Antifouling and Antioxidant Properties of PVDF Membrane Modified with Polyethylene Glycol Methacrylate and Propyl Gallate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Graft Copolymer
2.3. Fourier Transform Infrared Spectroscopy Characterization
2.4. Preparation of Membrane
2.5. Dynamic Water Contact Angle Measurement
2.6. Analysis of Scanning Electron Microscope
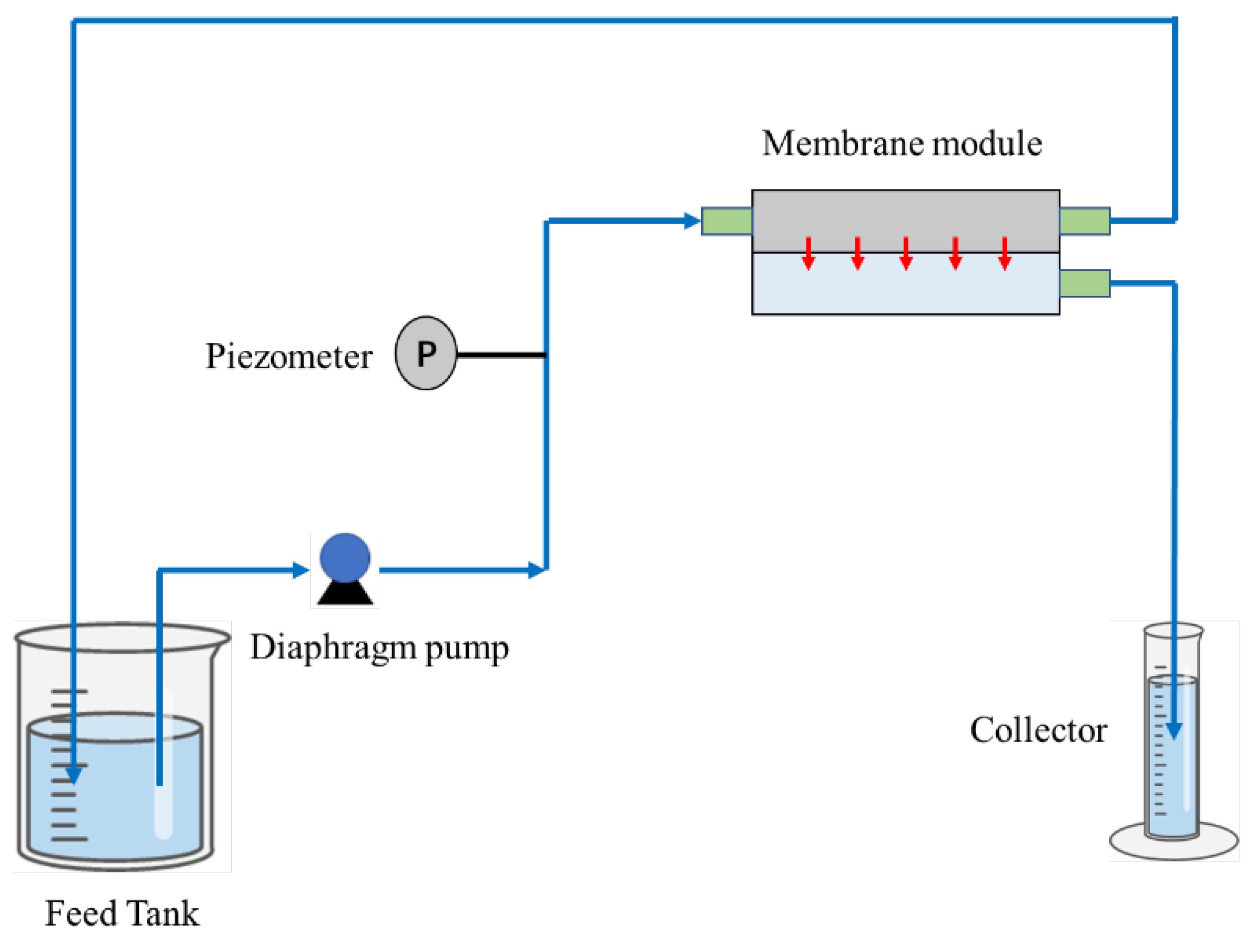
2.7. Filtering Experiment
2.8. Evaluation of Antifouling Performance of Membrane
2.9. Evaluation of the Oxidation Resistance of the Membrane
2.9.1. Free Radical Scavenging Effect
2.9.2. Oxidation Resistance of Membrane to NaClO Solution
3. Results and Discussion
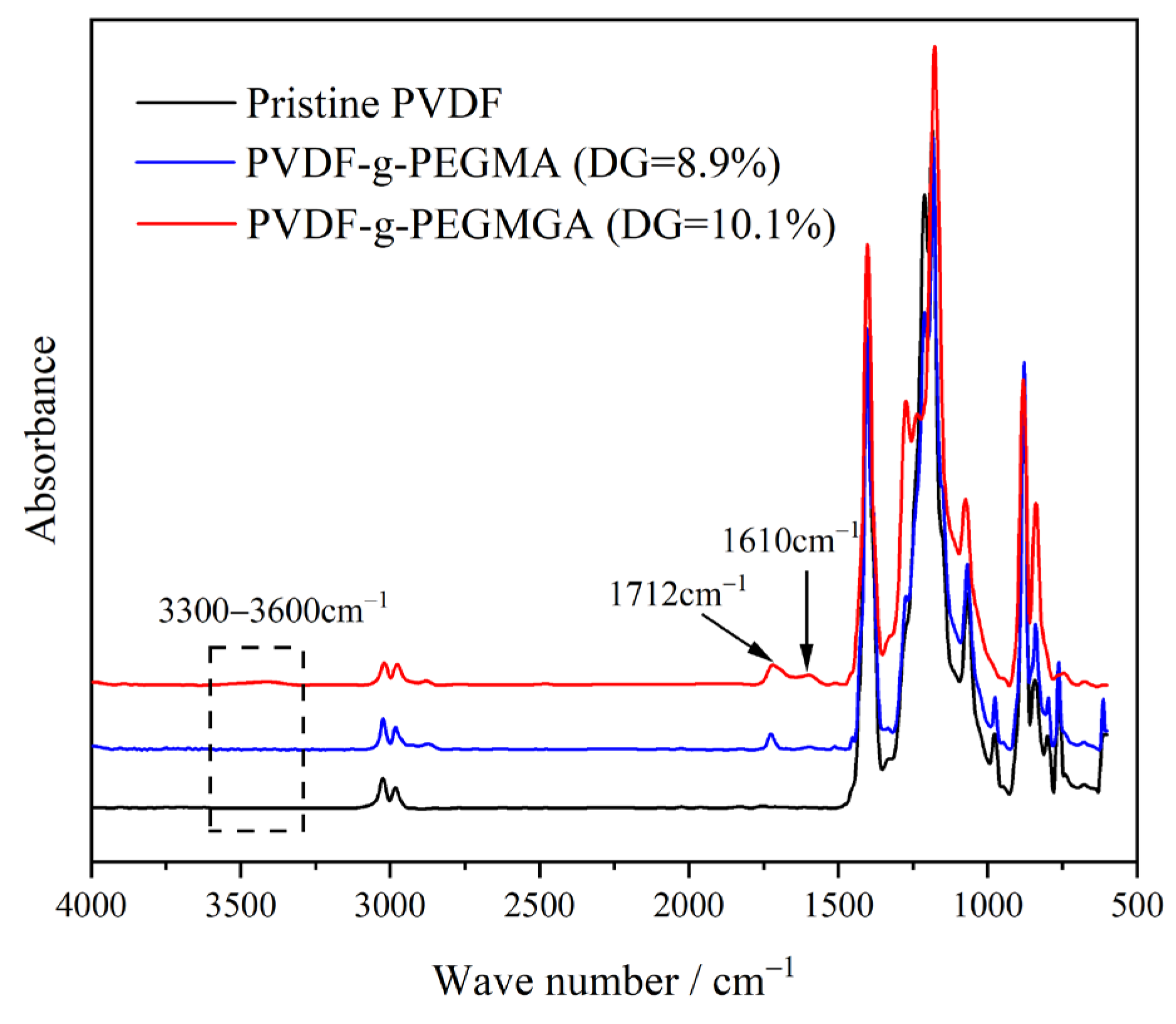
3.1. FTIR Spectra of Graft Copolymer
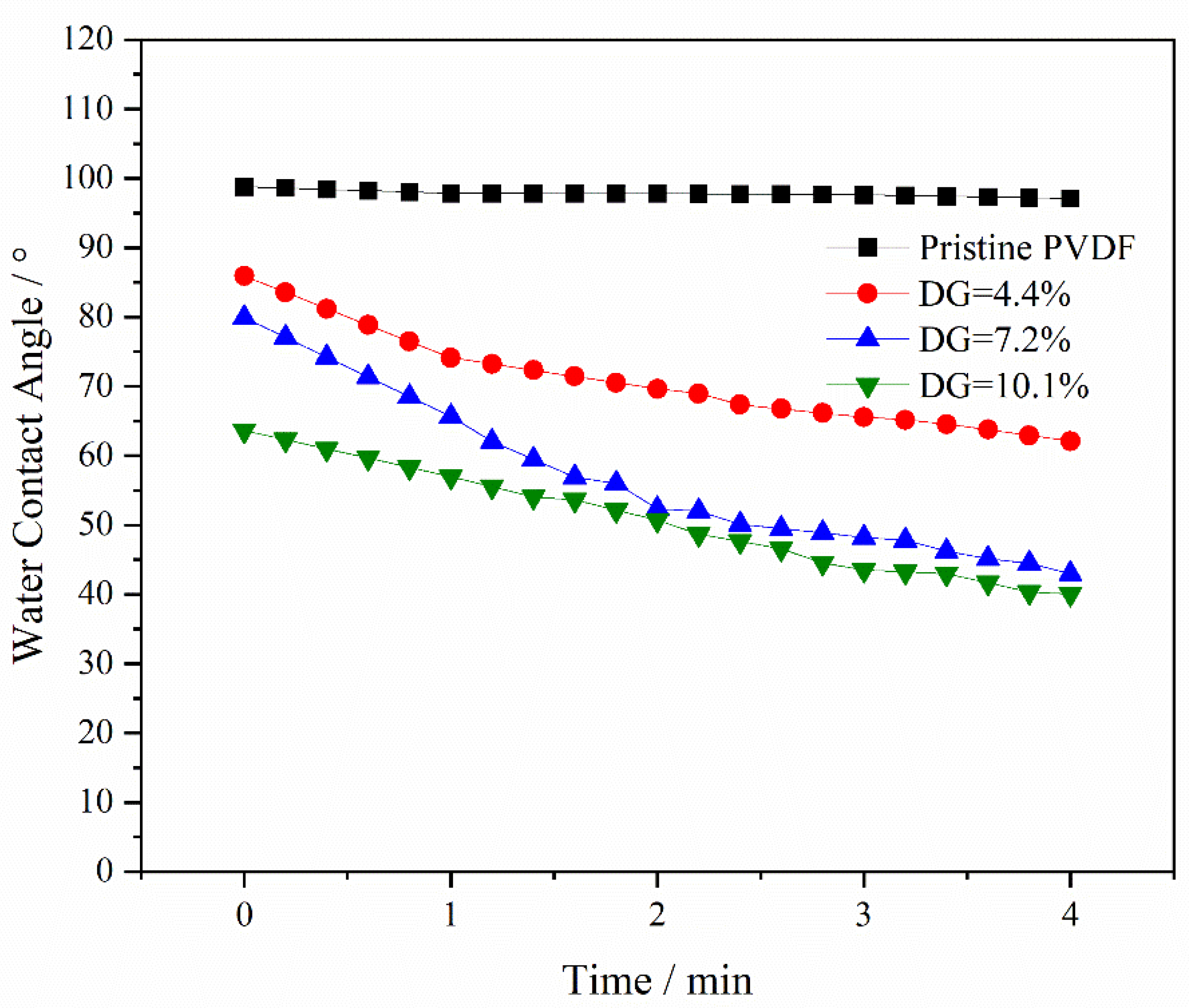
3.2. Dynamic Water Contact Angle Test
3.3. Morphology Characterization of Membrane
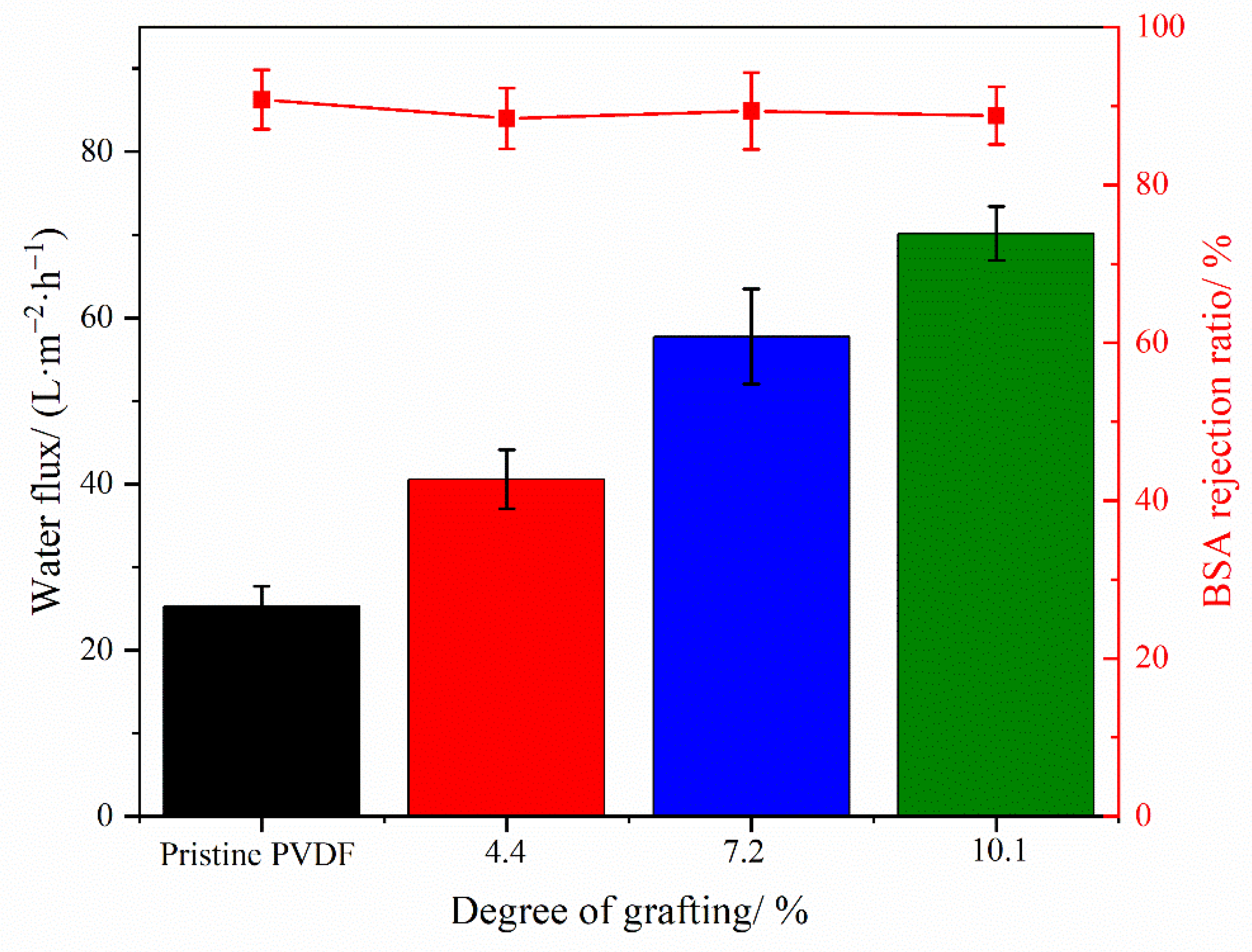
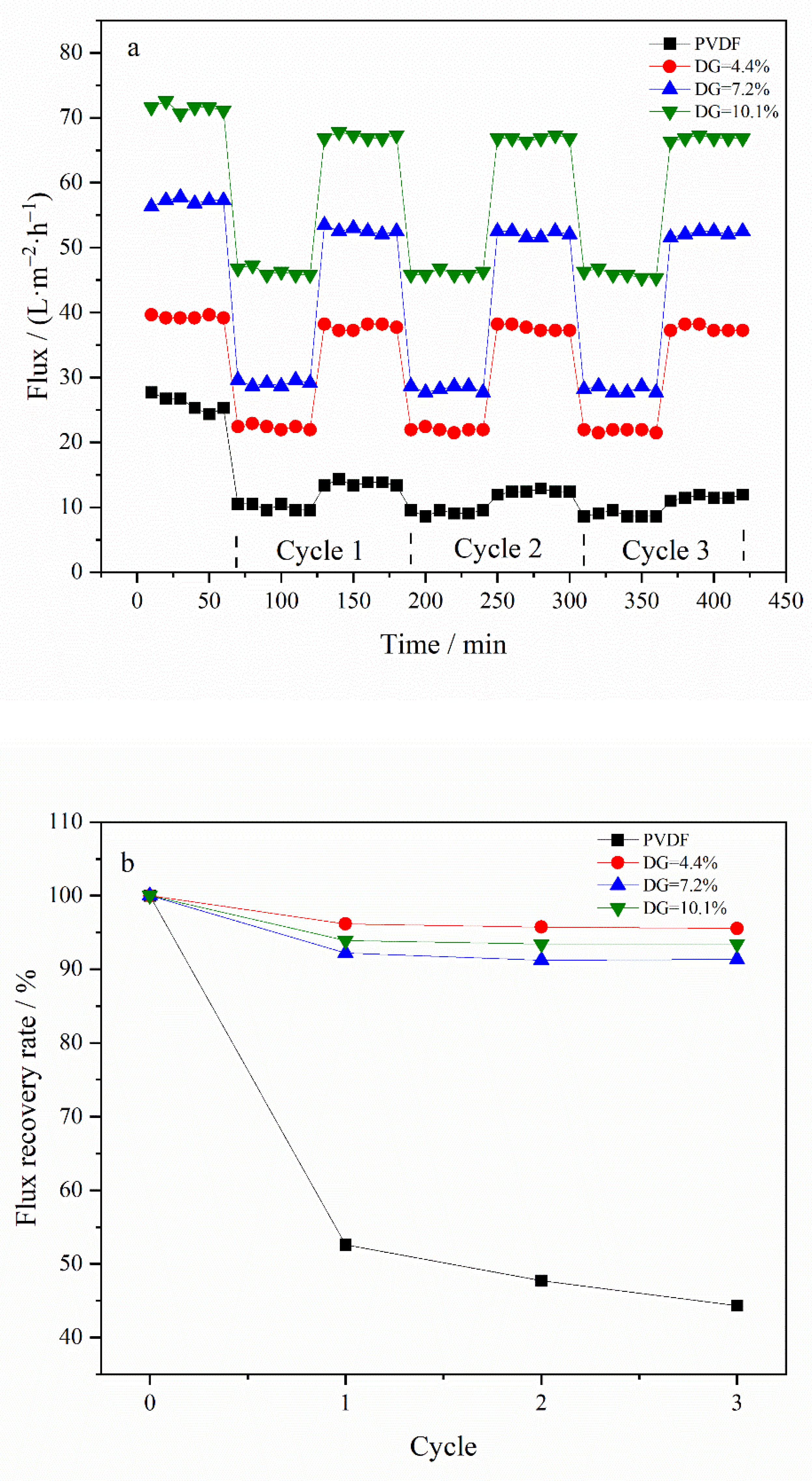
3.4. Evaluation of Filtration Performance and Antifouling Performance of Membrane
3.5. Oxidation Resistance of the Membrane
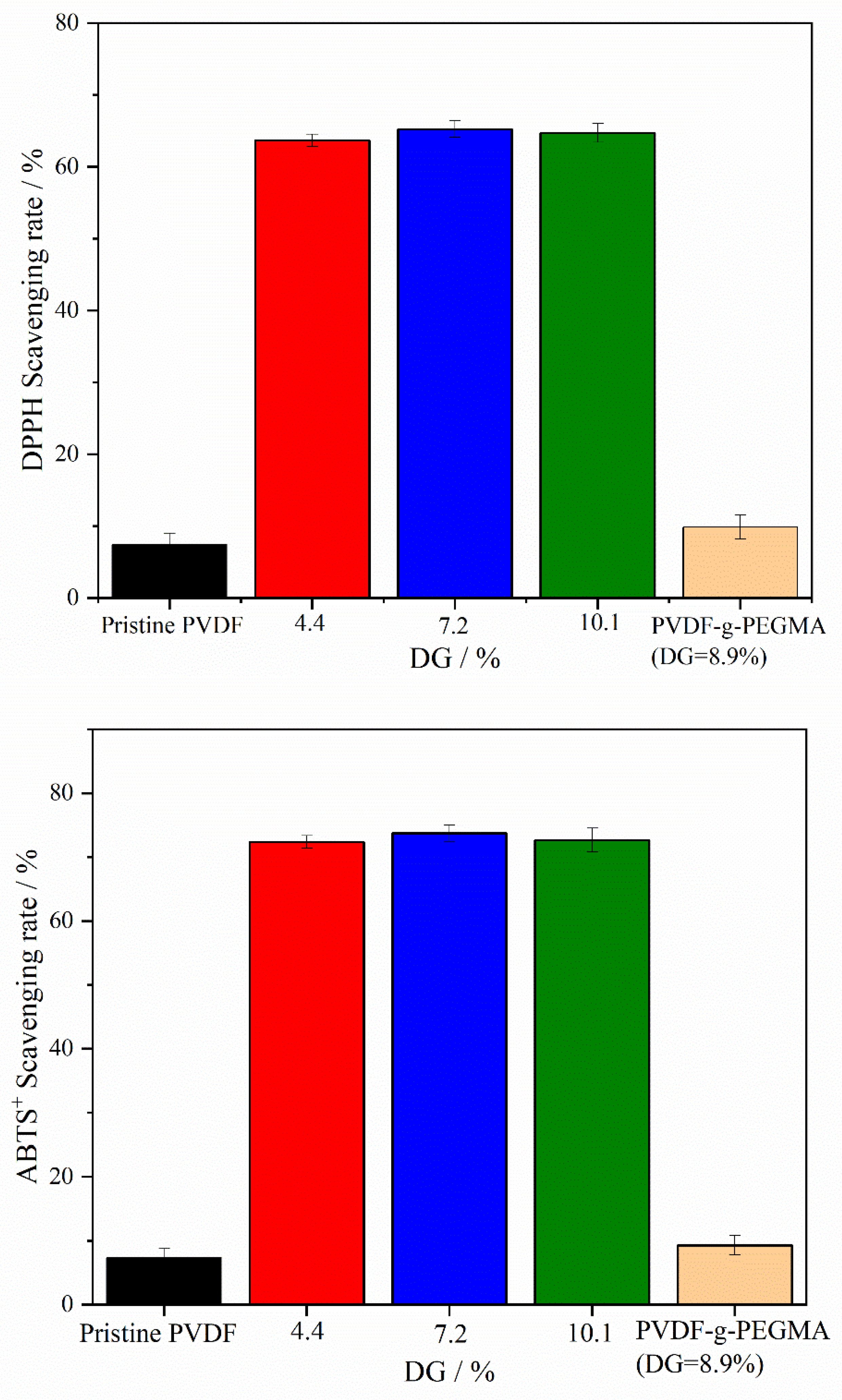
3.5.1. Free Radical Scavenging Effect of Membrane
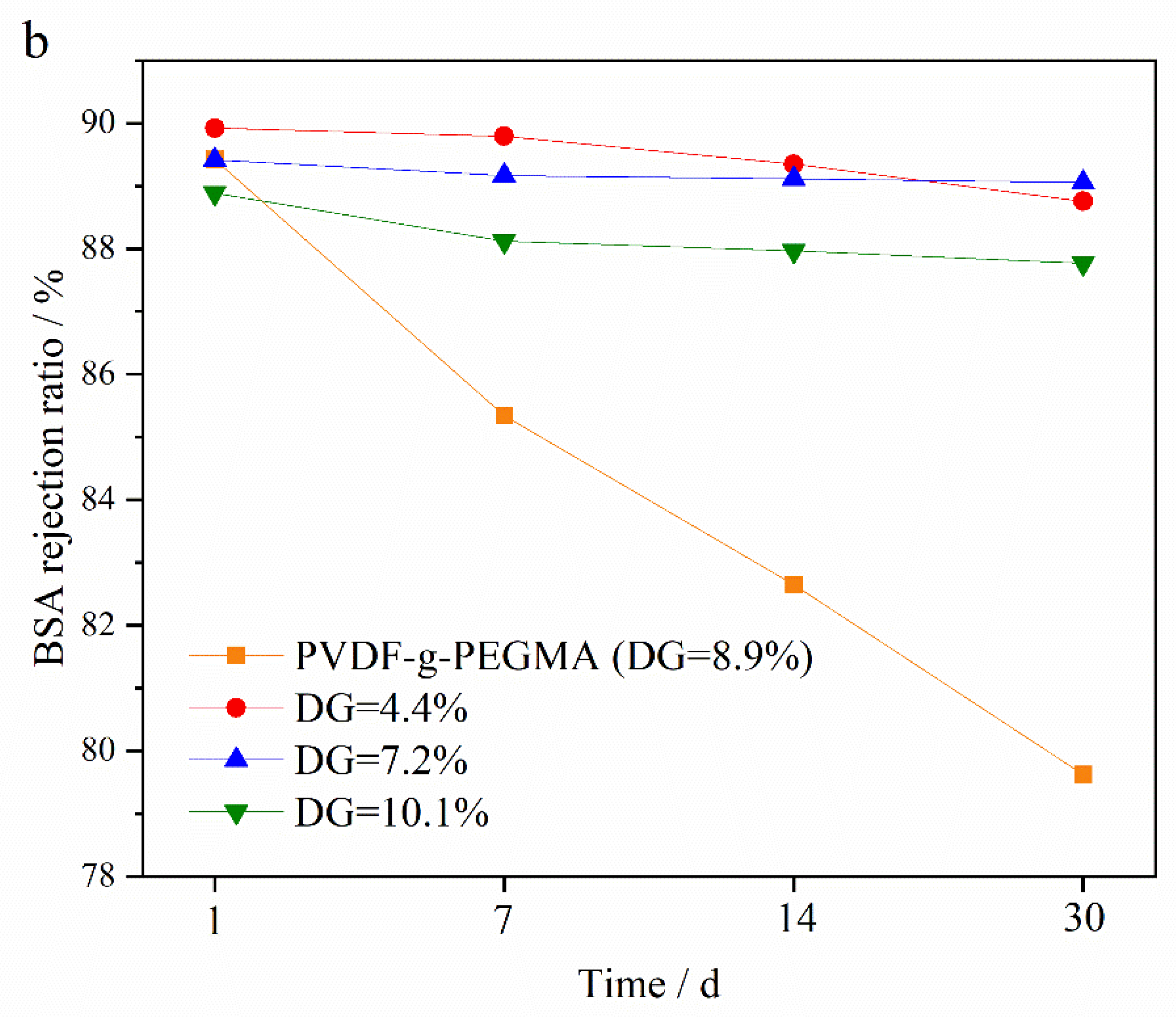
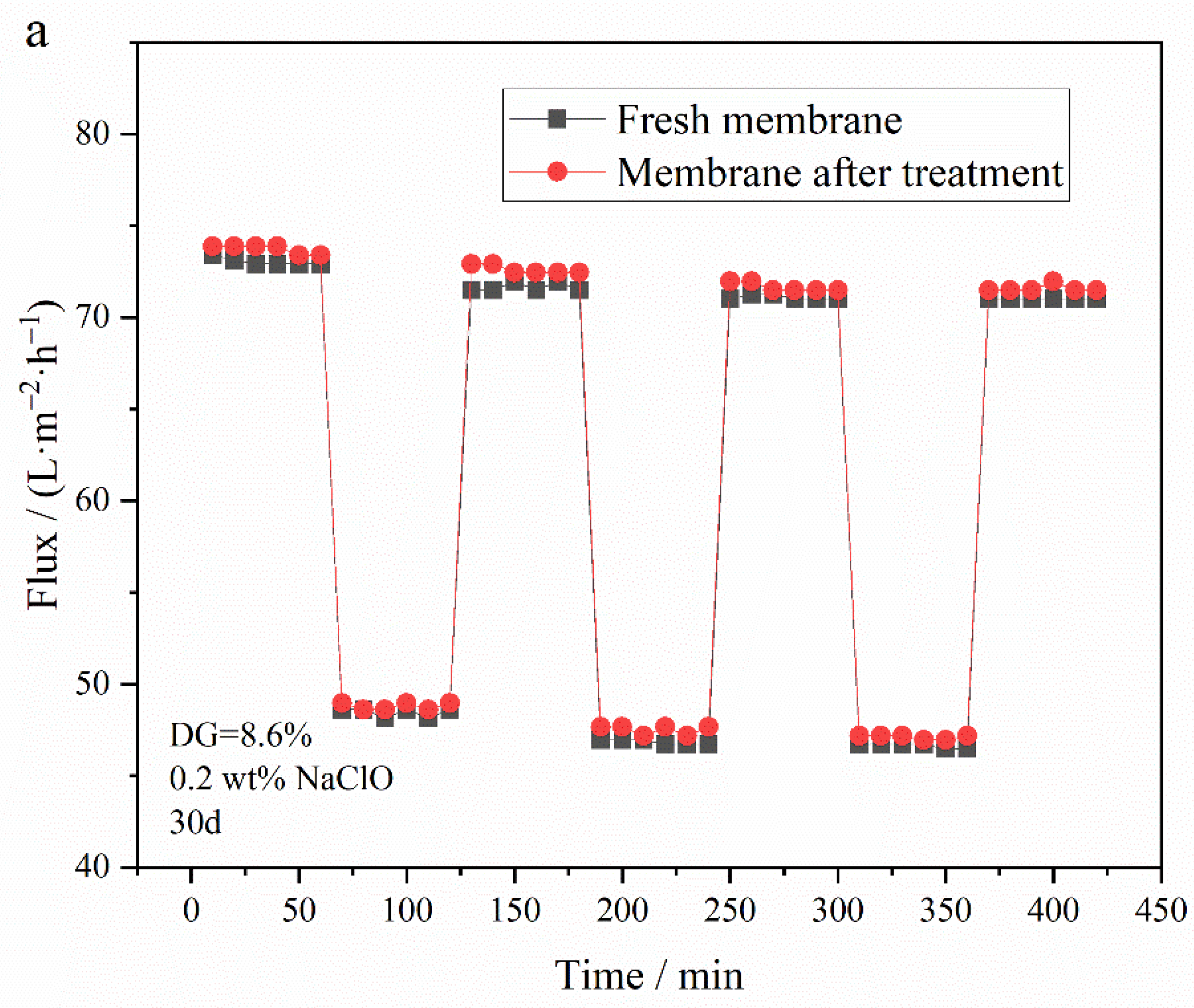
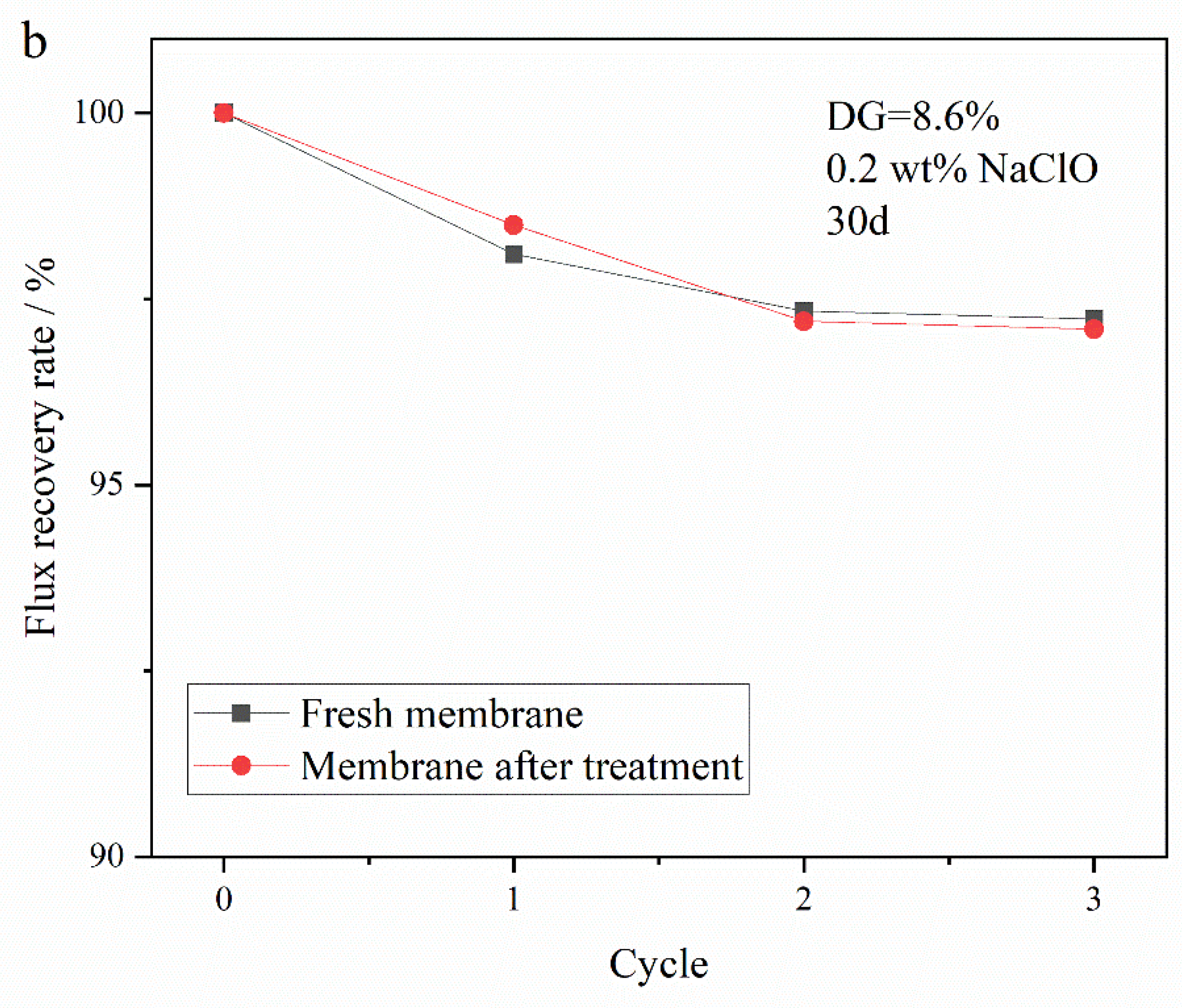
3.5.2. Oxidation Resistance of Membrane to NaClO Solution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Bi, Q.; Lin, H.-H.; Bian, L.-X.; Wang, X.-L. A Novel Ultrafiltration (UF) Membrane with Controllable Selectivity for Protein Separation. J. Membr. Sci. 2013, 427, 155–167. [Google Scholar] [CrossRef]
- Bai, T.; Zhao, K.; Lu, Z.; Liu, X.; Lin, Z.; Cheng, M.; Li, Z.; Zhu, D.; Zhang, L. Simple Fabrication of Cu2+ Doped Calcium Alginate Hydrogel Filtration Membrane with Excellent Anti-Fouling and Antibacterial Properties. Chin. Chem. Lett. 2021, 32, 1051–1054. [Google Scholar] [CrossRef]
- Imahase, H.; Sakamoto, Y.; Kusunose, M.; Koami, H.; Nishimura, Y.; Goto, A.; Yamashita, T.; Nakashima, A.; Iwamura, T.; Kutsukata, N. Examination of Blood Filtration Membrane Removal Ability of HMGB1. Crit. Care 2012, 16, P10. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-Based Nanomaterials Advance Biomedicine: A Review. Int. J. Mol. Sci. 2022, 23, 5405. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.; Ramakrishna, S. Applications of Polymer Nanofibers in Biomedicine and Biotechnology. ABAB 2005, 125, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Shen, Z. Nanocellulose Based Filtration Membrane in Industrial Waste Water Treatment: A Review. Materials 2021, 14, 5398. [Google Scholar] [CrossRef] [PubMed]
