A Study on the Removal of Impurity Elements Silicon and Zinc from Rubidium Chloride by Vacuum Distillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
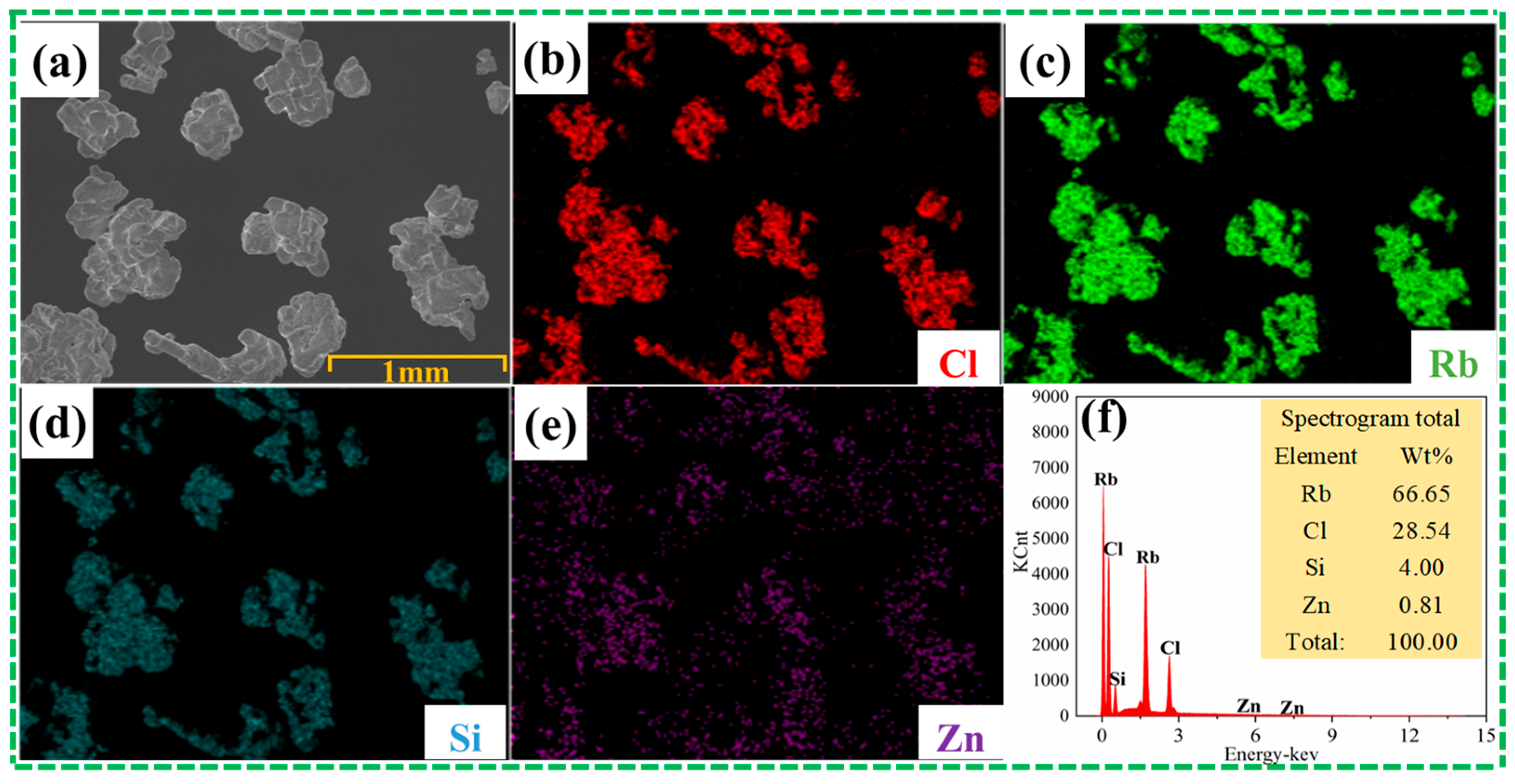
2.3. Characterization and Analysis
3. Results and Discussion
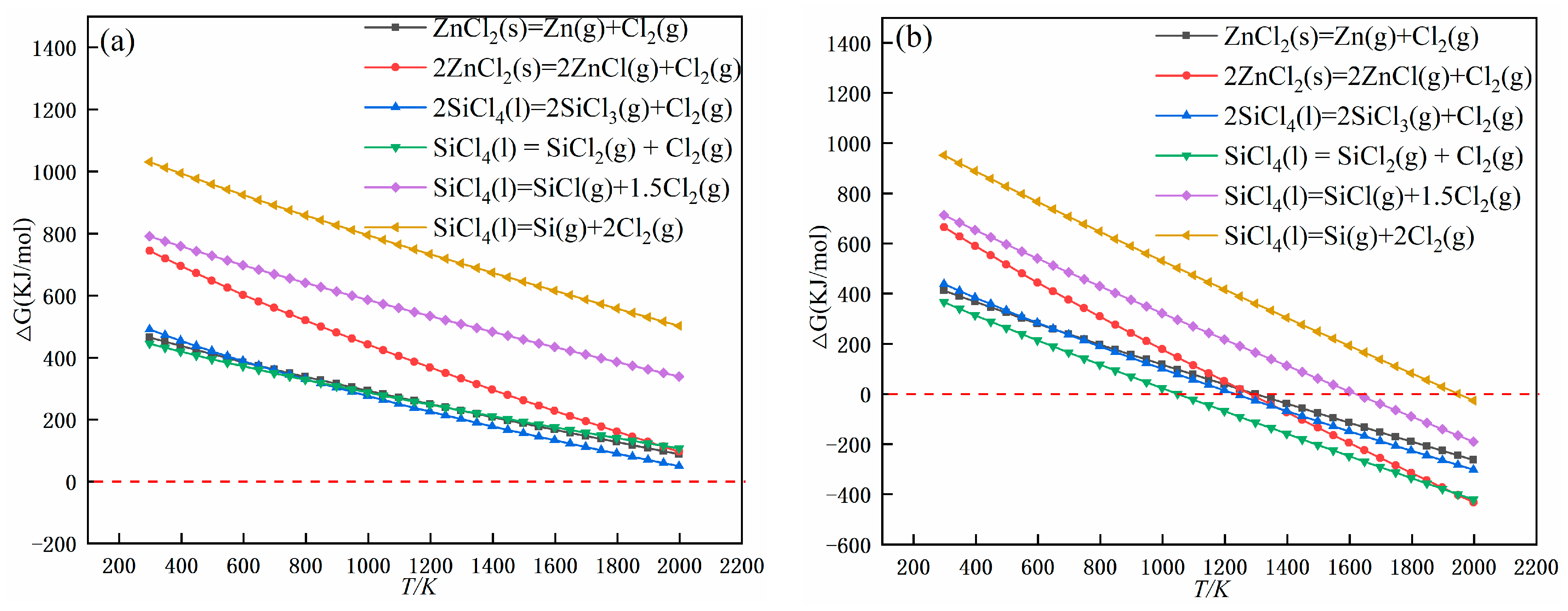
3.1. Theoretical Analyses
3.1.1. Saturated Vapour Pressure
3.1.2. Thermodynamic Calculations
3.1.3. AIMD Simulation Results of Decomposition of Double Salt Rb2SiCl6
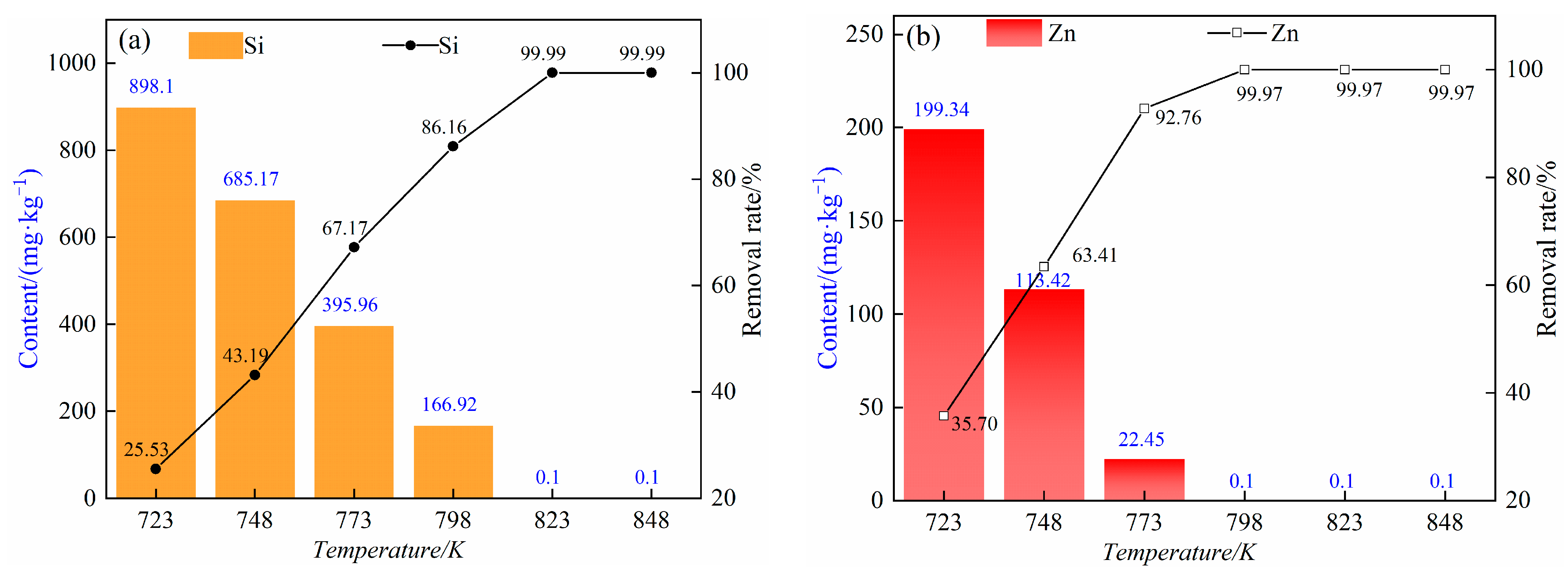
3.2. Effect of Distillation Temperature on the Effect of Removing Impurities
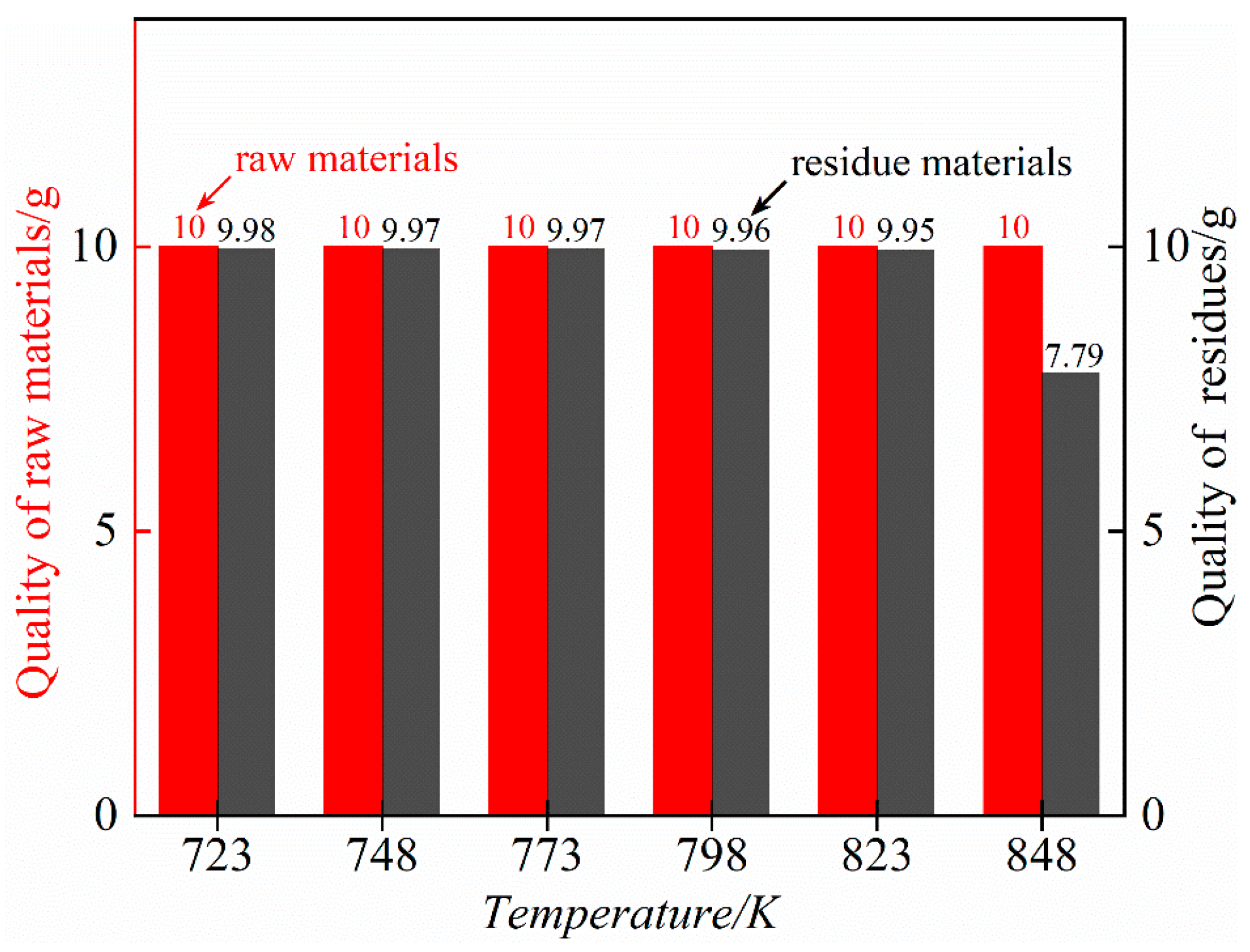
3.3. Effect of Distillation Temperature on the Effect of Removing Impurities
4. Conclusions
- The thermodynamic calculation and ab initio molecular dynamics results of this study show that the removal of impurity elements silicon and zinc in rubidium chloride is feasible. At a low temperature, the decomposition of the double salt Rb2SiCl6 is carried out step by step, first decomposed into one RbCl, and then with the increase in time, Rb2SiCl6 completely decomposed into two RbCl and one SiCl4. At higher temperatures, Rb2SiCl6 directly decomposed into two RbCl and one SiCl4, and increasing the temperature is more conducive to the decomposition of Rb2SiCl6.
- Under the pressure range of 5–10 Pa and the temperature range of 723~823 K, ZnCl2 is volatilized and removed in the form of gas phase, and silicon in the form of the double salt Rb2SiCl6 will decompose into SiCl4 and RbCl; SiCl4 is volatilized and removed in the form of gas phase, and RbCl can exist statically in the residue. At temperatures as high as 848 k, rubidium chloride will be volatile.
