Adsorption of Co2+ and Cr3+ in Industrial Wastewater by Magnesium Silicate Nanomaterials
Abstract
:1. Introduction
2. Experimental Section
2.1. Main Reagents and Instruments
2.2. Adsorption Kinetics Experiment
2.3. Adsorption Thermodynamics Experiment
3. Results and Discussions
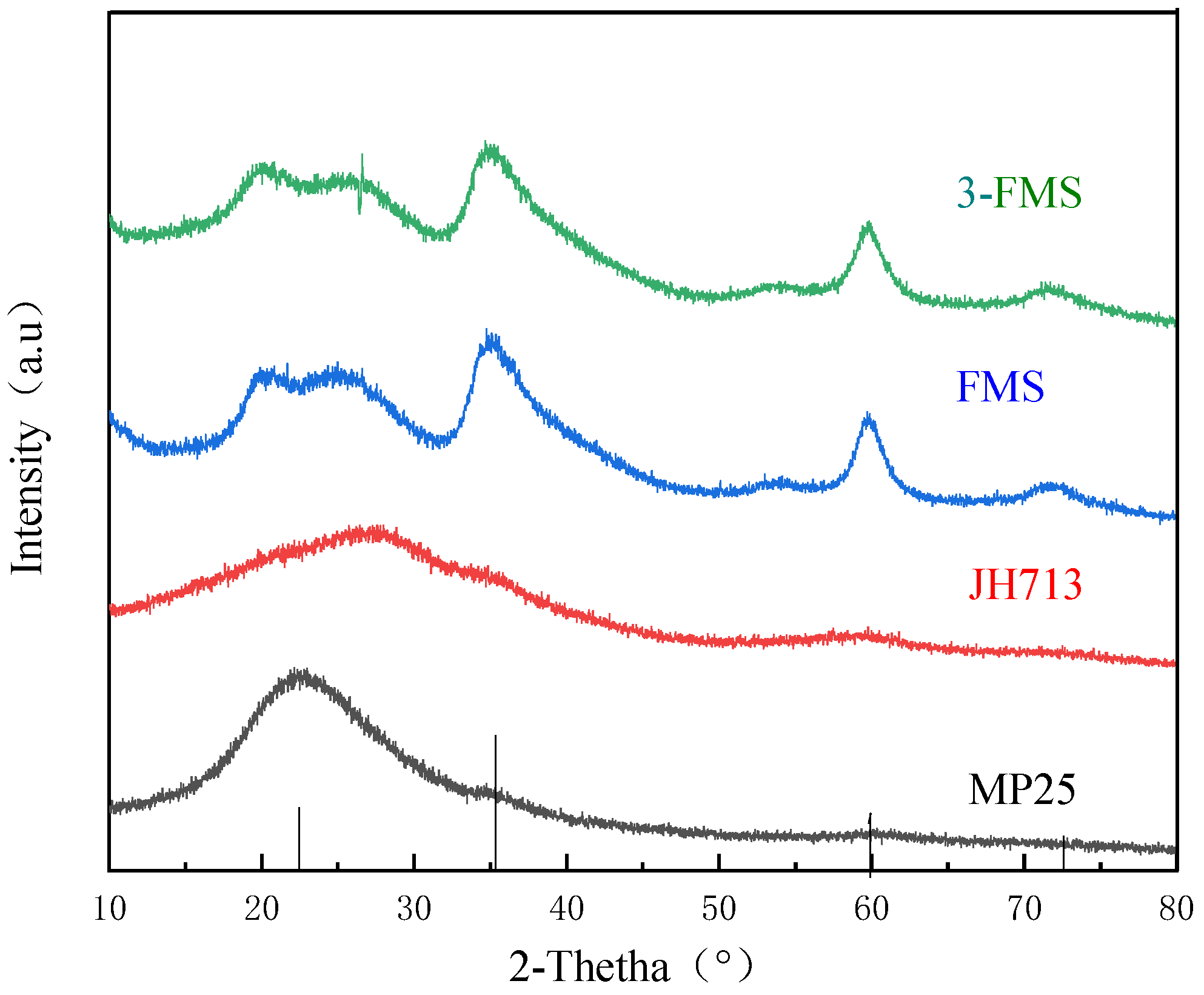
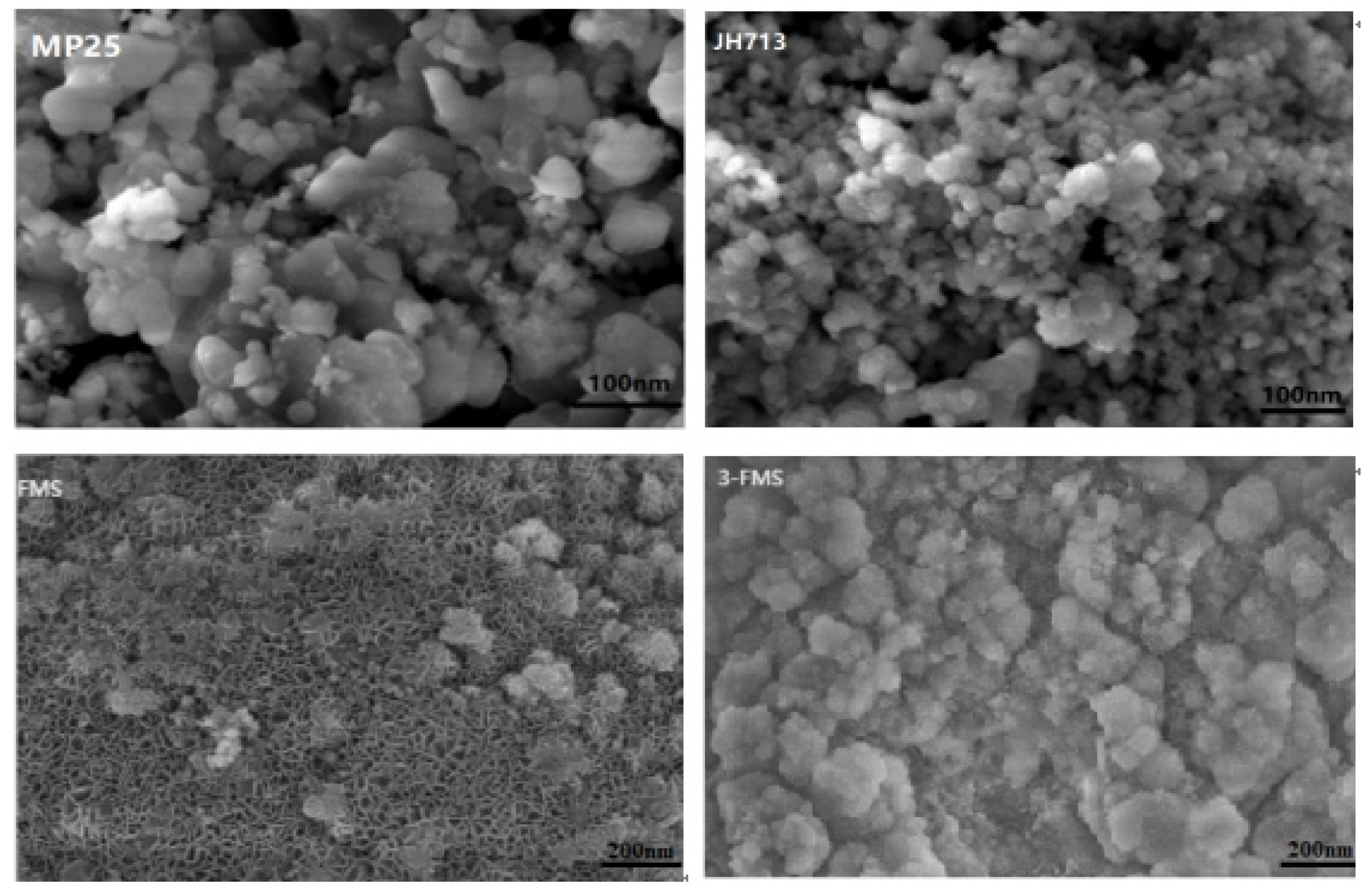
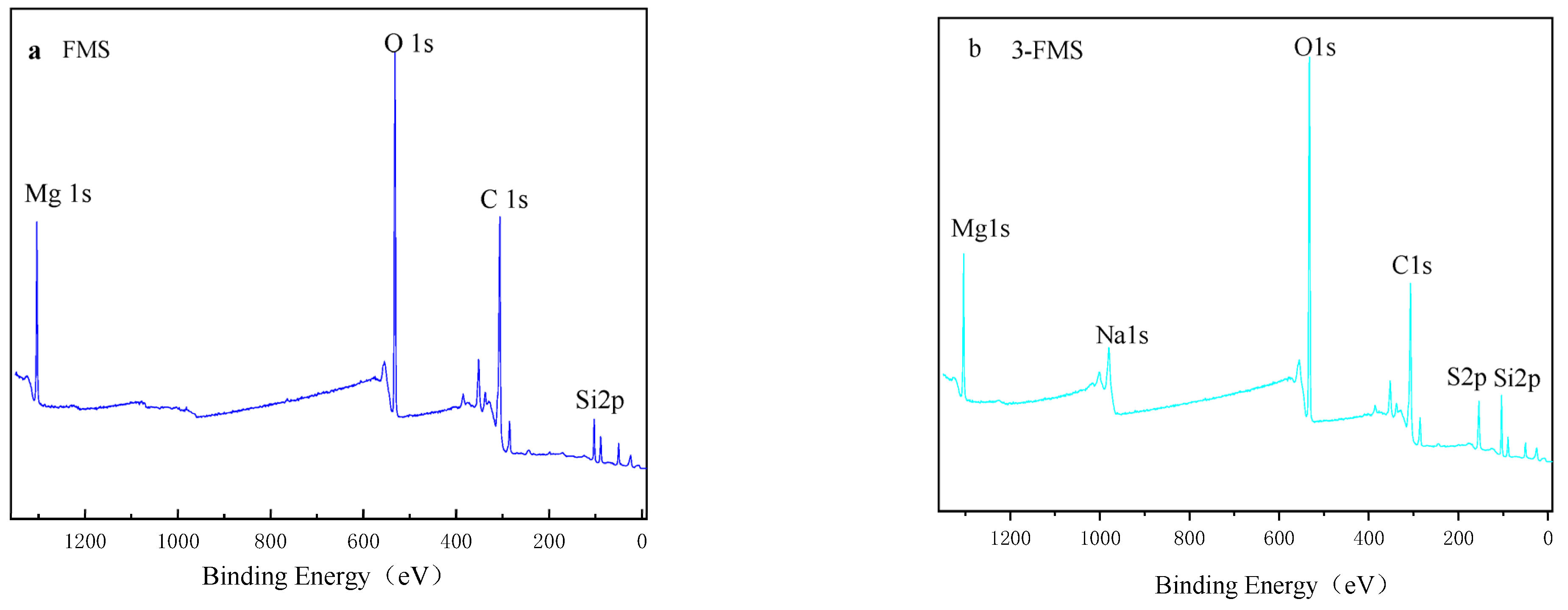
3.1. Characterization of Magnesium Silicate Nanomaterials
3.2. Adsorption Properties of Magnesium Silicate Nanomaterials for Co2+ and Cr3+
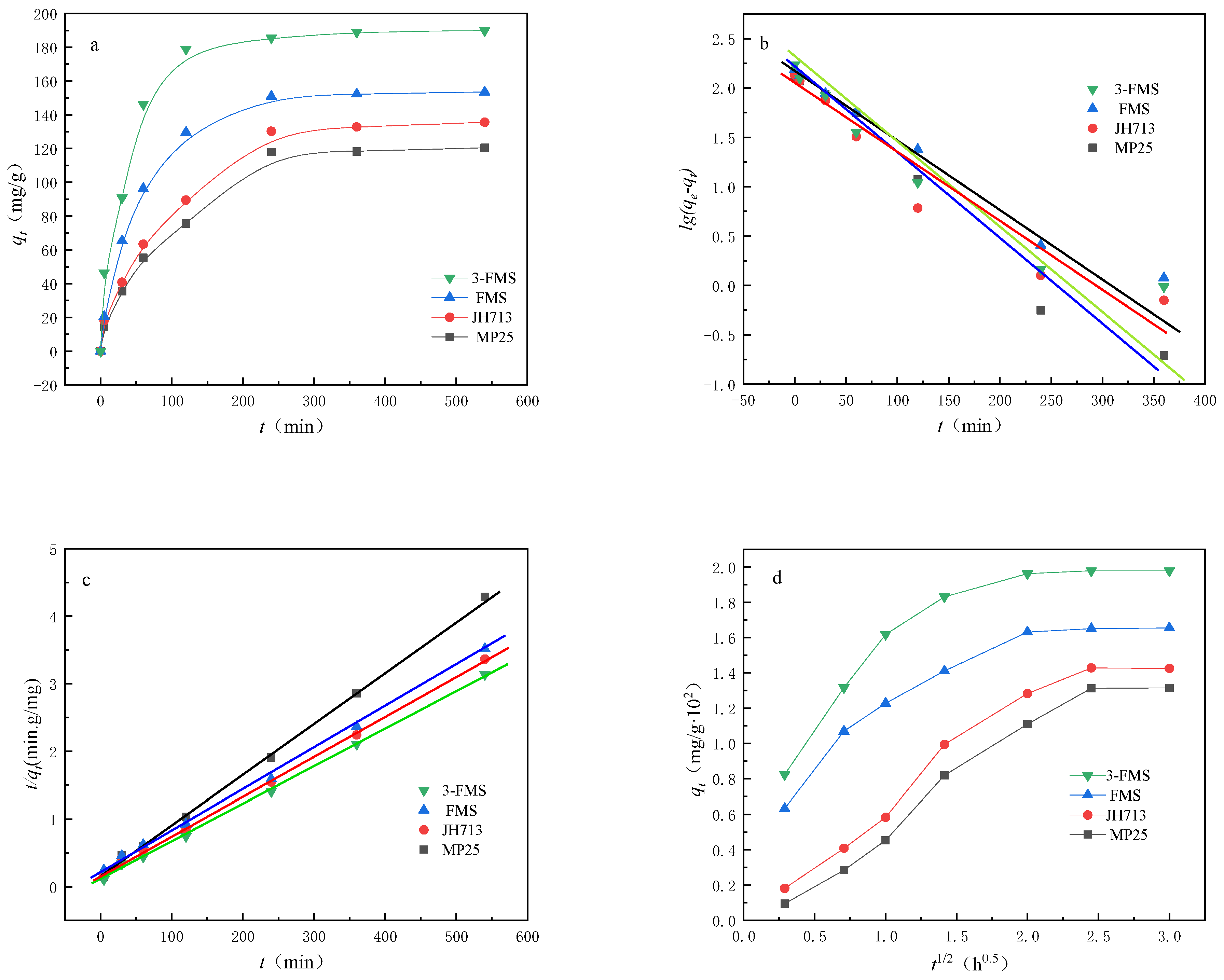
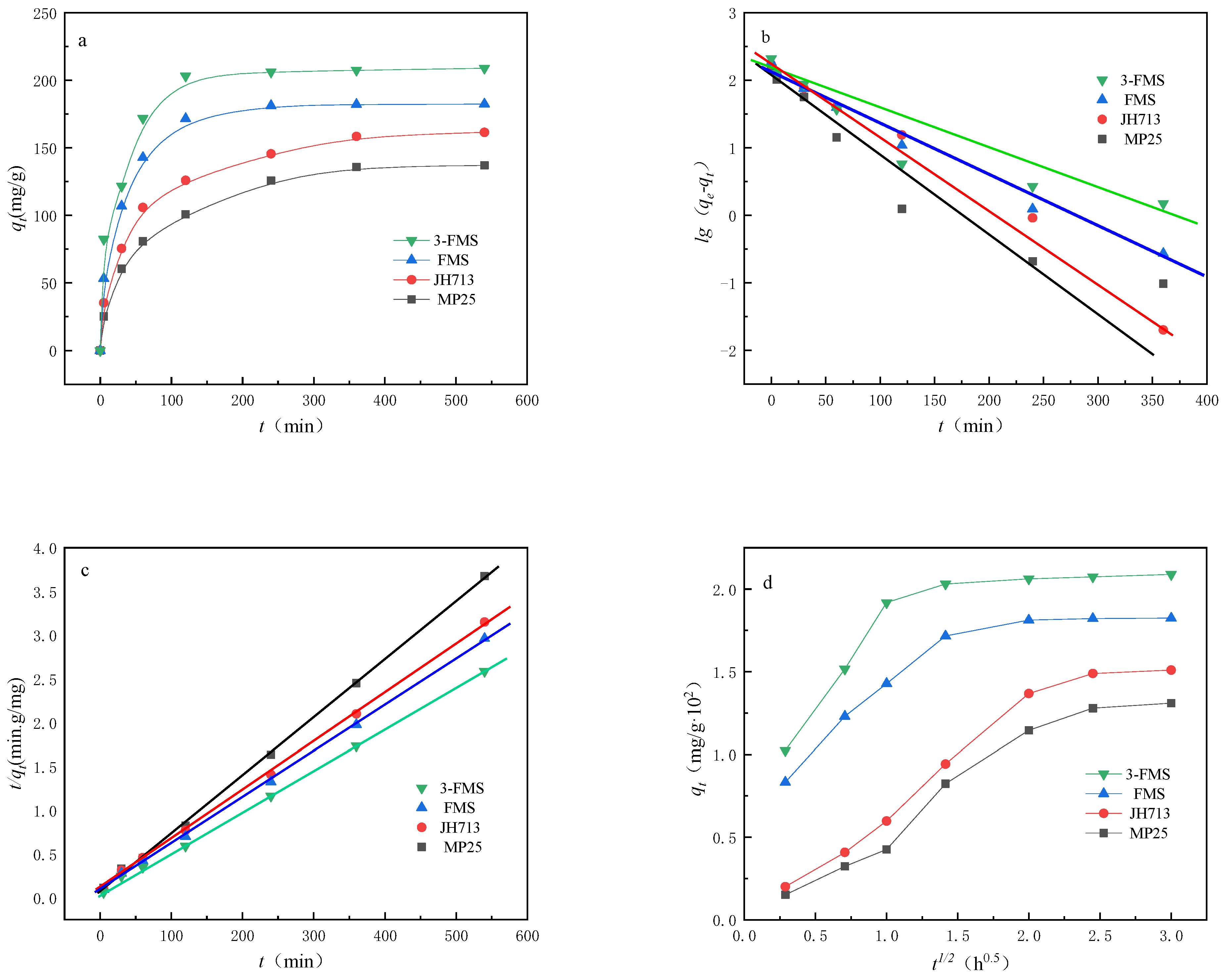
3.2.1. Adsorption Dynamics
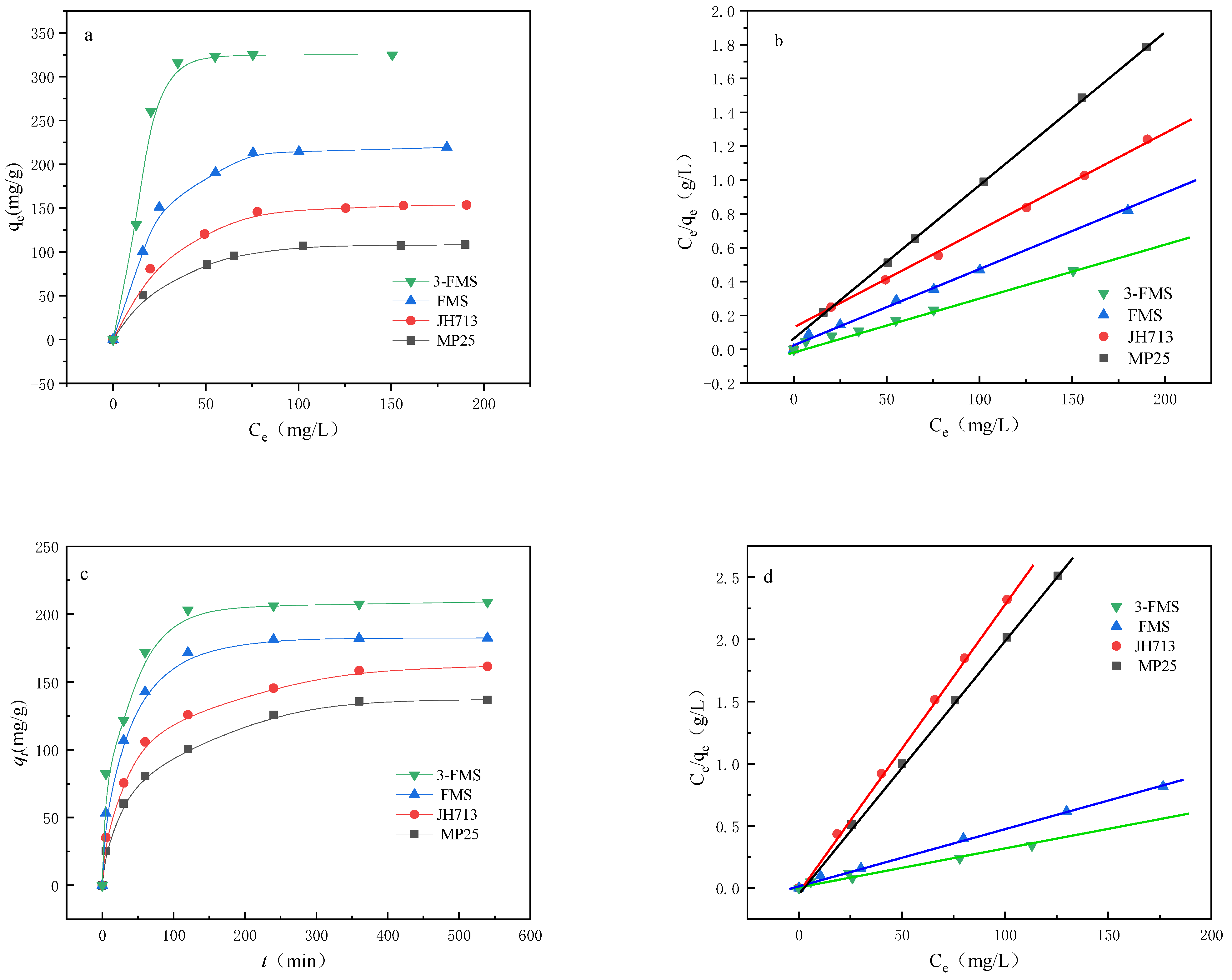
3.2.2. Adsorption Thermodynamics
3.3. Adsorption Mechanism of Heavy Metals Co2+ and Cr3+
3.3.1. Ion Exchange
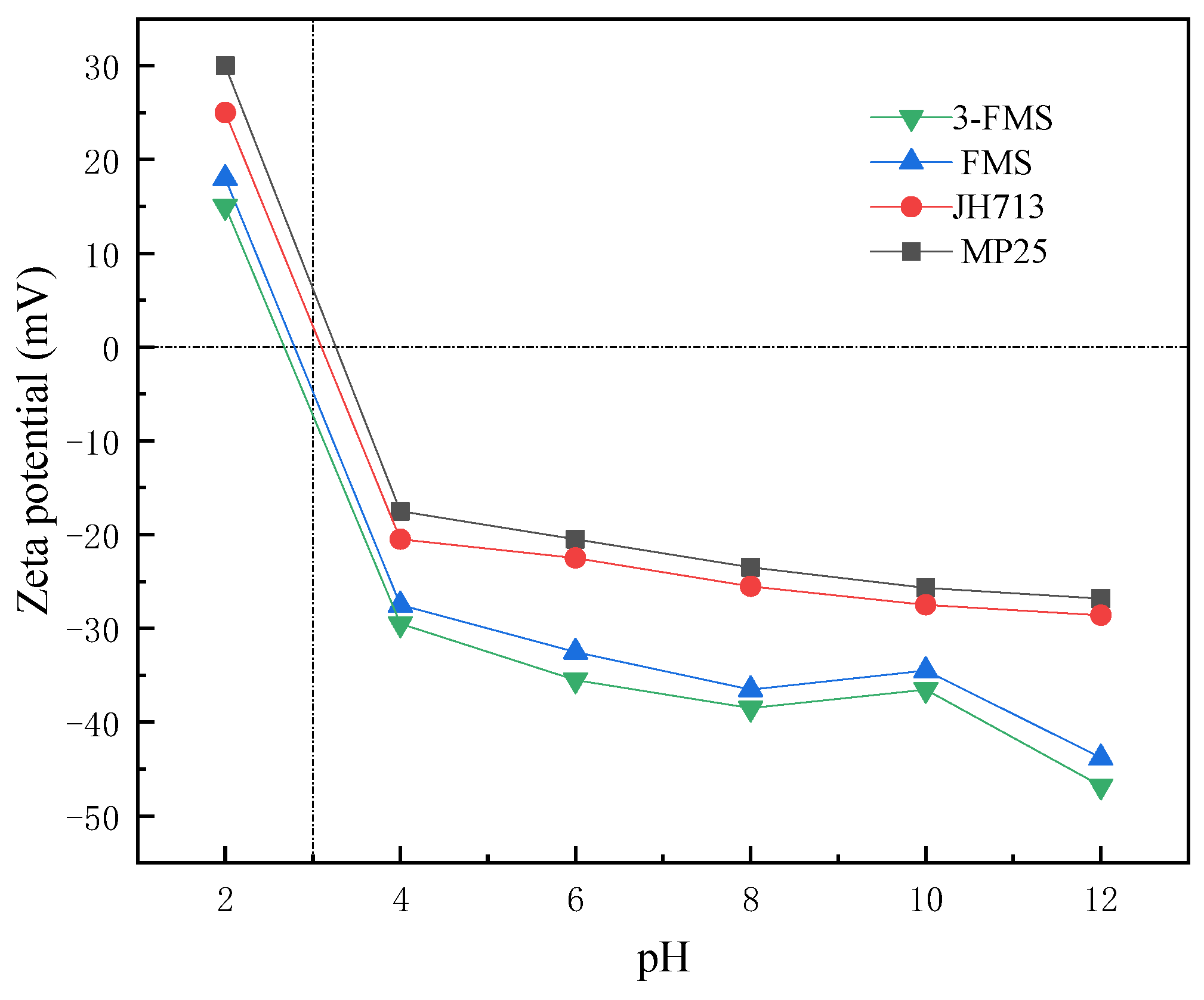
3.3.2. Electrostatic Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keshtkar, A.R.; Tabatabaeefar, A.; Vaneghi, A.S.; Moosavian, M.A. Electrospun polyvinylpyrrolidone/silica/3-aminopropyltriethoxysilane composite nanofiber adsorbent: Preparation, characterization and its application for heavy metal ions removal from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 1248–1258. [Google Scholar] [CrossRef]
- Cao, Y.X.; Zhao, M.J.; Ma, X.Y.; Song, Y.W.; Zuo, S.H.; Li, H.H.; Deng, W.Z. A critical review on the interactions of microplastics with heavy metals: Mechanism and their combined effect on organisms and humans. Sci. Total Environ. 2021, 788, 147620. [Google Scholar] [CrossRef] [PubMed]
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential use of algae for heavy metal bioremediation, a critical review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, Y.X. Removal of recalcitrant trivalent chromium complexes from industrial wastewater under strict discharge standards. Environ. Technol. Innov. 2021, 23, 101644. [Google Scholar] [CrossRef]
