Adsorption of Heavy Metal Ions on Alginate-Based Magnetic Nanocomposite Adsorbent Beads
Abstract
:1. Introduction
2. Materials and Methods
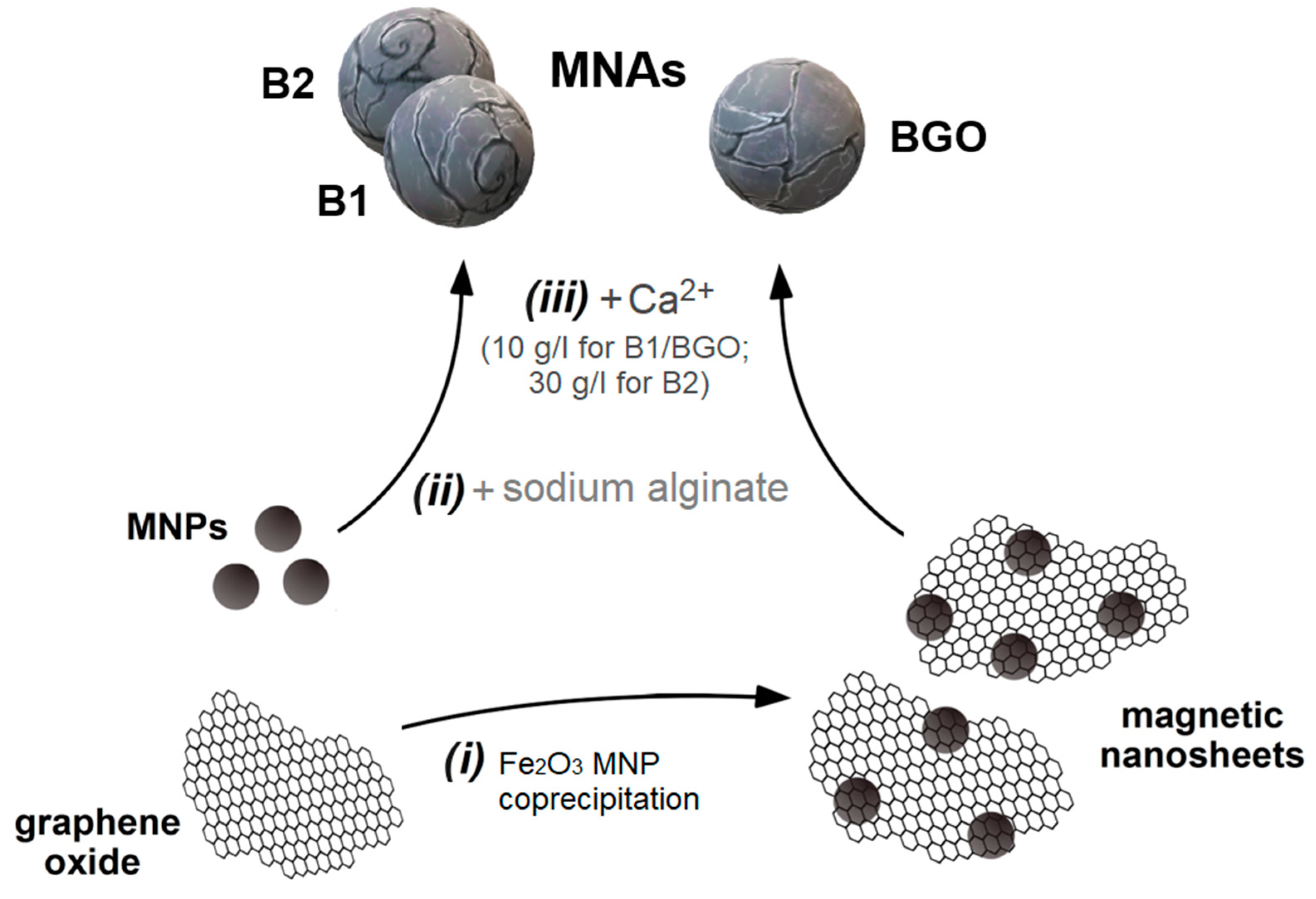
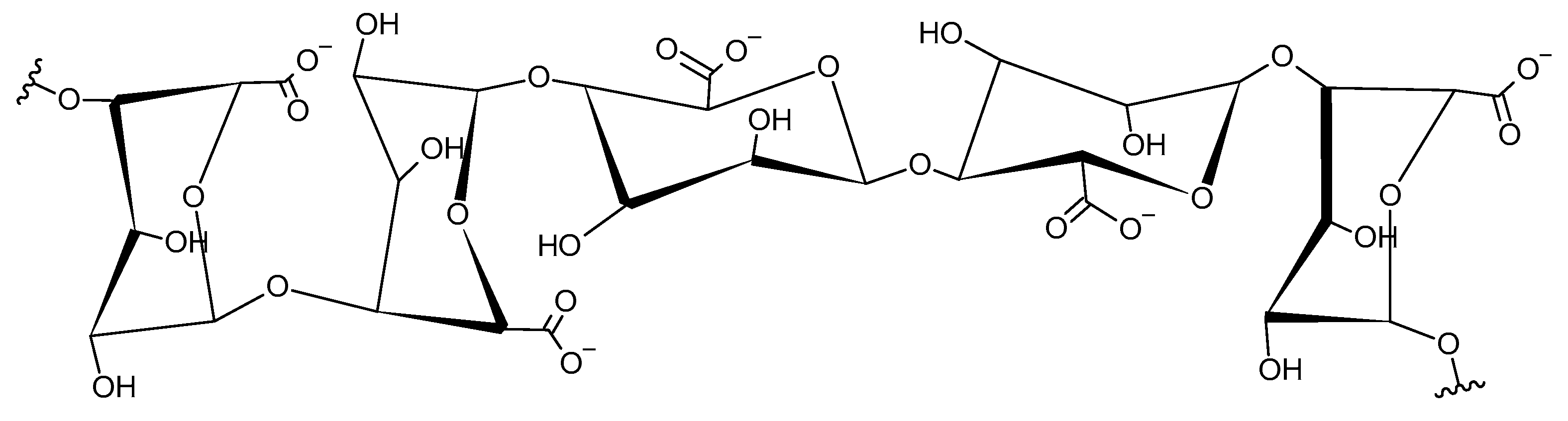
2.1. Alginate Bead Preparation
- (1)
- Dispersion of the pristine magnetite MNPs (5.0 mg/mL) in a sodium alginate (C6H9NaO7, Sigma-Aldrich Italy, Milan, Italy) solution prepared by dissolving the compound in deionized water at a concentration of 10.0 g/L.
- (2)
- Drop-wise adding of the previously prepared suspension in a cross-linking solution, obtained by dissolving either 10.0 g/L (for B1) or 30.0 g/L (for B2) of calcium chloride (CaCl2, Sigma-Aldrich) in deionized water. During the addition, the Ca2+ solution was kept under constant magnetic stirring.
- (3)
- Mechanical recovery from the solution, washing four times with deionized water, and storage in deionized water.
- (1)
- Dispersion of the GO-based magnetic nanosheets (2.5 mg/mL) in a sodium alginate (C6H9NaO7, Sigma-Aldrich) solution prepared by dissolving the compound in deionized water at a concentration of 10.0 g/L.
- (2)
- Drop-wise adding of the previously prepared suspension in a cross-linking solution, obtained by dissolving 10.0 g/L of calcium chloride (CaCl2, Sigma-Aldrich) in deionized water. In this case, during the addition, the Ca2+ solution was kept under constant magnetic stirring.
- (3)
- Mechanical recovery from the solution, washing four times with deionized water, and storage in deionized water.
2.2. Experimental Protocol
- Cu2+ (Copper(II) nitrate, CuNO3∙3H2O, 99% Sigma Aldrich);
- Ni2+ (Nickel(II) chloride, NiCl2∙6H2O, 99% Sigma Aldrich);
- Cr3+ (Chromium(III) chloride, CrCl3∙6H2O, 99% Sigma Aldrich).
