Synthesis and Catalytic Performance of High-Entropy Rare-Earth Perovskite Nanofibers: (Y0.2La0.2Nd0.2Gd0.2Sm0.2)CoO3 in Low-Temperature Carbon Monoxide Oxidation
Abstract
:1. Introduction
2. Materials and Method
2.1. Synthesis
2.2. Material Characterization
2.3. CO Oxidation Tests
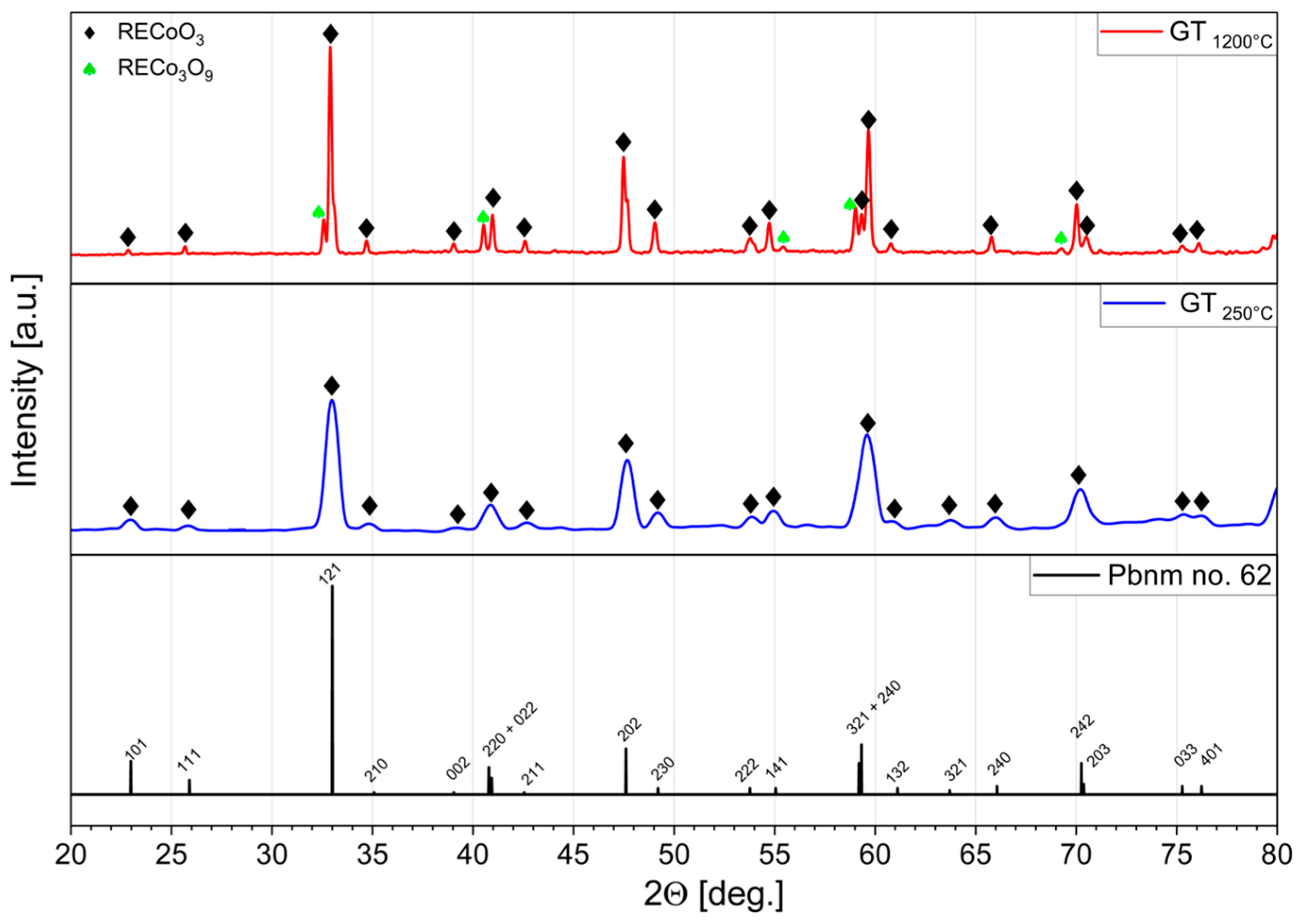
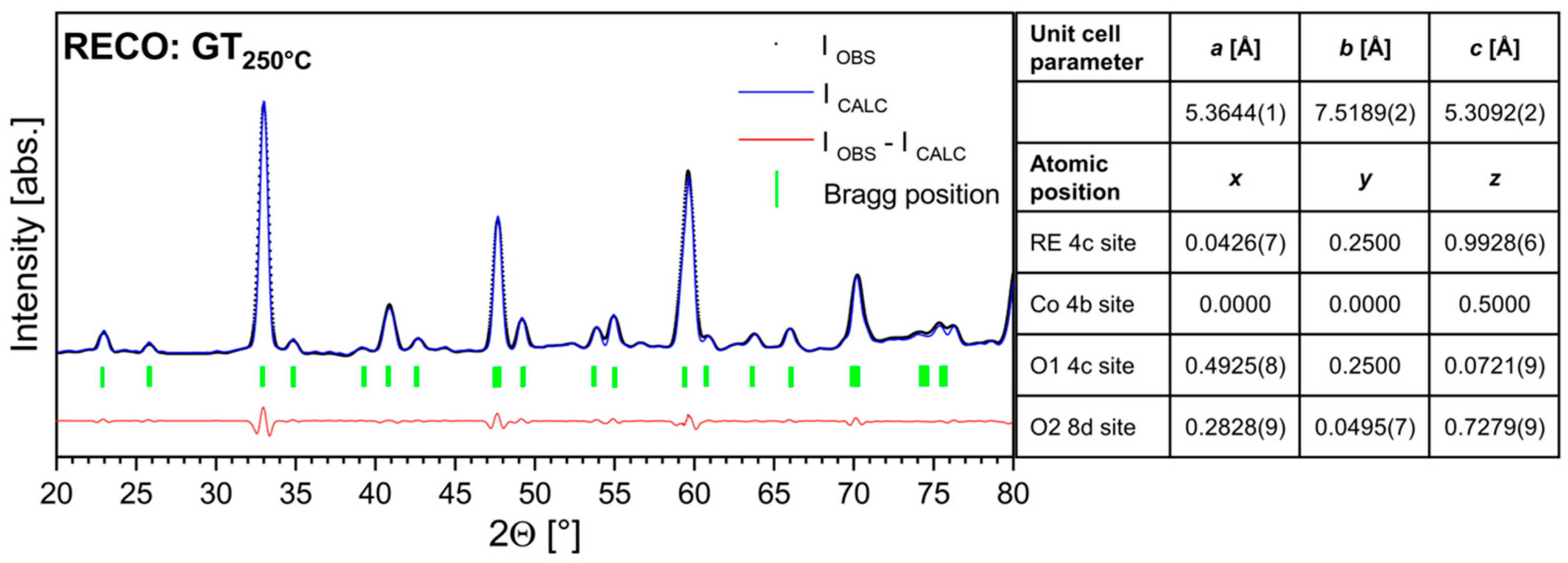
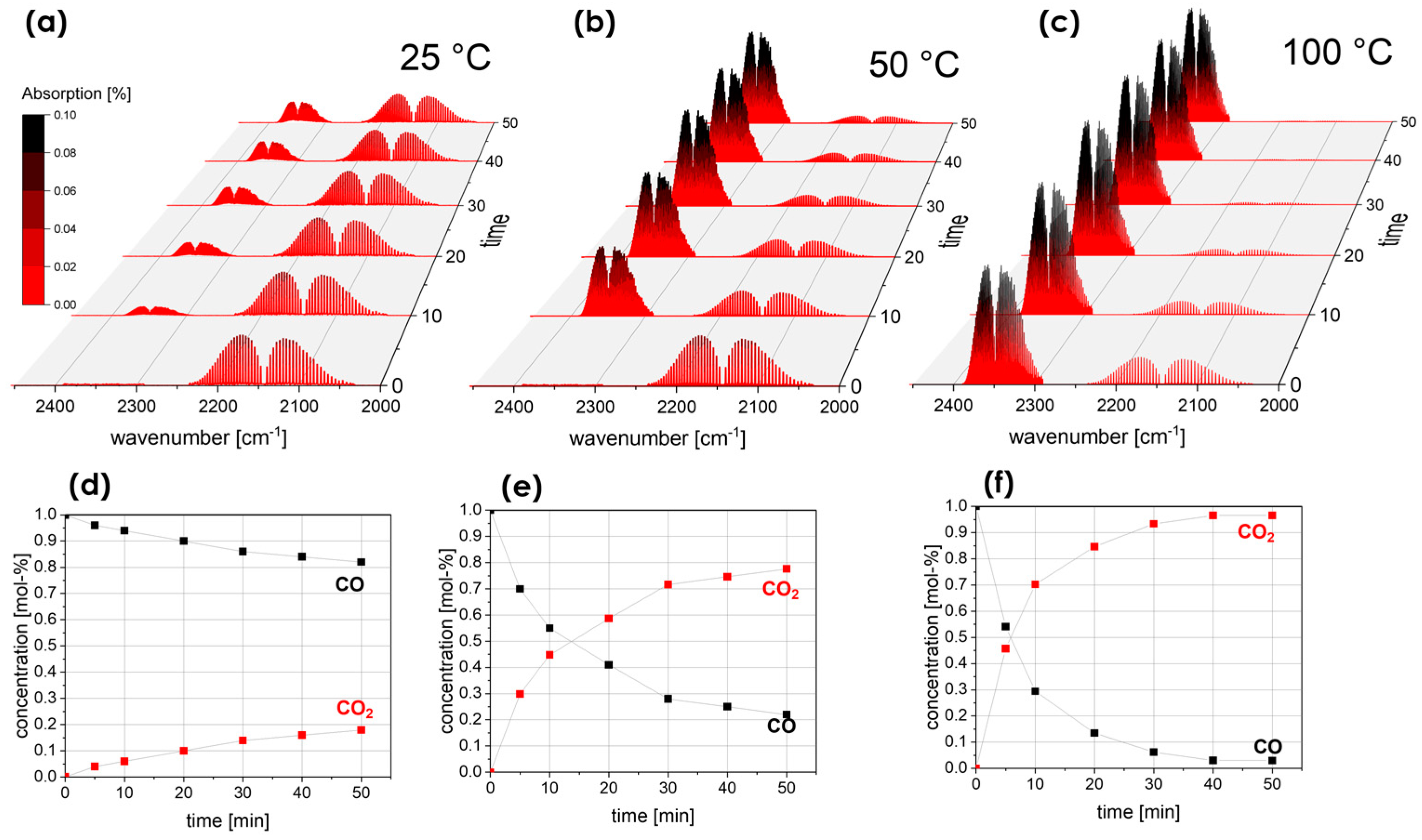
3. Results
4. Discussion
5. Conclusions
- The glycothermal method, carried out at a reduced temperature of only 250 °C, means a significant reduction in energy consumption of approximately 52% compared to typical high-temperature synthesis methods above 1000 °C. This low-temperature approach to synthesis demonstrates energy efficiency and sustainability, adapting to the growing demand for environmentally friendly synthesis methods.
- The employment of 1,4-butanediol and diethylene glycol in a glycothermal process shows significant potential for the fabrication of nanofibers from (Y0.2La0.2Nd0.2Gd0.2Sm0.2)CoO3 material. This nanostructuring method holds promise for the efficient and facile synthesis of high-entropy nanomaterials and composites.
- Catalytic evaluation highlights the effectiveness of (Y0.2La0.2Nd0.2Gd0.2Sm0.2)CoO3 in oxidizing CO under mild conditions, showing conversion rates of 78% and 97% at 50 °C and 100 °C, respectively, demonstrating its versatility in various catalytic processes. The inclusion of rare-earth elements in the high-entropy composition improves its catalytic properties, suggesting a synergistic effect that can significantly improve performance.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sherif, Z.; Sarfraz, S.; Jolly, M.; Salonitis, K. Identification of the Right Environmental KPIs for Manufacturing Operations: Towards a Continuous Sustainability Framework. Materials 2022, 15, 7690. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chowdhury, S.; Tiwary, C.S. High-Entropy-Based Nano-Materials for Sustainable Environmental Applications. Nanoscale 2024. [Google Scholar] [CrossRef] [PubMed]
