Exploring the Potential of a Novel Iodine-Based Material as an Alternative Contrast Agent in X-ray Imaging Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Computational Study
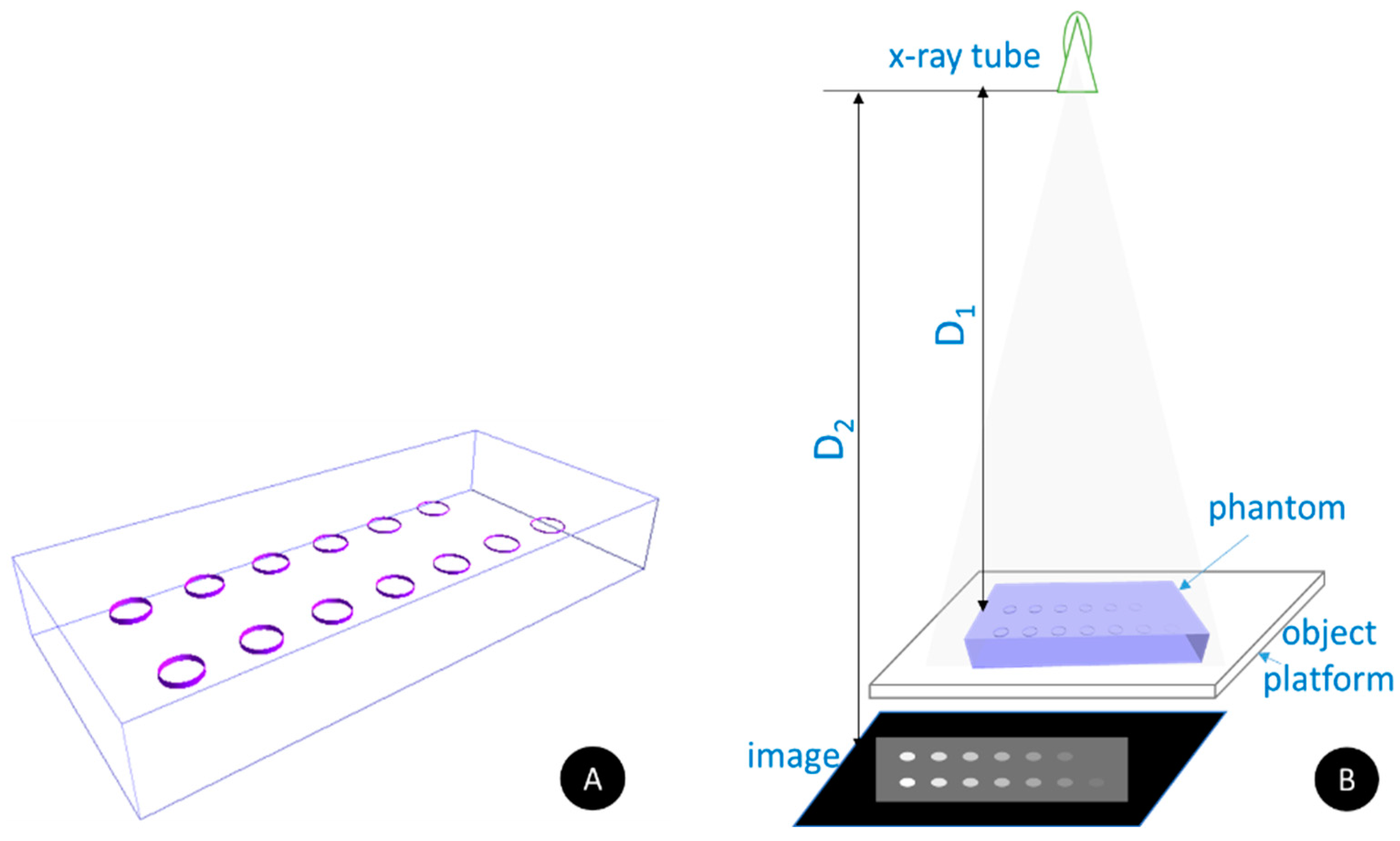
2.2. Experimental Study
- (1)
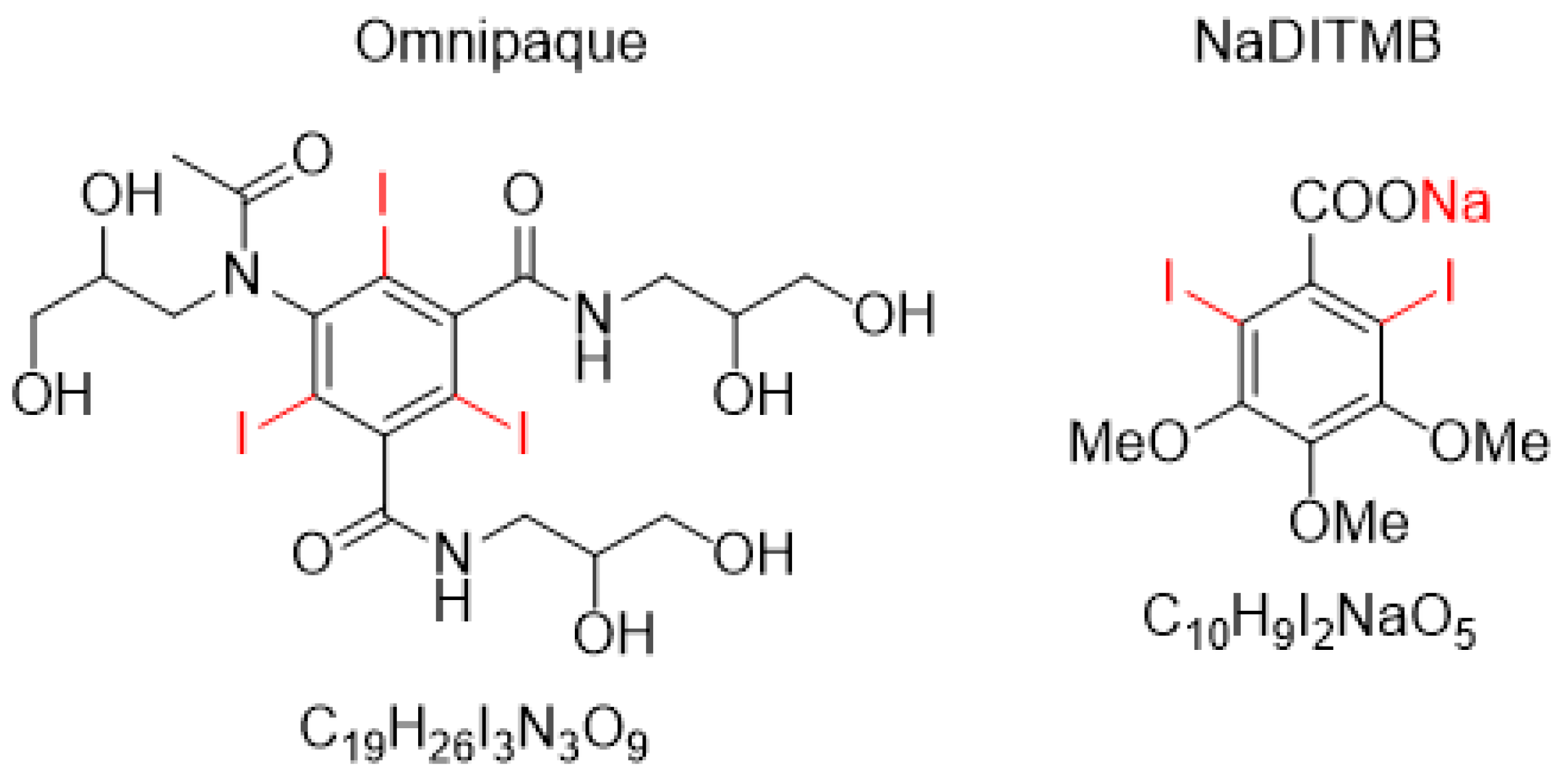
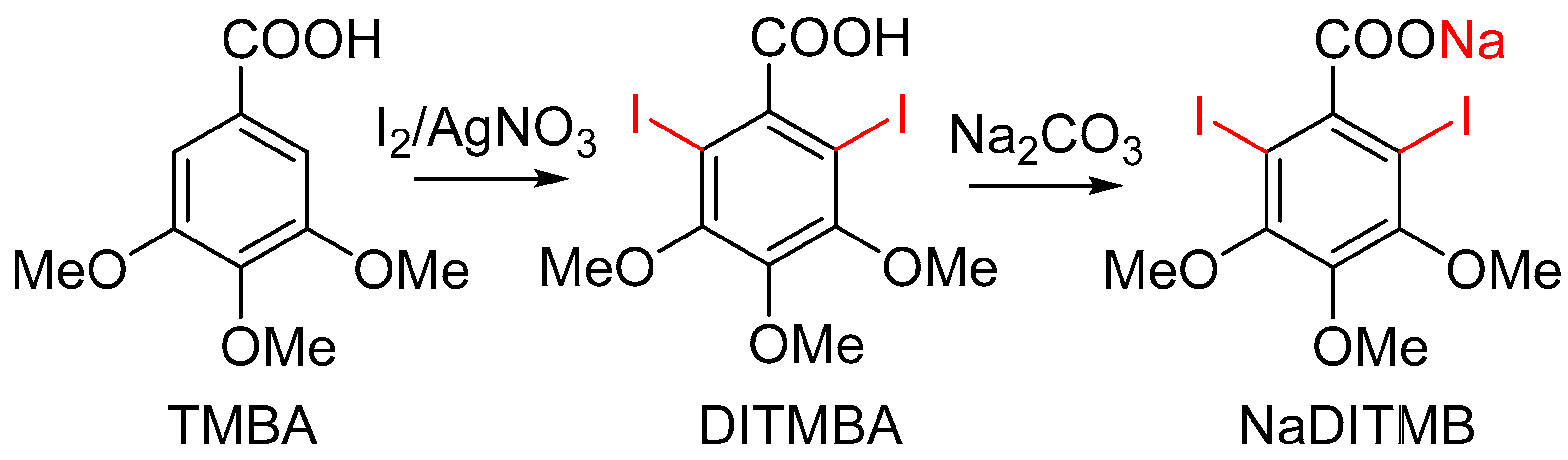
- Synthesis of 2,6-diiodo-3,4,5-trimethoxybenzoic acid [DITMBA] and its sodium 2,6-diiodo-3,4,5-trimethoxybenzoate solution [NaDITMB]
- (2)
- X-ray imaging
3. Results and Discussion
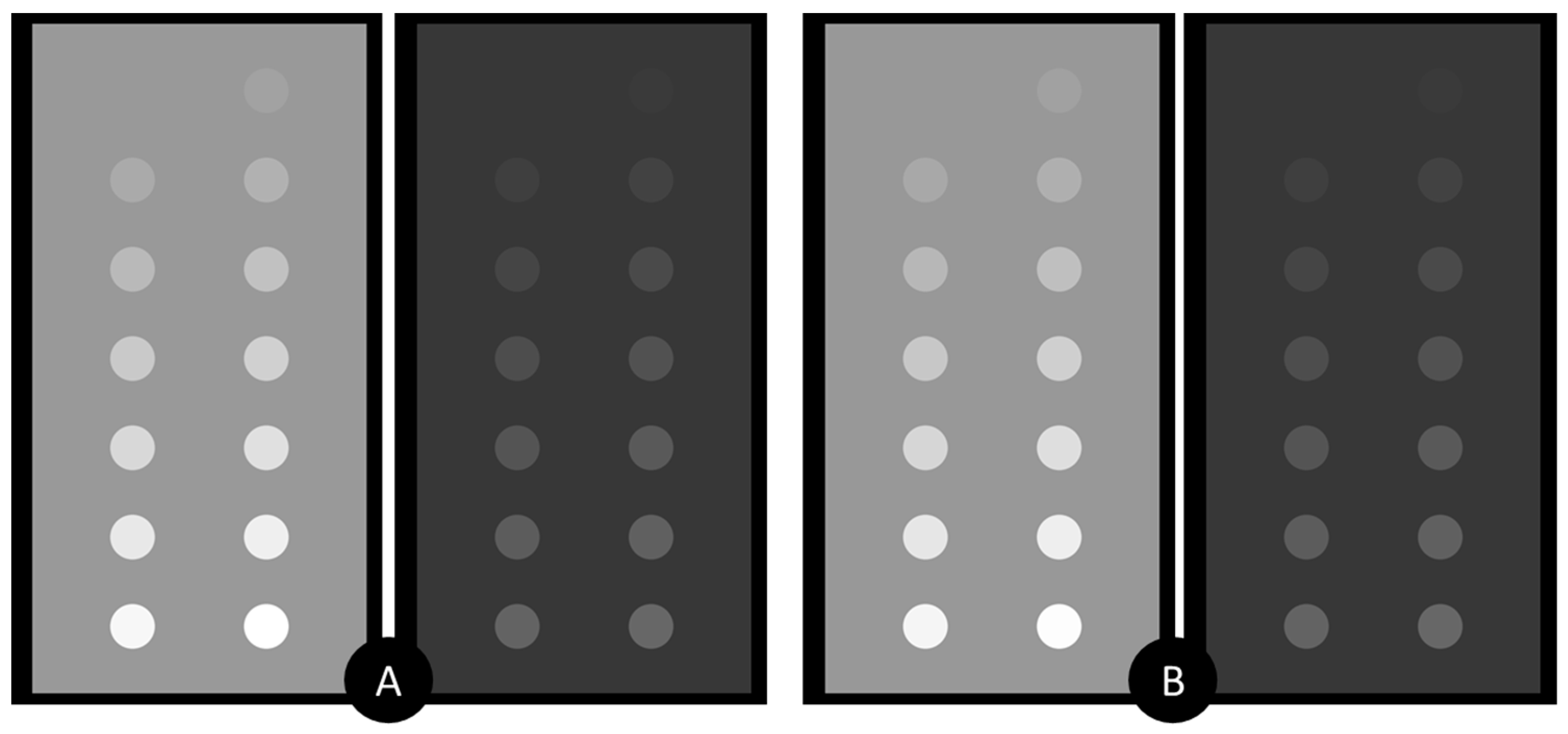
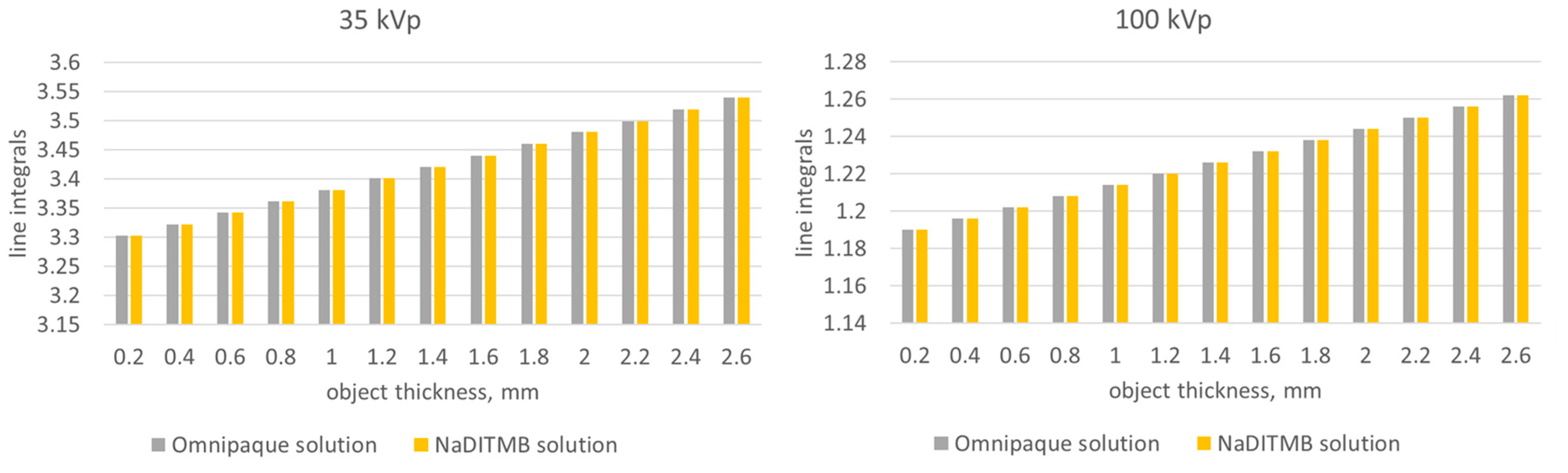
3.1. Simulation Results
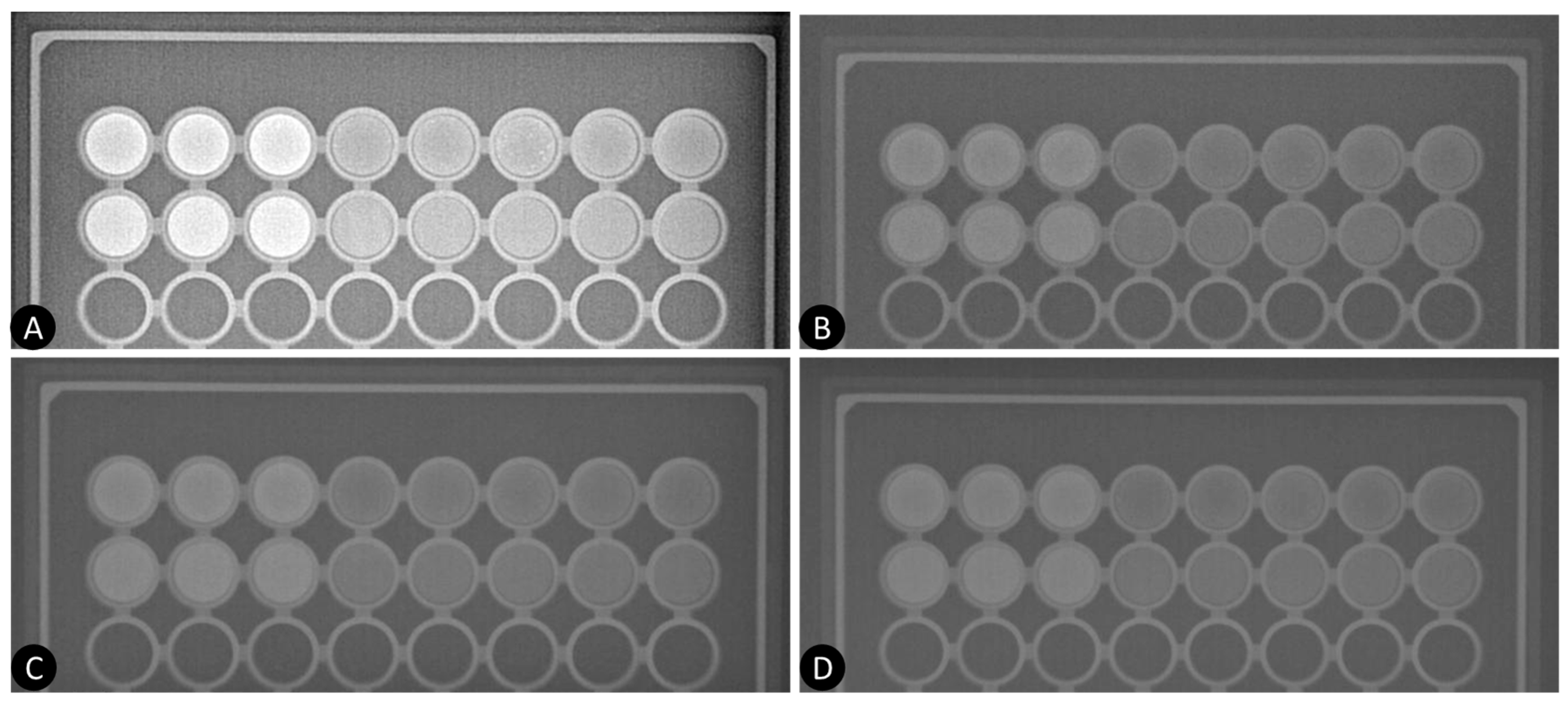
3.2. Experimental Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Lobbes, M.B.; Lalji, U.; Houwers, J.; Nijssen, E.C.; Nelemans, P.J.; van Roozendaal, L.; Smidt, M.L.; Heuts, E.; Wildberger, J.E. Contrast-enhanced spectral mammography in patients referred from the breast cancer screening programme. Eur. Radiol. 2014, 24, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Coffey, K.; Jochelson, M.S. Contrast-enhanced mammography in breast cancer screening. Eur. J. Radiol. 2022, 156, 110513. [Google Scholar] [CrossRef] [PubMed]
- Jochelson, M.S.; Lobbes, M.B.I. Contrast-enhanced Mammography: State of the Art. Radiology 2021, 299, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Dromain, C.; Thibault, F.; Muller, S.; Rimareix, F.; Delaloge, S.; Tardivon, A.; Balleyguier, C. Dual-energy contrast-enhanced digital mammography: Initial clinical results. Eur. Radiol. 