- Pronk, W.; Ding, A.; Morgenroth, E.; Derlon, N.; Desmond, P.; Burkhardt, M.; Wu, B.; Fane, A.G. Gravity-Driven Membrane Filtration for Water and Wastewater Treatment: A Review. Water Res. 2019, 149, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Madsen, H.T. Membrane Filtration in Water Treatment—Removal of Micropollutants. In Chemistry of Advanced Environmental Purification Processes of Water; Elsevier: Amsterdam, The Netherlands, 2014; pp. 199–248. ISBN 978-0-444-53178-0. [Google Scholar]
- Subasi, Y.; Cicek, B. Recent Advances in Hydrophilic Modification of PVDF Ultrafiltration Membranes—A Review: Part I. Membr. Technol. 2017, 2017, 7–12. [Google Scholar] [CrossRef]
- Al Aani, S.; Mustafa, T.N.; Hilal, N. Ultrafiltration Membranes for Wastewater and Water Process Engineering: A Comprehensive Statistical Review over the Past Decade. J. Water Process Eng. 2020, 35, 101241. [Google Scholar] [CrossRef]
- Ahmad, T.; Guria, C.; Mandal, A. A Review of Oily Wastewater Treatment Using Ultrafiltration Membrane: A Parametric Study to Enhance the Membrane Performance. J. Water Process Eng. 2020, 36, 101289. [Google Scholar] [CrossRef]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and Cleaning of Ultrafiltration Membranes: A Review. J. Water Process Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Arkhangelsky, E.; Kuzmenko, D.; Gitis, V. Impact of Chemical Cleaning on Properties and Functioning of Polyethersulfone Membranes. J. Membr. Sci. 2007, 305, 176–184. [Google Scholar] [CrossRef]
- Regula, C.; Carretier, E.; Wyart, Y.; Gésan-Guiziou, G.; Vincent, A.; Boudot, D.; Moulin, P. Chemical Cleaning/Disinfection and Ageing of Organic UF Membranes: A Review. Water Res. 2014, 56, 325–365. [Google Scholar] [CrossRef] [PubMed]
- Mahlicli, F.Y.; Altinkaya, S.A. Immobilization of Alpha Lipoic Acid onto Polysulfone Membranes to Suppress Hemodialysis Induced Oxidative Stress. J. Membr. Sci. 2014, 449, 27–37. [Google Scholar] [CrossRef]
