Physicochemical Changes in Root-Canal Sealers under Thermal Challenge: A Comparative Analysis of Calcium Silicate- and Epoxy-Resin-Based Sealers
Abstract
:1. Introduction
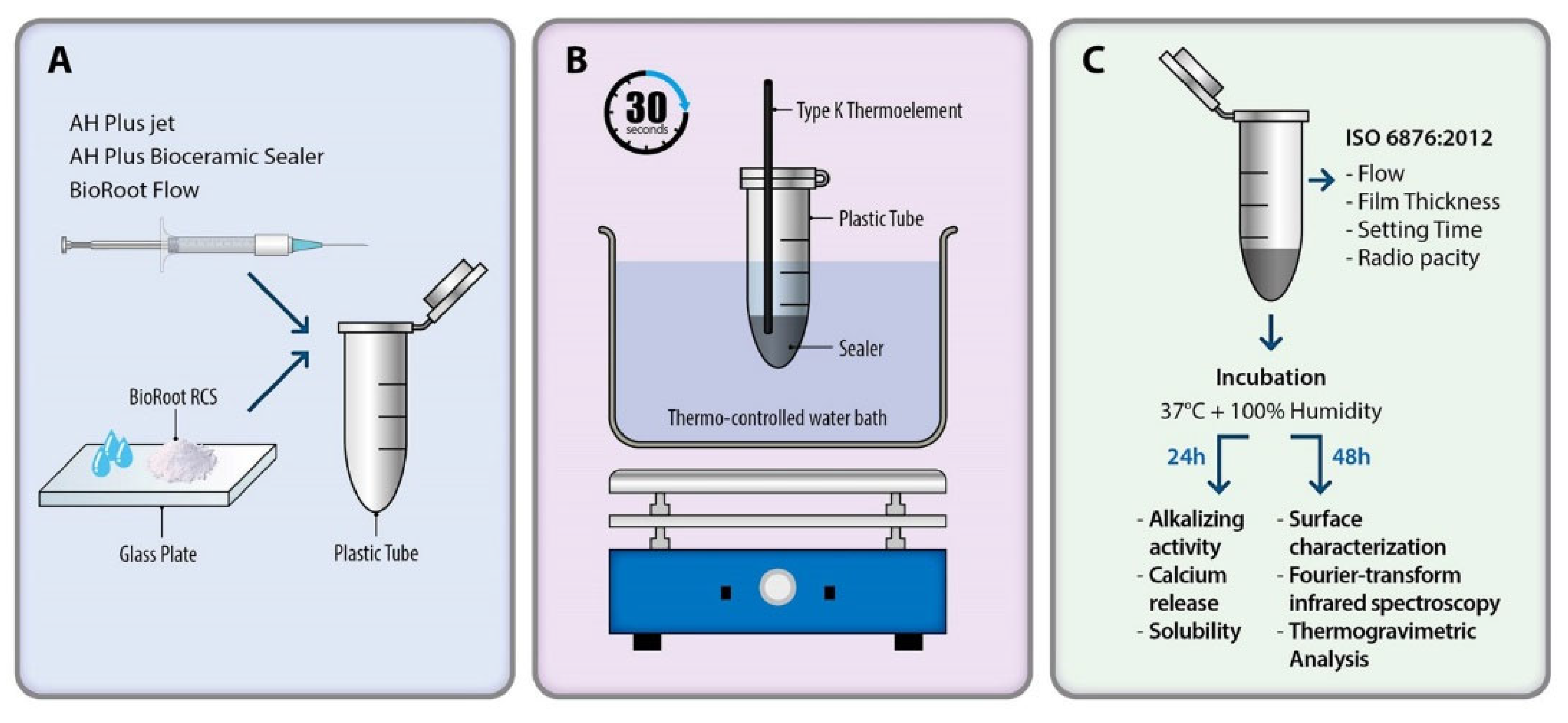
2. Materials and Methods
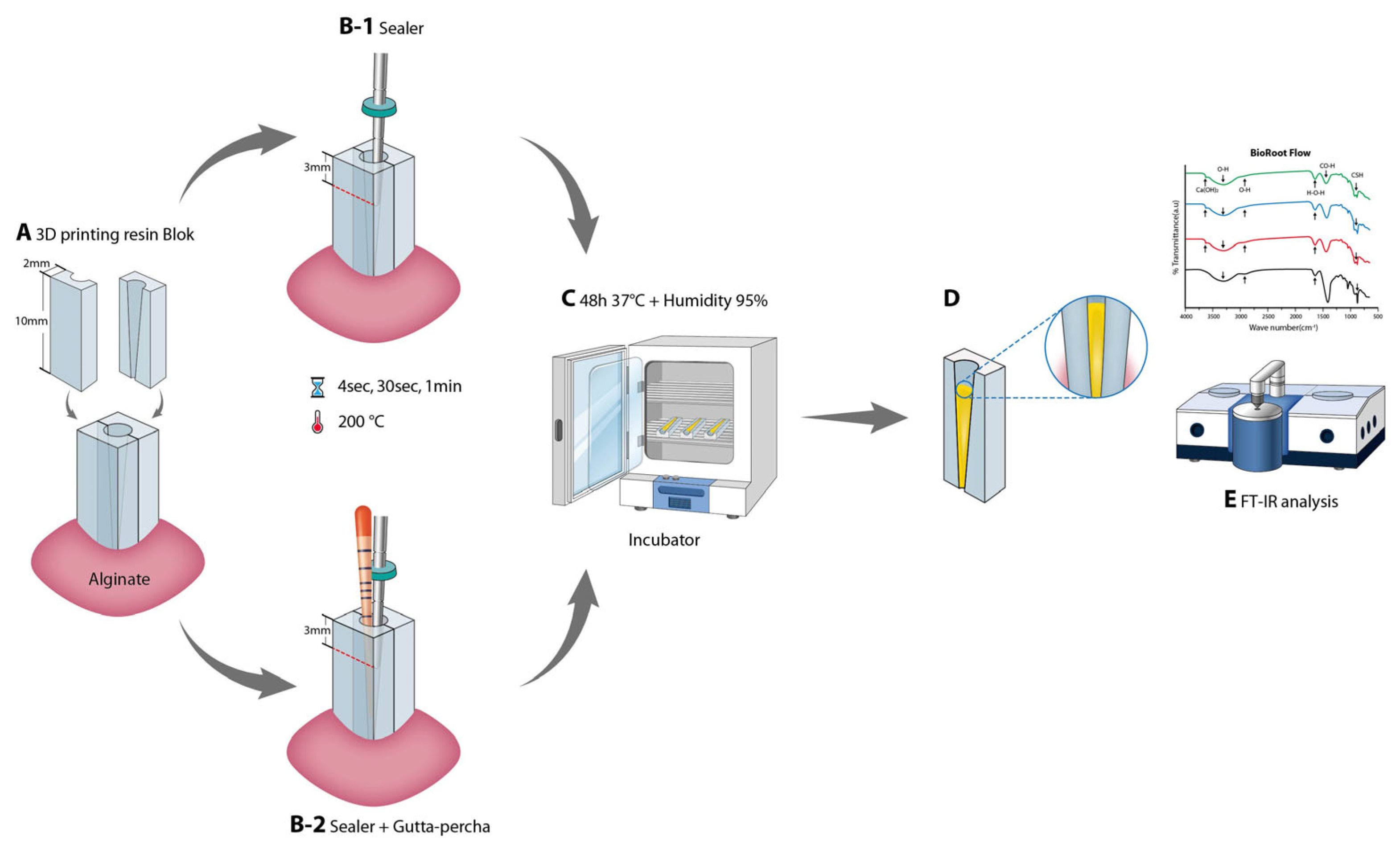
2.1. Heat Application on Sealer
2.2. Physical and Chemical Properties
2.2.1. Flow
2.2.2. Film Thickness
2.2.3. Setting Time
2.2.4. Calcium Ion Release
2.2.5. Solubility
2.2.6. pH Changes
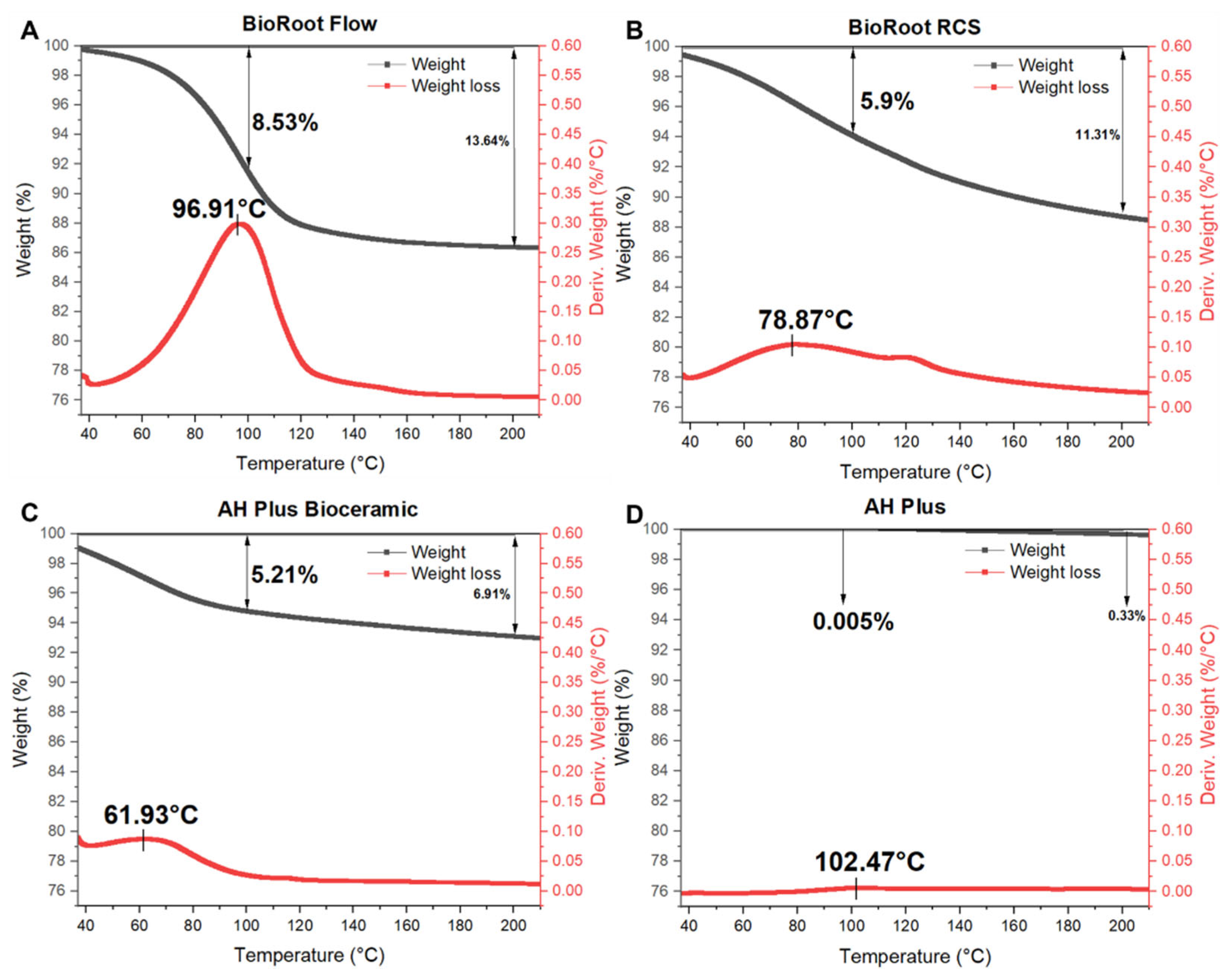
2.2.7. Thermogravimetric Analysis (TGA)
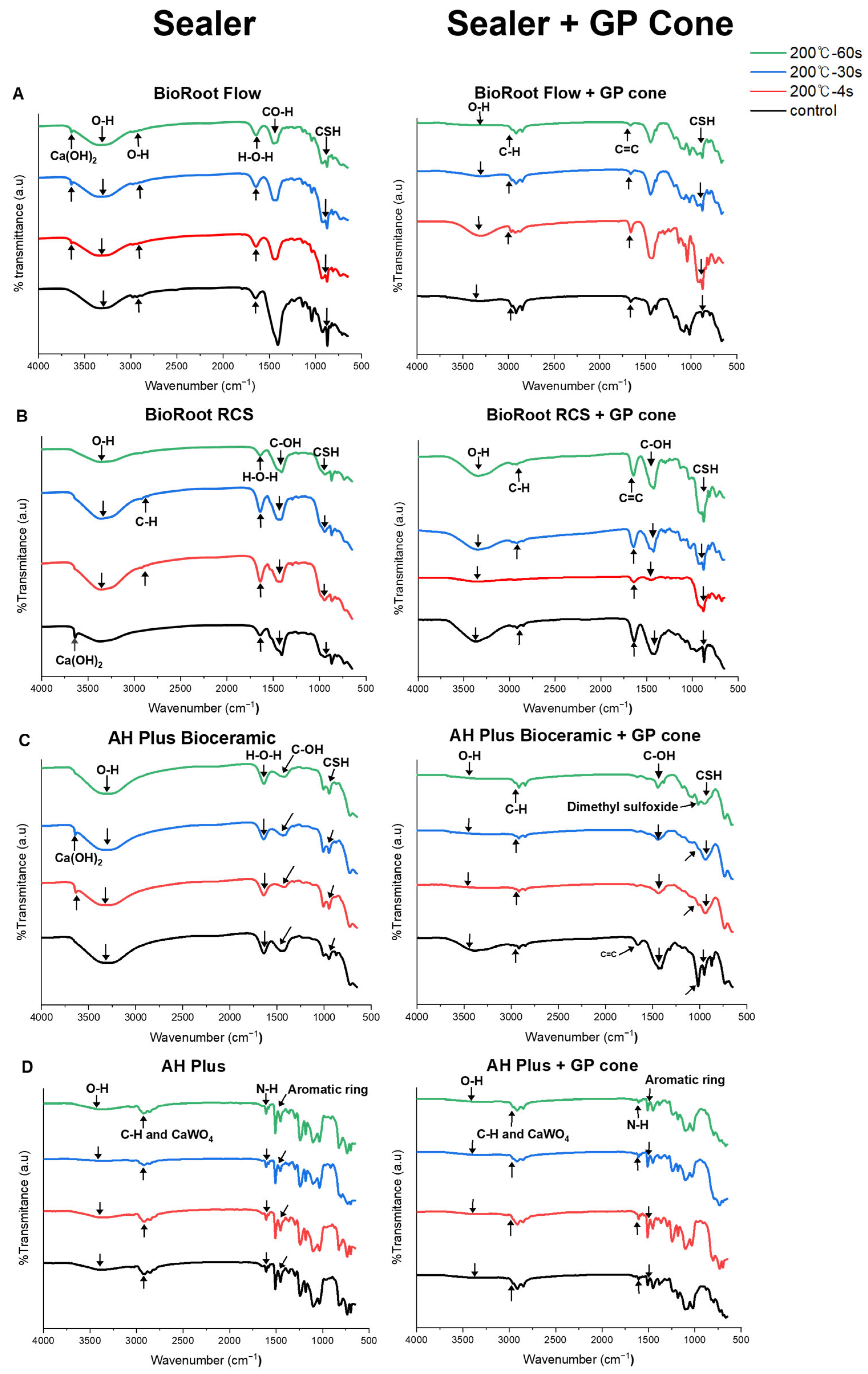
2.2.8. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.3. Surface Characterization