- Liu, G.J.; Cui, C.C.; Jiang, L.; Gao, H.; Gao, J. Visible light-induced hydrogels towards reversible adsorption and desorption based on trivalent chromium in aqueous solution. React. Funct. Polym. 2021, 163, 104886. [Google Scholar] [CrossRef]
- Lu, T.; Ma, R.G.; Ke, K.; Zhang, D.; Qi, D.M.; Zhao, H.T. Synthesis of gallic acid functionalized magnetic hydrogel beads for enhanced synergistic reduction and adsorption of aqueous chromium. Chem. Eng. J. 2021, 408, 127327. [Google Scholar] [CrossRef]
- Latif, A.; Sheng, D.; Sun, K.; Si, Y.B.; Azeem, M.; Abbas, A.; Bilal, M. Remediation of heavy metals polluted environment using Fe-based nanoparticles: Mechanisms, influencing factors, and environmental implications. Environ. Pollut. 2020, 264, 114728. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, B.; Venkatachalam, S.S.; Kiran, M.S.; Das, S.K. Rationally designed shewanella oneidensis biofilm toilored graphene-magnetite hybrid nanobiocomposite as reusable living functional nanomaterial for effective removal of trivalent chromium. Environ. Pollut. 2021, 278, 116847. [Google Scholar] [CrossRef]
- Chen, R.; Sheehan, T.; Ng, J.L.; Brucks, M.; Su, X. Capacitive deionization and electrosorption for heavy metal removal. Environ. Sci.-Water Res. Technol. 2020, 6, 258–282. [Google Scholar] [CrossRef]
- Demarco, C.F.; Quadro, M.S.; Carlos, F.S.; Pieniz, S.; Morselli, L.B.G.A.; Andreazza, R. Bioremediation of aquatic environments contaminated with heavy metals: A review of mechanisms, solutions and perspectives. Sustainability 2023, 15, 1411. [Google Scholar] [CrossRef]
