Sustainable Composite Materials Based on Carnauba Wax and Montmorillonite Nanoclay for Energy Storage
Abstract
:1. Introduction
2. Materials and Methods
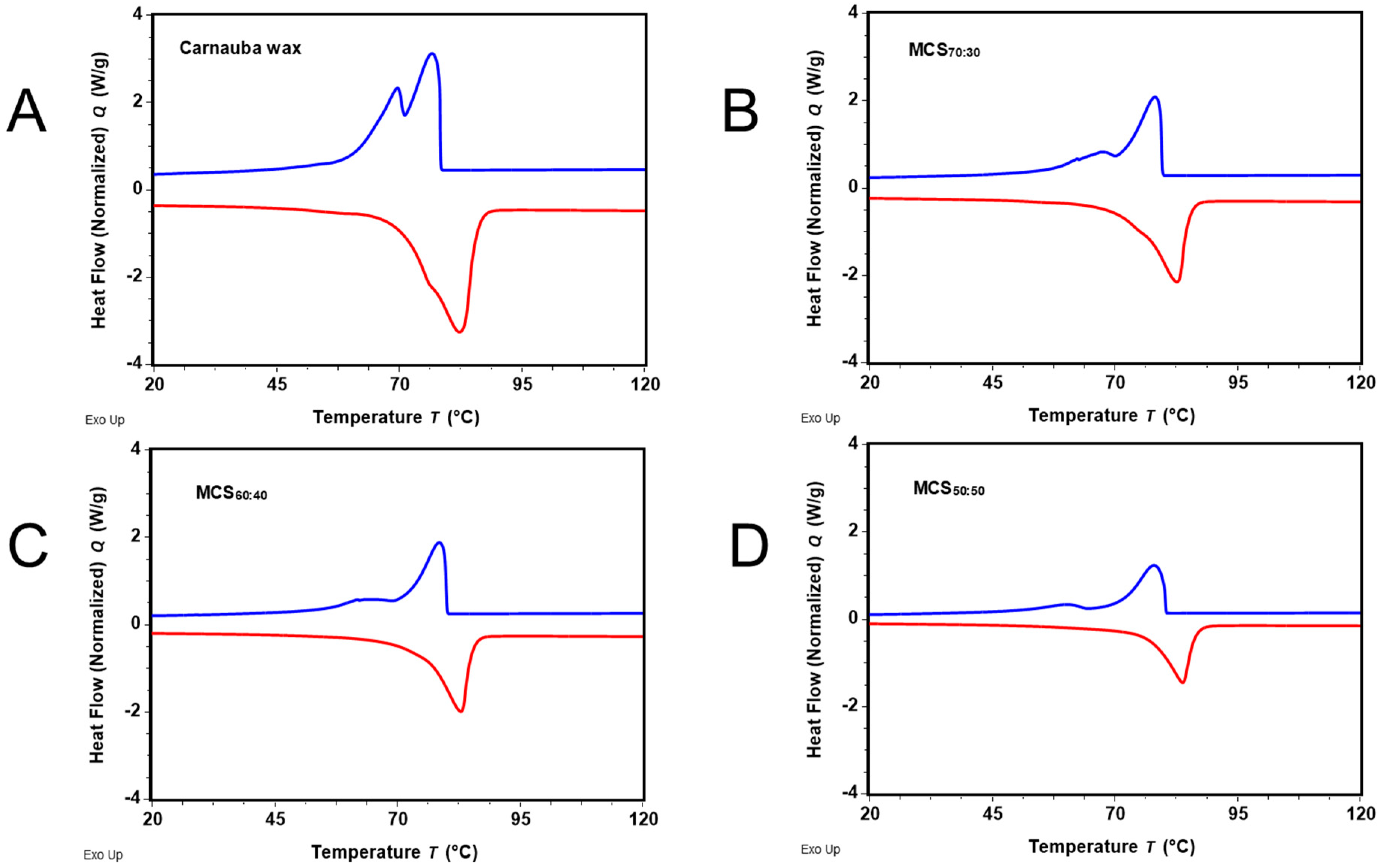
2.1. Differential Scanning Calorimetry (DSC)
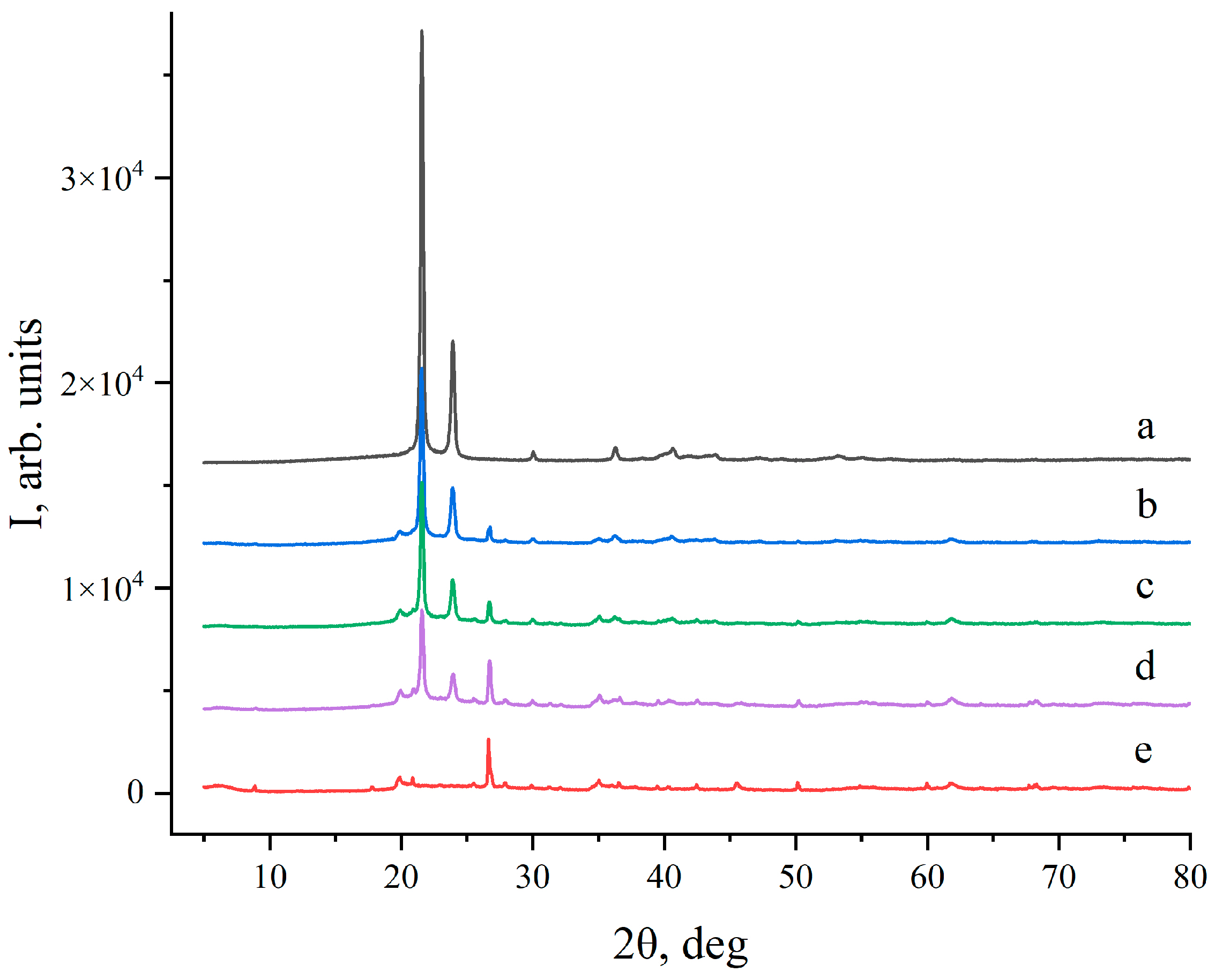
2.2. Powder X-ray Diffraction (PXRD)
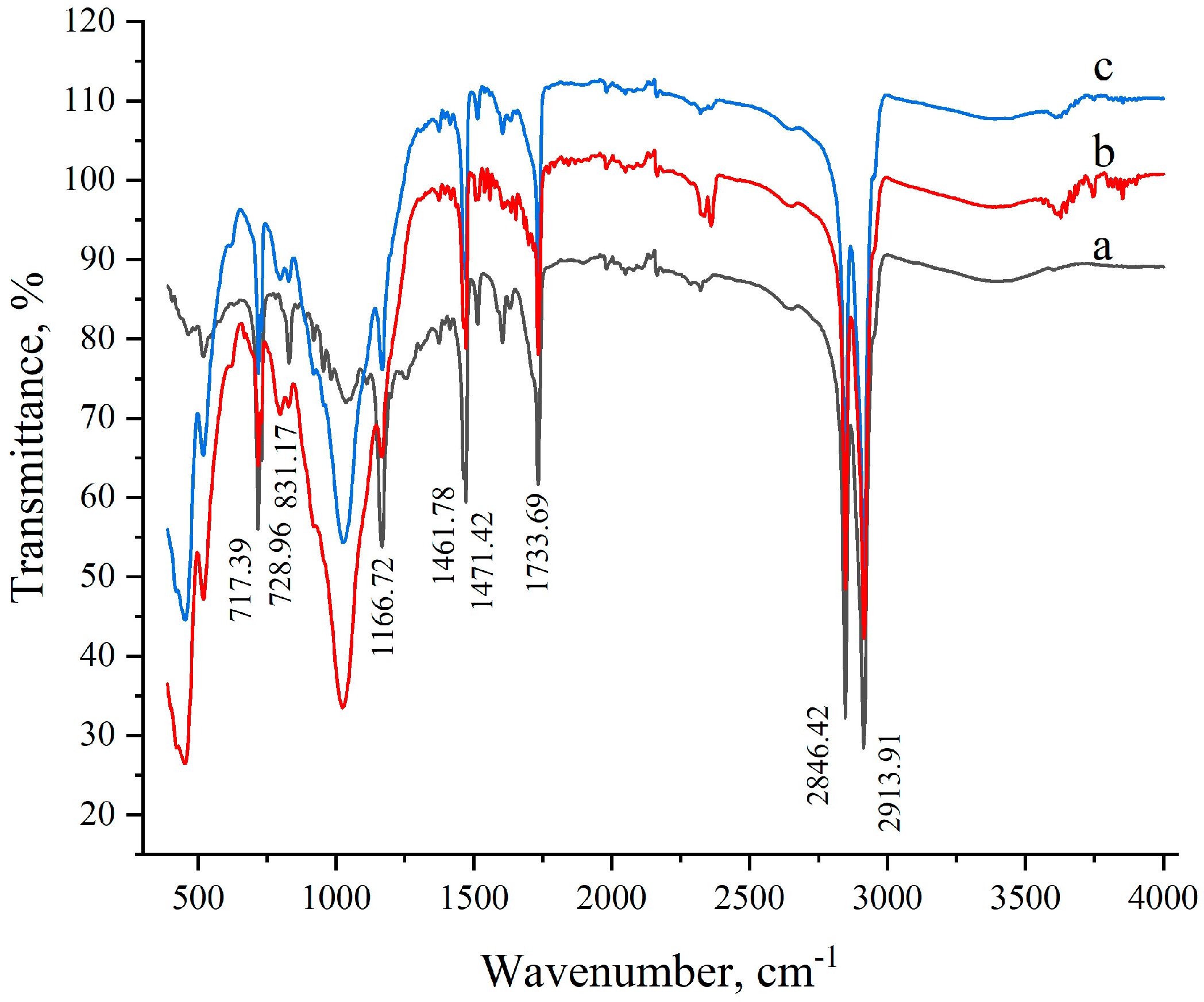
2.3. Infrared (IR) Spectroscopy
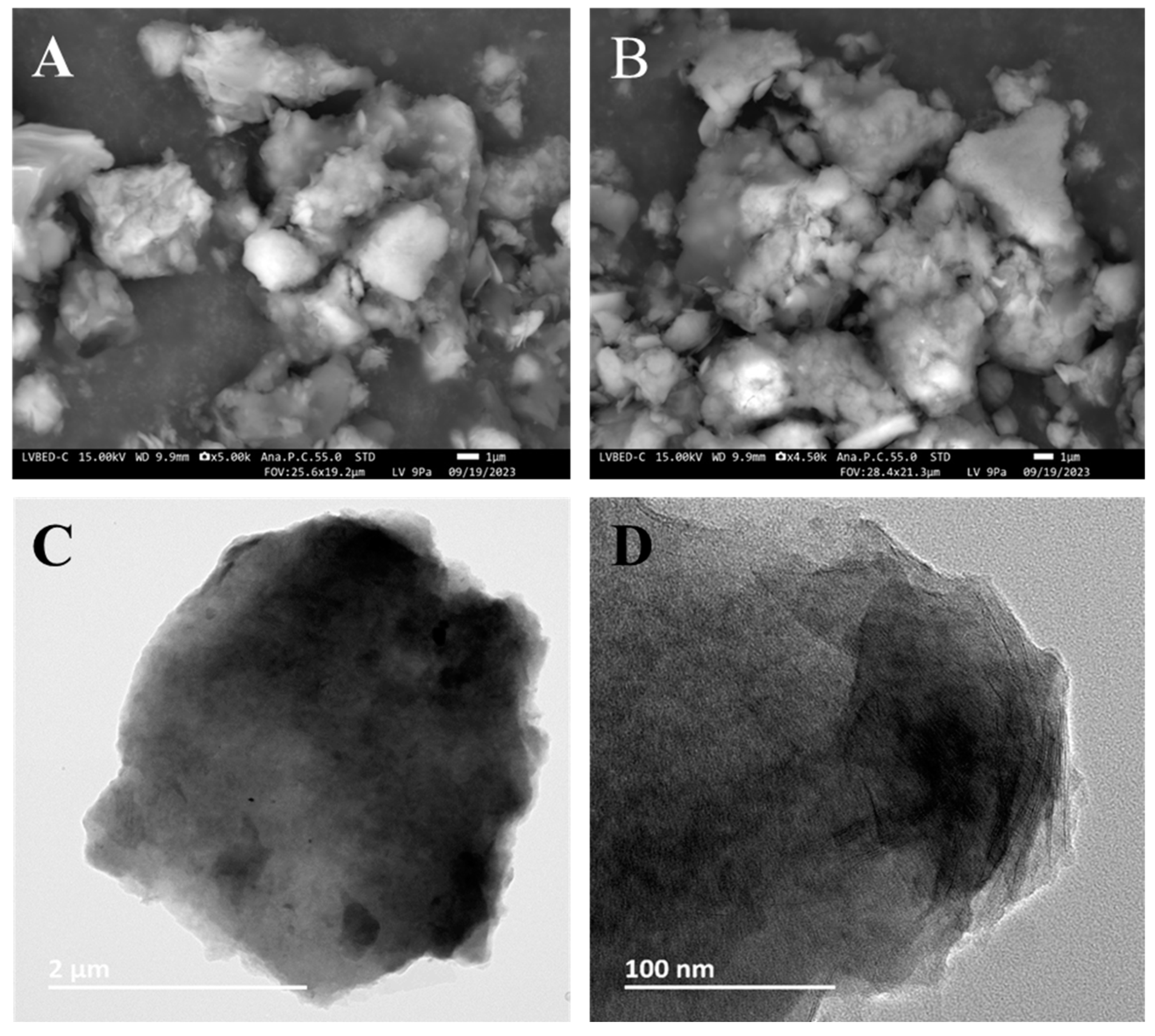
2.4. Transmission Electron Microscopy (TEM)
2.5. Scanning Electron Microscopy (SEM)
2.6. Solid-State NMR Spectroscopy
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vázquez-Núñez, E.; Avecilla-Ramírez, A.M.; Vergara-Porras, B.; Del López-Cuellar, M.R. Green composites and their contribution toward sustainability: A review. Polym. Polym. Compos. 2021, 29, S1588–S1608. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Hossain, M.; Shi, H.H.; Tariq, A.; Ramakrishna, S.; Umer, R. Additive manufacturing of sustainable biomaterials for biomedical applications. Asian J. Pharm. Sci. 2023, 18, 100812. [Google Scholar] [CrossRef]
- Aslam Khan, M.U.; Abd Razak, S.I.; Al Arjan, W.S.; Nazir, S.; Sahaya Anand, T.J.; Mehboob, H.; Amin, R. Recent Advances in Biopolymeric Composite Materials for Tissue Engineering and Regenerative Medicines: A Review. Molecules 2021, 26, 619. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.; Bras, J.; Mondragon, I.; Nechita, P.; Plackett, D.; Simon, P.; Svetec, D.G.; Virtanen, S.; Baschetti, M.G.; Breen, C.; et al. Renewable Fibers and Bio-based Materials for Packing Applications—A Review of Recent Develeopments. Bioresorces 2012, 7, 2506–2552. [Google Scholar] [CrossRef]
- Tajeddin, B. Packaging Composite Materials from Renewable Resources. In Handbook of Composites from Renewable Materials; Kumar Thakur, V., Kumari Thakur, M., Kessler, M.R., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2017. [Google Scholar]
- Xu, Z.; Zhang, Y.; Gou, W.; Liu, M.; Sun, Y.; Han, X.; Sun, W.; Li, C. The key role of concentrated Zn(OTF)2 electrolyte in the performance of aqueous Zn-S batteries. Chem. Commun. 2022, 58, 8145–8148. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Patil, R. The Road to Sustainable Tire Materials: Current State-of-the-Art and Future Prospectives. Environ. Sci. Technol. 2023, 57, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Ead, A.S.; Appel, R.; Alex, N.; Ayranci, C.; Carey, J.P. Life cycle analysis for green composites: A review of literature including considerations for local and global agricultural use. J. Eng. Fibers Fabr. 2021, 16, 155892502110269. [Google Scholar] [CrossRef]
