Characterization of Bioadsorbents from Organic Municipal Waste
Abstract
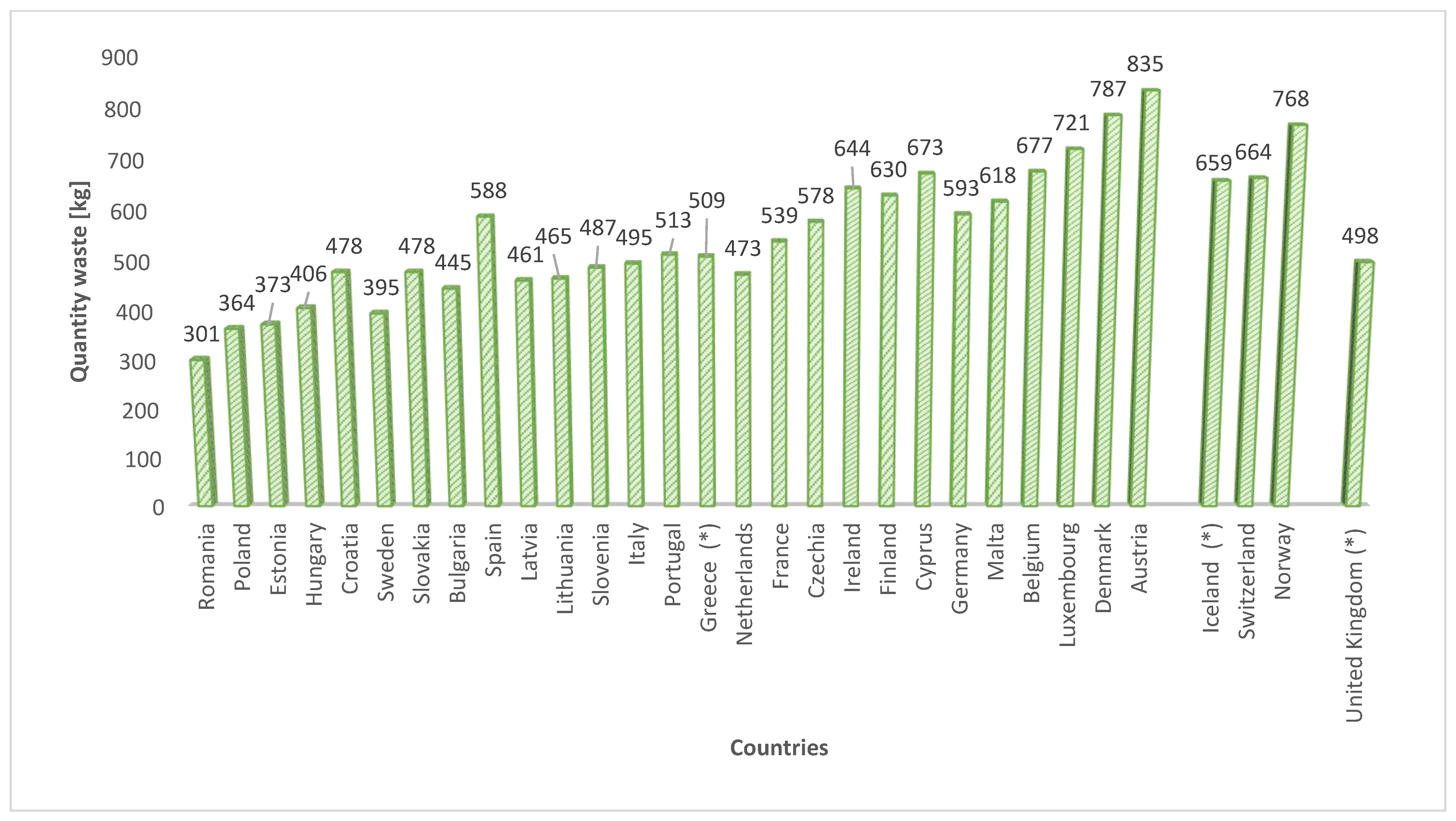
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Materials
Elemental Analysis of the Samples
3. Results and Discussions