- (1)
- A flask containing 1 mL of deionized water was prepared;
- (2)
- Then, 5 beads (B1, B2, or BGO, depending on the test) were manually added;
- (3)
- Successively, 1 mL of the tested salt solution (#i, double concentration) was added to reach the corresponding initial concentration reported in Table 1;
- (4)
- The flask was then mechanically shaken for 10 min at 25 °C.Such a time could seem very short, but it was chosen to ensure the possibility of realizing a real industrial application of the embedded nanoadsorbents (which must be used far from equilibrium conditions, characterized by poor adsorption kinetics). It was observed that such a time was sufficient to reach the “quasi equilibrium” concentration for copper for each bead type; therefore, it was used as reference contact time for all ions to make direct comparisons among the different adsorption bead performances;
- (5)
- At the end of the experiment, 1.5 mL of solution was transferred with a micropipette into a 2 mL vial for ICP analysis. All final sample concentrations were analyzed with an ICP-OES measurement.
2.3. Adsorption Parameters for Removal Characterization
- In a batch experiment, the starting concentration is not maintained; hence, the concentration gradient between the two phases is lower compared to the continuous one.
- In a batch experiment, the equilibrium is reached between the adsorbent and the final concentration, while in a continuous experiment the equilibrium is between the adsorbent and the initial concentration.
2.4. Metal Ion Speciation in Water
3. Results
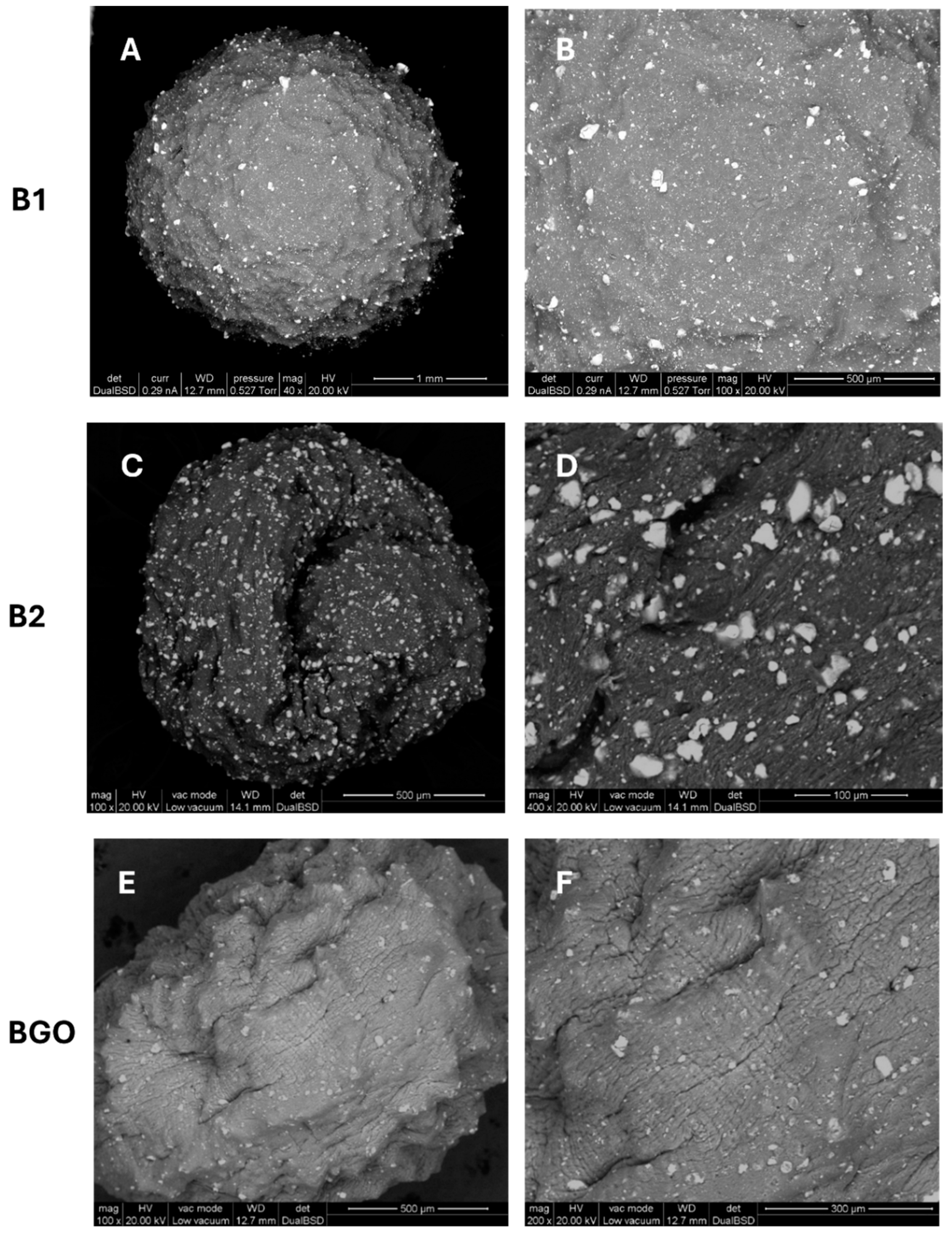
3.1. Bead Preparation and Characterization
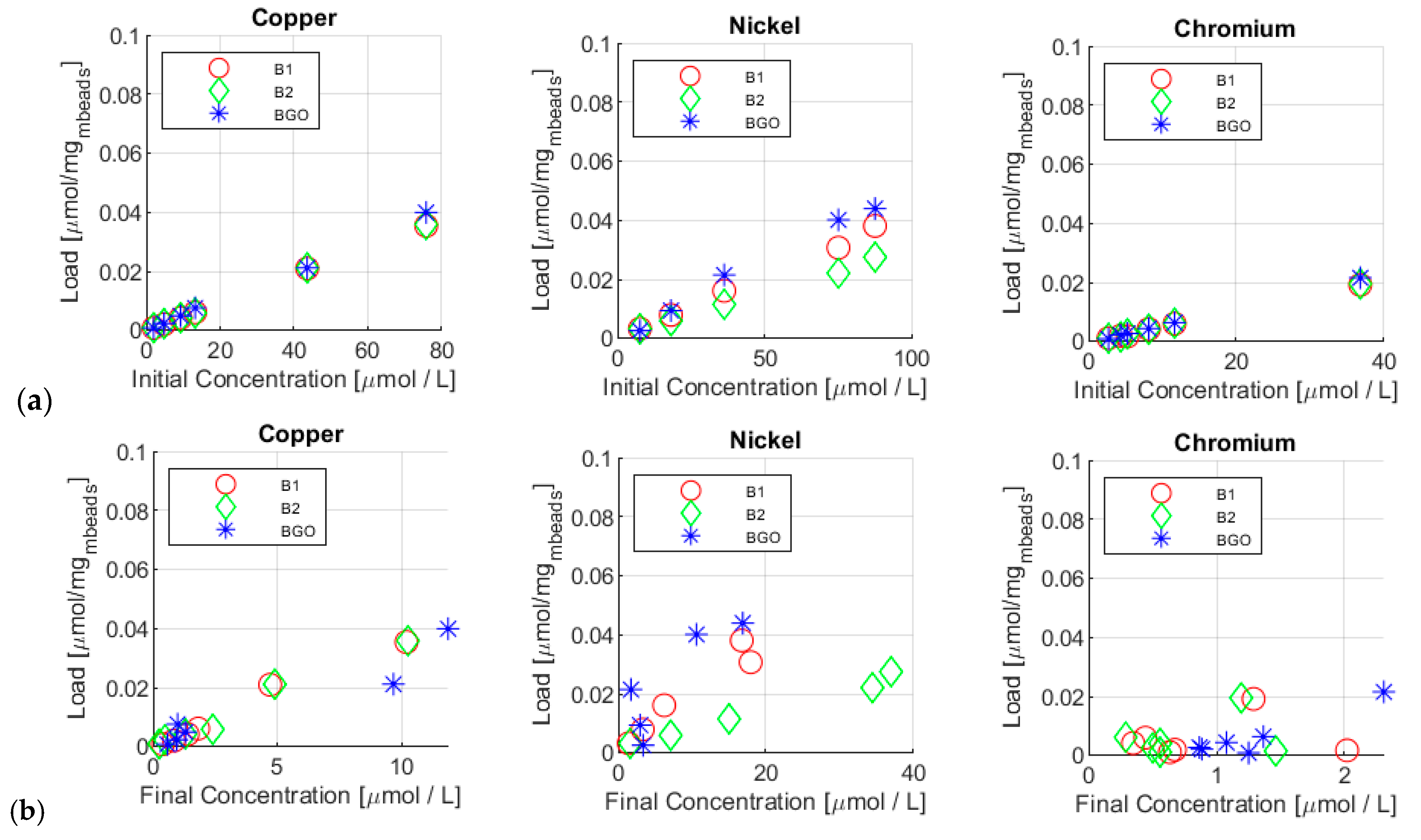
3.2. Adsorption Tests: Yield and Load Analysis