- Vidal, J.; Ponce, D.; Mija, A.; Rymarczyk, M.; Castell, P. Sustainable Composites from Nature to Construction: Hemp and Linseed Reinforced Biocomposites Based on Bio-Based Epoxy Resins. Materials 2023, 16, 1283. [Google Scholar] [CrossRef] [PubMed]
- Okogeri, O.; Stathopoulos, V.N. What about greener phase change materials? A review on biobased phase change materials for thermal energy storage applications. Int. J. Thermofluids 2021, 10, 100081. [Google Scholar] [CrossRef]
- Ould Amrouche, S.; Rekioua, D.; Rekioua, T.; Bacha, S. Overview of energy storage in renewable energy systems. Int. J. Hydrogen Energy 2016, 41, 20914–20927. [Google Scholar] [CrossRef]
- BZalba, J.M.; Marin, L.F.; Cabeza, H. Mehling. PII: S1359-4311(02)00192-8. Appl. Therm. Eng. 2002, 23, 251–283. [Google Scholar]
- Radouane, N. A Comprehensive Review of Composite Phase Change Materials (cPCMs) for Thermal Management Applications, Including Manufacturing Processes, Performance, and Applications. Energies 2022, 15, 8271. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Zhang, J.; Lin, L.; Shi, J. A Review of Composite Phase Change Materials Based on Biomass Materials. Polymers 2022, 14, 4089. [Google Scholar] [CrossRef]
- Mitran, R.-A.; Ioniţǎ, S.; Lincu, D.; Berger, D.; Matei, C. A Review of Composite Phase Change Materials Based on Porous Silica Nanomaterials for Latent Heat Storage Applications. Molecules 2021, 26, 241. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, P.; Tang, Z.; Xu, X.; Gao, H.; Wang, G. Carbon-Based Composite Phase Change Materials for Thermal Energy Storage, Transfer, and Conversion. Adv. Sci. 2021, 8, 2001274. [Google Scholar] [CrossRef]
- Yang, J.; Tang, L.-S.; Bai, L.; Bao, R.-Y.; Liu, Z.-Y.; Xie, B.-H.; Yang, M.-B.; Yang, W. High-performance composite phase change materials for energy conversion based on macroscopically three-dimensional structural materials. Mater. Horiz. 2019, 6, 250–273. [Google Scholar] [CrossRef]
- Yang, T.; King, W.P.; Miljkovic, N. Phase change material-based thermal energy storage. Cell Rep. Phys. Sci. 2021, 2, 100540. [Google Scholar] [CrossRef]
- Mishra, D.K.; Bhowmik, C.; Bhowmik, S.; Pandey, K.M. Property-enhanced paraffin-based composite phase change material for thermal energy storage: A review. Env. Sci. Pollut. Res. 2022, 29, 43556–43587. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Wert, N.A.; Gowd, E.B.; Patel, R. Recent Progress in PEG-Based Composite Phase Change Materials. Polym. Rev. 2023, 63, 1078–1129. [Google Scholar] [CrossRef]