- Chen, Q.; He, Y.; Zhao, Y.; Chen, L. Intervening Oxidative Stress Integrated with an Excellent Biocompatibility of Hemodialysis Membrane Fabricated by Nucleobase-Recognized Co-Immobilization Strategy of Tannic Acid, Looped PEtOx Brush and Heparin. J. Membr. Sci. 2021, 625, 119174. [Google Scholar] [CrossRef]
- Hasegawa, T.; Iwasaki, Y.; Ishihara, K. Preparation and Performance of Protein-Adsorption-Resistant Asymmetric Porous Membrane Composed of Polysulfone/Phospholipid Polymer Blend. Biomaterials 2001, 22, 243–251. [Google Scholar] [CrossRef]
- Qi, X.; Yang, N.; Luo, Y.; Jia, X.; Zhao, J.; Feng, X.; Chen, L.; Zhao, Y. Resveratrol as a Plant Type Antioxidant Modifier for Polysulfone Membranes to Improve Hemodialysis-Induced Oxidative Stress. Mater. Sci. Eng. C 2021, 123, 111953. [Google Scholar] [CrossRef]
- Myronchuk, V.; Dzyazko, Y.; Zmievskii, Y.; Ukrainets, A.; Bildukevich, A.; Kornienko, L.; Rozhdestvenskaya, L.; Palchik, A. Organic-Inorganic Membranes for Filtration of Corn Distillery. Acta Period. Technol. 2016, 47, 153–165. [Google Scholar] [CrossRef]
- Marjani, A.; Nakhjiri, A.T.; Adimi, M.; Jirandehi, H.F.; Shirazian, S. Effect of Graphene Oxide on Modifying Polyethersulfone Membrane Performance and Its Application in Wastewater Treatment. Sci. Rep. 2020, 10, 2049. [Google Scholar] [CrossRef]
- Jaber, L.; Almanassra, I.W.; Backer, S.N.; Kochkodan, V.; Shanableh, A.; Atieh, M.A. A Comparative Analysis of the Effect of Carbonaceous Nanoparticles on the Physicochemical Properties of Hybrid Polyethersulfone Ultrafiltration Membranes. Membranes 2022, 12, 1143. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Jaber, L.; Backer, S.N.; Chatla, A.; Kochkodan, V.; Al-Ansari, T.; Shanableh, A.; Atieh, M.A. Oxidized Carbide-Derived Carbon as a Novel Filler for Improved Antifouling Characteristics and Permeate Flux of Hybrid Polyethersulfone Ultrafiltration Membranes. Chemosphere 2023, 313, 137425. [Google Scholar] [CrossRef] [PubMed]
- Almanassra, I.W.; Jaber, L.; Chatla, A.; Abushawish, A.; Shanableh, A.; Ali Atieh, M. Unveiling the Relationship between MOF Porosity, Particle Size, and Polyethersulfone Membranes Properties for Efficient Decontamination of Dye and Organic Matter. Chem. Eng. J. 2023, 471, 144616. [Google Scholar] [CrossRef]
- Jaber, L.; Almanassra, I.W.; AbuShawish, A.; Chatla, A.; Ihsanullah, I.; Ali, M.M.; Manawi, Y.; Shanableh, A.; Atieh, M.A. Pioneering Biofouling Resistant PES UF Membrane with MnFe2O4/g-C3N4 Nanocomposite: Insight into Mechanisms and Fouling Dynamics. J. Membr. Sci. 2024, 691, 122259. [Google Scholar] [CrossRef]