- Akrami, S.; Edalati, P.; Fuji, M.; Edalati, K. High-Entropy Ceramics: Review of Principles, Production and Applications. Mater. Sci. Eng. R Rep. 2021, 146, 100644. [Google Scholar] [CrossRef]
- Rost, C.M. Entropy-Stabilized Oxides: Explorations of a Novel Class of Multicomponent Materials. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2016. [Google Scholar]
- Djenadic, R.; Sarkar, A.; Clemens, O.; Loho, C.; Botros, M.; Chakravadhanula, V.S.K.; Kübel, C.; Bhattacharya, S.S.; Gandhi, A.S.; Hahn, H. Multicomponent Equiatomic Rare Earth Oxides. Mater. Res. Lett. 2017, 5, 102–109. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, J.; Zhang, W.; Zheng, Y.; Zhao, W.; Xue, L.; Yang, F.; Chen, H. A Promising High-Entropy Thermal Barrier Material with the Formula (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12. Materials 2022, 15, 8079. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Chatterjee, S.; Ranjan, M.; Bhattacharya, T.; Mukherjee, S.; Jana, S.S.; Dwivedi, A.; Maiti, T. High-Entropy Perovskites: An Emergent Class of Oxide Thermoelectrics with Ultralow Thermal Conductivity. ACS Sustain. Chem. Eng. 2020, 8, 17022–17032. [Google Scholar] [CrossRef]
- Sarkar, A.; Djenadic, R.; Wang, D.; Hein, C.; Kautenburger, R.; Clemens, O.; Hahn, H. Rare Earth and Transition Metal Based Entropy Stabilised Perovskite Type Oxides. J. Eur. Ceram. Soc. 2018, 38, 2318–2327. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Wang, Q.; Schweidler, S.; Botros, M.; Fu, T.; Hahn, H.; Brezesinski, T.; Breitung, B. High-Entropy Energy Materials: Challenges and New Opportunities. Energy Environ. Sci. 2021, 14, 2883–2905. [Google Scholar] [CrossRef]
- Xu, Z.; Du, Z.; Zhang, R.; Zeng, F.; Meng, Z.; Hu, X.; Tian, H. Regulating the Lattice Strain Field by High-Entropy Strategy to Realize the Conformal Growth of Perovskites for Efficient Oxygen Evolution. Appl. Catal. B Environ. Energy 2024, 344, 123668. [Google Scholar] [CrossRef]