- Kaleni, A.; Lebelo, K.; Mochane, M.J.; Mokhena, T.C.; Motloung, M.T. Recent progress on the morphology and thermal cycle of phase change materials (PCMs)/conductive filler composites: A mini review. J. Polym. Eng. 2022, 42, 827–845. [Google Scholar] [CrossRef]
- Chang, Y.; Yao, X.; Chen, Y.; Huang, L.; Zou, D. Review on ceramic-based composite phase change materials: Preparation, characterization and application. Compos. Part B: Eng. 2023, 254, 110584. [Google Scholar] [CrossRef]
- Podara, C.V.; Kartsonakis, I.A.; Charitidis, C.A. Towards Phase Change Materials for Thermal Energy Storage: Classification, Improvements and Applications in the Building Sector. Appl. Sci. 2021, 11, 1490. [Google Scholar] [CrossRef]
- Saqib, M.; Andrzejczyk, R. A review of phase change materials and heat enhancement methodologies. WIREs Energy Environ. 2023, 12, e467. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, G.; Liu, X.; Shao, L.; Xu, Y.; Zhang, X.; Li, Y.; Yan, Z.; Zou, T. Minireview on Design of Flexible Composite Phase Change Materials to Various Energy Applications: Progresses and Perspectives. Energy Fuels 2023, 37, 6348–6364. [Google Scholar] [CrossRef]
- Salgueiro, T.; Samagaio, A.; Gonçalves, M.; Figueiredo, A.; Labrincha, J.; Silva, L. Incorporation of phase change materials in an expanded clay containing mortar for indoor thermal regulation of buildings. J. Energy Storage 2021, 36, 102385. [Google Scholar] [CrossRef]
- Gulfam, R.; Zhang, P.; Meng, Z. Advanced thermal systems driven by paraffin-based phase change materials—A review. Appl. Energy 2019, 238, 582–611. [Google Scholar] [CrossRef]
- Diani, A.; Moro, L.; Rossetto, L. Melting of Paraffin Waxes Embedded in a Porous Matrix Made by Additive Manufacturing. Appl. Sci. 2021, 11, 5396. [Google Scholar] [CrossRef]
- Trp, A. An experimental and numerical investigation of heat transfer during technical grade paraffin melting and solidification in a shell-and-tube latent thermal energy storage unit. Sol. Energy 2005, 79, 648–660. [Google Scholar] [CrossRef]
- Prabhu, B.; Valan Arasu, A. Stability analysis of TiO2–Ag nanocomposite particles dispersed paraffin wax as energy storage material for solar thermal systems. Renew. Energy 2020, 152, 358–367. [Google Scholar] [CrossRef]
- Kumar, K.; Sharma, K.; Verma, S.; Upadhyay, N. Experimental Investigation of Graphene-Paraffin Wax Nanocomposites for Thermal Energy Storage. Mater. Today Proc. 2019, 18, 5158–5163. [Google Scholar] [CrossRef]
- Mohamed, N.H.; Soliman, F.S.; El Maghraby, H.; Moustfa, Y.M. Thermal conductivity enhancement of treated petroleum waxes, as phase change material, by α nano alumina: Energy storage. Renew. Sustain. Energy Rev. 2017, 70, 1052–1058. [Google Scholar] [CrossRef]