3.3. Comparison with Nanosheet and MNPs without Alginate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guven, H.; Ersahin, M.E.; Ozgun, H.; Ozturk, I.; Koyuncu, I. Energy and Material Refineries of Future: Wastewater Treatment Plants. J. Environ. Manag. 2023, 329, 117130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Serventi, L. Sustainability of Dairy and Soy Processing: A Review on Wastewater Recycling. J. Clean. Prod. 2019, 237, 117821. [Google Scholar] [CrossRef]
- Lee, K.E.; Morad, N.; Teng, T.T.; Poh, B.T. Development, Characterization and the Application of Hybrid Materials in Coagulation/Flocculation of Wastewater: A Review. Chem. Eng. J. 2012, 203, 370–386. [Google Scholar] [CrossRef]
- Ali, I.; Basheer, A.A.; Mbianda, X.Y.; Burakov, A.; Galunin, E.; Burakova, I.; Mkrtchyan, E.; Tkachev, A.; Grachev, V. Graphene Based Adsorbents for Remediation of Noxious Pollutants from Wastewater. Environ. Int. 2019, 127, 160–180. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.M.; Al-Hazmi, H.E.; Śniatała, B.; Esmaeili, A.; Habibzadeh, S. Nanoparticles and Nanofiltration for Wastewater Treatment: From Polluted to Fresh Water. Environ. Res. 2023, 238, 117114. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-X.; Luo, D.; Li, M.-M.; Xing, X.-F.; Ma, Z.-Z.; Xu, H. Recyclable Fe3O4 Nanoparticles Catalysts for Aza-Michael Addition of Acryl Amides by Magnetic Field. Catalysts 2017, 7, 219. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, J.; Zhang, M.; Li, J.; Yu, J.; Lu, S.; Song, W.; Wei, Y.; Li, Z.; Liu, J. Electron Delocalization of Au Nanoclusters Triggered by Fe Single Atoms Boosts Alkaline Overall Water Splitting. ACS Appl. Mater. Interfaces 2023, 15, 10696–10708. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.; Wabaidur, S.M.; Khan, M.R.; Al-Resayes, S.I.; Islam, M.S. Removal of Chromium(III) and Cadmium(II) Heavy Metal Ions from Aqueous Solutions Using Treated Date Seeds: An Eco-Friendly Method. Molecules 2021, 26, 3718. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.N.; Lo, I.M.C. Magnetic Nanoparticles: Essential Factors for Sustainable Environmental Applications. Water Res. 2013, 47, 2613–2632. [Google Scholar] [CrossRef]