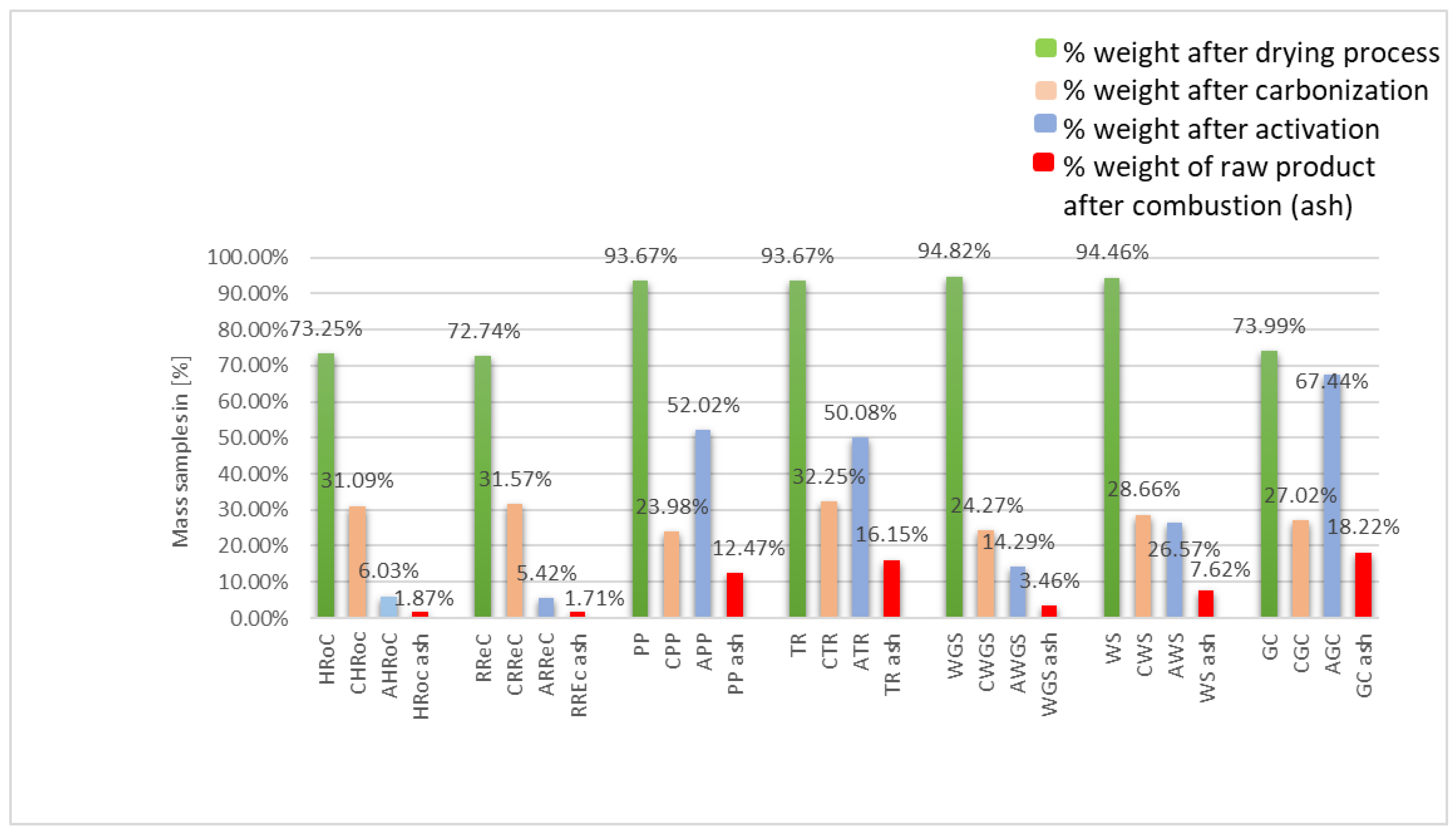
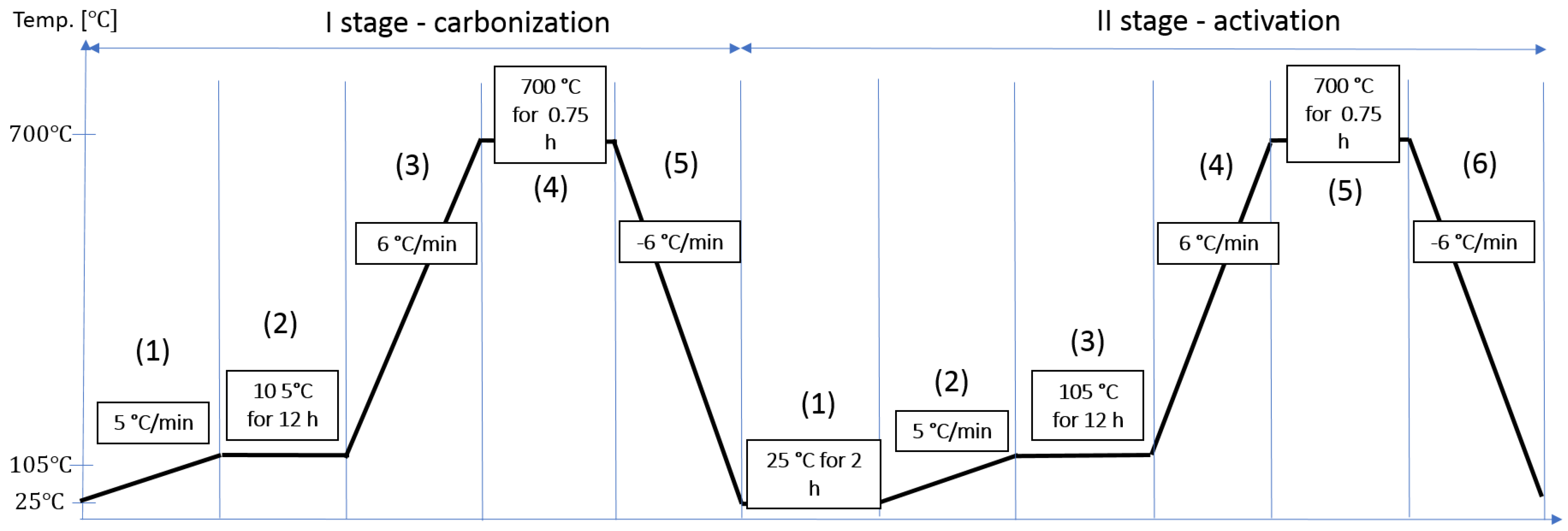
3.1. Synthesis Bioadsorbents
3.2. Characterization of Materials
3.2.1. Characterization of Chemical Composition
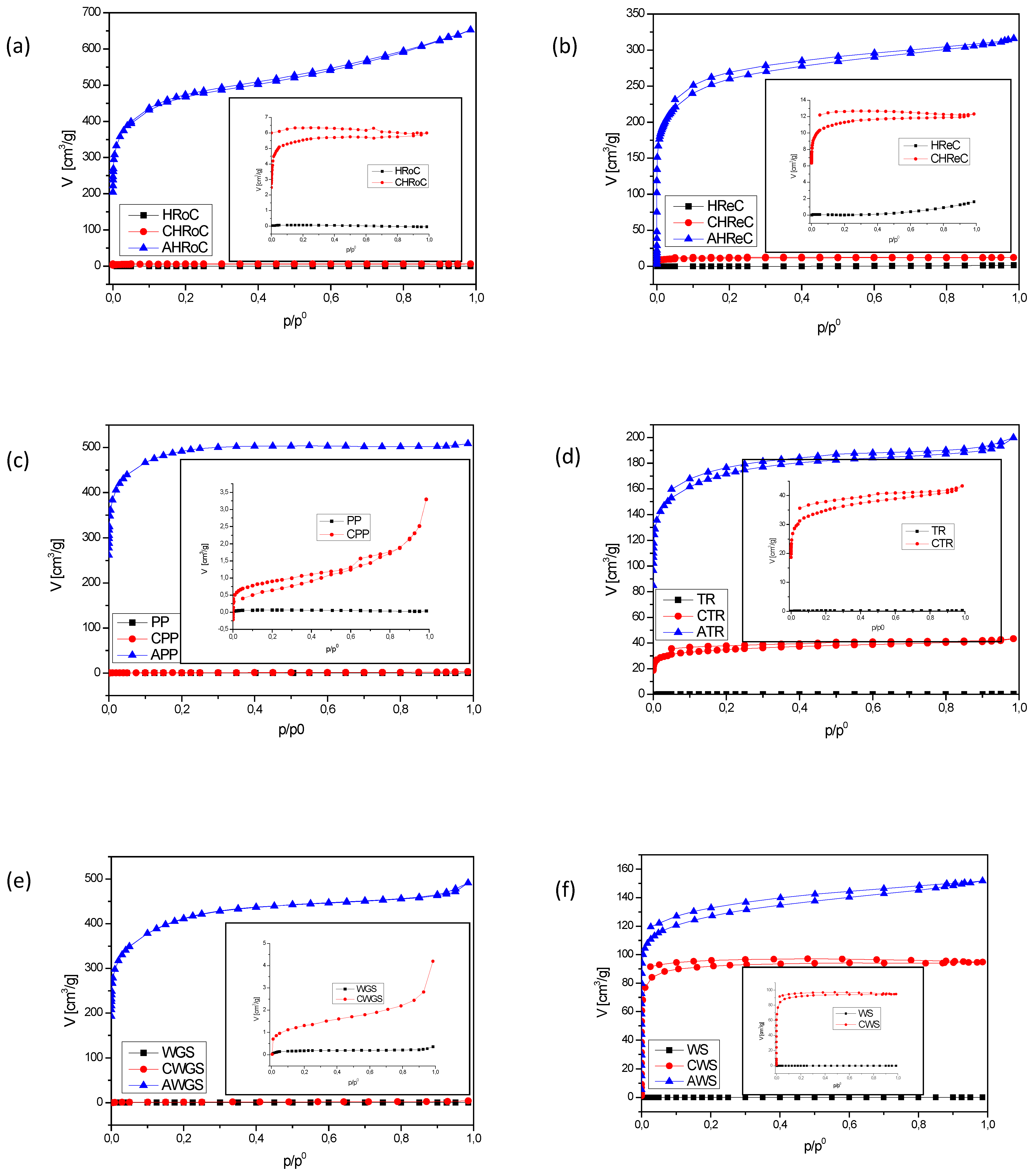