- Wang, P.; Tan, K.L.; Kang, E.T.; Neoh, K.G. Synthesis, Characterization and Anti-Fouling Properties of Poly(Ethylene Glycol) Grafted Poly(Vinylidene Fluoride) Copolymer Membranes. J. Mater. Chem. 2001, 11, 783–789. [Google Scholar] [CrossRef]
- Hester, J.F.; Banerjee, P.; Won, Y.-Y.; Akthakul, A.; Acar, M.H.; Mayes, A.M. ATRP of Amphiphilic Graft Copolymers Based on PVDF and Their Use as Membrane Additives. Macromolecules 2002, 35, 7652–7661. [Google Scholar] [CrossRef]
- Liu, B.; Chen, C.; Li, T.; Crittenden, J.; Chen, Y. High Performance Ultrafiltration Membrane Composed of PVDF Blended with Its Derivative Copolymer PVDF-g-PEGMA. J. Membr. Sci. 2013, 445, 66–75. [Google Scholar] [CrossRef]
- Wang, T.; Hou, Z.; Yang, H.; Hu, J. A PEGylated PVDF Antifouling Membrane Prepared by Grafting of Methoxypolyethylene Glycol Acrylate in Gama-Irradiated Homogeneous Solution. Materials 2024, 17, 873. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Shih, Y.-J.; Ruaan, R.-C.; Higuchi, A.; Chen, W.-Y.; Lai, J.-Y. Preparation of Poly(Vinylidene Fluoride) Microfiltration Membrane with Uniform Surface-Copolymerized Poly(Ethylene Glycol) Methacrylate and Improvement of Blood Compatibility. J. Membr. Sci. 2008, 309, 165–174. [Google Scholar] [CrossRef]
- Chang, Y.; Ko, C.-Y.; Shih, Y.-J.; Quémener, D.; Deratani, A.; Wei, T.-C.; Wang, D.-M.; Lai, J.-Y. Surface Grafting Control of PEGylated Poly(Vinylidene Fluoride) Antifouling Membrane via Surface-Initiated Radical Graft Copolymerization. J. Membr. Sci. 2009, 345, 160–169. [Google Scholar] [CrossRef]
- Wang, P.; Tan, K.L.; Kang, E.T.; Neoh, K.G. Plasma-Induced Immobilization of Poly(Ethylene Glycol) onto Poly(Vinylidene Fluoride) Microporous Membrane. J. Membr. Sci. 2002, 195, 103–114. [Google Scholar] [CrossRef]
- Liu, F.; Du, C.-H.; Zhu, B.-K.; Xu, Y.-Y. Surface Immobilization of Polymer Brushes onto Porous Poly(Vinylidene Fluoride) Membrane by Electron Beam to Improve the Hydrophilicity and Fouling Resistance. Polymer 2007, 48, 2910–2918. [Google Scholar] [CrossRef]
- Guo, H.; Li, C.; Zhu, L.; Wang, Y.; Zeng, Z.; Wang, L.; Xue, Q. Preparation of PEGylated Poly(Vinylidene Fluoride) Porous Membranes with Improved Antifouling Property. Surf. Topogr. Metrol. Prop. 2019, 7, 014002. [Google Scholar] [CrossRef]