- Reyes, A.; Henríquez-Vargas, L.; Rivera, J.; Sepúlveda, F. Theoretical and experimental study of aluminum foils and paraffin wax mixtures as thermal energy storage material. Renew. Energy 2017, 101, 225–235. [Google Scholar] [CrossRef]
- Mhike, W.; Focke, W.W.; Mofokeng, J.P.; Luyt, A.S. Thermally conductive phase-change materials for energy storage based on low-density polyethylene, soft Fischer–Tropsch wax and graphite. Thermochim. Acta 2012, 527, 75–82. [Google Scholar] [CrossRef]
- Ben Zaid, Z.; Tilioua, A.; Lamaamar, I.; Ansari, O.; Hamdi Alaoui, M.A. Thermal performance of clay-straw wall incorporating phase change materials in Errachidia city (South Eastern Morocco): A simulation approach. Case Stud. Constr. Mater. 2021, 15, e00786. [Google Scholar] [CrossRef]
- Tufvesson, L.M.; Börjesson, P. Wax production from renewable feedstock using biocatalysts instead of fossil feedstock and conventional methods. Int. J. Life Cycle Assess 2008, 13, 328–338. [Google Scholar] [CrossRef]
- Fei, T.; Ren, K.; Wang, T. The Friction and Wear Behaviors of Vegetable Oil-Based Waxes, Natural Beeswax, and Petroleum Paraffin Wax. J. Am. Oil Chem. Soc. 2020, 97, 1141–1150. [Google Scholar] [CrossRef]
- Winkler-Moser, J.K.; Anderson, J.; Byars, J.A.; Singh, M.; Hwang, H.-S. Evaluation of Beeswax, Candelilla Wax, Rice Bran Wax, and Sunflower Wax as Alternative Stabilizers for Peanut Butter. J. Am. Oil Chem. Soc. 2019, 96, 1235–1248. [Google Scholar] [CrossRef]
- Rudra Murthy, B.V.; Thanaiah, K.; Gumtapure, V. Experimental investigation of shellac wax as potential bio-phase change material for medium temperature solar thermal energy storage applications. Sol. Energy 2022, 231, 1002–1014. [Google Scholar] [CrossRef]
- Tangsiriratana, E.; Skolpap, W.; Patterson, R.J.; Sriprapha, K. Thermal properties and behavior of microencapsulated sugarcane wax phase change material. Heliyon 2019, 5, e02184. [Google Scholar] [CrossRef]
- Simeiko, K.V.; Sydorenko, M.A.; Brichka, S.Y. Heat Accumulation by Wax Polydisperse Nanomaterials with Phase Transition. Powder Metall. Met. Ceram. 2022, 61, 269–277. [Google Scholar] [CrossRef]
- Jacob, M.T. A Process for the Processing and Production of Carnauba Wax. International Patent Application No. PCT/BR93/00044, 10 December 1993. [Google Scholar]
- Junio, E.J.M.R.; Stephen, J.R.V.; Muthuvel, M.; Roy, A.; Rodrigue, P.A.; Filho, M.J.A.M.; Teixeira, R.A.; Barbosa, A.P.; Benjamin, S.R. Gums, Resins and Latexes of Plant Origin, 1st ed.; Murthy, H.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2022; ISBN 9783030913786. [Google Scholar]