- The results of different experimental conditions show that the optimal process parameters for removing impurity elements silicon and zinc in rubidium chloride under the pressure range of 5–10 Pa are as follows: a distillation temperature of 823 K and a distillation time of 60 min. Under such conditions, rubidium chloride will not lose. Through one-step vacuum distillation, the contents of the impurity elements silicon and zinc in rubidium chloride decreased from 1206 mg/kg and 310 mg/kg to less than 0.1 mg/kg and 0.1 mg/kg, respectively, with removal rates of 99.99% and 99.97%. Therefore, the content of the impurity elements silicon and zinc in rubidium chloride is effectively reduced by this process, which lays a foundation for further purification of rubidium chloride by vacuum distillation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaisler, V.A.; Derebezov, I.A.; Gaisler, A.V.; Dmitriev, D.V.; Bakarov, A.K.; Toropov, A.I.; Kachanova, M.M.; Zhivodkov, Y.A.; Latyshev, A.V.; Skvortsov, M.N.; et al. Vertical-cavity surface-emitting lasers for miniature quantum frequency standards. Optoelectron. Instrum. 2022, 57, 445–450. [Google Scholar] [CrossRef]
- Bandi, T.N. Advanced space rubidium atomic frequency standard for satellite navigation. GPS. Solut. 2022, 26, 54. [Google Scholar] [CrossRef]
- Glukhov, I.L.; Kamenski, A.A.; Ovsyannikov, V.D. Interaction of blackbody radiation with rubidium and caesium atoms in small-angular-momentum rydberg states. Quantum Electron. 2022, 52, 570–576. [Google Scholar] [CrossRef]
- Jung, J.; Cho, H.; Kim, I.; Kim, S.; Jo, W.; Kim, H. Dual functionalities of Rb cation in lean electrolyte lithium sulfur batteries. Energy Storage Mater. 2023, 63, 103040. [Google Scholar] [CrossRef]