- Zhang, M.; Nguyen, Q.T.; Ping, Z. Hydrophilic Modification of Poly (Vinylidene Fluoride) Microporous Membrane. J. Membr. Sci. 2009, 327, 78–86. [Google Scholar] [CrossRef]
- Gao, F.; Wang, J.; Zhang, H.; Hang, M.A.; Cui, Z.; Yang, G. Interaction Energy and Competitive Adsorption Evaluation of Different NOM Fractions on Aged Membrane Surfaces. J. Membr. Sci. 2017, 542, 195–207. [Google Scholar] [CrossRef]
- Li, S.; Chen, H.; Zhao, X.; Lucia, L.A.; Liang, C.; Liu, Y. Impact Factors for Flux Decline in Ultrafiltration of Lignocellulosic Hydrolysis Liquor. Sep. Purif. Technol. 2020, 240, 116597. [Google Scholar] [CrossRef]
- Robinson, S.; Abdullah, S.Z.; Bérubé, P.; Le-Clech, P. Ageing of Membranes for Water Treatment: Linking Changes to Performance. J. Membr. Sci. 2016, 503, 177–187. [Google Scholar] [CrossRef]
- Rabuni, M.F.; Nik Sulaiman, N.M.; Aroua, M.K.; Yern Chee, C.; Awanis Hashim, N. Impact of in Situ Physical and Chemical Cleaning on PVDF Membrane Properties and Performances. Chem. Eng. Sci. 2015, 122, 426–435. [Google Scholar] [CrossRef]
- Kumar, A.P.; Depan, D.; Singh Tomer, N.; Singh, R.P. Nanoscale Particles for Polymer Degradation and Stabilization—Trends and Future Perspectives. Prog. Polym. Sci. 2009, 34, 479–515. [Google Scholar] [CrossRef]
- Liu, W.-W.; Chai, S.-P.; Mohamed, A.R.; Hashim, U. Synthesis and Characterization of Graphene and Carbon Nanotubes: A Review on the Past and Recent Developments. J. Ind. Eng. Chem. 2014, 20, 1171–1185. [Google Scholar] [CrossRef]
- Yu, T.; Meng, L.; Zhao, Q.-B.; Shi, Y.; Hu, H.-Y.; Lu, Y. Effects of Chemical Cleaning on RO Membrane Inorganic, Organic and Microbial Foulant Removal in a Full-Scale Plant for Municipal Wastewater Reclamation. Water Res. 2017, 113, 1–10. [Google Scholar] [CrossRef]
- Hajibabania, S.; Antony, A.; Leslie, G.; Le-Clech, P. Relative Impact of Fouling and Cleaning on PVDF Membrane Hydraulic Performances. Sep. Purif. Technol. 2012, 90, 204–212. [Google Scholar] [CrossRef]
- Gao, F.; Wang, J.; Zhang, H.; Zhang, Y.; Hang, M.A. Effects of Sodium Hypochlorite on Structural/Surface Characteristics, Filtration Performance and Fouling Behaviors of PVDF Membranes. J. Membr. Sci. 2016, 519, 22–31. [Google Scholar] [CrossRef]
- Abdullah, S.Z.; Bérubé, P.R. Assessing the Effects of Sodium Hypochlorite Exposure on the Characteristics of PVDF Based Membranes. Water Res. 2013, 47, 5392–5399. [Google Scholar] [CrossRef] [PubMed]