- Lv, P.; Liu, C.; Rao, Z. Review on clay mineral-based form-stable phase change materials: Preparation, characterization and applications. Renew. Sustain. Energy Rev. 2017, 68, 707–726. [Google Scholar] [CrossRef]
- Luo, Y.; Xiong, S.; Huang, J.; Zhang, F.; Li, C.; Min, Y.; Peng, R.; Liu, Y. Preparation, characterization and performance of paraffin/sepiolite composites as novel shape-stabilized phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 231, 111300. [Google Scholar] [CrossRef]
- Emmerich, K.; Wolters, F.; Kahr, G.; Lagaly, G. Clay Profiling: The Classification of Montmorillonites. Clays Clay Miner. 2009, 57, 104–114. [Google Scholar] [CrossRef]
- Al Kausor, M.; Sen Gupta, S.; Bhattacharyya, K.G.; Chakrabortty, D. Montmorillonite and modified montmorillonite as adsorbents for removal of water soluble organic dyes: A review on current status of the art. Inorg. Chem. Commun. 2022, 143, 109686. [Google Scholar] [CrossRef]
- Chen, C.; Liu, X.; Liu, W.; Ma, M. A comparative study of myristic acid/bentonite and myristic acid/Eudragit L100 form stable phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2014, 127, 14–20. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.; Kao, H.; Tan, J. Experimental investigation of preparation and thermal performances of paraffin/bentonite composite phase change material. Energy Convers. Manag. 2011, 52, 3275–3281. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E.; Ozay, S.; Ozkilic, Y. Preparation of phase change material–montmorillonite composites suitable for thermal energy storage. Thermochim. Acta 2011, 524, 39–46. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Q.; Wang, X.; Cao, S.; Tang, N.; Wang, Q.; Lv, G.; Liao, L. Preparation of two-dimensional nano montmorillonite/stearic acid energy storage composites with excellent stability and heat storage property. Appl. Clay Sci. 2020, 191, 105614. [Google Scholar] [CrossRef]
- Xie, W.; Gao, Z.; Liu, K.; Pan, W.-P.; Vaia, R.; Hunter, D.; Singh, A. Thermal characterization of organically modified montmorillonite. Thermochim. Acta 2001, 367–368, 339–350. [Google Scholar]
- Gao, N.; Tang, T.; Xiang, H.; Zhang, W.; Li, Y.; Yang, C.; Xia, T.; Liu, X. Preparation and structure-properties of crosslinking organic montmorillonite/polyurethane as solid-solid phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 244, 111831. [Google Scholar] [CrossRef]