Field Emission Scanning Electron Microscope
2.4. Statistical Analysis
3. Results
3.1. Physicochemical Properties
3.1.1. Flow
3.1.2. Film Thickness
3.1.3. Setting Time
3.1.4. Calcium Ion Release
3.1.5. Solubility
3.1.6. Changes in pH
3.1.7. TGA
3.1.8. FT-IR
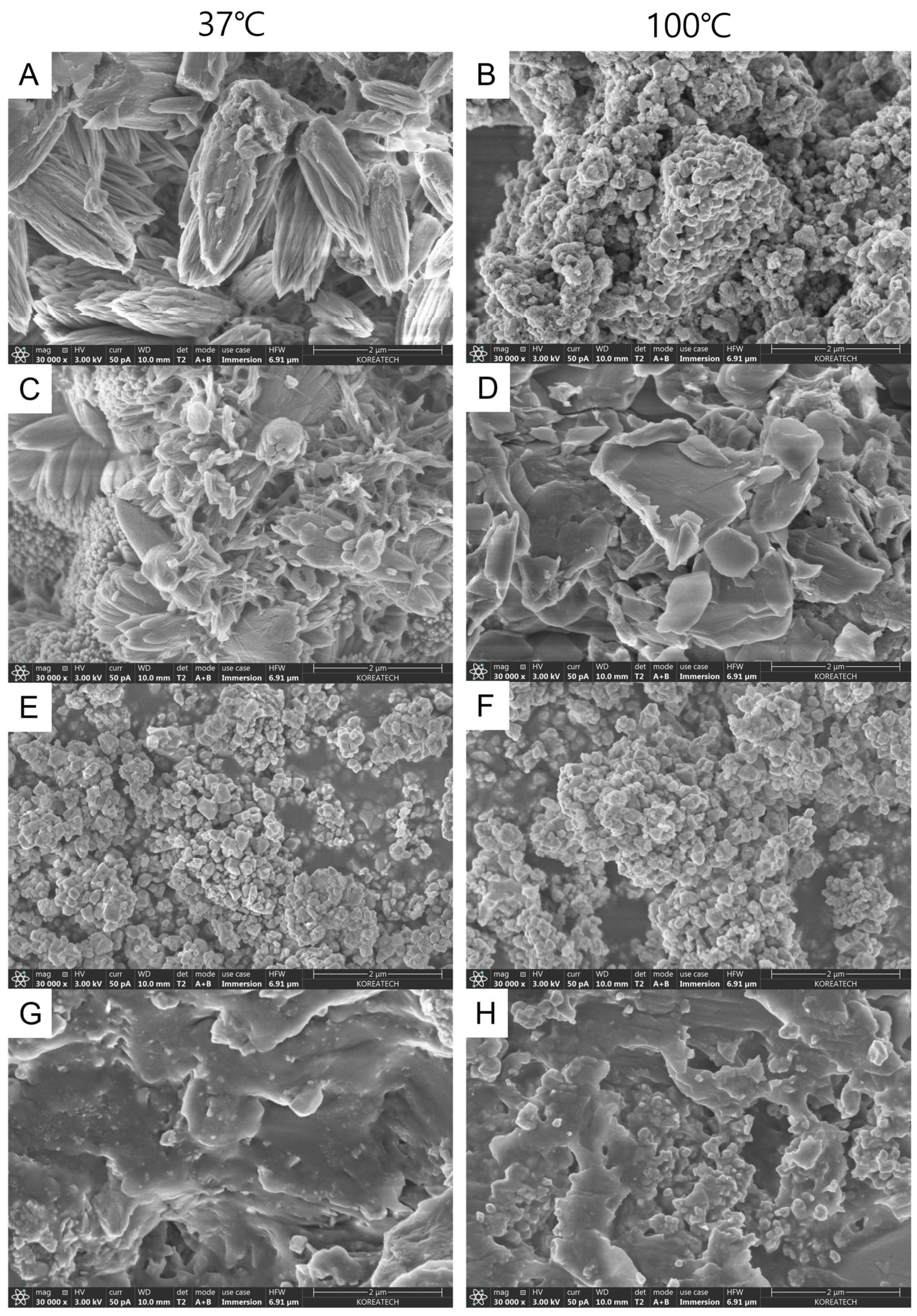
3.2. Field Emission Scanning Electron Microscope
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, Y.L.; Mann, V.; Rahbaran, S.; Lewsey, J.; Gulabivala, K. Outcome of primary root canal treatment: Systematic review of the literature—Part 2. Influence of clinical factors. Int. Endod. J. 2008, 41, 6–31. [Google Scholar] [CrossRef] [PubMed]