- Salian, A.; Mandal, S. Review on the Deposition, Structure and Properties of High-entropy Oxide Films: Current and Future Perspectives. Bull. Mater. Sci. 2022, 45, 49. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, Z.; Song, Y.; Yang, G.; Qian, W.; Yang, M.; Zhu, Y.; Ran, R.; Wang, W.; Zhou, W.; et al. High-Entropy Perovskite Oxide: A New Opportunity for Developing Highly Active and Durable Air Electrode for Reversible Protonic Ceramic Electrochemical Cells. Nano-Micro Lett. 2022, 14, 217. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Jiang, J.; Yan, C.; Lin, Y.; Mortazavi, M.; Kaul, A.B.; Jiang, Q. Progress and Application of Halide Perovskite Materials for Solar Cells and Light Emitting Devices. Nanomaterials 2024, 14, 391. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Ding, J.; Xi, G.; Li, H.; Yang, Q.; Tian, J.; Zhang, L. Controllable Chemical Composition in Double-Perovskite Bi0.5Sm0.5FeO3 Epitaxial Thin Films for Ferroelectric, Photovoltaic, and Ferromagnetic Properties. Chem. Eng. J. 2023, 453, 139726. [Google Scholar] [CrossRef]
- Zhang, P.; Shi, Y.; Zhang, Y.; Feng, S.; Shi, L.; Pan, J.; Cao, J.; Li, C. Self-Cleaning Transparent Pn Junction in CuAlO2/WO3 via High-entropy Perovskite Oxide La(Cu0.2Cr0.2Ni0.2Fe0.2Co0.2)O3 Transition Layer for Enhanced Photovoltaic Conversion. Chem. Eng. J. 2024, 487, 150727. [Google Scholar] [CrossRef]
- Witte, R.; Sarkar, A.; Kruk, R.; Eggert, B.; Brand, R.A.; Wende, H.; Hahn, H. High-Entropy Oxides: An Emerging Prospect for Magnetic Rare-Earth Transition Metal Perovskites. Phys. Rev. Mater. 2019, 3, 034406. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Liang, Z.; Ning, H.; Fu, X.; Xu, Z.; Qiu, T.; Xu, W.; Yao, R.; Peng, J. High-Entropy Oxides: Advanced Research on Electrical Properties. Coatings 2021, 11, 628. [Google Scholar] [CrossRef]
- Qi, H.; Chen, L.; Deng, S.; Chen, J. High-Entropy Ferroelectric Materials. Nat. Rev. Mater. 2023, 1–2. [Google Scholar] [CrossRef]
- Schweidler, S.; Botros, M.; Strauss, F.; Wang, Q.; Ma, Y.; Velasco, L.; Cadilha Marques, G.; Sarkar, A.; Kübel, C.; Hahn, H.; et al. High-Entropy Materials for Energy and Electronic Applications. Nat. Rev. Mater. 2024, 9, 266–281. [Google Scholar] [CrossRef]
- Esquius, J.R.; Liu, L. High-entropy Materials as Emerging Electrocatalysts for Hydrogen Production through Low-Temperature Water Electrolysis. Mater. Futures 2023, 2, 022102. [Google Scholar] [CrossRef]