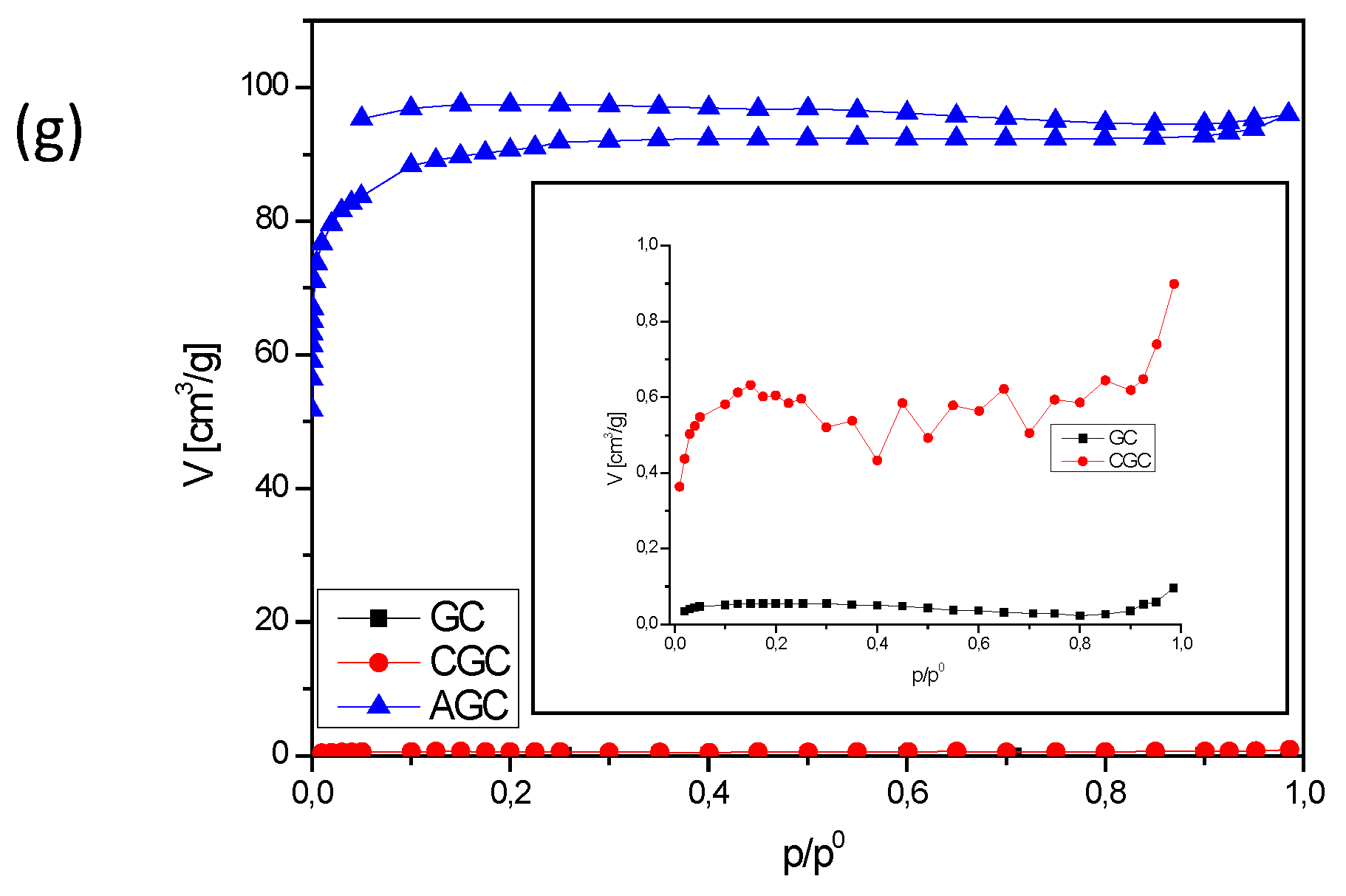
3.2.2. BET Analysis
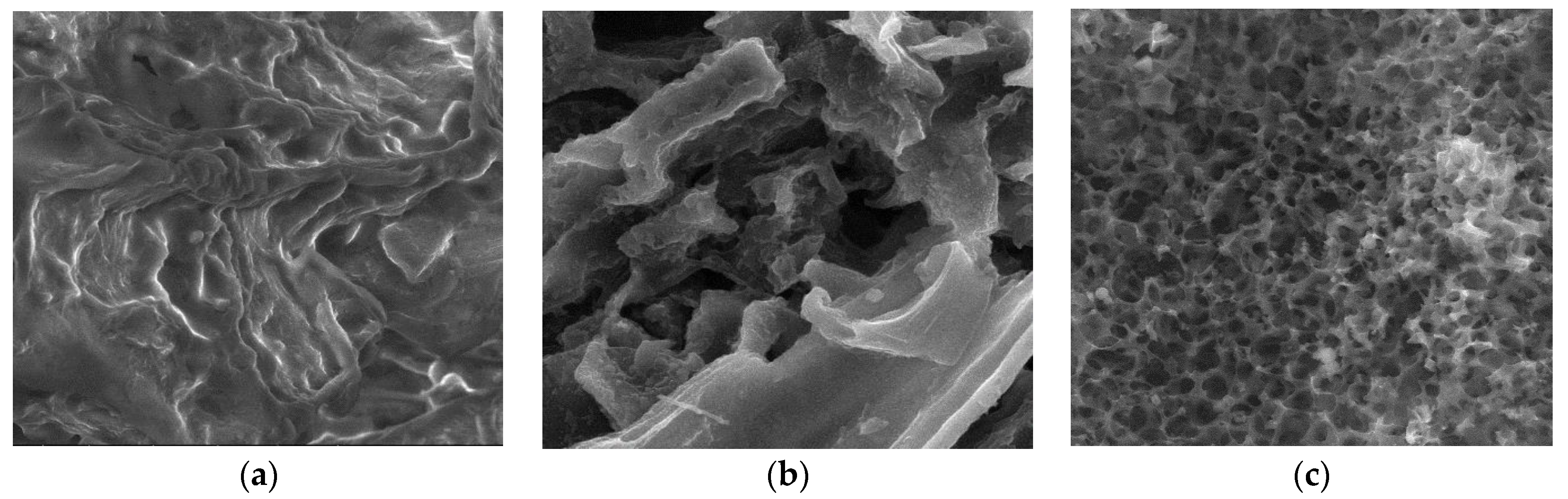
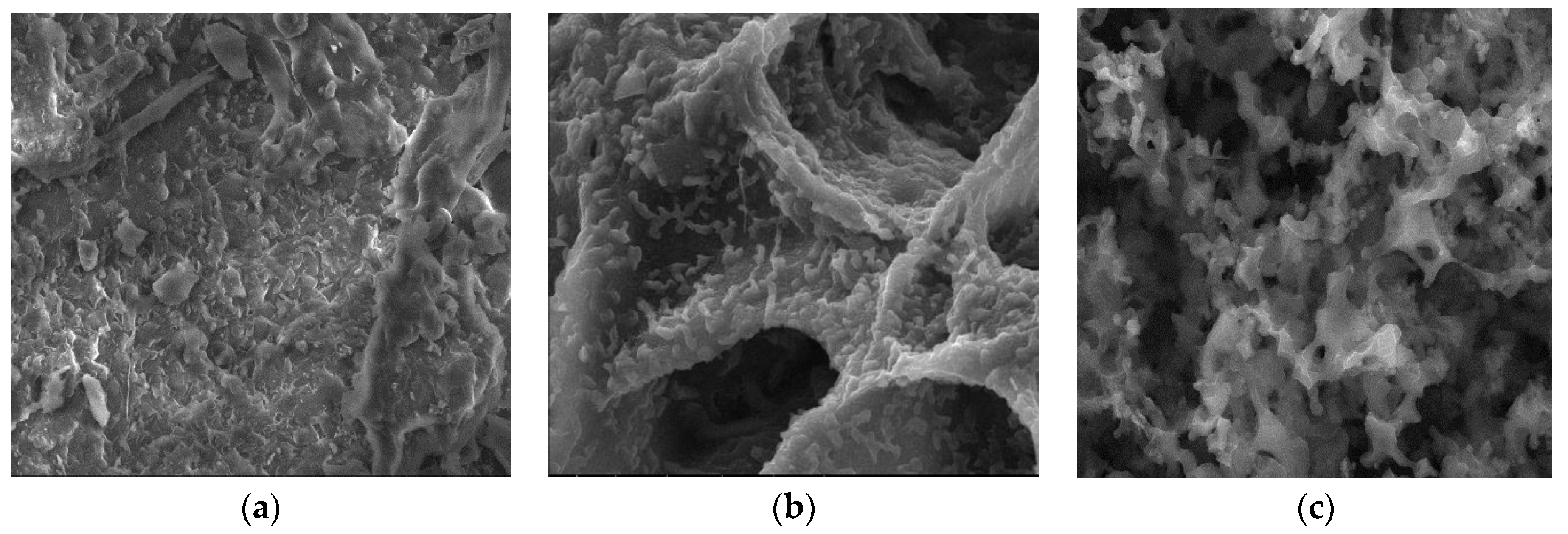
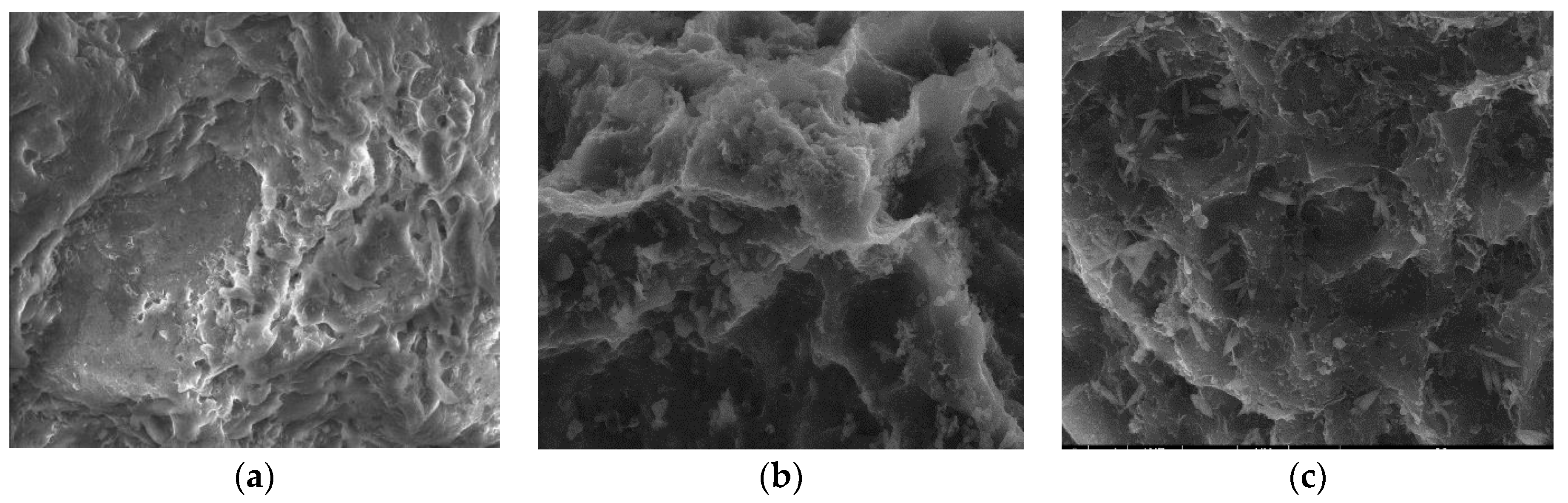
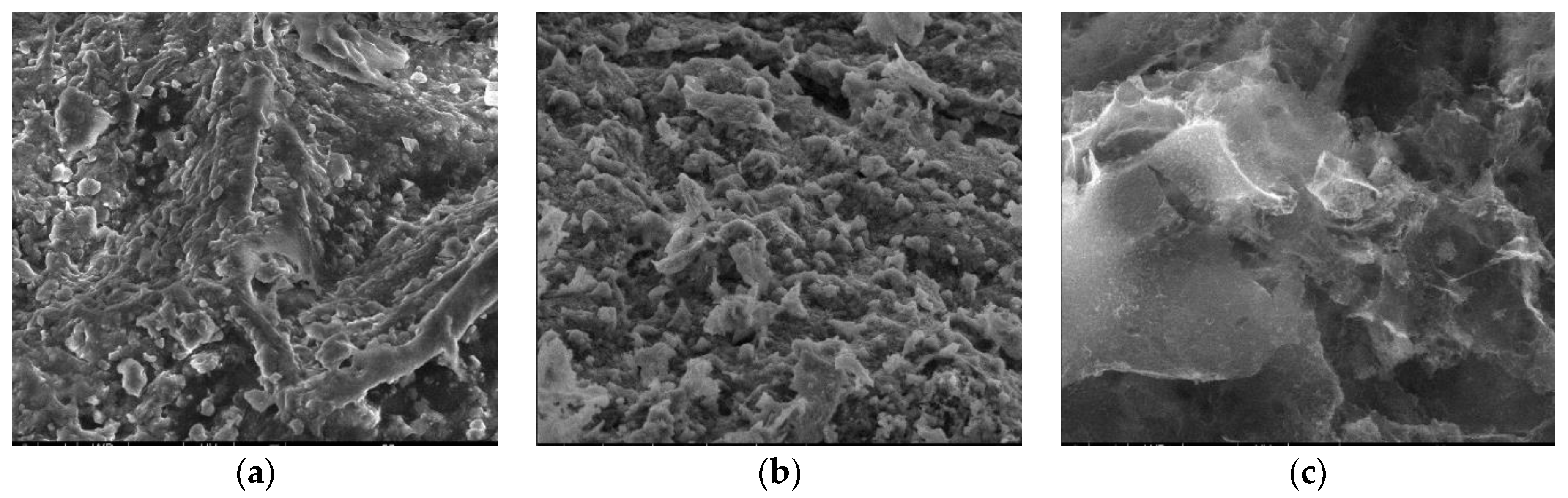