- Grossman, L. Obturation of the root canal. Endod. Pract. 1988, 242–270. [Google Scholar]
- Silva Almeida, L.H.; Moraes, R.R.; Morgental, R.D.; Pappen, F.G. Are Premixed Calcium Silicate–based Endodontic Sealers Comparable to Conventional Materials? A Systematic Review of In Vitro Studies. J. Endod. 2017, 43, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Viapiana, R.; Guerreiro-Tanomaru, J.M.; Hungaro-Duarte, M.A.; Tanomaru-Filho, M.; Camilleri, J. Chemical characterization and bioactivity of epoxy resin and Portland cement-based sealers with niobium and zirconium oxide radiopacifiers. Dent. Mater. 2014, 30, 1005–1020. [Google Scholar] [CrossRef]
- Faria-Júnior, N.B.; Tanomaru-Filho, M.; Berbert, F.L.; Guerreiro-Tanomaru, J.M. Antibiofilm activity, pH and solubility of endodontic sealers. Int. Endod. J. 2013, 46, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Naaman, A.; Camilleri, J. Properties of Tricalcium Silicate Sealers. J. Endod. 2016, 42, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Siboni, F.; Taddei, P.; Zamparini, F.; Prati, C.; Gandolfi, M.G. Properties of BioRoot RCS, a tricalcium silicate endodontic sealer modified with povidone and polycarboxylate. Int. Endod. J. 2017, 50 (Suppl. S2), e120–e136. [Google Scholar] [CrossRef] [PubMed]
- Prati, C.; Gandolfi, M.G. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent. Mater. 2015, 31, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Kodama, S.; Okiji, T. Evaluation of calcium-releasing and apatite-forming abilities of fast-setting calcium silicate-based endodontic materials. Int. Endod. J. 2015, 48, 124–130. [Google Scholar] [CrossRef]
- Jeanneau, C.; Giraud, T.; Laurent, P.; About, I. BioRoot RCS Extracts Modulate the Early Mechanisms of Periodontal Inflammation and Regeneration. J. Endod. 2019, 45, 1016–1023. [Google Scholar] [CrossRef]
- Dentsply Sirona USA. AH Plus Bioceramic Sealer. 2021. Available online: https://www.dentsplysirona.com/en-hk/categories/endodontics/ah-plus-bioceramic-sealer.html#downloads (accessed on 12 March 2024).