- Li, X.; Qiang, Z.; Han, G.; Guan, S.; Zhao, Y.; Lou, S.; Zhu, Y. Enhanced Redox Electrocatalysis in High-Entropy Perovskite Fluorides by Tailoring d–p Hybridization. Nano-Micro Lett. 2023, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Albedwawi, S.H.; AlJaberi, A.; Haidemenopoulos, G.N.; Polychronopoulou, K. High-entropy Oxides-Exploring a Paradigm of Promising Catalysts: A Review. Mater. Des. 2021, 202, 109534. [Google Scholar] [CrossRef]
- Xu, X.; Shao, Z.; Jiang, S.P. High-Entropy Materials for Water Electrolysis. Energy Technol. 2022, 10, 2200573. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef] [PubMed]
- Vashishtha, P.; Bishnoi, S.; Li, C.-H.A.; Jagadeeswararao, M.; Hooper, T.J.N.; Lohia, N.; Shivarudraiah, S.B.; Ansari, M.S.; Sharma, S.N.; Halpert, J.E. Recent Advancements in Near-Infrared Perovskite Light-Emitting Diodes. ACS Appl. Electron. Mater. 2020, 2, 3470–3490. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Song, Y.; Yu, J.; Tian, Y.; Robson, M.J.; Wang, J.; Zhang, Z.; Lin, X.; Zhou, G.; et al. High-Entropy Perovskites for Energy Conversion and Storage: Design, Synthesis, and Potential Applications. Small Methods 2023, 7, 2201138. [Google Scholar] [CrossRef] [PubMed]
- Vinnik, D.A.; Zhivulin, V.E.; Trofimov, E.A.; Gudkova, S.A.; Punda, A.Y.; Valiulina, A.N.; Gavrilyak, M.; Zaitseva, O.V.; Taskaev, S.V.; Khandaker, M.U.; et al. A-Site Cation Size Effect on Structure and Magnetic Properties of Sm(Eu,Gd)Cr0.2Mn0.2Fe0.2Co0.2Ni0.2O3 High-Entropy Solid Solutions. Nanomaterials 2022, 12, 36. [Google Scholar] [CrossRef]
- Zhang, R.-Z.; Reece, M.J. Review of High-entropy Ceramics: Design, Synthesis, Structure and Properties. J. Mater. Chem. A 2019, 7, 22148–22162. [Google Scholar] [CrossRef]
- Mao, A.; Xiang, H.-Z.; Zhang, Z.-G.; Kuramoto, K.; Yu, H.; Ran, S. Solution Combustion Synthesis and Magnetic Property of Rock-Salt (Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O High-Entropy Oxide Nanocrystalline Powder. J. Magn. Magn. Mater. 2019, 484, 245–252. [Google Scholar] [CrossRef]