- Awanis Hashim, N.; Liu, Y.; Li, K. Stability of PVDF Hollow Fibre Membranes in Sodium Hydroxide Aqueous Solution. Chem. Eng. Sci. 2011, 66, 1565–1575. [Google Scholar] [CrossRef]
- Rouaix, S.; Causserand, C.; Aimar, P. Experimental Study of the Effects of Hypochlorite on Polysulfone Membrane Properties. J. Membr. Sci. 2006, 277, 137–147. [Google Scholar] [CrossRef]
- Causserand, C.; Rouaix, S.; Lafaille, J.-P.; Aimar, P. Ageing of Polysulfone Membranes in Contact with Bleach Solution: Role of Radical Oxidation and of Some Dissolved Metal Ions. Chem. Eng. Process. Process Intensif. 2008, 47, 48–56. [Google Scholar] [CrossRef]
- Kwon, Y.; Leckie, J. Hypochlorite Degradation of Crosslinked Polyamide membranesII. Changes in Hydrogen Bonding Behavior and Performance. J. Membr. Sci. 2006, 282, 456–464. [Google Scholar] [CrossRef]
- Gaudichetmaurin, E.; Thominette, F. Ageing of Polysulfone Ultrafiltration Membranes in Contact with Bleach Solutions. J. Membr. Sci. 2006, 282, 198–204. [Google Scholar] [CrossRef]
- Wolff, S.H.; Zydney, A.L. Effect of Bleach on the Transport Characteristics of Polysulfone Hemodialyzers. J. Membr. Sci. 2004, 243, 389–399. [Google Scholar] [CrossRef]
- Mohammadi, T.; Madaeni, S.S.; Moghadam, M.K. Investigation of Membrane Fouling. Desalination 2003, 153, 155–160. [Google Scholar] [CrossRef]
- Trägårdh, G. Membrane Cleaning. Desalination 1989, 71, 325–335. [Google Scholar] [CrossRef]
- Cota, D.; Patil, D. Antibacterial Potential of Ellagic Acid and Gallic Acid against IBD Bacterial Isolates and Cytotoxicity against Colorectal Cancer. Nat. Prod. Res. 2023, 37, 1998–2002. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tang, G.; Zhang, C.; Wang, N.; Feng, Y. Gallic Acid and Diabetes Mellitus: Its Association with Oxidative Stress. Molecules 2021, 26, 7115. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic Acid: Pharmacological Activities and Molecular Mechanisms Involved in Inflammation-Related Diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Toledo, R.T. Major Flavonoids in Grape Seeds and Skins: Antioxidant Capacity of Catechin, Epicatechin, and Gallic Acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef]
- Kumaran, A.; Joel Karunakaran, R. Antioxidant and Free Radical Scavenging Activity of an Aqueous Extract of Coleus Aromaticus. Food Chem. 2006, 97, 109–114. [Google Scholar] [CrossRef]
