CrossRef]
- Zi, Z.; Sun, Y.; Zhu, X.; Yang, Z.; Dai, J.; Song, W. Synthesis and magnetic properties of CoFe2O4 ferrite nanoparticles. J. Magn. Magn. Mater. 2009, 321, 1251–1255. [Google Scholar] [CrossRef]
- Zhang, K.; Piao, S.H.; Choi, H.J. Hollow Structured Magnetic Particles of CoFe2O4 and Their Magnetorheological Characteristics. IEEE Trans. Magn. 2015, 51, 2005904. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, F.; Lu, Z.; Ma, Y.; Li, X.; Tong, Y.; Dong, X. Controlled synthesis of CoFe2O4/MoS2 nanocomposites with excellent sedimentation stability for magnetorheological fluid. J. Ind. Eng. Chem. 2019, 70, 439–446. [Google Scholar] [CrossRef]
- Gusynin, V.P.; Sharapov, S.G. Unconventional integer quantum Hall effect in graphene. Phys. Rev. Lett. 2005, 95, 146801. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Song, Y.; Zhang, X.; Ma, Y.; Liang, J.; Chen, Y. Room-temperature ferromagnetism of graphene. Nano Lett. 2009, 9, 220–224. [Google Scholar] [CrossRef]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; van der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable atomic membranes from graphene sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhi, L.; Mullen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Knapek, A.; Sobola, D.; Burda, D.; Danhel, A.; Mousa, M.; Kolarik, V. Polymer Graphite Pencil Lead as a Cheap Alternative for Classic Conductive SPM Probes. Nanomaterials 2019, 9, 1756. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Mishra, R.K.; Ha, S.K.; Huczko, A. Evolution of Graphene Oxide and Graphene: From Imagination to Industrialization. ChemNanoMat 2018, 4, 598–620. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef]
- Buchsteiner, A.; Lerf, A.; Pieper, J. Water dynamics in graphite oxide investigated with neutron scattering. J. Phys. Chem. B 2006, 110, 22328–22338. [Google Scholar] [CrossRef] [PubMed]
- Seredych, M.; Tamashausky, A.V.; Bandosz, T.J. Graphite oxides obtained from porous graphite: The role of surface chemistry and texture in ammonia retention at ambient conditions. Adv. Funct. Mater. 2010, 20, 1670–1679. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, X.; Li, S.; Zhang, N.; Wang, J. Magnetic reduced graphene oxide nanocomposite as an effective electromagnetic wave absorber and its absorbing mechanism. Ceram. Int. 2016, 42, 17116–17122. [Google Scholar] [CrossRef]
- Xie, Y.L.; An, J.X.; Shi, P.Z.; Ye, N.S. Determination of Lysozyme by Graphene Oxide-Polyethylene Glycol-Based Fluorescence Resonance Energy Transfer. Anal. Lett. 2017, 50, 148–160. [Google Scholar] [CrossRef]
- Yavuz, E.; Tokalioglu, S.; Sahan, H.; Kaçer, M.; Patat, S. Dispersive Solid-Phase Extraction of Rhodium from Water, Street Dust, and Catalytic Converters Using a Cellulose-Graphite Oxide Composite. Anal. Lett. 2017, 50, 63–79. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Choi, H.-Y.; Choi, J.-Y.; Park, H.-H.; Kim, T.-H.; Choi, H.J.; Lee, C.-S. Effect of reduced graphene oxide and MnFe2O4 nanoparticles on carbonyl iron for magnetorheological fluids. J. Ind. Eng. Chem. 2021, 98, 140–147. [Google Scholar] [CrossRef]
- Hack, R.; Correia, C.H.G.; Zanon, R.A.d.S.; Pezzin, S.H. Characterization of graphene nanosheets obtained by a modified Hummer’s method. Matéria 2018, 23, e-11988. [Google Scholar] [CrossRef]
- Chen, T.; Du, P.; Jiang, W.; Liu, J.; Hao, G.; Gao, H.; Xiao, L.; Ke, X.; Zhao, F.; Xuan, C. A facile one-pot solvothermal synthesis of CoFe2O4/RGO and its excellent catalytic activity on thermal decomposition of ammonium perchlorate. RSC Adv. 2016, 6, 83838–83847. [Google Scholar] [CrossRef]
- Dedi; Idayanti, N.; Kristiantoro, T.; Alarn, G.F.N.; Sudrajat, N. Magnetic Properties of Cobalt Ferrite Synthesized by Mechanical Alloying. In Proceedings of the 1st International Seminar on Metallurgy and Materials (ISMM), Jakarta, Indonesia, 24–25 October 2017. [Google Scholar] [CrossRef]
- Rao, S.; Upadhyay, J.; Polychronopoulou, K.; Umer, R.; Das, R. Reduced Graphene Oxide: Effect of Reduction on Electrical Conductivity. J. Compos. Sci. 2018, 2, 25. [Google Scholar] [CrossRef]
- Mitra, S.; Veluri, P.S.; Chakraborthy, A.; Petla, R.K. Electrochemical Properties of Spinel Cobalt Ferrite Nanoparticles with Sodium Alginate as Interactive Binder. ChemElectroChem 2014, 1, 1068–1074. [Google Scholar] [CrossRef]