- Okejiri, F.; Zhang, Z.; Liu, J.; Liu, M.; Yang, S.; Dai, S. Room-Temperature Synthesis of High-Entropy Perovskite Oxide Nanoparticle Catalysts through Ultrasonication-Based Method. ChemSusChem 2020, 13, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jie, K.; Jafta, C.J.; Yang, Z.; Yao, S.; Liu, M.; Zhang, Z.; Liu, J.; Chi, M.; Fu, J.; et al. An Ultrastable Heterostructured Oxide Catalyst Based on High-Entropy Materials: A New Strategy toward Catalyst Stabilization via Synergistic Interfacial Interaction. Appl. Catal. B Environ. 2020, 276, 119155. [Google Scholar] [CrossRef]
- Haupert, T.; Hanson, D.; Savio, L. The Application of New Diagnostic Protocols for the Condition-Based Assessment of High-Voltage Electrical Equipment. In Proceedings of the Conference Record of the 2000 IEEE International Symposium on Electrical Insulation (Cat. No.00CH37075), Graz, Austria, 12 July 2000; pp. 377–381. [Google Scholar]
- Wang, Y.; Mi, J.; Wu, Z.-S. Recent Status and Challenging Perspective of High-entropy Oxides for Chemical Catalysis. Chem. Catal. 2022, 2, 1624–1656. [Google Scholar] [CrossRef]
- Neha; Singh, H.; Singh, S.V. Insights into the Interface of NiCo2O4 Spinel/LaCoO3 Perovskite Nano-Composite for CO and Soot Oxidation. J. Environ. Sci. 2024, 138, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, P.A.; Jurczyszyn, M.; Pawlak, J.; Salamon, W.; Baran, P.; Kmita, A.; Gondek, Ł.; Sikora, M.; Kapusta, C.; Strączek, T.; et al. High-Entropy Perovskites as Multifunctional Metal Oxide Semiconductors: Synthesis and Characterization of (Gd0.2Nd0.2La0.2Sm0.2Y0.2)CoO3. ACS Appl. Electron. Mater. 2020, 2, 3211–3220. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Nishikawa, T.; Nakamura, T.; Inui, T. Glycothermal Reaction of Rare-Earth Acetate and Iron Acetylacetonate: Formation of Hexagonal ReFeO3. J. Am. Ceram. Soc. 1997, 80, 2157–2160. [Google Scholar] [CrossRef]
- Zhang, Y. Preparation Methods of High-Entropy Materials. In High-Entropy Materials: A Brief Introduction; Zhang, Y., Ed.; Springer: Singapore, 2019; pp. 65–75. ISBN 9789811385261. [Google Scholar]
- Rodriguez-Carvajal, J. Recent Developments of the Program FULLPROF, Commission on Powder Diffraction. IUCr Newsl. 2001, 26, 12–19. [Google Scholar]