- Qiu, M.Q.; Liu, L.J.; Ling, Q.; Cai, Y.W.; Yu, S.J.; Wang, S.Q.; Fu, D.; Hu, B.W.; Wang, X.K. Biochar for the removal of contaminants from soil and water: A review. Biochar 2022, 4, 19–27. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J. Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: A review. Environ. Chem. Lett. 2020, 18, 2055–2062. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Mu, Y.; Ai, Z.H.; Zhang, L.Z.; Song, F.H. Insight into core-shell dependent anoxic Cr (VI) removal with Fe@Fe2O3 nanowires: Indispensable role of surface bound Fe (II). ACS Appl. Mater. Interfaces 2015, 7, 1997–2005. [Google Scholar] [CrossRef]
- Bilal, M.; Ihsanullah, I.; Younas, M.; Bilal, M.; Ihsanullah, I.; Younas, M.; Shah, M.U.I.H. Recent advances in applications of low-cost adsorbents for the removal of heavy metals from water: A critical review. Sep. Purif. Technol. 2021, 278, 119510. [Google Scholar] [CrossRef]
- Bin, B.K.; Aljlil, S. Utilization of prepared nanocellulose as a biopolymer for adsorption kinetics of cobalt ions from wastewater. Polymers 2023, 15, 2143. [Google Scholar] [CrossRef]
- Yuan, G.Y.; Tu, H.; Li, M.; Liu, J.; Zhao, C.S.; Liao, J.L.; Yang, Y.Y.; Yang, J.J.; Liu, N. Glycine derivative-functionalized metal-organic framework (MOF) materials for Co(II) removal from aqueous solution. Appl. Surf. Sci. 2018, 466, 903–910. [Google Scholar] [CrossRef]
- He, M.Y.; Zhu, Y.; Yang, Y.; Han, B.P.; Zhang, Y.M. Adsorption of cobalt (II) ions from aqueous solutions by palygorskite. Appl. Clay Sci. 2011, 54, 292–296. [Google Scholar] [CrossRef]
- Lujaniene, G.; Novikau, R.; Karaleviciute, K.; Pakstas, V.; Talaikis, M.; Levinskaite, L.; Selskiene, A.; Selskis, A.; Mazeika, J.; Joksas, K. Chitosan-minerals-based composites for adsorption of caesium, cobalt and europium. J. Hazard. Mater. 2023, 462, 132747. [Google Scholar] [CrossRef]