- Ai, H.; Lv, L.; Chen, T.; Zhang, Y.; Dong, L.; Song, S. An eco-friendly and facile montmorillonite nanosheets aerogel based phase change materials for efficient solar-to-thermal energy conversion. Energy Convers. Manag. 2022, 253, 115172. [Google Scholar] [CrossRef]
- Jin, W.; Jiang, L.; Chen, L.; Gu, Y.; Guo, M.; Han, L.; Ben, X.; Yuan, H.; Lin, Z. Preparation and characterization of capric-stearic acid/montmorillonite/graphene composite phase change material for thermal energy storage in buildings. Constr. Build. Mater. 2021, 301, 124102. [Google Scholar] [CrossRef]
- Li, M.; Guo, Q.; Nutt, S. Carbon nanotube/paraffin/montmorillonite composite phase change material for thermal energy storage. Sol. Energy 2017, 146, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Ai, Z.; Zhao, Y.; Zhang, X.; Song, S. Design of 3D-network montmorillonite nanosheet/stearic acid shape-stabilized phase change materials for solar energy storage. Sol. Energy Mater. Sol. Cells 2020, 204, 110233. [Google Scholar] [CrossRef]
- Thanakkasaranee, S.; Seo, J. Effect of halloysite nanotubes on shape stabilities of polyethylene glycol-based composite phase change materials. Int. J. Heat Mass Transf. 2019, 132, 154–161. [Google Scholar] [CrossRef]
- Muzhanje, A.T.; Hassan, M.A.; Ookawara, S.; Hassan, H. An overview of the preparation and characteristics of phase change materials with nanomaterials. J. Energy Storage 2022, 51, 104353. [Google Scholar] [CrossRef]
- Wu, M.Q.; Wu, S.; Cai, Y.F.; Wang, R.Z.; Li, T.X. Form-stable phase change composites: Preparation, performance, and applications for thermal energy conversion, storage and management. Energy Storage Mater. 2021, 42, 380–417. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Chopra, K.; Sharma, R.K.; Pandey, A.K.; Tyagi, S.K.; Ahmad, M.S.; Sarı, A.; Kothari, R. A comprehensive review on phase change materials for heat storage applications: Development, characterization, thermal and chemical stability. Sol. Energy Mater. Sol. Cells 2022, 234, 111392. [Google Scholar] [CrossRef]
- Kameda, T.; Tamada, Y. Variable-temperature 13C solid-state NMR study of the molecular structure of honeybee wax and silk. Int. J. Biol. Macromol. 2009, 44, 64–69. [Google Scholar] [CrossRef]
- Anderson, J.E.; Slichter, W.P. Nuclear Spin Relaxation in Solid n-Alkanes. J. Phys. Chem. 1965, 69, 3099–3104. [Google Scholar] [CrossRef]
- Reynhardt, E.C.; Rieder, M. Structures and molecular dynamics of plant waxes. Eur. Biophys. J. 1994, 23, 59–70. [Google Scholar] [CrossRef]
- Chen, B.; Xing, B. Sorption and conformational characteristics of reconstituted plant cuticular waxes on montmorillonite. Environ. Sci. Technol. 2005, 39, 8315–8323. [Google Scholar] [CrossRef] [PubMed]