- Blasco, J.; Stankiewicz, J.; García, J. Phase Segregation in the Gd1−xSrxFeO3−δ Series. J. Solid State Chem. 2006, 179, 898–908. [Google Scholar] [CrossRef]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-Ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef] [PubMed]
- Morris, M. Standard X-ray Diffraction Powder Patterns: Section 21—Data for 92 Substances; National Institute of Standards and Technology, Gaithersburg, MD, USA, 1985.
- Nie, S.; Wu, L.; Zhao, L.; Zhang, P. Enthalpy-Change Driven Synthesis of High-Entropy Perovskite Nanoparticles. Nano Res. 2022, 15, 4867–4872. [Google Scholar] [CrossRef]
- Ball, J.M.; Lee, M.M.; Hey, A.; Snaith, H.J. Low-Temperature Processed Meso-Superstructured to Thin-Film Perovskite Solar Cells. Energy Environ. Sci. 2013, 6, 1739–1743. [Google Scholar] [CrossRef]
- Juhre, K. Long-Term Performance and Decomposition of Fluoronitrile-Containing Gas Mixtures in Gas-Insulated Systems. Available online: https://cigre.org.uk/web-cont1001/uploads/Long-term-performance-and-decomposition-of-Fluoronitrile-containing-gas-mixtures-in-gas-insulated-systems.pdf (accessed on 3 March 2024).
- Li, Y.; Zhang, X.; Zhang, J.; Xie, C.; Shao, X.; Wang, Z.; Chen, D.; Xiao, S. Study on the Thermal Decomposition Characteristics of C4F7N–CO2 Mixture as Eco-Friendly Gas-Insulating Medium. High Volt. 2020, 5, 46–52. [Google Scholar] [CrossRef]
- Tan, Q.; Song, B.; Wang, L.; Wu, S. Study on Characteristics Gases of Metal Protrusions Partial Discharge in 10-kV Air-Insulated Switchgear. IET Sci. Meas. Technol. 2023, 17, 221–229. [Google Scholar] [CrossRef]
- Inchausti, J.M.; Arostegui, J.; Sebastián, S.; De-La-Cruz, M.; Izcara, J.; Sanchez, J.A. Assessing real alternatives to MV SF6 gas-insulated switchgear. In Proceedings of the CIRED 2021—The 26th International Conference and Exhibition on Electricity Distribution, Online Conference, 20–23 September 2021; pp. 233–237. [Google Scholar] [CrossRef]
- Materials Compatibility Study of C4F7N/CO2 Gas Mixture for Medium-Voltage Switchgear|IEEE Journals & Magazine|IEEE Xplore. Available online: https://ieeexplore.ieee.org/abstract/document/9697079 (accessed on 4 March 2024).