- Madeeha, T.; Qasim, Z.; Huanqing, Y.; William, G.N.; Sameen, A.; Jinshuai, Z.; Lei, S. Fabrication of potassium-and rubidium-goped formamidinium lead bromide nanocrystals for surface defect passivation and improved photoluminescence stability. ACS Appl. Electron. Mater. 2024, 6, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Makarov, A.O.; Brazhnikov, D.V.; Goncharov, A.N. Observation of the strong magneto-optical rotation of the polarization of light in rubidium vapor for applications in atomic magnetometry. JETP Lett. 2023, 117, 509–516. [Google Scholar] [CrossRef]
- Yao, S.L. A Method for Preparing High Purity Metals Rubidium and Cesium. CN201711166131.7 9 August 2019. [Google Scholar]
- Niu, H.X. Research and development of extraction metallurgy and application of rubidium and its compounds. Rare Met. 2006, 30, 523–527. [Google Scholar] [CrossRef]
- Dai, Y.N.; Yang, B. Non-Ferrous Metallurg Vacuum Metallurg; Metallurgical Industry Press: Beijing, China, 2009; pp. 133–134. [Google Scholar]
- Park, S.; Kim, D.K.; Jeong, J.; Shin, J.H.; Kang, Y.; Liu, R.; Kim, T.S.; Song, M. Separation and recovery Nd and Dy from Mg-Rees alloy by vacuum distillation. J. Alloys Compd. 2023, 967, 171775. [Google Scholar] [CrossRef]
- Jeoung, H.J.; Lee, T.H.; Lee, J.Y.; Yi, K.W.; Kang, J. An electrolytic process using an Ag cathode and vacuum distillation for Mg metal production from MgO. J. Sustain. Metal. 2023, 9, 688–699. [Google Scholar] [CrossRef]
- Jeoung, H.J.; Lee, T.H.; Lee, J.Y.; Yi, K.W.; Kang, J. Production of high-purity Mg metal from dolomite through novel molten salt electrolysis and vacuum distillation. J. Magnes. Alloys 2023, 11, 1308–1320. [Google Scholar] [CrossRef]