- An, Y.-C.; Gao, X.-X.; Jiang, W.-L.; Han, J.-L.; Ye, Y.; Chen, T.-M.; Ren, R.-Y.; Zhang, J.-H.; Liang, B.; Li, Z.-L.; et al. A Critical Review on Graphene Oxide Membrane for Industrial Wastewater Treatment. Environ. Res. 2023, 223, 115409. [Google Scholar] [CrossRef]
- Mashangoane, B.F.; Chirwa, E.N.; Mahlathi, C. Kinetics and Isotherms of a Genetically Engineered Saccharomyces Cerevisiae EBY100 Strain Expressing Palladium Binding Peptides for the Biosorption of Pd (II) in a Batch Reactor. Chem. Eng. Trans. 2023, 99, 487–492. [Google Scholar] [CrossRef]
- Ho, Y.-S. Review of Second-Order Models for Adsorption Systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X. Adsorption Isotherm Models: Classification, Physical Meaning, Application and Solving Method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Asadi Haris, S.; Dabagh, S.; Mollasalehi, H.; Ertas, Y.N. Alginate Coated Superparamagnetic Iron Oxide Nanoparticles as Nanocomposite Adsorbents for Arsenic Removal from Aqueous Solutions. Sep. Purif. Technol. 2023, 310, 123193. [Google Scholar] [CrossRef]
- Barozzi, M.; Copelli, S.; Russo, E.; Sgarbossa, P.; Lavagnolo, M.C.; Sandon, A.; Morosini, C.; Sieni, E. Implementation of Magnetic Nanostructured Adsorbents for Heavy Metals Separation from Textile Wastewater. Sustainability 2022, 14, 11785. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-Induced Gelation of Alginate: Mechanisms and Applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Kayalvizhi, K.; Alhaji, N.M.I.; Saravanakkumar, D.; Mohamed, S.B.; Kaviyarasu, K.; Ayeshamariam, A.; Al-Mohaimeed, A.M.; AbdelGawwad, M.R.; Elshikh, M.S. Adsorption of Copper and Nickel by Using Sawdust Chitosan Nanocomposite Beads—A Kinetic and Thermodynamic Study. Environ. Res. 2022, 203, 111814. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Donald, A.M. The Use of Environmental Scanning Electron Microscopy for Imaging Wet and Insulating Materials. Nat. Mater. 2003, 2, 511–516. [Google Scholar] [CrossRef]
- Liu, H.; Shi, C.; Chen, Z.; Wang, S.; Yang, M.; Zhao, J.; Chen, C.; Song, Y.; Ling, Z. Turning gas hydrate nucleation with oxygen-containing groups on size selected graphene oxide flakes. J. Energy Chem. 2023, 87, 351–358. [Google Scholar] [CrossRef]
- Horváth, S.; Lukács, D.; Farsang, E.; Horváth, K. Unbiased Determination of Adsorption Isotherms by Inverse Method in Liquid Chromatography. Molecules 2023, 28, 1031. [Google Scholar] [CrossRef] [PubMed]






| Sample | Th. Conc. Cu [µg/L] | Eff. Conc. Cu [µg/L] | Th. Conc. Ni [µg/L] | Eff. Conc. Ni [µg/L] | Th. Conc. Cr [µg/L] | Eff. Conc. Cr [µg/L] |
|---|---|---|---|---|---|---|
| #1 | 100 | 123 ± 1 | 400 | 443 ± 1 | 100 | 139 ± 1 |
| #2 | 300 | 302 ± 1 | 1000 | 1060 ± 10 | 200 | 225 ± 1 |
| #3 | 600 | 587 ± 1 | 2000 | 2120 ± 10 | 300 | 273 ± 1 |
| #4 | 800 | 838 ± 1 | 4000 | 4394 ± 10 | 400 | 423 ± 1 |
| #5 | 3000 | 2770 ± 10 | 5000 | 5123 ± 10 | 600 | 605 ± 1 |
| #6 | 5000 | 4820 ± 10 | - | - | 2000 | 1915 ± 10 |
| # | B1 | B2 | BGO |
|---|---|---|---|
| Composition | MNP + Alginate | MNP + Alginate | MNP + Alginate + GO |
| Weight | 0.74 mg/bead | 0.75 mg/bead | 0.64 mg/bead |
| MNP | 0.22 mg/bead | 0.22 mg/bead | 0.10 mg/bead |
| Alginate | 0.52 mg/bead | 0.53 mg/bead | 0.53 mg/bead |
| GO | - | - | 0.010 mg/bead |
| # | B1 | B2 | BGO | |||
|---|---|---|---|---|---|---|
| Wet | Dry | Wet | Dry | Wet | Dry | |
| # of beads | 27 | 27 | 29 | 29 | 34 | 34 |
| Average Weight | 23.4 mg | 0.7 mg | 23.1 mg | 0.7 mg | 22.6 mg | 0.6 mg |