2011, 21, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Travieso-Aja, M.D.M.; Maldonado-Saluzzi, D.; Naranjo-Santana, P.; Fernández-Ruiz, C.; Severino-Rondón, W.; Rodríguez, M.R.; Benítez, V.V.; Pérez-Luzardo, O. Diagnostic performance of contrast-enhanced dual-energy spectral mammography (CESM): A retrospective study involving 644 breast lesions. Radiol. Med. 2019, 124, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Tagliafico, A.S.; Bignotti, B.; Rossi, F.; Signori, A.; Sormani, M.P.; Valdora, F.; Calabrese, M.; Houssami, N. Diagnostic performance of contrast-enhanced spectral mammography: Systematic review and meta-analysis. Breast 2016, 28, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Luczynska, E.; Heinze, S.; Adamczyk, A.; Rys, J.; Mitus, J.W.; Hendrick, E. Comparison of the Mammography, Contrast-Enhanced Spectral Mammography and Ultrasonography in a Group of 116 patients. Anticancer Res. 2016, 36, 4359–4366. [Google Scholar] [PubMed]
- Zhu, X.; Huang, J.-M.; Zhang, K.; Xia, L.-J.; Feng, L.; Yang, P.; Zhang, M.-Y.; Xiao, W.; Lin, H.-X.; Yu, Y.-H. Diagnostic Value of Contrast-Enhanced Spectral Mammography for Screening Breast Cancer: Systematic Review and Meta-analysis. Clin. Breast Cancer 2018, 18, e985–e995. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, C.; Ghammraoui, B.; Taguchi, K.; Glick, S.J. Theoretical comparison and optimization of cadmium telluride and gallium arsenide photon-counting detectors for contrast-enhanced spectral mammography. J. Med. Imaging 2023, 10, S22406. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, P.; Bravin, A.; Di Maggio, C.; Gennaro, G.; Sarnelli, A.; Taibi, A.; Gambaccini, M. Evaluation of the minimum iodine concentration for contrast-enhanced subtraction mammography. Phys. Med. Biol. 2006, 51, 4233–4251. [Google Scholar] [CrossRef] [PubMed]
- Calandrino, R.; Loria, A.; Panizza, P.; Taibi, A.; del Vecchio, A. State of art and optimization perspectives for breast imaging. Phys. Open 2021, 7, 100071. [Google Scholar] [CrossRef]
- Bliznakova, K. The advent of anthropomorphic three-dimensional breast phantoms for X-ray imaging. Phys. Med. 2020, 79, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Rossman, A.H.; Catenacci, M.; Zhao, C.; Sikaria, D.; Knudsen, J.E.; Dawes, D.; Gehm, M.E.; Samei, E.; Wiley, B.J.; Lo, J.Y.-C. Three-dimensionally-printed anthropomorphic physical phantom for mammography and digital breast tomosynthesis with custom materials, lesions, and uniform quality control region. J. Med. Imaging 2019, 6, 021604. [Google Scholar] [CrossRef] [PubMed]
- Contillo, A.; Di Domenico, G.; Cardarelli, P.; Gambaccini, M.; Taibi, A. A novel approach to background subtraction in contrast-enhanced dual-energy digital mammography with commercially available mammography devices: Polychromaticity correction. Med. Phys. 2015, 42, 6641–6650. [Google Scholar] [CrossRef]