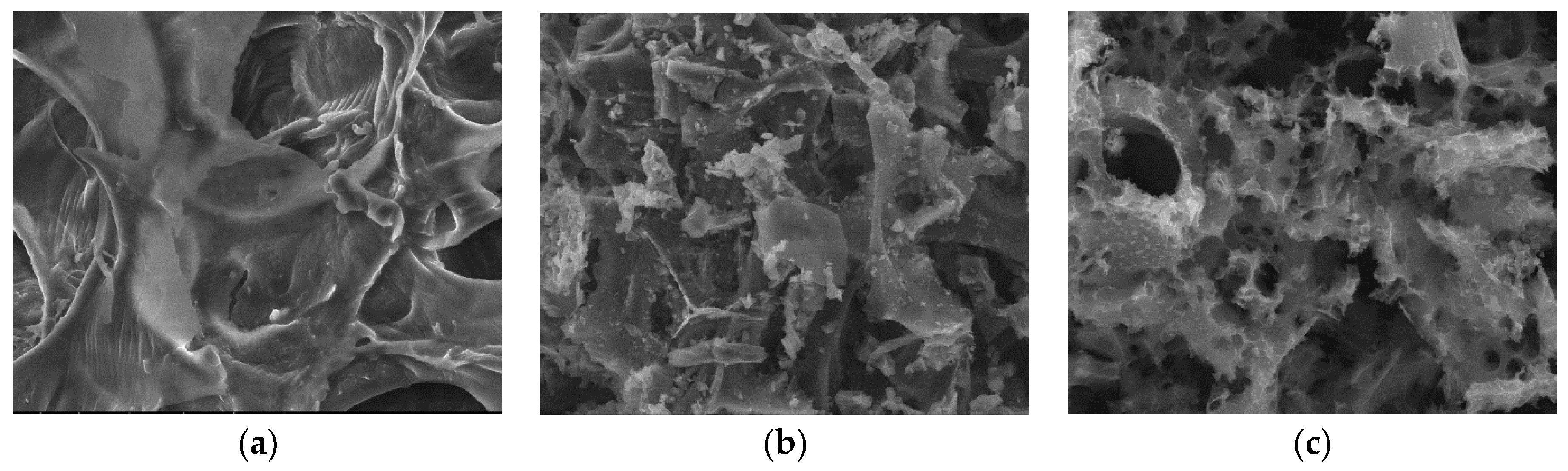
3.2.3. SEM
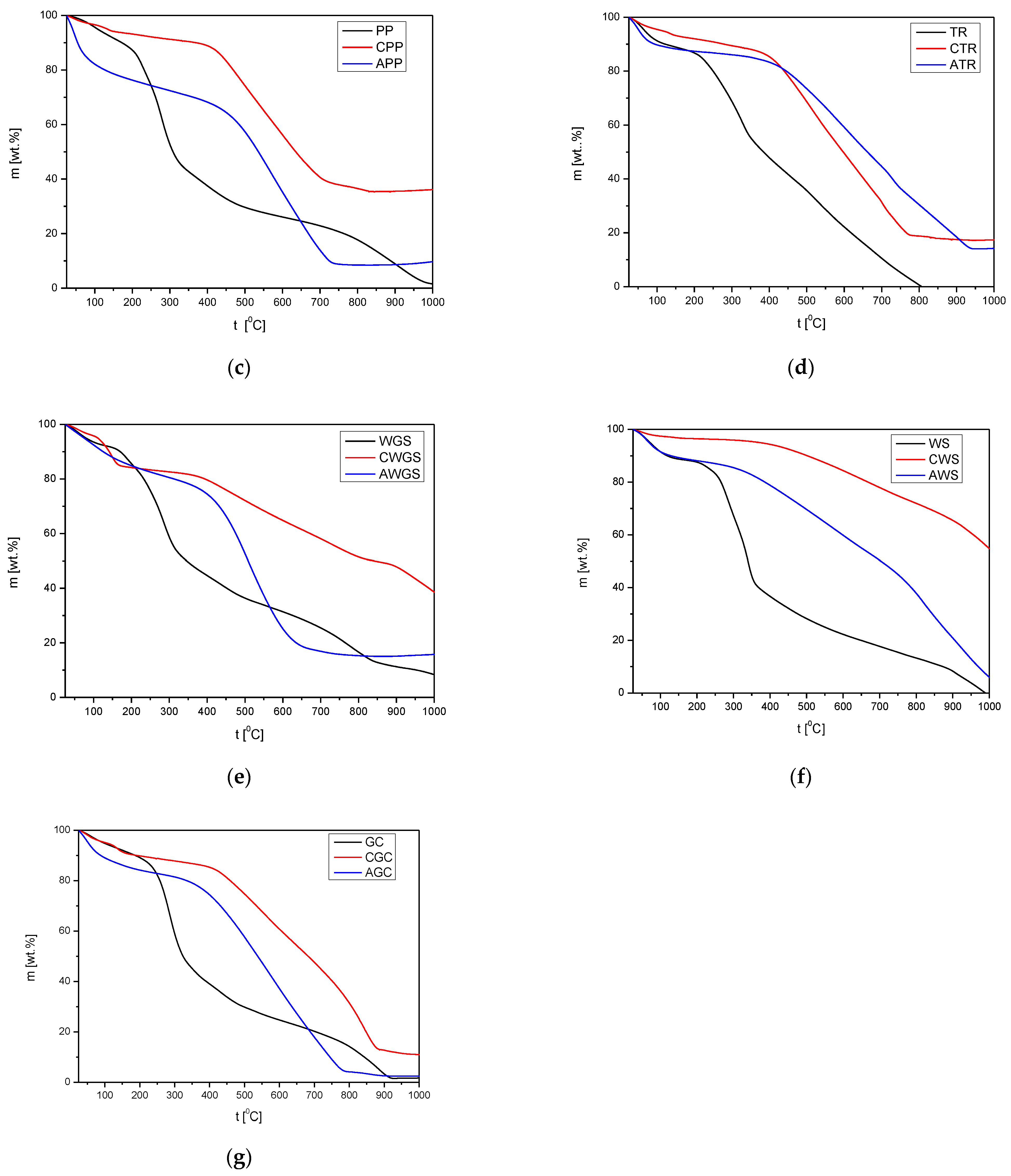
3.2.4. TG Profiles
3.2.5. XRD Analysis
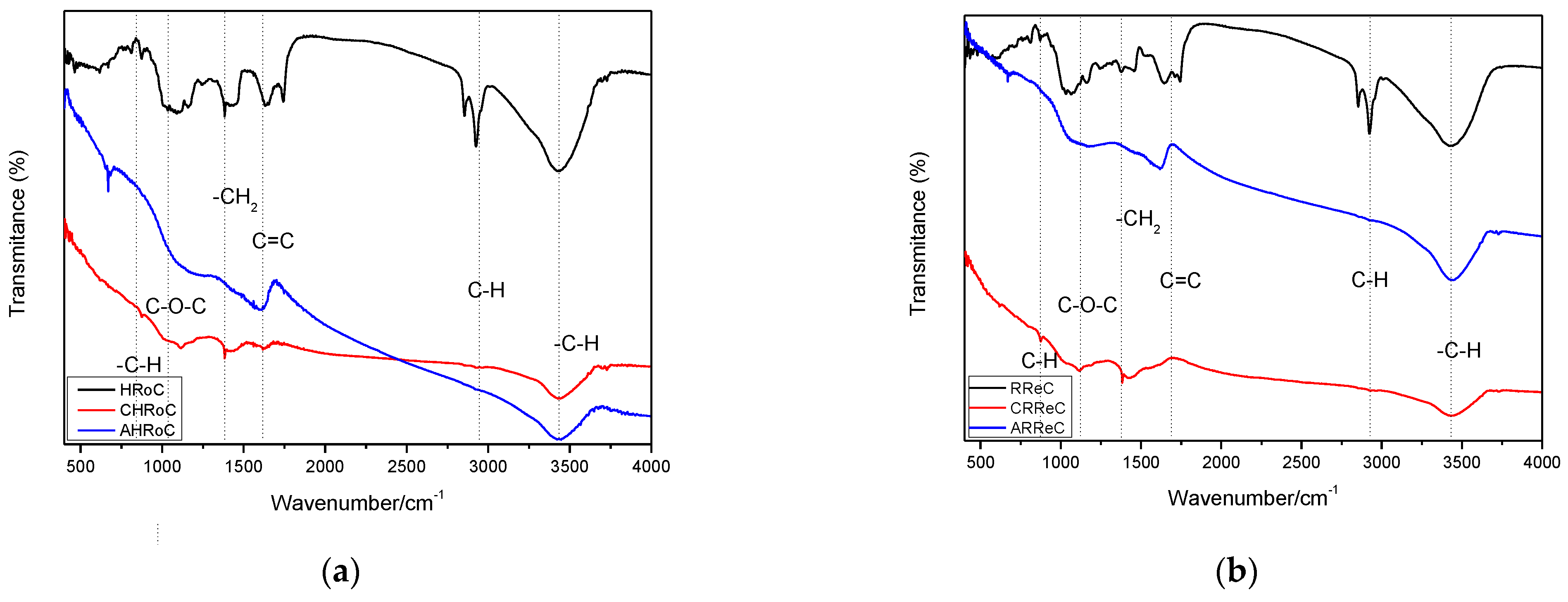
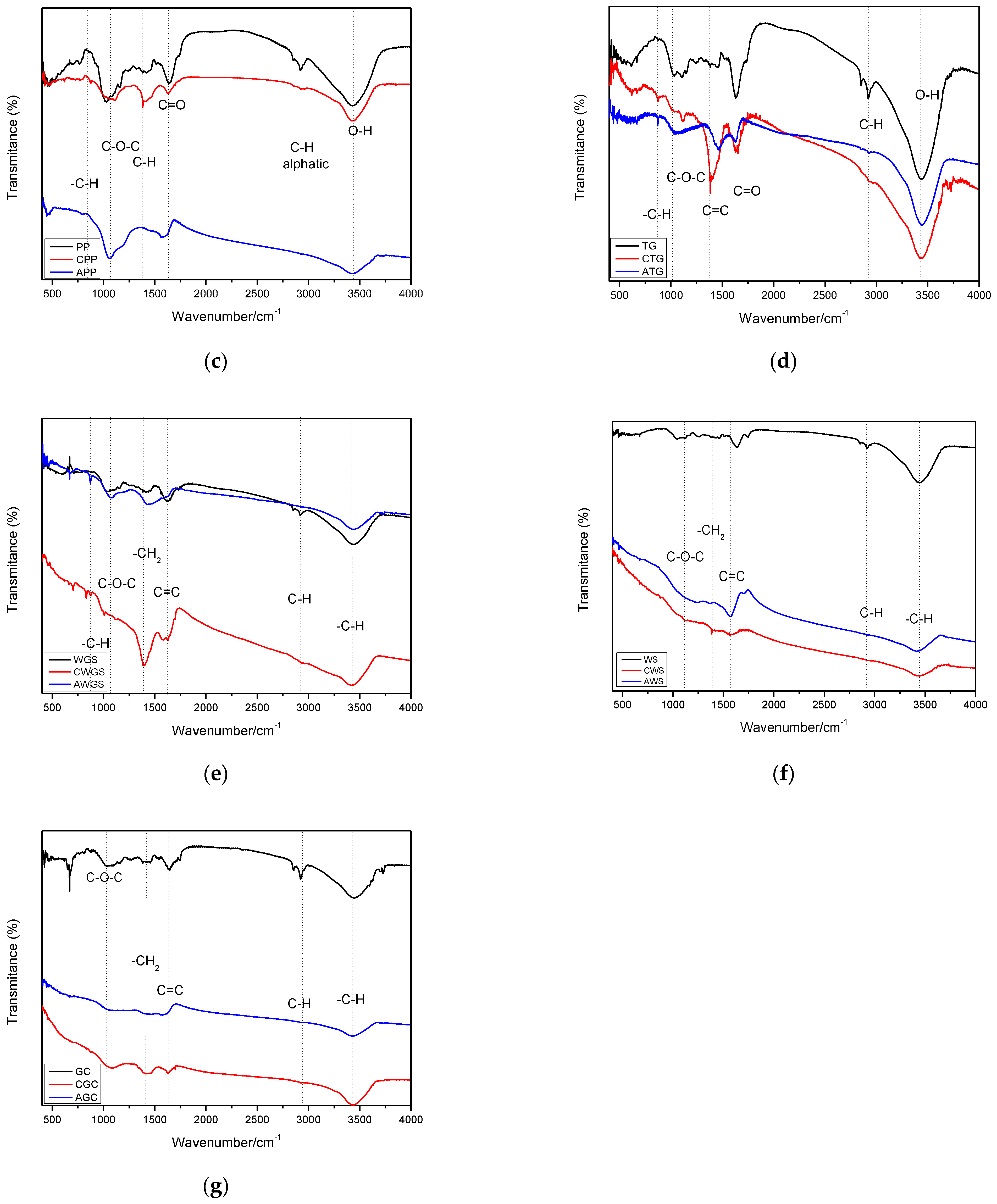
3.2.6. FT-IR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Municipal Waste by Waste Management Operations, Eurostat. Available online: https://ec.europa.eu/eurostat/databrowser/view/env_wasmun/default/table?lang=en (accessed on 1 December 2023).