- Gürü, M.; Venedik, D.; Murathan, A. Removal of trivalent chromium from water using low-cost natural diatomite. J. Hazard. Mater. 2008, 160, 318–323. [Google Scholar] [CrossRef]
- Iddou, A.; Ouali, M.S. Waste-activated sludge (WAS) as Cr(III) sorbent biosolid from wastewater effluent. Colloids Surf. B Biointerfaces 2008, 66, 240–245. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P.; Singh, V.K. Trivalent chromium removal from wastewater using low cost activated carbon derived from agricultural waste material and activated carbon fabric cloth. J. Hazard. Mater. 2005, 135, 280–295. [Google Scholar] [CrossRef]
- Kaur, I.; Mandiyal, D.; Singh, B.P.; Kumar, R.; Chawla, J. Amino-functionalized mesoporous MCM-41: An efficient adsorbent for the removal of chromium (III) ions from aqueous solution. J. Water Supply Res. Technol. 2016, 65, 480–493. [Google Scholar] [CrossRef]
- Gomez-Gonzalez, S.E.; Carbajal-Arizaga, G.G.; Manriquez-Gonzalez, R.; De la Cruz-Hernandez, W.; Gomez-Salazar, S. Trivalent chromium removal from aqueous solutions by a sol-gel synthesized silica adsorbent functionalized with sulphonic acid groups. Mater. Res. Bull. 2014, 59, 394–404. [Google Scholar] [CrossRef]
- Jin, X.L.; Yu, C.; Hu, H.Y. Preparation of novel nanoadsorbent based on organic inorganic hybrid and their adsorption for heavy metals and organic pollutants presented in water environment. J. Hazard. Mater. 2011, 186, 1672–1680. [Google Scholar] [CrossRef]