| Std. Dev. | 2.5 mg | 0.1 mg | 1.6 mg | 0.1 mg | 3.6 mg | 0.1 mg |
| Std. Dev. (%) | 10.5% | 12.4% | 6.8% | 13.1% | 16.0% | 13.4% |
| Element | Weight %—B1 | Weight %—B2 | Weight %—BGO |
|---|---|---|---|
| C | 22.08 | 26.06 | 12.77 |
| O | 61.65 | 55.57 | 83.21 |
| Ca | 5.87 | 5.62 | 2.03 |
| Fe | 10.40 | 12.76 | 1.99 |
| #1 | #2 | #3 | #4 | #5 | #6 | |
|---|---|---|---|---|---|---|
| Initial Cu Conc. | 123 ± 1 µg/L 1.94 µmol/L | 302 ± 1 µg/L 4.75 µmol | 587 ± 1 µg/L 9.24 µmol/L | 838 ± 1 µg/L 13.2 µmol/L | 2770 ± 10 µg/L 43.6 µmol/L | 4820 ± 10 µg/L 75.8 µmol/L |
| Bead type | B1 | B1 | B1 | B1 | B1 | B1 |
| Final Cu Conc. | 24 ± 1 µg/L 0.377 µmol/L | 53 ± 1 µg/L 0.833 µmol/L | 88 ± 1 µg/L 1.38 µmol/L | 115 ± 1 µg/L 1.81 µmol/L | 299 ± 1 µg/L 4.70 µmol/L | 648 ± 1 µg/L 10.2 µmol/L |
| Bead type | B2 | B2 | B2 | B2 | B2 | B2 |
| Final Cu Conc. | 15 ± 1 µg/L 0.236 µmol/L | 30 ± 1 µg/L 0.472 µmol/L | 82 ± 1 µg/L 1.29 µmol/L | 152 ± 1 µg/L 2.39 µmol/L | 311 ± 1 µg/L 4.89 µmol/L | 652 ± 1 µg/L 10.2 µmol/L |
| Bead type | BGO | BGO | BGO | BGO | BGO | BGO |
| Final Cu Conc. | 36 ± 1 µg/L 0.566 µmol/L | 60 ± 1 µg/L 0.94 µmol/L | 82 ± 1 µg/L 1.29 µmol/L | 62 ± 1 µg/L 0.976 µmol/L | 615 ± 1 µg/L 9.68 µmol/L | 755 ± 1 µg/L 11.9 µmol/L |
| #1 | #2 | #3 | #4 | #5 | |
|---|---|---|---|---|---|
| Initial Ni Conc. | 443 ± 1µg/L 7.55 µmol/L | 1060 ± 1 µg/L 18.1 µmol/L | 2120 ± 1 µg/L 36.1 µmol/L | 4394 ± 10 µg/L 74.9 µmol/L | 5123 ± 10 µg/L 87.3 µmol/L |
| Bead type | B1 | B1 | B1 | B1 | B1 |
| Final Ni Conc. | 82 ± 1 µg/L 1.40 µmol/L | 193 ± 1 µg/L 3.29 µmol/L | 364 ± 1 µg/L 6.20 µmol/L | 1053 ± 1 µg/L 17.9 µmol/L | 982 ± 1 µg/L 16.7 µmol/L |
| Bead type | B2 | B2 | B2 | B2 | B2 |
| Final Ni Conc. | 93 ± 1 µg/L 1.58 µmol/L | 415 ± 1 µg/L 7.07 µmol/L | 881 ± 1 µg/L 15.0 µmol/L | 2022 ± 1 µg/L 34.4 µmol/L | 2171 ± 1 µg/L 37.0 µmol/L |
| Bead type | BGO | BGO | BGO | BGO | BGO |