- Recycling: The Seventh Resource Manifesto, Global Recycling Day. 18 March 2018. Available online: https://www.bir.org/publications/facts-figures/item/recycling-the-seventh-resource-manifesto (accessed on 9 May 2022).
- Directive (EU) 2018/851 of the European Parliament and of the Council of 30 May 2018 Amending Directive 2008/98/EC on Waste. Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/?uri=CELEX%3A32018L0851 (accessed on 15 March 2024).
- National Waste Management Plan 2028. Available online: https://bip.mos.gov.pl/strategie-plany-programy/krajowy-plan-gospodarki-odpadami/ (accessed on 11 March 2023).
- Podstawy Gospodarki Odpadami; Publ. PWN, Ed. 6; Czesława Rosik-Dulkowska: Warsaw, Poland, 2023.
- European Parliament, Waste Management in the EU: Facts and Figures (Infographic), Last Update: 17/11/2023. Available online: https://www.europarl.europa.eu/topics/en/article/20181212STO21610/plastic-waste-and-recycling-in-the-eu-facts-and-figures (accessed on 13 February 2024).
- Central Statistical Office. Environmental Protection 2022, Statistical Analyses; Central Statistical Office: Warsaw, Poland, 2022. [Google Scholar]
- Green Economy, A Ton of Coffee Grounds Worth PLN 4000. Euro. Available online: https://zielonagospodarka.pl/tona-fusow-po-kawie-warta-4-tys-euro-start-up-ecobean-pracuje-nad-uruchomieniem-kawowej-biorafinerii-6397 (accessed on 13 May 2023).
- Center for Good Therapy, to Drink Tea or Not to Drink. Available online: https://www.centrumdobrejterapii.pl/materialy/herbata-pic-czy-nie-pic/ (accessed on 21 April 2023).
- Institute for the Development of Ecological Thought, Potato—The Undisputed Leader Among Vegetables on Polish Tables. Available online: https://irme.pl/ziemniak-niekwestionowany-krol-posrod-warzyw-na-polskich-stolach/ (accessed on 13 April 2023).
- Obeng, G.Y.; Amoah, D.Y.; Opoku, R.; Sekyere, C.K.; Adjei, E.A.; Mensah, E. Coconut wastes as bioresources for sustainable energy: Quantifying waste, calorific value and emissions in Ghana. Energies 2020, 13, 2178. [Google Scholar] [CrossRef]
- Spasówka, E. The Goodyear corporation aims to double the use of silica from rice husks in 2021. Polimery 2021, 66, 146. [Google Scholar]
- Wang, R.; Wang, G. Acceleration effect of rice husk ash on hydration of styrene-acrylic ester/cement composite pastes. Cement Wapno Beton 2018, 23, 5. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Brunetti, C.; Fidalgo, A.; Ciriminna, R.; Delisi, R.; Albanese, L.; Zabini, F.; Gori, A.; Nascimento, L.B.D.S.; De Carlo, A.; et al. Real-scale integral valorization of waste orange peel via hydrodynamic cavitation. Processes 2019, 7, 581. [Google Scholar] [CrossRef]
- Decofire, How to Use Coffee Grounds? Available online: https://decofire.pl/blog/jak-wykorzystac-fusy-z-kawy (accessed on 9 March 2024).
- Chen, J.; Yang, J.; Hu, X.; Li, Z.; Shen, S.; Radosz, M.; Fan, M. Enhanced CO2 capture capacity of nitrogen-doped biomass-derived porous carbons. ACS Sustain. Chem. Eng. 2016, 4, 1439–1445. [Google Scholar] [CrossRef]
- Ello, A.S.; Souza, L.K.C.; Trokorey, A.; Jaroniec, M. Coconut shell-based microporous carbons for CO2 capture. Microporous Mesoporous Mater. 2013, 180, 280–283. [Google Scholar] [CrossRef]
- He, S.; Chen, G.; Xiao, H.; Shi, G.; Ruan, C.; Ma, Y.; Dai, H.; Yuan, B.; Chen, X.; Yang, X. Facile preparation of N-doped activated carbon produced from rice husk for CO2 capture. J. Colloid Interface Sci. 2021, 582, 90–101. [Google Scholar] [CrossRef]
- Li, D.; Ma, T.; Zhang, R.; Tian, Y.; Qiao, Y. Preparation of porous carbons with high low-pressure CO2 uptake by KOH activation of rice husk char. Fuel 2015, 139, 68–70. [Google Scholar] [CrossRef]
- Ouzzine, M.; Serafin, J.; Sreńscek-Nazzal, J. Single step preparation of activated biocarbons derived from pomegranate peels and their CO2 adsorption performance. J. Anal. Appl. Pyrolysis 2021, 160, 105338. [Google Scholar] [CrossRef]
- Serafin, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Querejeta, N.; Gil, M.V.; Rubiera, F.; Pevida, C. Sustainable coffee-based CO2 adsorbents: Toward a greener production via hydrothermal carbonization. Greenh. Gases Sci. Technol. 2018, 8, 309–323. [Google Scholar] [CrossRef]
- Plaza, M.G.; González-Vázquez, M.P.; Pevida, C.; Pis, J.J.; Rubiera, F. Valorisation of spent coffee grounds as CO2 adsorbents for postcombustion capture applications. Appl. Energy 2012, 99, 272–279. [Google Scholar] [CrossRef]
- Tubers. PWN Dictionary of Biological Terms. Available online: https://sjp.pwn.pl/sjp/bulwa;2446675.html (accessed on 7 March 2022).
- Fonseka, D.C. Visionary leadership and the case of Dilmah. Sri Lankan J. Manag. 2009, 14, 1–16. [Google Scholar] [CrossRef]
- Zdyb, H. Walnut; National Agricultural and Forestry Publishing House: Warsaw, Poland, 2009; p. 182. ISBN 978-83-09-01042-5. [Google Scholar]
- PN-EN ISO 18134-1; Solid Biofuels. Determination of Moisture Content. Dryer Method. Vol. 1. Total Moisture. Reference Method. ISO: Geneva, Switzerland, 2015. Available online: https://www.iso.org/standard/61538.html (accessed on 7 March 2023).
- PN-EN ISO 16948:2015-07; Determination of Total Carbon, Hydrogen and Nitrogen Content in Solid Biofuels. System Cyfrowej Sprezedazy Produktow Uslug. ISO: Geneva, Switzerland, 2015. Available online: https://www.iso.org/obp/ui/#iso:std:iso:16948:ed-1:v1:en (accessed on 7 March 2023).