- Gotenbruck, M.; Curtolo, D.C.; Friedrich, S.; Friedrich, B. The effectiveness of cooled-finger and vacuum distillation processes in view of the removal of Fe, Si and Zn from aluminium. Metals 2022, 12, 2027. [Google Scholar] [CrossRef]
- Trebukhov, S.; Volodin, V.; Nitsenko, A.; Burabaeva, N.; Ruzakhunova, G. Recovery of zinc from the concentrate of domestic waste processing by vacuum distillation. Metals 2022, 12, 703. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Faisal, F.; Sattar, M.A.; Dexter, R.B. Standard diesel production from mixed waste plastics through thermal pyrolysis and vacuum distillation. Energy Rep. 2023, 9 (Suppl. S11), 540–545. [Google Scholar] [CrossRef]
- Ma, Z.; Ma, S.; Zhu, F.; Li, K.; Sheng, Z.; Li, Z.; Wang, Y. Preparation of high-purity magnesium from electrolytically produced crude magnesium via vacuum distillation. Metals 2023, 13, 811. [Google Scholar] [CrossRef]
- Pospiech, B.; Chagnes, A. Highly selective solvent extraction of Zn(II) and Cu(II) from acidic aqueous chloride solutions with mixture of cyanex 272 and aliquat 336. Sep. Sci. Technol. 2015, 50, 1302–1309. [Google Scholar] [CrossRef]
- Sui, N.; Huang, K.; Zheng, H.; Lin, J.Y.; Wang, X.Q.; Xiao, C.X.; Liu, H.Z. Three-liquid-phase extraction and separation of rare earths and Fe, Al, and Si by a novel mixer-settler-mixer three-chamber integrated extractor. Ind. Eng. Chem. Res. 2014, 53, 16033–16043. [Google Scholar] [CrossRef]
- Saloni, P.; Kumar, P.; Sharma, P.; Ranjan, P.; Chakraborty, T. Electronic and optical properties of lead-free double perovskites A2BCl6 (A = Rb, Cs; B = Si, Ge, Sn) for solar cell applications: A systematic computational study. J. Phys. Org. Chem. 2023, 36, e4492. [Google Scholar] [CrossRef]