- Leithner, R.; Knogler, T.; Homolka, P. Development and production of a prototype iodine contrast phantom for CEDEM. Phys. Med. Biol. 2013, 58, N25–N35. [Google Scholar] [CrossRef] [PubMed]
- Carton, A.K.; Ullberg, C.; Maidment, A.D. Optimization of a dual-energy contrast-enhanced technique for a photon-counting digital breast tomosynthesis system: II. An experimental validation. Med. Phys. 2010, 37, 5908–5913. [Google Scholar] [CrossRef] [PubMed]
- Aminu, A.; Chen, W.; Yin, Z.; Kuniewicz, M.; Walocha, J.; Perde, F.; Molenaar, P.; Iaizzo, P.A.; Dobrzynski, H.; Atkinson, A.J. Novel micro-computed tomography contrast agents to visualise the human cardiac conduction system and surrounding structures in hearts from normal, aged, and obese individuals. Transl. Res. Anat. 2022, 27, 100175. [Google Scholar] [CrossRef]
- Zhu, J.; Weng, H.; Xie, S.; Cheng, J.; Zhu, J. A novel CT contrast agent for intestinal-targeted imaging through rectal administration. e-Polymers 2021, 21, 754–762. [Google Scholar] [CrossRef]
- Gomez, C.; Hallot, G.; Laurent, S.; Port, M. Medical Applications of Metallic Bismuth Nanoparticles. Pharmaceutics 2021, 13, 1793. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, R.; Capozza, M.; Carrera, C.; Terreno, E. Bi-HPDO3A as a novel contrast agent for X-ray computed tomography. Sci. Rep. 2023, 13, 16747. [Google Scholar] [CrossRef] [PubMed]