- Dey, S.; Mehta, N.S. Low Temperature Catalytic Conversion of Carbon Monoxide by the Application of Novel Perovskite Catalysts. Sci. One Health 2022, 1, 100002. [Google Scholar] [CrossRef]
- Wang, X.; Huang, K.; Yuan, L.; Xi, S.; Yan, W.; Geng, Z.; Cong, Y.; Sun, Y.; Tan, H.; Wu, X.; et al. Activation of Surface Oxygen Sites in a Cobalt-Based Perovskite Model Catalyst for CO Oxidation. J. Phys. Chem. Lett. 2018, 9, 4146–4154. [Google Scholar] [CrossRef] [PubMed]
- Glisenti, A.; Vittadini, A. On the Effects of Doping on the Catalytic Performance of (La,Sr)CoO3. A DFT Study of CO Oxidation. Catalysts 2019, 9, 312. [Google Scholar] [CrossRef]
- Liu, W.; Flytzani-Stephanopoulos, M. Transition Metal-Promoted Oxidation Catalysis by Fluorite Oxides: A Study of CO Oxidation over CuCeO2. Chem. Eng. J. Biochem. Eng. J. 1996, 64, 283–294. [Google Scholar] [CrossRef]
- Aamlid, S.S.; Oudah, M.; Rottler, J.; Hallas, A.M. Understanding the Role of Entropy in High-entropy Oxides. J. Am. Chem. Soc. 2023, 145, 5991–6006. [Google Scholar] [CrossRef] [PubMed]
- Fracchia, M.; Coduri, M.; Ghigna, P.; Anselmi-Tamburini, U. Phase Stability of High-entropy Oxides: A Critical Review. J. Eur. Ceram. Soc. 2024, 44, 585–594. [Google Scholar] [CrossRef]
- Dreyer, M.; Krebs, M.; Najafishirtari, S.; Rabe, A.; Friedel Ortega, K.; Behrens, M. The Effect of Co Incorporation on the CO Oxidation Activity of LaFe1−xCoxO3 Perovskites. Catalysts 2021, 11, 550. [Google Scholar] [CrossRef]
- Ruh, T.; Buchinger, R.; Lindenthal, L.; Schrenk, F.; Rameshan, C. CO Oxidation Capabilities of La- and Nd-Based Perovskites. Fuels 2022, 3, 31–43. [Google Scholar] [CrossRef]