- Fritsch, D.; Ederer, C. First-principles calculation of magnetoelastic coefficients and magnetostriction in the spinel ferrites CoFe2O4 and NiFe2O4. Phys. Rev. B 2012, 86, 014406. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, Z.; Zhang, D.; Peng, W.; Sun, H.; Wang, S. Magnetic CoFe2O4–Graphene Hybrids: Facile Synthesis, Characterization, and Catalytic Properties. Ind. Eng. Chem. Res. 2012, 51, 6044–6051. [Google Scholar] [CrossRef]
- Liu, C.; Rondinone, A.J.; Zhang, Z.J. Synthesis of magnetic spinel ferrite CoFe2O4 nanoparticles from ferric salt and characterization of the size-dependent superparamagnetic properties. Pure Appl. Chem. 2000, 72, 37–45. [Google Scholar] [CrossRef]
- Hu, L.; Li, M.; Cheng, L.; Jiang, B.; Ai, J. Solvothermal synthesis of octahedral and magnetic CoFe2O4-reduced graphene oxide hybrids and their photo-Fenton-like behavior under visible-light irradiation. RSC Adv. 2021, 11, 22250–22263. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.C.; Chia, C.H.; Zakaria, S.; Yusoff, M.; Haw, C.Y.; Ahmadi, S.; Huang, N.M.; Lim, H.N. Hydrothermal preparation of high saturation magnetization and coercivity cobalt ferrite nanocrystals without subsequent calcination. Mater. Chem. Phys. 2010, 120, 31–35. [Google Scholar] [CrossRef]
- Mazarji, M.; Esmaili, H.; Bidhendi, G.N.; Mahmoodi, N.M.; Minkina, T.; Sushkova, S.; Mandzhieva, S.; Barakhov, A.; Moghtaderi, H.; Bhatnagar, A. Green synthesis of reduced graphene oxide-CoFe2O4 nanocomposite as a highly efficient visible-light-driven catalyst in photocatalysis and photo Fenton-like reaction. Mater. Sci. Eng. B 2021, 270, 115223. [Google Scholar] [CrossRef]
- Devi, L.G.; Srinivas, M. Hydrothermal synthesis of reduced graphene oxide-CoFe2O4 heteroarchitecture for high visible light photocatalytic activity: Exploration of efficiency, stability and mechanistic pathways. J. Environ. Chem. Eng. 2017, 5, 3243–3255. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Zhang, J.; Kong, Y.N.; Cuan, H.L. Dynamical Simulation of Magnetorheological Fluid Characteristics. Adv. Mater. Res. 2011, 255–260, 3505–3509. [Google Scholar] [CrossRef]
- Agirre-Olabide, I.; Berasategui, J.; Elejabarrieta, M.J.; Bou-Ali, M.M. Characterization of the linear viscoelastic region of magnetorheological elastomers. J. Intell. Mater. Syst. Struct. 2014, 25, 2074–2081. [Google Scholar] [CrossRef]
- Kim, Y.J.; Liu, Y.D.; Seo, Y.; Choi, H.J. Pickering-emulsion-polymerized polystyrene/Fe2O3 composite particles and their magnetoresponsive characteristics. Langmuir 2013, 29, 4959–4965. [Google Scholar] [CrossRef] [PubMed]
- Schwarzl, F.R. Numerical calculation of storage and loss modulus from stress relaxation data for linear viscoelastic materials. Rheol. Acta 1971, 10, 165–173. [Google Scholar] [CrossRef]
- Versaci, M.; Palumbo, A. Magnetorheological Fluids: Qualitative comparison between a mixture model in the Extended Irreversible Thermodynamics framework and an Herschel–Bulkley experimental elastoviscoplastic model. Int. J. Non-Linear Mech. 2020, 118, 103228. [Google Scholar] [CrossRef]
- Maurya, C.S.; Sarkar, C. Effect of Fe3O4 Nanoparticles on Magnetorheological Properties of Flake-Shaped Carbonyl Iron Water-Based Suspension. IEEE Trans. Magn. 2020, 56, 4600608. [Google Scholar] [CrossRef]
- Choi, H.J.; Cho, M.S.; Kim, J.W.; Kim, C.A.; Jhon, M.S. A yield stress scaling function for electrorheological fluids. Appl. Phys. Lett. 2001, 78, 3806–3808. [Google Scholar] [CrossRef]
- Arief, I.; Mukhopadhyay, P.K. Dynamic and rate-dependent yielding behavior of Co0.9Ni0.1 microcluster based magnetorheological fluids. J. Magn. Magn. Mater. 2016, 397, 57–63. [Google Scholar] [CrossRef]
- Lopez-Lopez, M.T.; Gomez-Ramirez, A.; Rodriguez-Arco, L.; Duran, J.D.; Iskakova, L.; Zubarev, A. Colloids on the frontier of ferrofluids. Rheological properties. Langmuir 2012, 28, 6232–6245. [Google Scholar] [CrossRef]


| H/(kA/m) | K | ||
|---|---|---|---|
| 68 | 12 | 27 | 0.78 |
| 103 | 25 | 41 | 0.63 |
| 137 | 42 | 53 | 0.59 |
| 205 | 84 | 76 | 0.58 |
| 274 | 150 | 89 | 0.56 |
| 342 | 200 | 97 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Gong, C.; Dong, Y.; Choi, H.J. Synthesis of rGO/CoFe2O4 Composite and Its Magnetorheological Characteristics. Materials 2024, 17, 1859. https://doi.org/10.3390/ma17081859
Lv Y, Gong C, Dong Y, Choi HJ. Synthesis of rGO/CoFe2O4 Composite and Its Magnetorheological Characteristics. Materials. 2024; 17(8):1859. https://doi.org/10.3390/ma17081859
Chicago/Turabian StyleLv, Yang, Chengjie Gong, Yuzhen Dong, and Hyoung Jin Choi. 2024. "Synthesis of rGO/CoFe2O4 Composite and Its Magnetorheological Characteristics" Materials 17, no. 8: 1859. https://doi.org/10.3390/ma17081859