- Amato, C.; Klein, L.; Wehrse, E.; Rotkopf, L.T.; Sawall, S.; Maier, J.; Ziener, C.H.; Schlemmer, H.-P.; Kachelrieß, M. Potential of contrast agents based on high-Z elements for contrast-enhanced photon-counting computed tomography. Med. Phys. 2020, 47, 6179–6190. [Google Scholar] [CrossRef] [PubMed]
- Amato, C.; Susenburger, M.; Lehr, S.; Kuntz, J.; Gehrke, N.; Franke, D.; Thüring, T.; Briel, A.; Brönnimann, C.; Kachelrieß, M.; et al. Dual-contrast photon-counting micro-CT using iodine and a novel bismuth-based contrast agent. Phys. Med. Biol. 2023, 68, 135001. [Google Scholar] [CrossRef] [PubMed]
- Waller, J.; DeStefano, K.; Chiu, B.; Jang, I.; Cole, Y.; Agyemang, C.; Miao, T.; Shah, J.; Martin, C.; Umair, M. An update on nanoparticle usage in breast cancer imaging. Nano Sel. 2022, 3, 1103–1111. [Google Scholar] [CrossRef]
- King, B.F.; Hartman, G.W.; Williamson, B., Jr.; LeRoy, A.J.; Hattery, R.R. Low-Osmolality Contrast Media: A Current Perspective. Mayo Clin. Proc. 1989, 64, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Kolev, I.; Dimova, T.; Iliev, I.; Rogozherov, M.; Bodensteiner, M. Further findings concerning 2,6-diiodo-3,4,5-trimethoxybenzoic acid (Part II). J. Mol. Struct. 2023, 1294, 136388. [Google Scholar] [CrossRef]