| Final Ni Conc. | 196 ± 1 µg/L 3.34 µmol/L | 172 ± 1 µg/L 2.93 µmol/L | 100 ± 1 µg/L 1.70 µmol/L | 621 ± 1 µg/L 10.6 µmol/L | 990 ± 1 µg/L 16.9 µmol/L |
| #1 | #2 | #3 | #4 | #5 | #6 | |
|---|---|---|---|---|---|---|
| Initial Cr Conc. | 139 ± 1 µg/L 2.67 µmol/L | 225 ± 1 µg/L 4.33 µmol/L | 273 ± 1 µg/L 5.25 µmol/L | 423 ± 1 µg/L 8.13 µmol/L | 605 ± 1 µg/L 11.6 µmol/L | 1915 ± 1 µg/L 36.8 µmol/L |
| Bead type | B1 | B1 | B1 | B1 | B1 | B1 |
| Final Cr Conc. | 33 ± 1 µg/L 0.635 µmol/L | 35 ± 1 µg/L 0.673 µmol/L | 105 ± 1 µg/L 2.02 µmol/L | 18 ± 1 µg/L 0.346 µmol/L | 23 ± 1 µg/L 0.44 µmol/L | 67 ± 1 µg/L 1.29 µmol/L |
| Bead type | B2 | B2 | B2 | B2 | B2 | B2 |
| Final Cr Conc. | 29 ± 1 µg/L 0.558 µmol/L | 76 ± 1 µg/L 1.46 µmol/L | 26 ± 1 µg/L 0.500 µmol/L | 29 ± 1 µg/L 0.558 µmol/L | 15 ± 1 µg/L 0.288 µmol/L | 62 ± 1 µg/L 1.19 µmol/L |
| Bead type | BGO | BGO | BGO | BGO | BGO | BGO |
| Final Cr Conc. | 65 ± 1 µg/L 1.25 µmol/L | 46 ± 1 µg/L 0.885 µmol/L | 45 ± 1 µg/L 0.865 µmol/L | 56 ± 1 µg/L 1.08 µmol/L | 71 ± 1 µg/L 1.36 µmol/L | 120 ± 1 µg/L 2.31 µmol/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, E.; Sgarbossa, P.; Gelosa, S.; Copelli, S.; Sieni, E.; Barozzi, M. Adsorption of Heavy Metal Ions on Alginate-Based Magnetic Nanocomposite Adsorbent Beads. Materials 2024, 17, 1942. https://doi.org/10.3390/ma17091942
Russo E, Sgarbossa P, Gelosa S, Copelli S, Sieni E, Barozzi M. Adsorption of Heavy Metal Ions on Alginate-Based Magnetic Nanocomposite Adsorbent Beads. Materials. 2024; 17(9):1942. https://doi.org/10.3390/ma17091942
Chicago/Turabian StyleRusso, Eleonora, Paolo Sgarbossa, Simone Gelosa, Sabrina Copelli, Elisabetta Sieni, and Marco Barozzi. 2024. "Adsorption of Heavy Metal Ions on Alginate-Based Magnetic Nanocomposite Adsorbent Beads" Materials 17, no. 9: 1942. https://doi.org/10.3390/ma17091942