- Lata, S.; Samadder, S.R. Removal of arsenic from water using nano adsorbents and challenges: A review. J. Environ. Manag. 2016, 166, 387–406. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatmentpurposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Castro-Munoz, R. A critical review on electrospun membranes containing 2D materials for seawater desalination. Desalination 2023, 555, 116528. [Google Scholar] [CrossRef]
- Basheer, A. New generation nano-adsorbents for the removal of emerging contaminants in water. J. Mol. Liq. 2018, 261, 583–593. [Google Scholar] [CrossRef]
- Anjum, M.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M.A. Remediation of wastewater using various nano-materials. Arab. J. Chem. 2019, 12, 4897–4919. [Google Scholar] [CrossRef]
- Jawed, A.; Saxena, V.; Pandey, L.M. Engineered nanomaterials and their surface functionalization for the removal of heavy metals: A review. J. Water Process. Eng. 2020, 33, 101009. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yang, Y.; Xiang, G.L.; Wang, X. Magnesium silicate hollow nanostructures as highly efficient absorbents for toxic metal ions. J. Phys. Chem. C 2009, 113, 10441–10445. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Sun, Z.W.; Huang, D.; Duan, X.H.; Hong, W.; Liang, J.S. Functionalized nanoflower-like hydroxyl magnesium silicate for effective adsorption of aflatoxin B1. J. Hazard. Mater. 2020, 387, 122185. [Google Scholar] [CrossRef]
- Qu, J.; Li, W.; Cao, C.Y.; Yin, X.J.; Zhao, L.; Bai, J.; Qin, Z.; Song, W.G. Metal silicate nanotubes with nanostructured walls as superb adsorbents for uranyl ions and lead ions in water. J. Mater. Sci. 2012, 22, 17222. [Google Scholar] [CrossRef]
- Bao, J.; Feng, Y.J.; Pan, Y.; Jiang, J.C. Modified approaches to prepare nano-magnesium silicates with hierarchical pore structure and their performance towards adsorption of Cd2+. Environ. Sci. Pollut. R 2023, 30, 89784–89793. [Google Scholar] [CrossRef]