- Ferrari, C.K.B.; Torres, E.A.F.S. Biochemical Pharmacology of Functional Foods and Prevention of Chronic Diseases of Aging. Biomed. Pharmacother. 2003, 57, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, C.; Rosso, R.; Santos-Silva, M.C.; De Souza, C.A.; Licínio, M.A.; Leal, P.; Bazzo, M.L.; Yunes, R.A.; Creczynski-Pasa, T.B. Ester Derivatives of Gallic Acid with Potential Toxicity toward L1210 Leukemia Cells. Bioorg. Med. Chem. 2008, 16, 3791–3799. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, H.; Pal, A.; Bai, Y.; Shao, L. Biomimetic Silicification on Membrane Surface for Highly Efficient Treatments of Both Oil-in-Water Emulsion and Protein Wastewater. ACS Appl. Mater. Interfaces 2018, 10, 29982–29991. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Wang, Z.X.; Zhang, Y.; Zhang, Y.; Ma, J.; Shao, L. Bio-Inspired Loose Nanofiltration Membranes with Optimized Separation Performance for Antibiotics Removals. J. Membr. Sci. 2018, 554, 385–394. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Z. A Loose Nano-Filtration Membrane Prepared by Coating HPAN UF Membrane with Modified PEI for Dye Reuse and Desalination. J. Membr. Sci. 2017, 524, 214–224. [Google Scholar] [CrossRef]
- Rajesh, S.; Murthy, Z.V.P. Synthesis, Characterization and Application of Antioxidants Nanoparticles Incorporated Polymeric Membranes. Sep. Sci. Technol. 2019, 54, 247–257. [Google Scholar] [CrossRef]
- Teotia, R.; Verma, S.K.; Kalita, D.; Singh, A.K.; Dahe, G.; Bellare, J. Porosity and Compatibility of Novel Polysulfone-/Vitamin E-TPGS-Grafted Composite Membrane. J. Mater. Sci. 2017, 52, 12513–12523. [Google Scholar] [CrossRef]
- Buchmüller, Y.; Wokaun, A.; Gubler, L. Polymer-Bound Antioxidants in Grafted Membranes for Fuel Cells. J. Mater. Chem. A 2014, 2, 5870–5882. [Google Scholar] [CrossRef]
- Noble, J.E.; Bailey, M.J.A. Chapter 8 Quantitation of Protein. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 463, pp. 73–95. ISBN 978-0-12-374536-1. [Google Scholar]