- Morozov, E.V.; Nizovtseva, P.V.; Martyanov, O.N. From Components to Phase-Dependent Dynamics of Diffusivity in Wax Solutions Subjected to Fluid–Solid Phase Transition: Insights from Pulsed Field Gradient NMR. Energy Fuels 2022, 36, 14696–14709. [Google Scholar] [CrossRef]
- Guzman-Juarez, B.; Abdelaal, A.B.; Reven, L. NMR Characterization of Nanoscale Surface Patterning in Mixed Ligand Nanoparticles. ACS Nano 2022, 16, 20116–20128. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T. Molecular structure of crude beeswax studied by solid-state 13C NMR. J. Insect Sci. 2004, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Brychka, S. Heat Accumulation with Montmorillonite/Carnauba Wax Nanomaterials. Energ. Tech. Res. Sav. 2022, 3, 58–69. [Google Scholar] [CrossRef]
- Langer, B.; Schnell, I.; Spiess, H.W.; Grimmer, A.-R. Temperature Calibration under Ultrafast MAS Conditions. J. Magn. Resoinance 1999, 138, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.E.; Rienstra, C.M.; Auger, M.; Lakshmi, K.V.; Griffin, R.G. Heteronuclear decoupling in rotating solids. J. Chem. Phys. 1995, 103, 6951–6958. [Google Scholar] [CrossRef]
- de Freitas, C.A.S.; de Sousa, P.H.M.; Soares, D.J.; Da Silva, J.Y.G.; Benjamin, S.R.; Guedes, M.I.F. Carnauba wax uses in food—A review. Food Chem. 2019, 291, 38–48. [Google Scholar] [CrossRef]
- Basson, I.; Reynhardt, E.C. An investigation of the structures and molecular dynamics of natural waxes: II. Carnauba Wax. J. Phys. D Appl. Phys. 1988, 21, 1429–1433. [Google Scholar] [CrossRef]
- Park, D.-H.; Yang, J.-H.; Vinu, A.; Elzatahry, A.; Choy, J.-H. X-ray diffraction and X-ray absorption spectroscopic analyses for intercalative nanohybrids with low crystallinity. Arab. J. Chem. 2016, 9, 190–205. [Google Scholar] [CrossRef]
- Jouhara, H.; Żabnieńska-Góra, A.; Khordehgah, N.; Ahmad, D.; Lipinski, T. Latent thermal energy storage technologies and applications: A review. Int. J. Thermofluids 2020, 5–6, 100039. [Google Scholar] [CrossRef]
- Colthup, N.B.; Daly, L.H.; Wiberley, S.E. (Eds.) Introduction to Infrared and Raman Spectroscopy; Academic Press, Ink., Ltd.: London, UK, 1975; ISBN 0121825523. [Google Scholar]
- MacKenzie, K.J.D.; Smith, M.E. Multinuclear Solid State NMR Spectroscopy of Inorganic Materials; Pergamon Press Series: Oxford, UK, 2002; ISBN 9780080437873. [Google Scholar]