- Kolev, I.N.; Hadzhieva, N.B.; Rogozherov, M.I. Spectral behavior peculiarities of the carboxyl functional group in iodine encirclement—The case of 2,6-diiodo-3,4,5-trimethoxybenzoic acid. J. Mol. Struct. 2020, 1226, 129303. [Google Scholar] [CrossRef]
- Salomon, E.; Homolka, P.; Semturs, F.; Figl, M.; Gruber, M.; Hummel, J. Comparison of a personalized breast dosimetry method with standard dosimetry protocols. Sci. Rep. 2019, 9, 5866. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.-L.; Chu, T.-C.; Lan, G.-Y.; Lin, Y.-C.; Yeh, Y.-H.; Chuang, K.-S. Development of an adjustable model breast for mammographic dosimetry assessment in Taiwanese women. Am. J. Roentgenol. 2011, 196, W476–W481. [Google Scholar] [CrossRef]
- Bliznakova, K.; Speller, R.; Horrocks, J.; Liaparinos, P.; Kolitsi, Z.; Pallikarakis, N. Experimental validation of a radiographic simulation code using breast phantom for X-ray imaging. Comput. Biol. Med. 2010, 40, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Lazos, D.; Bliznakova, K.; Kolitsi, Z.; Pallikarakis, N. An integrated research tool for X-ray imaging simulation. Comput. Methods Programs Biomed. 2002, 70, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Bliznakova, K.; Suryanarayanan, S.; Karellas, A.; Pallikarakis, N. Evaluation of an improved algorithm for producing realistic 3D breast software phantoms: Application for mammography. Med. Phys. 2010, 37, 5604–5617. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.H. Photon mass attenuation and mass energy-absorption coefficients for H, C, N, O, Ar, and seven mixtures from 0.1 keV to 20 MeV. Radiat. Res. 1977, 70, 58–81. [Google Scholar] [CrossRef] [PubMed]