- Zhang, T.; Vandeperre, L.J.; Cheeseman, C.R. Formation of magnesium silicate hydrate (M-S-H) cement pastes using sodium hexametaphosphate. Cem. Concr. Res. 2014, 65, 8–14. [Google Scholar] [CrossRef]
- Liao, L.B.; Lv, G.C.; Cai, D.X.; Wu, L.M. The sequential intercalation of three types of surfactants into sodium montmorillonite. Appl. Clay Sci. 2016, 119, 82–86. [Google Scholar] [CrossRef]
- Rutkai, G.; Ható, Z.; Kristóf, T. Stability of the kaolinite-guest molecule intercalation system: A molecular simulation. Fluid Phase Equilibr. 2016, 409, 434–438. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Q.F.; Cheng, H.F.; Gao, F.; Liu, C.; Teppen, B.J. Mechanism responsible for intercalation of dimethyl sulfoxide in kaolinite: Molecular dynamics simulations. Appl. Clay Sci. 2018, 151, 46–53. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Narasimharao, K.; Ali, T.T.; Bawaked, S.; Basahel, S. Effect of si precursor on structural and catalytic properties of nanosize magnesium silicates. Appl. Catal. A Gen. 2014, 488, 208–218. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dang, L.Y.; Zhang, M.C.; Lu, Q.S.; Zhao, S.F. Characterization of mesoporous magnesium silicate with hierarchical structure and its adsorption performance for dye and lead ion. Surf. Interfaces 2017, 8, 112–118. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Hui, K.S.; Chao, C.Y.; Kot, S.C. Removal of mixed heavy metal ions in wastewater by Zeolite 4a and residual products from recycled coal fly ash. J. Hazard. Mater. 2005, 127, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Kooh, M.R.R.; Dahri, M.K.; Lim, L.B.L. Removal of methyl violet 2B dye from aqueous solution using Nepenthes rafflflesiana pitcher and leaves. Appl. Water Sci. 2017, 7, 3859–3868. [Google Scholar] [CrossRef]