- Baiker, A.; Marti, P.E.; Keusch, P.; Fritsch, E.; Reller, A. Influence of the A-Site Cation in ACoO3 (A = La, Pr, Nd, and Gd) Perovskite-Type Oxides on Catalytic Activity for Methane Combustion. J. Catal. 1994, 146, 268–276. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C. Catalytic Conversion of Carbon Monoxide into Carbon Dioxide over Spinel Catalysts: An Overview. Mater. Sci. Energy Technol. 2019, 2, 575–588. [Google Scholar] [CrossRef]
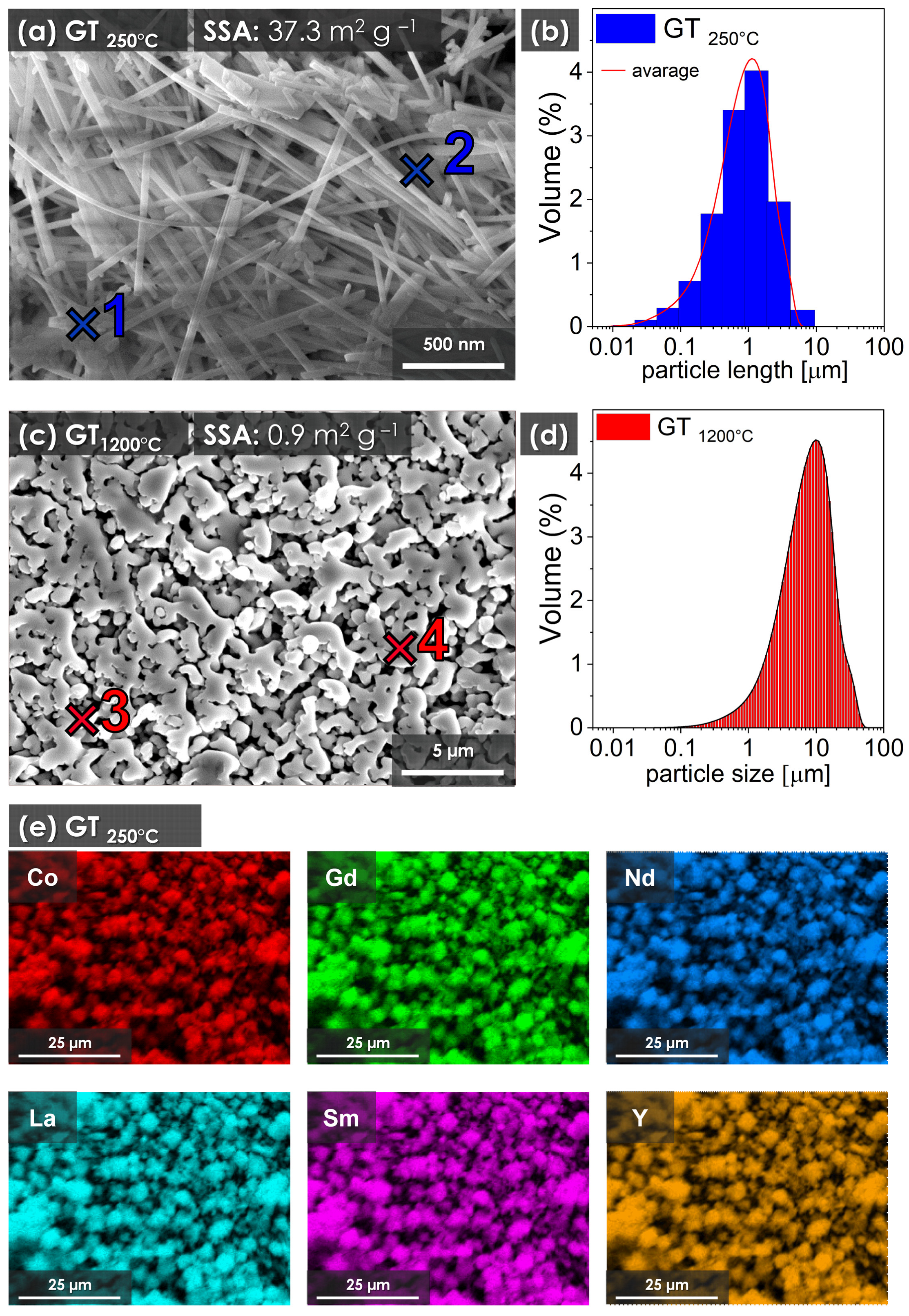
| Sample | Co | Y | La | Nd | Gd | Sm | |
|---|---|---|---|---|---|---|---|
| at-% | |||||||
| RECO GT250°C | 1 | 51.1 | 9.6 | 9.4 | 9.8 | 10.0 | 10.1 |
| 2 | 50.7 | 10.6 | 9.6 | 9.6 | 9.9 | 9.7 | |
| x- | 50.8 ± 1.0 | 9.8 ± 0.4 | 9.5 ± 0.5 | 10.2 ± 0.5 | 10.3 ± 0.5 | 9.4 ± 0.4 | |
| RECO GT1200°C | 3 | 49.7 | 10.1 | 10.3 | 9.7 | 10.6 | 9.6 |
| 4 | 50.5 | 9.1 | 10.1 | 10.8 | 10.6 | 8.9 | |
| x- | 50.3 ± 1.6 | 9.9 ± 0.6 | 9.4 ± 0.8 | 10.3 ± 0.9 | 10.2 ± 0.7 | 9.9 ± 0.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawczyk, P.A.; Wyrwa, J.; Kubiak, W.W. Synthesis and Catalytic Performance of High-Entropy Rare-Earth Perovskite Nanofibers: (Y0.2La0.2Nd0.2Gd0.2Sm0.2)CoO3 in Low-Temperature Carbon Monoxide Oxidation. Materials 2024, 17, 1883. https://doi.org/10.3390/ma17081883
Krawczyk PA, Wyrwa J, Kubiak WW. Synthesis and Catalytic Performance of High-Entropy Rare-Earth Perovskite Nanofibers: (Y0.2La0.2Nd0.2Gd0.2Sm0.2)CoO3 in Low-Temperature Carbon Monoxide Oxidation. Materials. 2024; 17(8):1883. https://doi.org/10.3390/ma17081883
Chicago/Turabian StyleKrawczyk, Paweł A., Jan Wyrwa, and Władysław W. Kubiak. 2024. "Synthesis and Catalytic Performance of High-Entropy Rare-Earth Perovskite Nanofibers: (Y0.2La0.2Nd0.2Gd0.2Sm0.2)CoO3 in Low-Temperature Carbon Monoxide Oxidation" Materials 17, no. 8: 1883. https://doi.org/10.3390/ma17081883