- Septodont USA. Bioroot Flow Brochure (Version 16). 2022. Available online: https://www.septodontusa.com/wp-content/uploads/2022/11/Bioroot-Flow-Brochure-V16.pdf?x56063/ (accessed on 12 March 2024).
- Lea, C.S.; Apicella, M.J.; Mines, P.; Yancich, P.P.; Parker, M.H. Comparison of the Obturation Density of Cold Lateral Compaction Versus Warm Vertical Compaction Using the Continuous Wave of Condensation Technique. J. Endod. 2005, 31, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.I.; Sonntag, D.; Peters, O.A. Homogeneity of root canal fillings performed by undergraduate students with warm vertical and cold lateral techniques. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2010, 110, e41–e49. [Google Scholar] [CrossRef] [PubMed]
- Bhandi, S.; Mashyakhy, M.; Abumelha, A.S.; Alkahtany, M.F.; Jamal, M.; Chohan, H.; Raj, A.T.; Testarelli, L.; Reda, R.; Patil, S. Complete Obturation-Cold Lateral Condensation vs. Thermoplastic Techniques: A Systematic Review of Micro-CT Studies. Materials 2021, 14, 4013. [Google Scholar] [CrossRef] [PubMed]
- Atmeh, A.R.; AlShwaimi, E. The Effect of Heating Time and Temperature on Epoxy Resin and Calcium Silicate–based Endodontic Sealers. J. Endod. 2017, 43, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- Viapiana, R.; Baluci, C.A.; Tanomaru-Filho, M.; Camilleri, J. Investigation of chemical changes in sealers during application of the warm vertical compaction technique. Int. Endod. J. 2015, 48, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Viapiana, R.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M.; Camilleri, J. Investigation of the Effect of Sealer Use on the Heat Generated at the External Root Surface during Root Canal Obturation Using Warm Vertical Compaction Technique with System B Heat Source. J. Endod. 2014, 40, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Donnermeyer, D.; Urban, K.; Bürklein, S.; Schäfer, E. Physico-chemical investigation of endodontic sealers exposed to simulated intracanal heat application: Epoxy resins and zinc oxide-eugenols. Int. Endod. J. 2020, 53, 690–697. [Google Scholar] [CrossRef]
- Donnermeyer, D.; Ibing, M.; Bürklein, S.; Weber, I.; Reitze, M.P.; Schäfer, E. Physico-Chemical Investigation of Endodontic Sealers Exposed to Simulated Intracanal Heat Application: Hydraulic Calcium Silicate-Based Sealers. Materials 2021, 14, 728. [Google Scholar] [CrossRef] [PubMed]
- Heran, J.; Khalid, S.; Albaaj, F.; Tomson, P.L.; Camilleri, J. The single cone obturation technique with a modified warm filler. J. Dent. 2019, 89, 103181. [Google Scholar] [CrossRef]
- Donnermeyer, D.; Schemkämper, P.; Bürklein, S.; Schäfer, E. Short and Long-Term Solubility, Alkalizing Effect, and Thermal Persistence of Premixed Calcium Silicate-Based Sealers: AH Plus Bioceramic Sealer vs. Total Fill BC Sealer. Materials 2022, 15, 7320. [Google Scholar] [CrossRef]
- ISO 6876; International Standard: Dentistry—Root Canal Sealing Materials. International Organization for Standardization: Geneva, Switzerland, 2012.