- Wongsakulphasatch, S.; Kiatkittipong, W.; Saiswat, J.; Oonkhanond, B.; Striolo, A.; Assabumrungrat, S. The adsorption aspect of Cu2+ and Zn2+ on MCM-41 and SDS-modified MCM-41. Inorg. Chem. Commun. 2014, 46, 301. [Google Scholar] [CrossRef]
- Huang, R.Y.; Wu, M.J.; Zhang, T.; Li, D.Q.; Tang, P.G.; Feng, Y.J. Template-free synthesis of large-pore-size porous magnesium silicate hierarchical nanostructures for removal of heavy metal ions. ACS Sustain. Chem. Eng. 2017, 27, 2774–2780. [Google Scholar] [CrossRef]
- Gui, C.X.; Wang, Q.Q.; Hao, S.M.; Qu, J.; Huang, P.P. Sandwich like magnesium silicate/reduced graphene oxide nanocomposite for enhanced Pb2+ and methylene blue adsorption. ACS Appl. Mater. Interfaces 2014, 6, 14653. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.D.; Mo, Z.L.; Li, L.; Chen, F.; Wu, Q.J.; Qi, L. Efficient removal of copper (II) and malachite green from aqueous solution by magnetic magnesium silicate composite. J. Chem. Eng. Data 2017, 62, 3036. [Google Scholar] [CrossRef]
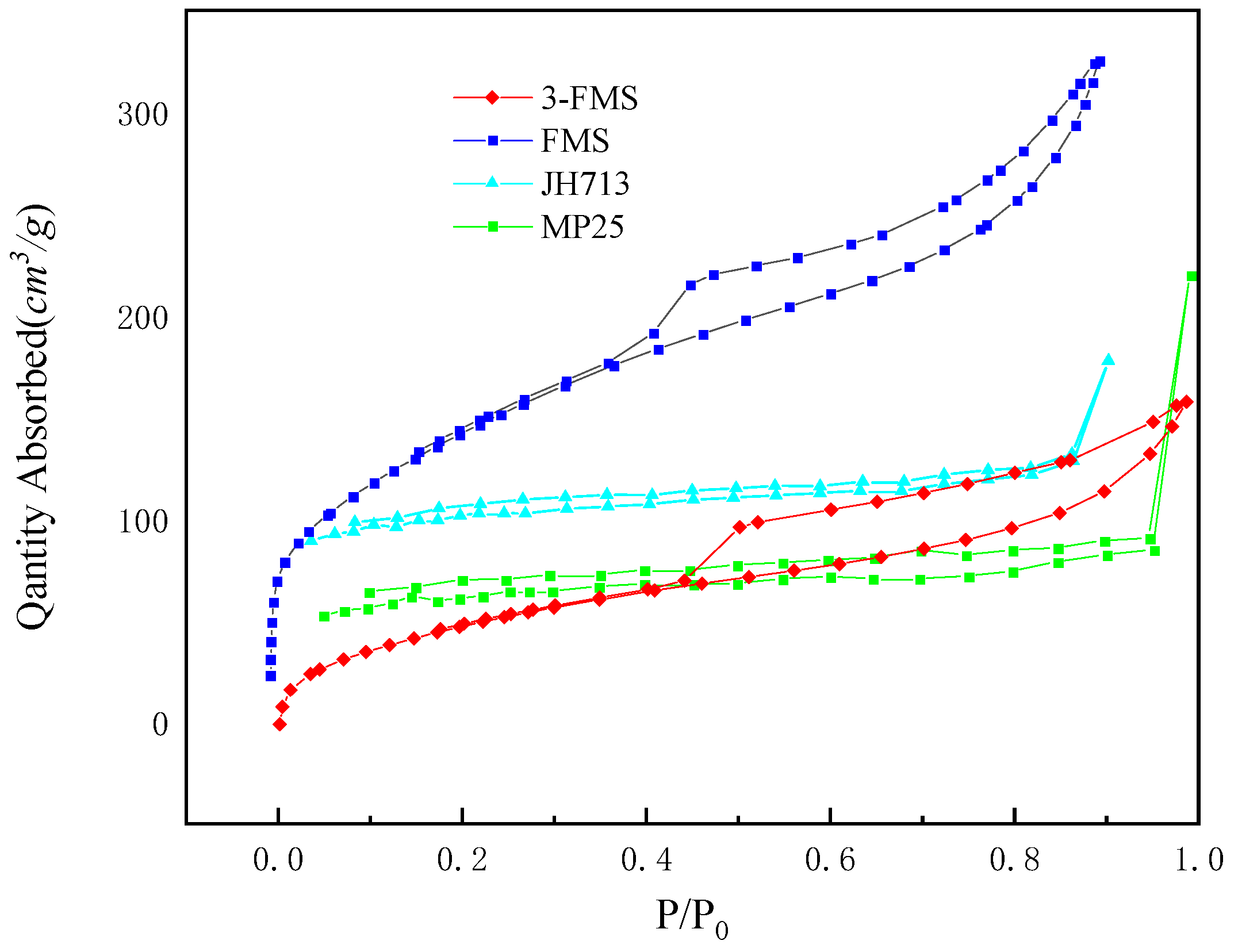



| Sample | Surface Area (m2/g) | Average Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| MP25 | 230.59 | 2.42 | 0.14 |
| FMS | 241.75 | 4.33 | 0.26 |
| JH713 | 367.45 | 3.01 | 0.27 |
| 3-FMS | 502.42 | 7.28 | 0.63 |
| Material | O (%) | Mg (%) | Si (%) | S (%) | Na (%) | C (%) |
|---|---|---|---|---|---|---|
| FMS | 52.12 | 18.37 | 17.04 | / | / | 12.46 |
| 3-FMS | 51.11 | 17.86 | 16.57 | 0.61 | 0.36 | 13.50 |
| Material | qe,exp (mg/g) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| qe,cal (mg·g−1) | k1 (min−1) | R2 | qe,cal (mg·g−1) | k2(g·mg−1·min−1) | R2 | ||