- Dubey, P.; Sharma, P.; Kumar, V. Structural profiling of wax biopolymer from Pinus roxburghii Sarg. needles using spectroscopic methods. Int. J. Biol. Macromol. 2017, 104, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Speight, R.J.; Rourke, J.P.; Wong, A.; Barrow, N.S.; Ellis, P.R.; Bishop, P.T.; Smith, M.E. 1H and 13C solution- and solid-state NMR investigation into wax products from the Fischer-Tropsch process. Solid State Nucl. Magn. Reson. 2011, 39, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Gitsas, A.; Floudas, G.; Dietz, M.; Mondeshki, M.; Spiess, H.W.; Wegner, G. Self-Assembly and Molecular Dynamics of Copolymers of γ-Methyl-l-glutamate and Stearyl-l-glutamate. Macromolecules 2007, 40, 8311–8322. [Google Scholar] [CrossRef]
- Gitsas, A.; Floudas, G.; Mondeshki, M.; Spiess, H.W.; Aliferis, T.; Iatrou, H.; Hadjichristidis, N. Control of Peptide Secondary Structure and Dynamics in Poly(γ-benzyl-l-glutamate)-b-polyalanine Peptides. Macromolecules 2008, 41, 8072–8080. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, J.; Liu, M. Interphase in Polymer Nanocomposites. JACS Au 2022, 2, 280–291. [Google Scholar] [CrossRef]
- Long, D.; Sotta, P. Stress relaxation of large amplitudes and long timescales in soft thermoplastic and filled elastomers. Rheol. Acta 2007, 46, 1029–1044. [Google Scholar] [CrossRef]
- Quantitative and discriminative analysis of carnauba waxes by reactive pyrolysis-GC in the presence of organic alkali using a vertical microfurnace pyrolyzer. J. Anal. Appl. Pyrolysis 2001, 58–59, 525–537.
- Zhou, W.-X.; Cheng, Y.; Chen, K.-Q.; Xie, G.; Wang, T.; Zhang, G. Thermal Conductivity of Amorphous Materials. Adv. Funct. Mater. 2020, 30, 1903829. [Google Scholar] [CrossRef]
- Allen, P.B.; Feldman, J.L.; Fabian, J.; Wooten, F. Diffusons, locons and propagons: Character of atomic vibrations in amorphous Si. Philos. Mag. B 1999, 79, 1715–1731. [Google Scholar] [CrossRef]
- Simoncelli, M.; Marzari, N.; Mauri, F. Unified theory of thermal transport in crystals and glasses. Nat. Phys. 2019, 15, 809–813. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brychka, S.; Brychka, A.; Hedin, N.; Mondeshki, M. Sustainable Composite Materials Based on Carnauba Wax and Montmorillonite Nanoclay for Energy Storage. Materials 2024, 17, 1978. https://doi.org/10.3390/ma17091978
Brychka S, Brychka A, Hedin N, Mondeshki M. Sustainable Composite Materials Based on Carnauba Wax and Montmorillonite Nanoclay for Energy Storage. Materials. 2024; 17(9):1978. https://doi.org/10.3390/ma17091978
Chicago/Turabian StyleBrychka, Serhii, Alla Brychka, Niklas Hedin, and Mihail Mondeshki. 2024. "Sustainable Composite Materials Based on Carnauba Wax and Montmorillonite Nanoclay for Energy Storage" Materials 17, no. 9: 1978. https://doi.org/10.3390/ma17091978