- Antunes, T.B.M.; Janini, A.C.P.; Pelepenko, L.E.; Abuna, G.F.; Paiva, E.M.; Sinhoreti, M.A.C.; Raimundo, I.M., Jr.; Gomes, B.; de-Jesus-Soares, A.; Marciano, M.A. Heating stability, physical and chemical analysis of calcium silicate-based endodontic sealers. Int. Endod. J. 2021, 54, 1175–1188. [Google Scholar] [CrossRef]
- Chen, B.; Haapasalo, M.; Mobuchon, C.; Li, X.; Ma, J.; Shen, Y. Cytotoxicity and the Effect of Temperature on Physical Properties and Chemical Composition of a New Calcium Silicate–based Root Canal Sealer. J. Endod. 2020, 46, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, O.S.; Meier, M.M.; Carvalho, E.M.; Ferreira, P.V.C.; Gavini, G.; Zago, P.M.W.; Grazziotin-Soares, R.; Menezes, A.S.d.; Carvalho, C.N.; Bauer, J. Synthesis and characterization of experimental endodontic sealers containing bioactive glasses particles of NbG or 45S5. J. Mech. Behav. Biomed. Mater. 2022, 125, 104971. [Google Scholar] [CrossRef] [PubMed]
- Aksel, H.; Makowka, S.; Bosaid, F.; Guardian, M.G.; Sarkar, D.; Azim, A.A. Effect of heat application on the physical properties and chemical structure of calcium silicate-based sealers. Clin. Oral Investig. 2021, 25, 2717–2725. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.A.H.; Ordinola-Zapata, R.; Bernardes, R.A.; Bramante, C.M.; Bernardineli, N.; Garcia, R.B.; de Moraes, I.G. Influence of Calcium Hydroxide Association on the Physical Properties of AH Plus. J. Endod. 2010, 36, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-m.; Shen, Y.; Zheng, W.; Li, L.; Zheng, Y.-f.; Haapasalo, M. Physical Properties of 5 Root Canal Sealers. J. Endod. 2013, 39, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Versiani, M.A.; Abi Rached-Junior, F.J.; Kishen, A.; Pécora, J.D.; Silva-Sousa, Y.T.; de Sousa-Neto, M.D. Zinc Oxide Nanoparticles Enhance Physicochemical Characteristics of Grossman Sealer. J. Endod. 2016, 42, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.C.d.; Neves, G.S.T.; Kirkpatrick, T.; Letra, A.; Silva, R. Physicochemical and Biological Properties of AH Plus Bioceramic. J. Endod. 2023, 49, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Xuereb, M.; Sorrentino, F.; Damidot, D.; Camilleri, J. Development of novel tricalcium silicate-based endodontic cements with sintered radiopacifier phase. Clin. Oral Investig. 2016, 20, 967–982. [Google Scholar] [CrossRef]
- Angkasuvan, V.; Panichuttra, A.; Nawachinda, M.; Ratisoontorn, C. Evaluation of pH and calcium ion release at the simulated external root resorption cavities of teeth obturated with bioceramic sealer. Clin. Exp. Dent. Res. 2022, 8, 900–905. [Google Scholar] [CrossRef]
- Grant, S.A.; Boitnott, G.E.; Korhonen, C.J.; Sletten, R.S. Effect of temperature on hydration kinetics and polymerization of tricalcium silicate in stirred suspensions of CaO-saturated solutions. Cem. Concr. Res. 2006, 36, 671–677. [Google Scholar] [CrossRef]