- Ketelhut, S.; Büermann, L.; Hilgers, G. Catalog of x-ray spectra of Mo-, Rh-, and W-anode-based x-ray tubes from 10 to 50 kV. Phys. Med. Biol. 2021, 66, 115013. [Google Scholar] [CrossRef] [PubMed]
- Campillo-Rivera, G.E.; Torres-Cortes, C.O.; Vazquez-Bañuelos, J.; Garcia-Reyna, M.G.; Marquez-Mata, C.A.; Vasquez-Arteaga, M.; Vega-Carrillo, H.R. X-ray spectra and gamma factors from 70 to 120 kV X-ray tube voltages. Radiat. Phys. Chem. 2021, 184, 109437. [Google Scholar] [CrossRef]
- Boone, J.M.; Seibert, J.A. An accurate method for computer-generating tungsten anode x-ray spectra from 30 to 140 kV. Med. Phys. 1997, 24, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Conde, E.; Cadahía, E.; Garcia-Vallejo, M.C. HPLC analysis of flavonoids and phenolic acids and aldehydes in Eucalyptus spp. Chromatographia 1995, 41, 657–660. [Google Scholar] [CrossRef]
- Carton, A.K.; Ullberg, C.; Lindman, K.; Acciavatti, R.; Francke, T.; Maidment, A.D.A. Optimization of a dual-energy contrast-enhanced technique for a photon-counting digital breast tomosynthesis system: I. A theoretical model. Med. Phys. 2010, 37, 5896–5907. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.L.; Mainprize, J.G.; Puong, S.; Carton, A.