- Gan, Y.X. Activated Carbon from Biomass Sustainable Sources. C—J. Carbon Res. 2021, 7, 39. [Google Scholar] [CrossRef]
- Gil, M.V.; Riaza, J.; Álvarez, L.; Pevida, C.; Rubiera, F. Biomass devolatilization at high temperature under N2 and CO2: Char morphology and reactivity. Energy 2015, 91, 655–662. [Google Scholar] [CrossRef]
- Ukanwa, K.S.; Patchigolla, K.; Sakrabani, R.; Anthony, E.; Mandavgane, S. A review of chemicals to produce activated carbon from agricultural waste biomass. Sustainability 2019, 11, 6204. [Google Scholar] [CrossRef]
- Kim, M.J.; Choi, S.W.; Kim, H.; Mun, S.; Lee, K.B. Simple synthesis of spent coffee ground-based microporous carbons using K2CO3 as an activation agent and their application to CO2 capture. Chem. Eng. J. 2020, 397, 125404. [Google Scholar] [CrossRef]
- Hojjati-Najafabadi, A.; Farahbakhsh, E.; Gholamalian, G.; Feng, P.; Davar, F.; Aminabhavi, T.M.; Vasseghian, Y.; Kamyab, H.; Rahimi, H. Controllable synthesis of nanostructured flower-like cadium sulfides for photocatalytic degradation of methyl orange under different light sources. J. Water Process. Eng. 2024, 59, 105002. [Google Scholar] [CrossRef]
- Yousaf, B.; Guijian, L.; Qumber, A.; Ruwei, W.; Ali, M.U.; Ullah, H.; Liu, R.; Zhou, C. Systematic investigation on combustion characteristics and emission-reduction mechanism of potentially toxic elements in biomass-and biochar-coal co-combustion systems. Appl. Energy 2017, 208, 142–157. [Google Scholar] [CrossRef]
- Bader, N.; Abdelmottaleb, O. CO2 activation of olive bagasse for hydrogen storage. Environ. Prog. Sustain. Energy 2017, 36, 315–324. [Google Scholar] [CrossRef]
- McEnaney, B. Estimation of the dimensions of micropores in active carbons using the Dubinin-Radushkevich equation. Carbon 1987, 25, 69–75. [Google Scholar] [CrossRef]
- Gomis-Berenguer, A.; Iniesta, J.; Moro, A.; Maurino, V.; Lima, J.C.; Ania, C.O. Boosting visible light conversion in the confined pore space of nanoporous carbons. Carbon 2016, 96, 98–104. [Google Scholar] [CrossRef]
- Rouzitalab, Z.; Maklavany, D.M.; Jafarinejad, S.; Rashidi, A. Lignocellulose-based adsorbents: A spotlight review of the effective parameters on carbon dioxide capture process. Chemosphere 2020, 246, 125756. [Google Scholar] [CrossRef] [PubMed]
- Abuelnoor, N.; AlHajaj, A.; Khaleel, M.; Vega, L.F.; Abu-Zahra, M.R.M. Activated carbons from biomass-based sources for CO2 capture applications. Chemosphere 2021, 282, 131111. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.; Srinivas, G.; Zhengxiao, G. Superior CO2 adsorption from waste coffee ground derived carbons. RSC Adv. 2015, 5, 29558–29562. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, X.; Liu, Y.; Zhou, P.; Ma, G.; Lei, Z.; Lei, L. A low cost and highly efficient adsorbent (activated carbon) prepared from waste potato residue. J. Taiwan Inst. Chem. Eng. 2015, 49, 206–211. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Hojjati-Najafabadi, A.; Esfahani, P.N.; Davar, F.; Aminabhavi, T.M.; Vasseghian, Y. Adsorptive removal of malachite green using novel GO@ZnO-NiFe2O4-αAl2O3 nanocomposites. Chem. Eng. J. 2023, 471, 144485. [Google Scholar] [CrossRef]
- Mohammed, A.; Abdullah, A. Scanning electron microscopy (SEM): A review. In Proceedings of the 2018 International Conference on Hydraulics and Pneumatics—HERVEX, Băile Govora, Romania, 7–9 November 2018; pp. 7–9, ISSN 1454-8003. [Google Scholar]
- Yargicoglu, E.N.; Sadasivam, B.Y.; Reddy, K.R.; Spokas, K. Physical and chemical characterization of waste wood derived biochars. Waste Manag. 2015, 36, 256–268. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, B. Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour. Technol. 2013, 146, 485–493. [Google Scholar] [CrossRef]
- Zhou, W.; Apkarian, R.; Wang, Z.L.; Joy, D. Fundamentals of scanning electron microscopy (SEM). Scanning Microsc. Nanotechnol. Tech. Appl. 2006, 1–40. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Liu, X.; Lv, M.; Yang, K.; Meng, J. Co-gelation synthesis of porous graphitic carbons with high surface area and their applications. Carbon 2011, 49, 161–169. [Google Scholar] [CrossRef]
- Mallesh, D.; Anbarasan, J.; Kumar, P.M.; Upendar, K.; Chandrashekar, P.; Rao, B.V.S.K.; Lingaiah, N. Synthesis, characterization of carbon adsorbents derived from waste biomass and its application to CO2 capture. Appl. Surf. Sci. 2020, 530, 147226. [Google Scholar] [CrossRef]
- Lahijani, P.; Mohammadi, M.; Mohamed, A.R. Metal incorporated biochar as a potential adsorbent for high capacity CO2 capture at ambient condition. J. CO2 Util. 2018, 26, 281–293. [Google Scholar] [CrossRef]
- Singh, G.; Lakhi, K.S.; Ramadass, K.; Kim, S.; Stockdale, D.; Vinu, A. A combined strategy of acid-assisted polymerization and solid state activation to synthesize functionalized nanoporous activated biocarbons from biomass for CO2 capture. Microporous Mesoporous Mater. 2018, 271, 23–32. [Google Scholar] [CrossRef]
- Reffas, A.; Bernardet, V.; David, B.; Lehocine, M.B.; Dubois, M.; Batisse, N.; Duclaux, L. Carbons prepared from coffee grounds by H3PO4 activation: Characterization and adsorption of methylene blue and Nylosan Red N-2RBL. J. Hazard Mater. 2010, 175, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Adan-Mas, A.; Alcaraz, L.; Arévalo-Cid, P.; López-Gómez, F.A.; Montemor, F. Coffee-derived activated carbon from second biowaste for supercapacitor applications. Waste Manag. 2021, 120, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Boudrahem, F.; Soualah, A.; Aissani-Benissad, F. Pb (II) and Cd (II) removal from aqueous solutions using activated carbon developed from coffee residue activated with phosphoric acid and zinc chloride. J. Chem. Eng. Data 2011, 56, 1946–1955. [Google Scholar] [CrossRef]
- Idrees, M.; Rangari, V.; Jeelani, S. Sustainable packaging waste-derived activated carbon for carbon dioxide capture. J. CO2 Util. 2018, 26, 380–387. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Preparation of waste tea activated carbon using potassium acetate as an activating agent for adsorption of Acid Blue 25 dye. Chem. Eng. J. 2011, 171, 502–509. [Google Scholar] [CrossRef]
- Rattanaphan, S.; Rungrotmongkol, T.; Kongsune, P.P. Biogas improving by adsorption of CO2 on modified waste tea activated carbon. Renew. Energy 2020, 145, 622–631. [Google Scholar] [CrossRef]