- Vitti, R.P.; Prati, C.; Silva, E.J.N.L.; Sinhoreti, M.A.C.; Zanchi, C.H.; de Souza e Silva, M.G.; Ogliari, F.A.; Piva, E.; Gandolfi, M.G. Physical Properties of MTA Fillapex Sealer. J. Endod. 2013, 39, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Cardoso, M.L.; Rodrigues, J.P.; De-Deus, G.; Fidalgo, T. Solubility of bioceramic- and epoxy resin-based root canal sealers: A systematic review and meta-analysis. Aust. Endod. J. 2021, 47, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Elyassi, Y.; Moinzadeh, A.T.; Kleverlaan, C.J. Characterization of Leachates from 6 Root Canal Sealers. J. Endod. 2019, 45, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.P.; Sousa-Neto, M.D.; Versiani, M.A.; Rached-Júnior, F.A.; De-Deus, G.; Miranda, C.E.; Pécora, J.D. Changes in the surface of four calcium silicate-containing endodontic materials and an epoxy resin-based sealer after a solubility test. Int. Endod. J. 2012, 45, 419–428. [Google Scholar] [CrossRef]
- Prüllage, R.-K.; Urban, K.; Schäfer, E.; Dammaschke, T. Material Properties of a Tricalcium Silicate–containing, a Mineral Trioxide Aggregate–containing, and an Epoxy Resin–based Root Canal Sealer. J. Endod. 2016, 42, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- Okabe, T.; Sakamoto, M.; Takeuchi, H.; Matsushima, K. Effects of pH on Mineralization Ability of Human Dental Pulp Cells. J. Endod. 2006, 32, 198–201. [Google Scholar] [CrossRef]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its Role in Root Canal Treatment Failure and Current Concepts in Retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef]
- Saber, S.; Galal, M.M.; Ismail, A.G.; Hamdy, T.M. Thermal, chemical and physical analysis of VDW.1Seal, Fill Root ST, and ADseal root canal sealers. Sci. Rep. 2023, 13, 14829. [Google Scholar] [CrossRef]

| Material | 1 Day | 7 Day | 14 Day | 21 Day | 28 Day | Time x Group | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 37 °C | 100 °C | 37 °C | 100 °C | 37 °C | 100 °C | 37 °C | 100 °C | 37 °C | 100 °C | ||
| Flow | 10.61 ± 0.09 a | 10.71 ± 0.09 a | 10.53 ± 0.17 a | 10.26 ± 0.17 a | 10.45 ± 0.07 b | 11.12 ± 0.07 a | 10.01 ± 0.23 a | 9.02 ± 0.23 b | 9.64 ± 0.03 a | 8.48 ± 0.03 b | <0.05 * |
| RCS | 11.24 ± 0.04 a | 10.34 ± 0.04 b | 9.86 ± 0.08 a | 9.64 ± 0.08 a | 10.66 ± 0.14 b | 10.02 ± 0.14 a | 10.17 ± 0.14 b | 8.54 ± 0.14 a | 9.65 ± 0.04 a | 8.07 ± 0.04 b | <0.05 * |
| AH Bio | 9.86 ± 0.05 a | 9.41 ± 0.05 b | 9.41 ± 0.19 a | 8.61 ± 0.19 b | 9.56 ± 0.03 b | 9.63 ± 0.03 a | 8.61 ± 0.20 a | 7.79 ± 0.20 b | 8.6 ± 0.12 a | 7.74 ± 0.12 b | <0.05 * |
| AH Plus | 7.81 ± 0.14 a | 7.22 ± 0.14 b | 7.84 ± 0.06 a | 7.45 ± 0.06 b | 7.67 ± 0.1 a | 7.50 ± 0.1 a | 7.59 ± 0.07 a | 7.53 ± 0.07 a | 7.38 ± 0.14 a | 7.62 ± 0.14 a | >0.05 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-I.; Jang, Y.-E.; Kim, Y.; Kim, B.S. Physicochemical Changes in Root-Canal Sealers under Thermal Challenge: A Comparative Analysis of Calcium Silicate- and Epoxy-Resin-Based Sealers. Materials 2024, 17, 1932. https://doi.org/10.3390/ma17081932
Kim H-I, Jang Y-E, Kim Y, Kim BS. Physicochemical Changes in Root-Canal Sealers under Thermal Challenge: A Comparative Analysis of Calcium Silicate- and Epoxy-Resin-Based Sealers. Materials. 2024; 17(8):1932. https://doi.org/10.3390/ma17081932
Chicago/Turabian StyleKim, Hye-In, Young-Eun Jang, Yemi Kim, and Bom Sahn Kim. 2024. "Physicochemical Changes in Root-Canal Sealers under Thermal Challenge: A Comparative Analysis of Calcium Silicate- and Epoxy-Resin-Based Sealers" Materials 17, no. 8: 1932. https://doi.org/10.3390/ma17081932