- Xing, P.; Wang, C.Y.; Chen, Y.Q.; Ma, B.Z. Rubidium extraction from mineral and brine resources: A review. Hydrometallurgy 2021, 203, 105644. [Google Scholar] [CrossRef]
- Hehre, W.J. Ab initio molecular orbital theory. Acc. Chem. Res. 1976, 9, 399–406. [Google Scholar] [CrossRef]
- Liu, M.; Ruiz, P.L. Mechanisms and energetics of calcium aluminosilicate glass dissolution through ab initio molecular dynamics-metadynamics simulations. Npj Mater. Degrad. 2024, 8, 27. [Google Scholar] [CrossRef]
- Weck, P.F.; Kim, E. High-temperature chromium diffusion in austenitic stainless steel: Ab initio molecular dynamics simulations. Chem. Phys. Lett. 2024, 836, 141036. [Google Scholar] [CrossRef]
- Weck, P.F.; Moore, N.W. Pressure-induced phase transformations in Nb2O5 from ab initio molecular dynamics simulations. Chem. Phys. Lett. 2023, 831, 140851. [Google Scholar] [CrossRef]
- PerezBeltran, S.; Zaheer, W.; Sun, Z.; Defliese, W.F.; Banerjee, S.; Grossman, E.L. Density functional theory and ab initio molecular dynamics reveal atomistic mechanisms for carbonate clumped isotope reordering. Sci. Adv. 2024, 9, eadf1701. [Google Scholar] [CrossRef] [PubMed]








| No. | Reaction Equation | Theoretical Reaction Temperature/K | ||||
|---|---|---|---|---|---|---|
| 105 Pa | 100 Pa | 50 Pa | 10 Pa | 5 Pa | ||
| 1 | RbCl(s) = RbCl(g) | 1636 K | 1025 K | 1007 K | 924 K | 906 K |
| 2 | 2RbCl(s) = 2Rb(g) + Cl2(g) | — | — | — | 1821 K | 1791 K |
| 3 | 2RbCl(g) = 2Rb(g) + Cl2(g) | — | — | — | 1900 K | 1819 K |
| No. | Reaction Equation | Theoretical Reaction Temperature/K | |
|---|---|---|---|
| 105 Pa | 5 Pa | ||