-K.; Iordache, R.; Muller, S.; Yaffe, M.J. Impact of image acquisition timing on image quality for dual energy contrast-enhanced breast tomosynthesis. In Proceedings of the Progress in Biomedical Optics and Imaging—SPIE, San Diego, CA, USA, 5–8 February 2012. [Google Scholar]




| λ1 | λ2 | λ3 | λ4 | λ5 | |
|---|---|---|---|---|---|
| [10:31.17) keV | |||||
| Omnipaque | 33.7567 | 4.5735 | −117.5345 | −180.0997 | 0.9613 |
| NaDITMB | 32.8947 | 4.5145 | −114.4335 | −175.7637 | 1.0488 |
| [31.17:150) keV | |||||
| Omnipaque | 0.5985 | 1.9259 | 14.0620 | 11.3637 | 0.1039 |
| NaDITMB | 0.5286 | 1.9310 | 13.8811 | 11.1156 | 0.1016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bliznakova, K.; Kolev, I.; Dukov, N.; Dimova, T.; Bliznakov, Z. Exploring the Potential of a Novel Iodine-Based Material as an Alternative Contrast Agent in X-ray Imaging Studies. Materials 2024, 17, 2059. https://doi.org/10.3390/ma17092059
Bliznakova K, Kolev I, Dukov N, Dimova T, Bliznakov Z. Exploring the Potential of a Novel Iodine-Based Material as an Alternative Contrast Agent in X-ray Imaging Studies. Materials. 2024; 17(9):2059. https://doi.org/10.3390/ma17092059
Chicago/Turabian StyleBliznakova, Kristina, Iliyan Kolev, Nikolay Dukov, Tanya Dimova, and Zhivko Bliznakov. 2024. "Exploring the Potential of a Novel Iodine-Based Material as an Alternative Contrast Agent in X-ray Imaging Studies" Materials 17, no. 9: 2059. https://doi.org/10.3390/ma17092059