| MP25 | 120.48 | 107.73 | 0.27 | 0.941 | 119.80 | 0.53 | 0.999 |
| JH713 | 135.56 | 110.45 | 0.32 | 0.935 | 137.56 | 0.65 | 0.999 |
| FMS | 153.48 | 113.56 | 0.47 | 0.952 | 159.05 | 0.81 | 0.999 |
| 3-FMS | 190.01 | 172.87 | 0.69 | 0.956 | 195.38 | 1.02 | 0.998 |
| Material | qe,exp (mg/g) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| qe,cal (mg·g−1) | k1 (min−1) | R2 | qe,cal (mg·g−1) | k2 (g·mg−1·min−1) | R2 | ||
| MP25 | 136.98 | 100.84 | 0.54 | 0.936 | 135.73 | 0.14 | 0.998 |
| JH713 | 161.48 | 145.54 | 0.33 | 0.973 | 163.25 | 0.76 | 0.999 |
| FMS | 182.40 | 150.79 | 0.47 | 0.945 | 190.45 | 0.83 | 0.998 |
| 3-FMS | 208.89 | 169.89 | 0.61 | 0.972 | 220.63 | 1.04 | 0.999 |
| Material | KL (L/mg) | qm (mg/g) | R2 |
|---|---|---|---|
| MP25 | 0.56 | 150.85 | 0.999 |
| JH713 | 0.69 | 162.52 | 0.999 |
| FMS | 0.58 | 185.14. | 0.999 |
| 3-FMS | 1.07 | 207.62 | 0.999 |
| Material | KL (L/mg) | qm (mg/g) | R2 |
|---|---|---|---|
| MP25 | 0.47 | 162.43 | 0.999 |
| JH713 | 0.74 | 180.72 | 0.999 |
| FMS | 0.53 | 200.71 | 0.999 |
| 3-FMS | 1.03 | 230.85 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, J.; Feng, Y.; Pan, Y.; Jiang, J. Adsorption of Co2+ and Cr3+ in Industrial Wastewater by Magnesium Silicate Nanomaterials. Materials 2024, 17, 1946. https://doi.org/10.3390/ma17091946
Bao J, Feng Y, Pan Y, Jiang J. Adsorption of Co2+ and Cr3+ in Industrial Wastewater by Magnesium Silicate Nanomaterials. Materials. 2024; 17(9):1946. https://doi.org/10.3390/ma17091946
Chicago/Turabian StyleBao, Jing, Yongjun Feng, Yong Pan, and Juncheng Jiang. 2024. "Adsorption of Co2+ and Cr3+ in Industrial Wastewater by Magnesium Silicate Nanomaterials" Materials 17, no. 9: 1946. https://doi.org/10.3390/ma17091946