- Moreno-Barbosa, J.J.; López-Velandia, C.; Maldonado, A.D.P.; Giraldo, L.; Loreno-Piraján, J.C. Removal of lead (II) and zinc (II) ions from aqueous solutions by adsorption onto activated carbon synthesized from watermelon shell and walnut shell. Adsorp. 2013, 19, 675–685. [Google Scholar] [CrossRef]







| Raw Material | Coconut Shells | Rice Husks | Pomegranate Peels | Carrot Peels | Coffee Residue | |||
|---|---|---|---|---|---|---|---|---|
| annual production | >25 Mtons (Southeast Asia and the Asia-Pacific region) [11] | ~700 Mtons (global production) [12,13] | ~30 Mtons (global production) [14] | ~15 Mtons (global production) [15] | ||||
| activation process | two-stage activation process involving urea and KOH [16] | single-stage physical activation process using carbon dioxide | two-stage activation process involving KOH and chitosan | single-stage activation the organic waste with KOH | one-stage activation with potassium hydroxide | activation with the same chemical compound | one-stage physical | one-stage chemical activation with KOH |
| adsorbent specific properties achieved | 1687 m2/g 83% of C | 1357 m2/g | 1500 m2/g, 82% of C | 2695 m2/g | 2144 m2/g | 1376 m2/g | 534 m2/g 82% of C | 840 m2/g |
| Ref. | [16] | [17] | [18] | [19] | [20] | [21] | [22] | [23] |
| No. | Organic Waste | Designation | Designation of Organic Waste after Carbonation | Designation of Organic Waste after Activation |
|---|---|---|---|---|
| 1. | Heavily roasted coffee residue | HRoC | CHRoC | AHRoC |
| 2. | Regular roasted coffee residue | RReC | CRReC | ARReC |
| 3. | Potato peelings | PP | CPP | APP |
| 4. | Tea residue | TR | CTR | ATR |
| 5. | Walnut green shells | WGS | CWGS | AWGS |
| 6. | Walnut shells | WS | CWS | AWS |
| 7. | Green coffee residue | GC | CGC | AGC |
| Sample | Unit | HRoC | RReC | PP | TR | WGS | WS | GC | |
|---|---|---|---|---|---|---|---|---|---|
| Element | C | wt.% | 44.75 | 48.59 | 42.85 | 43.93 | 43.94 | 45.28 | 38.82 |
| H | wt.% | 5.88 | 6.31 | 5.89 | 5.43 | 5.38 | 5.85 | 5.60 | |
| N | wt.% | 2.08 | 2.32 | 2.37 | 2.89 | 0.77 | 0.39 | 1.72 | |
| S | wt.% | 0.00 | 0.01 | 0.05 | 0.08 | 0.01 | 0.00 | 0.09 | |
| O | wt.% | 47.29 | 42.77 | 49.34 | 49.83 | 49.90 | 48.48 | 53.77 |
| Sample | Unit | CHRoC | CRReC | CPP | CTR | CWGS | CWS | WGC | |
|---|---|---|---|---|---|---|---|---|---|
| Element | C | wt.% | 66.69 | 77.04 | 61.20 | 72.76 | 40.72 | 95.58 | 69.80 |
| H | wt.% | 1.31 | 1.55 | 1.22 | 1.47 | 1.26 | 1.39 | 1.24 | |
| N | wt.% | 2.92 | 3.88 | 2.23 | 2.95 | 0.69 | 0.58 | 2.73 | |
| S | wt.% | 0.00 | 0.01 | 0.01 | 0.06 | 0.01 | 0.00 | 0.06 | |
| O | wt.% | 29.08 | 17.52 | 34.34 | 22.76 | 57.32 | 5.45 | 26.17 |
| Sample | Unit | AHRoC | ARReC | APP | ATR | AWGS | AWS | AGC | |
|---|---|---|---|---|---|---|---|---|---|
| Element | C | wt.% | 68.89 | 79.90 | 63.44 | 72.59 | 45.56 | 93.52 | 76.41 |
| H | wt.% | 1.84 | 1.78 | 3.13 | 2.04 | 2.18 | 2.18 | 1.59 | |
| N | wt.% | 5.36 | 6.23 | 2.40 | 3.82 | 3.11 | 3.38 | 2.89 | |
| S | wt.% | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.02 | |
| O | wt.% | 23.91 | 12.09 | 31.03 | 21.54 | 49.15 | 0.92 | 19.09 |
| Textural Parameters | ||
|---|---|---|
| Total pore volume | Vp | Amount of N2 adsorbed at a relative pressure of 0.99 |
| Surface area | SBET | Brunauer-Emmett-Teller equation [32] |
| Micropore volume | W0 | N2 isotherms: Dubinin-Radushkevich (DR) equation assuming a density of the adsorbed phase of 0.808 cm3 g−1 and a cross sectional area of 0.162 nm2 [36] |
| Average micropore width | L0 | Stoeckli-Ballerini equation [37] |
| Sample | N2 Adsorption (at −196 ° C) | |||
|---|---|---|---|---|
| SBET | Vp | W0 | L0 | |
| m2/g | cm3/g | cm3/g | nm | |
| HRoC | 0.21 | 0.00006 | n/a | 1.54 |
| CHRoC | 18 | 0.008 | 0.004 | 1.36 |
| AHRoC | 1580 | 0.84 | 0.5 | 0.96 |
| RReC | 0.04 | 0.0024 | n/a | n/a |
| CRReC | 37 | 0.02 | 0.01 | 1.6 |
| ARReC | 863 | n/a | n/a | n/a |
| PP | 0.21 | 0.00005 | 0.00003 | 1.36 |
| CPP | 3.17 | 0.004 | 0.001 | 1.36 |
| APP | 1604 | 0.65 | 0.32 | 1.36 |
| TR | 0.52 | 0.0004 | 0.0002 | 1.60 |
| CTR | 115 | 0.05 | 0.02 | 1.27 |
| ATR | 564 | 0.25 | 0.12 | 0.86 |
| WGS | 0.62 | 0.00 | n/a | n/a |
| CWGS | 4.55 | 0.00 | 0.00 | 3.07 |
| AWGS | 1376 | 0.64 | 0.34 | 1.21 |
| WS | 0.17 | 0.0002 | 0.0001 | 1.6 |
| CWS | 289 | 0.12 | 0.12 | 0.93 |
| AWS | 416 | 0.20 | 0.18 | 0.86 |
| GC | n/a | n/a | n/a | n/a |
| CGC | 0.18 | 0.00013 | 0.00 | n/a |
| AGC | 293 | 0.12 | 0.05 | 1.18 |
| Precursor | Activation Conditions | Textural Parameters | |||||
|---|---|---|---|---|---|---|---|
| Activation Precursor | T [°C] | SBET [m2/g] | Vp [cm3/g] | Wo [cm3/g] | Lo [nm] | Ref. | |
| Coffee grounds | H3PO4 | 450 | 925 | 0.718 | n/a | n/a | [52] |
| Coffee grounds | CO2 | 700 | 593 | 0.24 | 0.24 | 0.8 | [23] |
| Coffee grounds | Steam | 800 | 981.12 | 1.03 | n/a | 4.19 | [53] |
| Coffee residue | H3PO4 | 600 | 1003 | 0.618 | n/a | n/a | [54] |
| Coffee residue | KOH | 700 | 1624 | 0.662 | n/a | n/a | [35] |
| Coffee residue | KOH | 700 | 1580 | 0.84 | 0.5 | 0.96 | This study |
| Waste potato | ZnCl2 | 600 | 1357 | 1.065 | n/a | n/a | [42] |
| Waste potato | KOH | 700 | 1383 | 0.736 | 0.234 | n/a | [55] |
| Waste potato | KOH | 700 | 1604 | 0.65 | 0.32 | 1.36 | This study |
| Tea residue | C2H3O2K | 800 | 820 | 0.219 | n/a | n/a | [56] |
| Tea residue | KOH | 500 | 256.45 | n/a | n/a | n/a | [57] |
| Tea residue | KOH | 700 | 564 | 0.25 | 0.12 | 0.86 | This study |
| Walnut shell | H3PO4 | 500 | 789 | 0.304 | n/a | n/a | [58] |
| Walnut shell | KOH | 700 | 416 | 0.2 | 0.18 | 0.86 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sołtysik, M.; Majchrzak-Kucęba, I.; Wawrzyńczak, D. Characterization of Bioadsorbents from Organic Municipal Waste. Materials 2024, 17, 1954. https://doi.org/10.3390/ma17091954
Sołtysik M, Majchrzak-Kucęba I, Wawrzyńczak D. Characterization of Bioadsorbents from Organic Municipal Waste. Materials. 2024; 17(9):1954. https://doi.org/10.3390/ma17091954
Chicago/Turabian StyleSołtysik, Marcelina, Izabela Majchrzak-Kucęba, and Dariusz Wawrzyńczak. 2024. "Characterization of Bioadsorbents from Organic Municipal Waste" Materials 17, no. 9: 1954. https://doi.org/10.3390/ma17091954