- Uğuzdoğan, E.; Denkbaş, E.B.; Öztürk, E.; Tuncel, S.A.; Kabasakal, O.S. Preparation and Characterization of Polyethyleneglycolmethacrylate (PEGMA)-Co-Vinylimidazole (VI) Microspheres to Use in Heavy Metal Removal. J. Hazard. Mater. 2009, 162, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Gálico, D.A.; Nova, C.V.; Guerra, R.B.; Bannach, G. Thermal and Spectroscopic Studies of the Antioxidant Food Additive Propyl Gallate. Food Chem. 2015, 182, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Smolders, C.A.; Reuvers, A.J.; Boom, R.M.; Wienk, I.M. Microstructures in Phase-Inversion Membranes. Part 1. Formation of Macrovoids. J. Membr. Sci. 1992, 73, 259–275. [Google Scholar] [CrossRef]
- Lee, J.; Park, B.; Kim, J.; Park, S.B. Effect of PVP, Lithium Chloride, and Glycerol Additives on PVDF Dual-Layer Hollow Fiber Membranes Fabricated Using Simultaneous Spinning of TIPS and NIPS. Macromol. Res. 2015, 23, 291–299. [Google Scholar] [CrossRef]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; Di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the Non-Solvent Induced Phase Separation (NIPS) Effect during the Fabrication of Microporous PVDF Membranes via Thermally Induced Phase Separation (TIPS). J. Membr. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Yu, L.; Yang, F.; Xiang, M. Phase Separation in a PSf/DMF/Water System: A Proposed Mechanism for Macrovoid Formation. RSC Adv. 2014, 4, 42391–42402. [Google Scholar] [CrossRef]
- Ying, L.; Kang, E.T.; Neoh, K.G. Covalent Immobilization of Glucose Oxidase on Microporous Membranes Prepared from Poly(Vinylidene Fluoride) with Grafted Poly(Acrylic Acid) Side Chains. J. Membr. Sci. 2002, 208, 361–374. [Google Scholar] [CrossRef]




| No. | PEGMA/g | TsOH/g | PG/g | PVDF/g | DMF/g | Total/g |
|---|---|---|---|---|---|---|
| 1 | 3 | 0.08 | 4.24 | 5 | 37.68 | 50 |
| 2 | 5 | 0.08 | 7.07 | 5 | 32.85 | 50 |
| 3 | 9 | 0.08 | 12.72 | 5 | 24 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Hu, J.; Hou, Z.; Yang, H. Antifouling and Antioxidant Properties of PVDF Membrane Modified with Polyethylene Glycol Methacrylate and Propyl Gallate. Materials 2024, 17, 1867. https://doi.org/10.3390/ma17081867
Wang T, Hu J, Hou Z, Yang H. Antifouling and Antioxidant Properties of PVDF Membrane Modified with Polyethylene Glycol Methacrylate and Propyl Gallate. Materials. 2024; 17(8):1867. https://doi.org/10.3390/ma17081867
Chicago/Turabian StyleWang, Ting, Jun Hu, Zhengchi Hou, and Haijun Yang. 2024. "Antifouling and Antioxidant Properties of PVDF Membrane Modified with Polyethylene Glycol Methacrylate and Propyl Gallate" Materials 17, no. 8: 1867. https://doi.org/10.3390/ma17081867