| 1 | ZnCl2(s) = Zn(g) + Cl2(g) | — | 1298 K |
| 2 | 2ZnCl2(s) = 2ZnCl(g) + Cl2(g) | — | 1280 K |
| 3 | 2SiCl4(l) = 2SiCl3(g) + Cl2(g) | — | 1233 K |
| 4 | SiCl4(l) = SiCl2(g) + Cl2(g) | — | 1049 K |
| 5 | SiCl4(l) = SiCl(g) + 1.5Cl2(g) | — | 1623 K |
| 6 | SiCl4(l) = Si(g) + 2Cl2(g) | — | 1949 K |
| Bond | Rb2SiCl6 System (0 ps) | Rb2SiCl6 System (723K, 5.222 ps) | Rb2SiCl6 System (823K, 5.238 ps) | |||
|---|---|---|---|---|---|---|
| Bond Length/Å | Population | Bond Length/Å | Population | Bond Length/Å | Population | |
| Cl2-Rb1 | 2.11052 | 0.14 | — | — | — | — |
| Cl3-Rb1 | 2.82006 | 0.15 | 2.88196 | 0.11 | ||
| Cl4-Rb1 | 2.12621 | 0.2 | — | — | — | — |
| Cl1-Rb2 | 2.96456 | 0.04 | 3.13225 | 0.02 | ||
| Si1-Cl2 | 2.0938 | 0.5 | 2.06541 | 0.58 | 2.08553 | 0.53 |
| Si1-Cl4 | 2.24446 | 0.4 | 2.0386 | 0.6 | 2.16396 | 0.55 |
| Si1-Cl5 | 2.09975 | 0.53 | 1.9909 | 0.6 | 2.01125 | 0.56 |
| Si1-Cl6 | 2.12136 | 0.49 | 1.97522 | 0.61 | 2.10425 | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Zhang, W.; Ji, R.; Yang, M.; Wang, S.; Qu, T. A Study on the Removal of Impurity Elements Silicon and Zinc from Rubidium Chloride by Vacuum Distillation. Materials 2024, 17, 1960. https://doi.org/10.3390/ma17091960
Cui X, Zhang W, Ji R, Yang M, Wang S, Qu T. A Study on the Removal of Impurity Elements Silicon and Zinc from Rubidium Chloride by Vacuum Distillation. Materials. 2024; 17(9):1960. https://doi.org/10.3390/ma17091960
Chicago/Turabian StyleCui, Xi, Wenzheng Zhang, Rui Ji, Mingliang Yang, Shichao Wang, and Tao Qu. 2024. "A Study on the Removal of Impurity Elements Silicon and Zinc from Rubidium Chloride by Vacuum Distillation" Materials 17, no. 9: 1960. https://doi.org/10.3390/ma17091960





